Abstract
Lipoxins A4 (LxA4) and B4 (LxB4), two lipoxygenase-generated icosanoids of arachidonic acid metabolism, were found to have a distinct biological profile. Both LxA4 and LxB4 slowly contracted pulmonary parenchymal strips isolated from guinea pigs, rabbits, and rats in a concentration-dependent manner over the range 0.1-1 microM. This bronchoconstrictor effect was not associated with release of peptide leukotrienes or thromboxane A2, nor was it blocked by lipoxygenase inhibitors or thromboxane receptor antagonists, suggesting it is a direct effect of lipoxins. However, the leukotriene D4 (LTD4) receptor antagonist LY-171883 reduced the LxA4 response, indicating that LTD4 and LxA4 may share the same receptor. LxA4 and LxB4 also exerted an endothelium-dependent vasorelaxation in guinea pig, rat, and, to a lesser extent, rabbit aortic vascular smooth muscle. In contrast to other vasoactive icosanoids, LxA4 and LxB4 failed to aggregate rat, rabbit, or guinea pig platelets or to inhibit ADP-induced aggregation. LxA4 also enhanced the release of liver lysosomal hydrolases in a liver large granule fraction, indicating a lysosomal labilizing action of LxA4. LxA4 and LxB4 share a similar biological profile. It is not clear yet whether the lipoxins could be mediators of circulatory or pulmonary disease states.
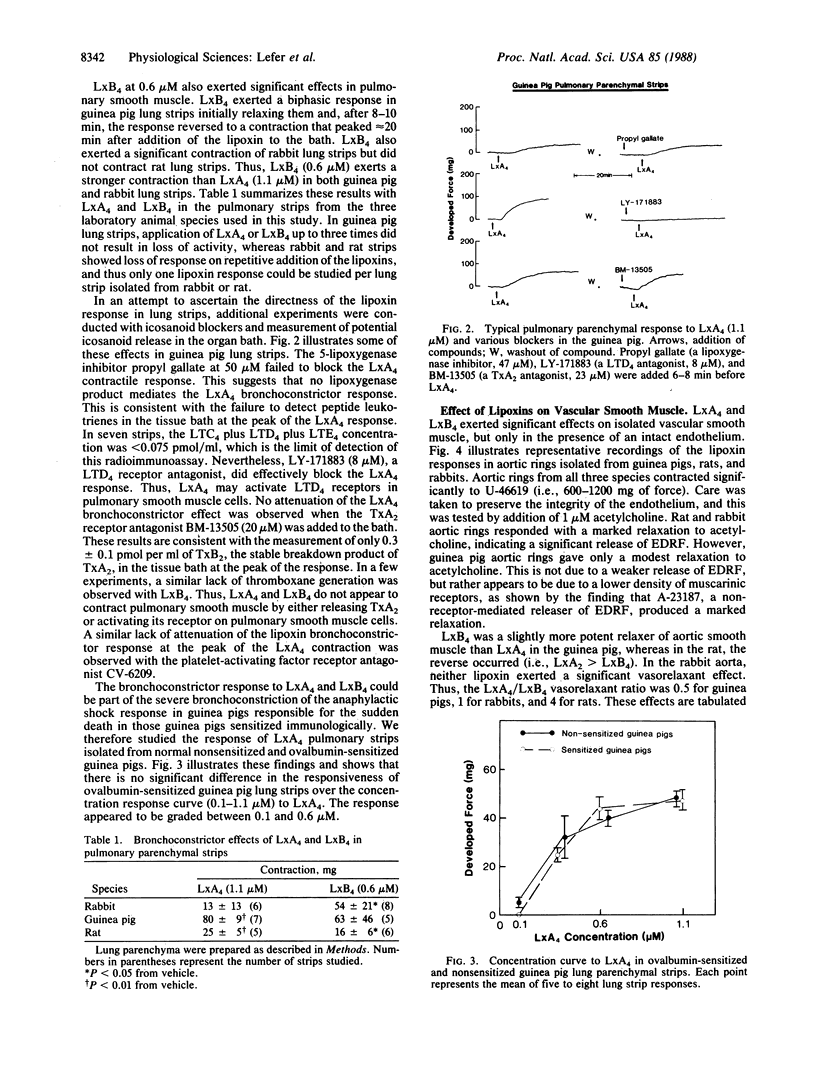
Full text
PDF

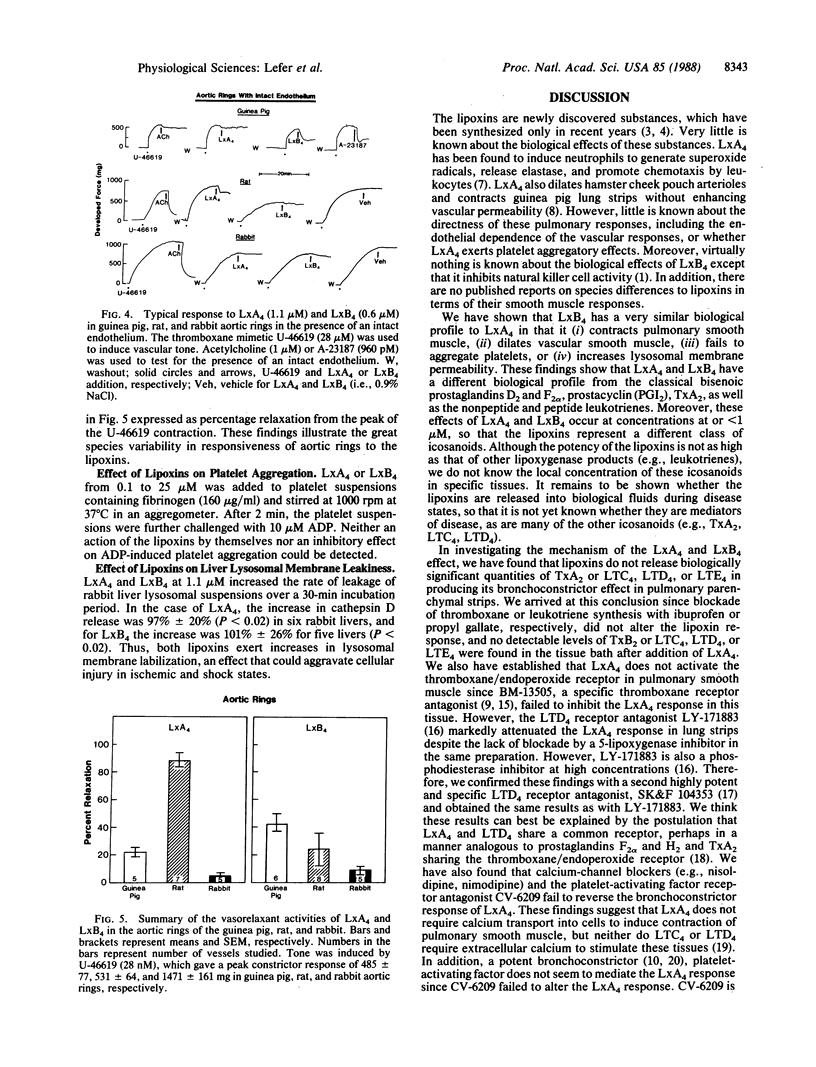

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharony D., Dobson P., Bernstein P. R., Kusner E. J., Krell R. D., Smith J. B. Determination of SRS-A release from guinea-pig lungs by a radioimmunoassay. Biochem Biophys Res Commun. 1983 Dec 16;117(2):574–579. doi: 10.1016/0006-291x(83)91239-1. [DOI] [PubMed] [Google Scholar]
- Badr K. F., Serhan C. N., Nicolaou K. C., Samuelsson B. The action of lipoxin-A on glomerular microcirculatory dynamics in the rat. Biochem Biophys Res Commun. 1987 May 29;145(1):408–414. doi: 10.1016/0006-291x(87)91337-4. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Bridenbaugh G. A., Flynn J. T., Lefer A. M. Arachidonic acid in splanchnic artery occlusion shock. Am J Physiol. 1976 Jul;231(1):112–119. doi: 10.1152/ajplegacy.1976.231.1.112. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Raud J., Serhan C. N., Björk J., Samuelsson B. Biological activities of lipoxin A include lung strip contraction and dilation of arterioles in vivo. Acta Physiol Scand. 1987 Aug;130(4):643–647. doi: 10.1111/j.1748-1716.1987.tb08187.x. [DOI] [PubMed] [Google Scholar]
- Darius H., Lefer D. J., Smith J. B., Lefer A. M. Role of platelet-activating factor-acether in mediating guinea pig anaphylaxis. Science. 1986 Apr 4;232(4746):58–60. doi: 10.1126/science.3082008. [DOI] [PubMed] [Google Scholar]
- Fleisch J. H., Rinkema L. E., Haisch K. D., Swanson-Bean D., Goodson T., Ho P. P., Marshall W. S. LY171883, 1-less than 2-hydroxy-3-propyl-4-less than 4-(1H-tetrazol-5-yl) butoxy greater than phenyl greater than ethanone, an orally active leukotriene D4 antagonist. J Pharmacol Exp Ther. 1985 Apr;233(1):148–157. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Halushka P. V., Mais D. E., Saussy D. L., Jr Platelet and vascular smooth muscle thromboxane A2/prostaglandin H2 receptors. Fed Proc. 1987 Jan;46(1):149–153. [PubMed] [Google Scholar]
- Hansson A., Serhan C. N., Haeggström J., Ingelman-Sundberg M., Samuelsson B. Activation of protein kinase C by lipoxin A and other eicosanoids. Intracellular action of oxygenation products of arachidonic acid. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1215–1222. doi: 10.1016/0006-291x(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Muccitelli R. M., Tucker S. S., Vickery-Clark L. M., Wilson K. A., Gleason J. G., Hall R. F., Wasserman M. A., Torphy T. J. Pharmacologic profile of SK&F 104353: a novel, potent and selective peptidoleukotriene receptor antagonist in guinea pig and human airways. J Pharmacol Exp Ther. 1987 Nov;243(2):474–481. [PubMed] [Google Scholar]
- Ingerman-Wojenski C., Silver M. J., Smith J. B., Macarak E. Bovine endothelial cells in culture produce thromboxane as well as prostacyclin. J Clin Invest. 1981 May;67(5):1292–1296. doi: 10.1172/JCI110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer A. M. Eicosanoids as mediators of ischemia and shock. Fed Proc. 1985 Feb;44(2):275–280. [PubMed] [Google Scholar]
- Lefer A. M., Leprán I., Roth D. M., Smith J. B. Specificity of anti-leukotriene actions of nicardipine. Pharmacol Res Commun. 1984 Dec;16(12):1141–1150. doi: 10.1016/s0031-6989(84)80079-x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Hamberg M., Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Hamberg M., Samuelsson B., Morris J., Wishka D. G. On the stereochemistry and biosynthesis of lipoxin B. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1983–1987. doi: 10.1073/pnas.83.7.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashita Z., Imura Y., Takatani M., Tsushima S., Nishikawa K. CV-6209, a highly potent antagonist of platelet activating factor in vitro and in vivo. J Pharmacol Exp Ther. 1987 Jul;242(1):263–268. [PubMed] [Google Scholar]
- Yanagisawa A., Smith J. A., Brezinski M. E., Lefer A. M. Mechanism of antagonism of thromboxane receptors in vascular smooth muscle. Eur J Pharmacol. 1987 Jan 6;133(1):89–96. doi: 10.1016/0014-2999(87)90209-3. [DOI] [PubMed] [Google Scholar]



