Diminished tissue sensitivity to the metabolic actions of insulin is a characteristic feature of various pathological conditions termed the “cardiometabolic syndrome.”1–3 Factors that contribute to the complex interaction of genetic and environmental factors required for impaired insulin signaling include obesity, inactivity, and aging. Recent research has underscored the importance of heightened activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, oxidative stress, inflammation, and mitochondrial functional abnormalities in promoting insulin resistance.1–3 In addition to the negative effects of these factors on insulin metabolic signaling in conventionally insulin-sensitive tissue, such as skeletal muscle, there is a contemporaneous negative effect on metabolic signaling in cardiovascular tissue. These negative effects include reduced insulin stimulation of endothelial cell NO production and increased NO destruction with resulting endothelial dysfunction and hypertension.1,2 Indeed, both pharmacological and nonpharmacological strategies to improve insulin metabolic signaling generally also improve endothelial function and lower blood pressure. In this regard, the current report from Ruggenenti et al4 is an important translational contribution that provides a potential mechanism by which oral provision of a critical mitochondrial substrate, L-carnitine, can correct insulin resistance and lower blood pressure through improvements in mitochondrial free fatty acid use.
This investigative team prospectively studied 2 cohorts of insulin-resistant subjects with the presence of other cardiometabolic risk factors, such as body mass index >25 kg/m2 and hypertension. Eligible subjects underwent a euglycemic, hyperinsulinemic clamp to determine glucose disposal rate (GDR) and were divided into those with and without a low GDR (≤7.9 or >7.9 mg/kg per minute). The 2 groups underwent an oral glucose tolerance test and measurement of blood pressure, as well as other metabolic markers. Subjects were then given 24 weeks of acetyl-L-carnitine treatment, followed by a 16-week recovery period off of treatment, and metabolic and blood pressure measurements were repeated after acetyl-L-carnitine treatment and recovery.
Their work was based on the premise that the product of metabolism of acetyl-L-carnitine, L-carnitine, improves glucose use through improved intracellular free fatty acid and lipid metabolism and facilitates glycolysis in skeletal muscle tissue.5–7 L-Carnitine serves as an obligatory cofactor for mitochondrial β-fatty acid oxidation to facilitate transport of long-chain fatty acids across the mitochondrial membrane, thus resulting in more efficient mitochondrial oxidative phosphorylation and glucose use. This improved mitochondrial function, in turn, would also improve both endothelial cell and skeletal muscle insulin-stimulated NO bioavailability and glucose use and ultimately improve endothelial function and systemic insulin sensitivity (Figure).
Figure.
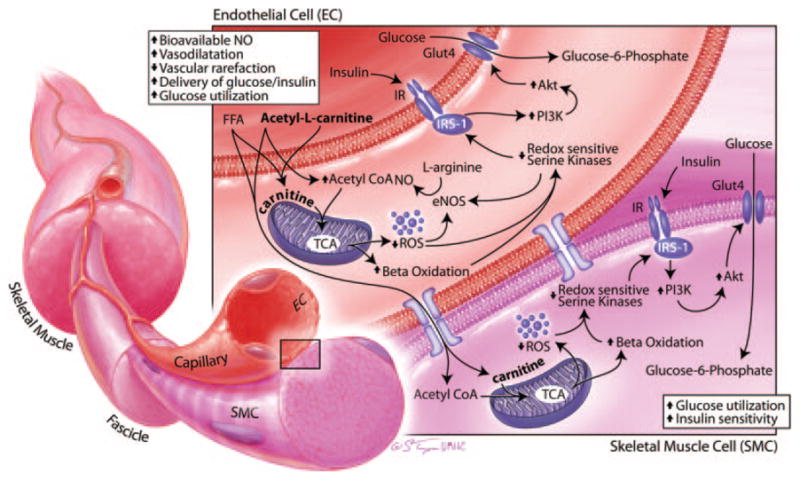
Potential actions of acetyl-L-carnitine on endothelial cell (EC) and skeletal muscle cell (SMC) insulin metabolic signaling. The product of metabolism of acetyl-L-carnitine, L-carnitine, serves as an obligatory cofactor for mitochondrial β-fatty acid oxidation to facilitate the transport of long-chain fatty acids across the mitochondrial membrane, thus resulting in more efficient mitochondrial oxidative phosphorylation and glucose use. This improves mitochondrial function, also improves both EC and SMC insulin-stimulated NO bioavailability and glucose use, and ultimately improves endothelial function and systemic insulin sensitivity. Akt indicates protein kinase B; eNOS, endothelial NO synthase; FFA, free fatty acids; GLUT4, glucose transporter 4; IR, insulin receptor; IRS-1, insulin resistance substrate 1; PI3-K, phosphoinositol 3-kinase; ROS, reactive oxygen species; TCA, tricarboxylic acid.
The authors report that the subjects with a lower GDR (≤7.9 mg/kg per minute) displayed characteristics of the cardiometabolic syndrome and consistently had higher systolic blood pressures when compared to those with a higher GDR (>7.9 mg/kg per minute). Treatment with acetyl-L-carnitine over 24 weeks resulted in improvement in GDR (insulin sensitivity) only in those with low GDR but lowered systolic blood pressure in both study groups. These effects in these overweight/obese subjects occurred independent of changes in diet and body weight. Interestingly, the authors observed that, in the recovery period off of therapy, systolic blood pressure in both groups returned to baseline, suggesting that acetyl-L-carnitine may have an effect on BP reduction partly independent of its actions on insulin sensitivity.
Endothelial dysfunction is commonly present in concert with insulin resistance. Several vascular metabolic abnormalities have been documented in obese, insulin-resistant subjects. These abnormalities include impaired insulin-stimulated glucose uptake, similar to what is seen in traditional insulin-sensitive tissues (eg, skeletal muscle, fat, and liver). Typically, insulin-dependent glucose use is partly dependent on insulin-mediated increases in blood flow and substrate delivery to tissues. In insulin resistance, there is decreased insulin stimulation of NO bioactivity (decreased endothelial NO synthase activation and increased NO destruction), diminished vasodilatation, and substrate delivery.8 Increased generation of reactive oxygen species plays an important role in this decrease in insulin metabolic signaling, in part by increasing redox-sensitive serine kinase activation. These activated kinases decrease insulin receptor 1 levels and engagement with phosphoinositol 3-kinase, with resulting diminution of protein kinase B and atypical protein kinase activation of glucose transport (Figure).1,8 As a result, insulin-resistant individuals with obesity are more prone to endothelial dysfunction and subsequent development of hypertension.
In the context of the current report in the journal, oral therapy with acetyl-L-carnitine improved insulin resistance in persons with low GDR and reduced systolic blood pressure in the entire study population with obesity.4 Carnitine, derived from orally administered acetyl-L-carnitine, acts as a substrate to facilitate free fatty acid and lipid metabolism through improvements in mitochondrial trafficking of acetyl-esters to generate ATP in the tricarboxylic acid/Krebs cycle (Figure).5,6 Importantly, the insulin-resistant state is associated with mitochondrial dysfunction and accumulation of fatty acid metabolites, diacylglycerol, and long-chain fatty acyl-coenzyme A that are associated with a reduction in β-fatty acid oxidation and oxidative stress. However, the precise mechanism by which L-carnitine improved endothelium function and reduced systolic blood pressure is less well known, because the role for mitochondrial oxidative phosphorylation in mediating endothelial function is poorly understood.1
It is conceivable that carnitine improves endothelial cell function by facilitating mitochondrial oxidative phosphorylation, reducing mitochondrial and NADPH oxidase generation of reactive oxygen species, and thereby increasing bioavailable NO. The reductions in reactive oxygen species generation also mediate improvements in redox-sensitive serine kinase activation and associated reductions in insulin metabolic signaling (Figure). Presumably, this improvement in insulin metabolic signaling in endothelial cells would lead to vasodilatation, improved delivery of glucose and insulin and glucose use, and reduced vascular rarefaction in skeletal muscle tissue.1
In summary, the report by Ruggenenti et al4 in the present issue of Hypertension provides a first report that oral administration of acetyl-L-carnitine over 24 weeks improved insulin-stimulated glucose use and lowered systolic blood pressure in persons with insulin resistance and obesity (ie, the cardiometabolic syndrome). The results of this pilot study suggest the potential to use oral acetyl-L-carnitine to prevent the development of diabetes mellitus and as adjunctive therapy for the treatment of hypertension in patients with the cardiometabolic syndrome. However, these preliminary results need to be confirmed and supported by prospective, randomized, placebo-controlled, multicentered clinical trials in a more heterogeneous population that includes persons of African, Asian, and Hispanic heritage. Nevertheless, the current study presents a new paradigm of targeting mitochondrial dysfunction as it relates to endothelial dysfunction and hypertension in the setting of insulin resistance and obesity.
Acknowledgments
We thank Stacy Turpin for her illustration contribution.
Sources of Funding
This work was supported by NIH RO1-HL073101-02 and VA Merit 0018.
Footnotes
The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Sowers JR, Whaley-Connell, Epstein M. The emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggenenti P, Cattaneo D, Loriga G, Ledda F, Motterlini N, Gherardi G, Orisio S, Remuzzi G. Ameliorating hypertension and insulin resistance in subjects at increased cardiovascular risk: effects of acetyl-L-carnitine therapy. Hypertension. 2009;54:567–574. doi: 10.1161/HYPERTENSIONAHA.109.132522. [DOI] [PubMed] [Google Scholar]
- 5.Mingrone G. Carnitine in type 2 diabetes. Ann N Y Acad Sci. 2004;1033:99–107. doi: 10.1196/annals.1320.009. [DOI] [PubMed] [Google Scholar]
- 6.Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- 7.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007;581:431–444. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–H2023. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]


