Abstract
Background
The Model for End-Stage Liver Disease (MELD) predicts events in cirrhotic subjects undergoing major surgery and may offer similar prognostication in LVAD candidates with comparable degrees of multisystem dysfunction.
Methods and Results
Preoperative MELD scores were calculated for subjects enrolled into the University of Michigan Health System (UMHS) mechanical circulatory support database. Univariate and multiple regression analyses were performed to investigate the ability of patient characteristics, laboratory data (including MELD scores), and hemodynamic measurements to predict total perioperative blood product exposure (TBPE) and operative mortality. The ability of preoperative MELD scores to predict operative mortality was evaluated in subjects enrolled into the Interagency Registry of Mechanically-Assisted Circulatory Support (INTERMACS), and results were compared to the UMHS cohort.
The mean±standard deviation MELD scores for the UMHS (n=211) and INTERMACS (n=324) cohorts were 13.7±6.1 and 15.2±5.8, respectively, with 29 (14%) and 19 (6%) perioperative deaths. In the UMHS cohort, median [25th,75th] TBPE was 74 [44,120] units. Each 5-unit MELD increase was associated with 15 [7.6,23] units of TBPE. Each 10-unit increase in TBPE increased the odds of operative death; OR [95%CI] =1.05 [1.01,1.10].
Odds ratios, measuring the ability of MELD scores to predict perioperative mortality, were 1.5 [1.1,2.0] and 1.5 [1.1,2.1] per 5 MELD-units for the UMHS and INTERMACS cohorts, respectively. When MELD scores were dichotomized as ≥17 and <17, risk-adjusted Cox proportional hazard ratios for 6-month mortality were 2.5 [1.2,5.3] and 2.5 [1.1,5.4] for the UMHS and INTERMACS cohorts, respectively.
Conclusions
The MELD score identified LVAD candidates at high risk for perioperative bleeding and mortality.
Keywords: hemorrhage, heart-assist device, heart failure, mortality
Introduction
Bleeding during implantation of mechanical circulatory support (MCS) is the most common perioperative complication, with cumulative incidences of 29-53% in devices placed for the bridge to transplant indication1,2. Causes of bleeding include both intrinsic (biological) and surgery-related factors. Intrinsic bleeding diatheses can result from preoperative renal insufficiency leading to uremic platelet dysfunction, congestive hepatopathy secondary to right ventricular (RV) dysfunction, and hepatic ischemia from poor cardiac output. Surgery-induced bleeding diatheses may be secondary to anticoagulation and hemodilution of clotting factors during cardiopulmonary bypass, low-grade postoperative disseminated intravascular coagulation in the setting of multisystem organ failure, as well as device-induced coagulopathies1,2.
In addition to the obvious need for increased blood product transfusions, bleeding significantly increases left ventricular assist device (LVAD) operative morbidity. Bleeding subjects up to 60% of LVAD recipients to the associated risks of reoperation1. Massive blood transfusions can trigger cytokine storms that may provoke respiratory insufficiency and reactive pulmonary vascular hypertension with resultant RV failure1. Blood transfusions are also associated with increased risk for nosocomial infections3, blood-borne diseases, and allosensitization4-6. However, accurately assessing perioperative bleeding risk in LVAD candidates is difficult and scant literature exists on the topic.
The Model for End-stage Liver Disease (MELD) was originally developed to assess prognosis in subjects with cirrhosis undergoing transjugular, intrahepatic portosystemic shunts (TIPS)7. The UNOS-modified MELD score is a weighted sum of serum creatinine, bilirubin, and the international normalized ratio (INR) with a minimum score set at 6 and no set maximum8. The clinical utility of the MELD score has been extended to prioritization of liver transplants8,9 and to predicting operative morbidity and mortality in cirrhotics undergoing major surgeries10-13.
While the MELD was originally derived for a different patient population, the variables comprising the score are markers of multisystem dysfunction and coagulopathy. To date, no study has evaluated the ability of the MELD to predict outcomes in LVAD candidates who often have similar degrees of end-organ dysfunction. The aims of this study are to 1) better assess the morbidities and mortality associated with perioperative LVAD transfusion requirements; 2) assess the utility of preoperative MELD scores in predicting perioperative transfusions; and 3) assess the ability of the MELD to predict operative morbidity and mortality in LVAD recipients.
Methods
The UMHS-MCS database, containing 211 initial LVAD implants (1996-2007), was analyzed. Demographic, clinical, preoperative pharmacologic and laboratory data were recorded prospectively while perioperative transfusions were tallied retrospectively from the UMHS blood-bank database. Perioperative transfusions were defined as products administered intraoperatively, ≤24 hours following LVAD implant, or within 24 hours of reoperation for sternal closure or LVAD-associated bleeding. Transfusions were at the discretion of the treating physician. General indications for transfusions included signs or symptoms of active bleeding, severe anemia (hemoglobin ≤8.0 mg/dL), thrombocytopenia (platelet count <100,000 K/mm3 intraoperatively or <50,000 K/mm3 ≤48 hours after surgery), or profuse coagulopathy (INR >1.8 or PTT >45 seconds without anticoagulant therapy).
Institution of preoperative extracorporeal RV- or LV-MCS was clinically driven or was in place at the time of arrival to UMHS. Typically, extracorporeal MCS is implemented in those with advanced multisystem organ failure who require rapid circulatory support or those with RV or LV failure refractory to inotrope or balloon-pump support. Our strategy is to transition subjects to intracorporeal support after subsequent hemodynamic and end-organ stabilization, taking into account limitations placed on extracorporeal support duration due to infectious and thromboembolic risks.
In the operating room, subjects received intravenous vitamin K 1mg/hour at the commencement of surgery, bolus-dose heparin (goal ACTs >480 sec) and aprotinin upon initiation of cardiopulmonary bypass, and protamine at bypass termination. Recombinant Factor VIIa and aminocaproic acid were only administered for significant intraoperative bleeding.
MELD Score Calculation
Laboratories were obtained <24 hours prior to LVAD implant and UNOS-modified MELD scores were calculated according to the formula8:
Per the UNOS modification, variable lower limits were set at 1.0 and the creatinine upper limit was set at 4.0 mg/dL. Subjects receiving preoperative renal replacement therapy were assigned a creatinine of 4.0 mg/dL. For example, the MELD score for an individual on preoperative dialysis with a creatinine=2.2 mg/dL, INR=1.2 seconds, and bilirubin =2.2 mg/dL is 25.
Study Aims
The primary aim of the study was to assess the ability of preoperative MELD scores to predict “total perioperative blood product exposures (TBPE),” defined as the sum of packed red blood cells (PRBCs), platelets, fresh frozen plasma (FFP), and cryoprecipitate units administered perioperatively. Secondary aims focused on the ability of the MELD to predict 1) operative death (defined as intraoperative death, death ≤30 days after LVAD implantation, or prior to hospital discharge), 2) 6-month survival (censoring for transplant or LVAD wean), and 3) postoperative morbidities. Morbidities included RV failure requiring RV-MCS (extracorporeal membrane oxygenation or RV assist device), renal failure requiring renal replacement therapy, intensive care unit (ICU) and total index hospital lengths of stay, device infection (≤3 months after implant), device malfunction (≤1 month after implant), and cerebrovascular events (transient ischemic attack, stroke, or new seizure ≤1 month after implant).
MELD Validation using INTERMACS
The MELD's ability to predict death (operative and 6 month) was validated using the INTERMACS registry (2006-2008), a national database containing prospectively collected information on implantations of FDA approved MCS intended for chronic use. No subject in the cohort of study had preoperative MCS in place. Complete data for MELD score calculation was available in 324 of 372 subjects. Because traditional operative death criteria, as defined above, are not tallied in INTERMACS, operative death for the purpose of this analysis was defined as death within 1 month of LVAD implant. Likewise, information on preoperative dialysis requirements was not available, so uncorrected creatinine measures were used for MELD calculation.
Statistical Analysis
Descriptive statistics for the primary dependent and independent variables of the UMHS and INTERMACS cohorts were calculated, along with other preoperative clinical, demographic, laboratory, and hemodynamic variables. Data analysis was performed using SAS (Cary, NC) v9.1.
Bleeding Analyses
Logistic regression was used in the UMHS cohort to investigate operative morbidity and mortality odds ratios associated with increasing transfusion requirements. Simple linear regression (PROC REG in SAS) was used to evaluate the ability of preoperative MELD scores and other covariates to predict perioperative transfusion requirements in the UMHS cohort, such that the regression coefficient represents the per-unit increase in blood product exposure per applicable unit increase in the covariate. Manual stepwise linear regression using PROC GLM in SAS was then employed to assess for independent predictors of TBPE. Entry criteria for the covariates (including MELD score) identified on univariable analysis were set at p ≤0.15. Covariates clinically relevant to the bleeding analysis (body surface area, age, prior sternotomy, preoperative right atrial pressure and pulmonary vascular resistance, preoperative mechanical circulatory support, and intraoperative heparin dose and cardiopulmonary bypass time)1 were forced into modeling. During model development, the likelihood ratio test and Akaike Information Criterion (for non-nested data) were used to optimize fit and the p-value criteria for covariate model exit was set at <0.05.
Morbidity and Mortality Analyses
Baseline characteristics, preoperative laboratories, and cardiopulmonary hemodynamic data were compared between operative deaths and survivors in the UMHS cohort using odds ratios calculated by logistic regression. In both the UMHS and INTERMACS cohorts, logistic regression was used to calculate unadjusted morbidity and mortality odds ratios associated with increasing MELD scores. Subjects in both cohorts were then categorized into either “high MELD” or “low MELD” groups. Dichotomization was determined a priori and was based on the UMHS 75th MELD percentile. Kaplan-Meier survival curves were then generated for the strata and risk-adjusted Cox mortality hazard ratios (95% confidence interval) were calculated. Proportional hazards assumptions were confirmed with visual inspection of log-minus-log plots and by assessing for time-dependent interactions with the MELD strata.
Using PROC REG in SAS, MELD scores in the UMHS cohort were then regressed on TBPE, yielding residuals which represented the unique component of MELD scores that was unrelated to subject TBPE. These residuals, along with the clinically relevant covariates age, preoperative vasopressor requirement, and sex,14 were then entered manually into stepwise logistic regression to examine for operative mortality associations (covariate exit criteria = p < 0.05).
Unless otherwise specified, data are expressed as mean±standard error of the mean (SEM) or median [25th, 75th percentiles] for normal and non-normal data, respectively. For all analyses, a p≤0.05 was considered statistically significant.
This study was approved by the UMHS Institutional Review Board. Written informed consent was obtained prior to subject participation. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Implanted devices (n=211) at UMHS included the first generation HeartMate (IP1000, VE, XVE; n=163, 77%) and HeartMate II (n=29, 14%, Thoratec Corp., Pleasanton, CA), Micromed-Debakey (n=2, 1%, MicroMed Cardiovascular Inc, Houston, TX) and Novacor LVADs (n=4, 2%, World Heart Inc., Oakland, CA), and Thoratec pVADs and IVADs (n=13, 6%, Thoratec Corp. Pleasanton, CA).
Perioperative Transfusion Requirements and Perioperative Morbidity and Mortality
Median perioperative transfusion requirements for the UMHS cohort were 13[8,22] PRBCs units, 14[9,20] FFP units, 30[20,50] platelets units, and 14[6,26] cryoprecipitate units, yielding a median 74[44,120] units of TBPE. In the UMHS cohort, there were 182 survivors and 29 (14%) perioperative deaths. The odds of death increased 30% per 5-unit transfusion of PRBCs (unadjusted OR=1.3[1.1,1.5]) and 5% (unadjusted OR=1.05[1.01,1.10]) per 10-units of TBPE (table I). Mortality was not significantly increased with escalating transfusions of other individual blood products.
Table I.
Transfusion requirements (median [25th, 75th]) by perioperative outcome and univariable odds ratios [95% CI] for death and postoperative morbidity based on transfusion requirements in the UMHS cohort.
| Transfusion units | Odds Ratio*† | Odds Ratio*† | Odds Ratio*† | Odds Ratio*† | ||
|---|---|---|---|---|---|---|
| Alive (n=182) |
Dead (n=29) |
Death (n=29) |
Renal Failure (n=40) |
RV-MCS (n=34) |
Neurologic Event (n=42) |
|
| PRBC | 13 [7,20] | 22 [13,40] | 1.3[1.1,1.5]‡ | 1.3[1.1,1.4]‡ | 1.3[1.2,1.5]‡ | 1.2[1.1,1.3]‡ |
| FFP | 13 [9,20] | 15 [10,24] | 1.2[0.96,1.4] | 1.3[1.1,1.5]‡ | 1.3[1.1,1.5]‡ | 1.2[1.1,1.4]‡ |
| Platelets | 30 [16,50] | 35 [20,80] | 1.1[1.0,1.1] | 1.08[1.04,1.12]‡ | 1.08[1.03,1.11]‡ | 1.05[1.01,1.08]‡ |
| Cryo | 14 [6,26] | 16 [8,27] | 1.0[0.93,1.1] | 1.0[0.97,1.1] | 1.06[0.98,1.13] | 1.0[0.95,1.1] |
| TBPE | 73 [40,115] | 86 [60,172] | 1.05[1.01,1.10]‡ | 1.08[1.04,1.13]‡ | 1.08[1.04,1.14]‡ | 1.05[1.01,1.10]‡ |
Odds ratios generated by logistic regression.
odds ratio per 5-unit transfusion of individual blood product.
odds ratio per 10-unit TBPE.
p≤0.050. Cryo= cryoprecipitate, FFP= fresh frozen plasma, PRBC= packed red blood cells, RV-MCS = right ventricular mechanical circulatory support, TBPE= total blood product exposure.
Morbidities in the UMHS cohort associated with perioperative transfusion requirements are displayed in table I. In short, blood product exposures were associated with increased univariable odds of developing postoperative RV failure, renal failure (30%, 30% and 8% per 5-unit transfusion of PRBCs, FFP, and platelets, respectively, for both morbidities) and neurological events (20% per 5-units of PRBCs) (all p≤0.050). Transfusion requirements were not related to the development of postoperative device malfunction (n=3, p>0.050 data not shown).
MELD Score Predicts Perioperative Transfusion Requirements
The mean±standard deviation MELD score for the UMHS cohort (n=211) was 13.7±6.1 (median 12 [9,16]), with a mean creatinine, bilirubin, and INR of 1.8±1.3 mg/dL, 1.8±2.8 mg/dL, and 1.2±0.3 seconds, respectively. Figure 1 shows the scatterplot for TBPE score regressed on MELD (Pearson correlations coefficient 0.33, p<0.001). Each 5-unit increase in MELD score was associated with an additional (unadjusted β±SE) 20±4.0 units of TBPE (R2=0.11, p<0.001), and 3.3±0.89 (R2=0.07, p<0.001) units of PRBCs, 2.0±0.55 (R2=0.06, p<0.001) units of FFP, 11±2.2 (R2=0.10, p<0.001) units of platelets, and 4.3±1.2 (R2=0.06, p<0.001) additional units of cryoprecipitate. Other predictors of perioperative bleeding are shown in table II.
Figure 1.

Scatter plot of preoperative MELD score versus total perioperative blood produce exposure (TBPE). Fitted line based on regression analysis is also shown.
Table II.
Predictors of bleeding (TBPE) in subjects undergoing LVAD.
| Unadjusted TBPE ± standard error | p- value | Adjusted TBPE ± standard error | p-value | |
|---|---|---|---|---|
| Body surface area, | -14.2±22.4 | 0.53 | --- | --- |
| Age, years | -0.12±0.39 | 0.76 | --- | --- |
| Preop. RV MCS | 48±17 | 0.0047 | --- | --- |
| Preop. LV MCS | 43±12 | 0.0004 | --- | --- |
| Preop. renal replacement | 87±18 | <0.0001 | --- | --- |
| Preop. ventilatory support | 67±11 | <0.0001 | 46±12 | 0.0001 |
| Preop. postcardiotomy shock | 32±19 | 0.085 | --- | --- |
| Prior sternotomy | 12±11 | 0.26 | --- | --- |
| Preop. RA pressure, per mmHg | 1.3±0.89 | 0.15 | --- | --- |
| Preop. PVR, per WU | -4.2±3.2 | 0.20 | --- | --- |
| Preop. MELD, per 5 units | 20±4.0 | <0.0001 | 15.1±3.8 | 0.0001 |
| Preop. hemoglobin, per mg/dL | -6.7±2.6 | 0.012 | -9.7±2.3 | <0.0001 |
| Preop. platelets, per K/mm3 | -0.28±0.06 | <0.0001 | -0.16±0.06 | 0.0043 |
| Preop. PTT, per sec. | 0.24±0.26 | 0.34 | --- | --- |
| Intraop. heparin, per 1000 units | 0.23±0.57 | 0.68 | --- | --- |
| Cardiopulmonary bypass time, per minute | 0.90±13 | <0.0001 | --- | --- |
LV= left ventricular, MCS= mechanical circulatory support, Preop= preoperative, PTT= partial thromboplastin time, PVR= pulmonary vascular resistance, RA=right atrial, RV= right ventricular, TBPE = total blood product exposure.
While the relationship between MELD score and TBPE by univariable linear regression analysis was not strong (R2 0.11), the MELD remained a predictor of perioperative bleeding on multivariable analysis (table III). Each 5-unit increase in preoperative MELD score increased TBPE (adjusted β±SE) by 15.0±3.8 units (p<0.001). Other predictors of bleeding included the need for preoperative ventilatory support (45±12 units, p<0.001), lower preoperative serum hemoglobin (-9.7±2.3 per mg/dL, p<0.001) and lower platelet counts (-0.16±0.05 per K/mm3, p=0.0043) (table III, model p<0.001, adjusted R2= 0.27). While subjects with higher MELD scores were more likely to have had other known risks for operative bleeding, including requirements for preoperative RV (unadjusted OR=2.0[1.4,2.8] per 5-MELD units) and/or LV-MCS (unadjusted OR=1.6[1.2,2.1] per 5-MELD units), elevated preoperative right atrial pressures (by 1.5±0.33 mmHg per 5-MELD units), and longer cardiopulmonary bypass times (6±2 minutes for each 5-units MELD, p=0.002), these variables were not predictive of operative bleeding on multivariable analysis (table III).
Table III.
Predictors of operative mortality in LVAD patients.*
| Unadjusted Odds Ratio [95% CI] | P value | Adjusted Odds Ratio [95% CI] | P value | |
|---|---|---|---|---|
| MELD residual, per 5 units | 1.4 [1.03, 1.9] | 0.034 | 1.6 [1.1, 2.3] | 0.013 |
| Preop. vasopressor use | 5.2 [2.2, 13] | 0.0003 | 4.9 [1.8, 13] | 0.002 |
| Female sex | 2.4 [1.01, 5.6] | 0.046 | 4.4 [1.6, 12] | 0.004 |
| Age, per 10 years | 1.04 [1.01, 1.08] | 0.023 | 2.0 [1.3, 3.1] | 0.002 |
unadjusted odds ratios for covariates not included in the multivariable analysis are shown in the online supplementary table.
MELD Score Predicts Perioperative Mortality
Baseline characteristics, preoperative laboratory and cardiopulmonary hemodynamic data for the 29 (14%) operative deaths and 182 survivors in the UMHS cohort are shown in the supplementary table (available online). In general, operative deaths tended to occur in older, female patients with evidence of multisystem organ dysfunction. Operative deaths had significantly longer times on cardiopulmonary bypass (128±7.3 minutes) than survivors (103±2.6 minutes, p=0.003) and received fewer units of heparin (25000 vs. 31000 units, p=0.03) but there were no significant differences in the use or dose of other intraoperative procoagulants or anticoagulants (data not shown, all p>0.05).
The mean MELD scores for operative deaths and survivors in the UMHS cohort were 14[11,22] and 12[9,15], respectively (p=0.008). The unadjusted odds of perioperative death increased 50% [1.5(1.1,2.0)] per 5-unit increase in preoperative MELD score. In the INTERMACS cohort, there were 19 (6%) operative deaths and 305 survivors with mean±SEM MELD scores of 18±1.8 and 15±0.33, respectively (p=0.036). Each 5-unit increase in MELD in the INTERMACS cohort increased the unadjusted odds of perioperative death by 1.5 (1.1,2.1).
In the UMHS cohort, after taking into account female sex, age, and need for preoperative vasopressor requirement, MELD score remained a significant predictor of operative death (table III). A 5-unit increase in the unique component of MELD (not shared with its ability to predict bleeding) increased the odds of operative mortality by 60% (adjusted OR=1.6 [1.1,2.3] per 5-MELD units, model adjusted R2=0.25, model p<0.001).
MELD Predicts Perioperative Morbidity in the UMHS Cohort
Preoperative MELD scores were also predictive of perioperative morbidity in the UMHS cohort. Each 5-unit MELD increment increased the unadjusted odds of requiring postoperative renal replacement therapy by 2.1[1.5,2.8] and RV-MCS by 2.1[1.6,2.8], with scores ≥17 affording equivalent 5.0-fold greater odds (both p<0.001) of developing the aforementioned complications. Similarly, each 5-unit increase in MELD was associated with 1.7[1.2,2.5] greater odds of developing an LVAD infection (unadjusted OR=3.9 [1.4,12] for MELD ≥17), and an additional 3±1 days in the ICU and 6±2 days of total hospital stay (both p=0.001).
Six-month survival by MELD score
Six-month Kaplan-Meier survival curves for the UMHS and INTERMACS cohorts are shown in Figure 2. In the UMHS cohort, survival at 6 months for subjects with MELD scores ≥17 and <17 was 74±6% and 88±3%, respectively (log rank p=0.009, figure 2a). The risk-adjusted hazard ratio for death in subjects with MELD scores ≥17 was 2.5 [1.2,5.3] times that of those with lower scores. In the INTERMACs cohort, 6-month survival for INTERMACS subjects with MELD scores ≥17 was 67±5% compared with 82±3% in subjects with lower scores (log rank p=0.032, figure 2b). The risk-adjusted hazard ratio for death during the 6-month follow-up period for INTERMACS patients with MELD scores ≥17 was 2.5 [1.1,5.4].
Figure 2.
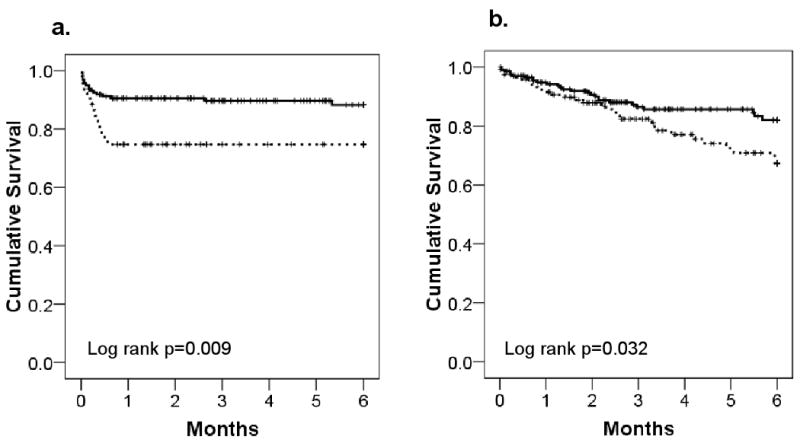
Kaplan-Meier Survival Curves for the a) UMHS and b) INTERMACS cohorts by MELD strata.
Discussion
Bleeding in the LVAD perioperative period is associated with increased morbidity and mortality. In this cohort, each 10-unit TBPE increased the odds of perioperative death by 5% with equivalent 8% increases in the odds of developing postoperative RV and renal failure. This is the first tool to accurately assess an LVAD candidate's risk of bleeding in the perioperative period. In the UMHS cohort, the MELD was a univariable predictor of hospital stay and the development of postoperative device infections, RV failure, and renal failure. In both the UMHS and INTERMACS cohorts, the MELD was predictive of operative and 6-month mortality.
The MELD was original developed to assess mortality in subjects with cirrhosis undergoing TIPS procedures7. In 2002, the utility of the MELD was extended to UNOS liver transplant allocation, with MELD scores >17 conferring improved survival with transplant8,9. By coincidence, using this same threshold (based on the UMHS MELD 75th percentile) in an entirely different patient population and study design, a MELD ≥17 was associated with increased perioperative LVAD events in both the UMHS and INTERMACS cohorts.
Outside of liver transplantation, the MELD has also been employed to assess operative mortality in individuals with cirrhosis undergoing cardiac11,12 and noncardiac surgeries10,12,13. In a study of 44 cirrhotic subjects undergoing cardiopulmonary bypass for cardiac interventions (coronary bypass, valve, pericardiectomy), the mean±standard deviation preoperative MELD score in operative deaths versus survivors was 18.3±6.9 and 10.2±3.7, respectively (p=0.004)11. In our analysis of subjects without known cirrhosis, mean preoperative MELD scores for perioperative LVAD deaths in the UMHS and INTERMACS cohorts were comparable at 16.7±7.0 and 17.9±7.7, respectively, but survivors had slightly higher MELD scores (13.2±0.4 and 15.0±5.7) than in the aforementioned study, reflecting a greater severity of end-organ dysfunction in these LVAD cohorts. In another study examining 772 surgical patients with cirrhosis (n=79 underwent cardiac surgery), subjects (n=58) with MELD scores >15 had approximately 5-fold higher odds of 30-day death than those (n=714) with lower scores12. Using a slightly different threshold, we found that preoperative MELD scores ≥17 offered 3-fold increased odds of perioperative LVAD death in the UMHS cohort, with hazard ratios for death at 6 months of 2.5 in both cohorts.
Cirrhotics with higher preoperative MELD scores have been shown to have greater perioperative PRBC transfusion requirements15,16. In LVAD candidates, perioperative transfusions have been associated with increased risks for various postoperative morbidities1,2. Similar to prior studies17, our results demonstrated increased odds of developing postoperative RV and renal failure in subjects requiring greater numbers of perioperative transfusions. In the UMHS cohort, there was an independent, linear relationship between preoperative MELD score and transfusions, with each 5-unit increase in MELD affording an additional 15 units of TBPE. Further, each 5-unit increase in MELD score was associated with an identical 200% increase in the univariable odds of requiring postoperative RV-MCS and renal replacement therapy.
The associations between perioperative transfusion requirements, preoperative MELD score and the development of postoperative RV and renal failure are likely multifactorial. UMHS patients with high MELD scores had greater transfusion requirements and longer times on bypass. Thus, postoperative RV failure in subjects with high MELD scores may result from increased RV preload following perioperative blood product administration and from increased pulmonary vasoreactivity secondary to cytokine-release during transfusions and prolonged bypass1,18. The association between MELD score and renal dysfunction is in large part due to the fact that creatinine is a component of the MELD score calculation. However, an association was also noted in the UMHS cohort between RV failure and renal failure (odds ratio for renal failure in subjects with RV failure= 3.4). Thus, the MELD may be predictive of postoperative renal failure because it identifies those at risk for cardiorenal syndrome from RV failure in the setting of high transfusion requirements.
In this analysis, we found a 70% increase in the odds of developing a postoperative device infection for each 5-unit increase in MELD. It can be surmised that the association between MELD score and infection may be related to increased microbial exposures during patient instrumentation and management of a critically ill state. Prolonged hospital stays have also been associated with elevated MELD scores in liver transplants19 and high transfusion requirements in LVAD recipients20. In this analysis, higher MELD scores were associated with prolonged ICU and total hospital stays, again increasing risks for microbial inoculations.
Last, studies have demonstrated increased allosensitization risk in subjects receiving greater numbers of transfusions during LVAD support4-6. While measures of allosensitization were not available in this sample, the positive relationship between preoperative MELD score and transfusions is of importance. Allosensitization prior to transplant has been associated with longer time on the transplant wait-list and worse posttransplant outcomes21, and studies are needed to determine if higher MELD scores are predictive of allosensitization, more frequent post-transplant graft dysfunction, and reduced transplant survival.
Clinical Utility of the MELD in LVAD Candidates
While subjects in this cohort were not known to have hepatic cirrhosis, the MELD score succeeded as an LVAD risk assessment tool likely because it is a marker of multisystem dysfunction (renal, hepatic, cardiac) and coagulopathy. While the association between multisystem organ dysfunction and LVAD perioperative morbidity and mortality is not in itself novel, the utility of the MELD lies in its ability to provide important patient-specific measures of LVAD perioperative risk in the preoperative setting. The score is composed of routinely collected laboratories and is available for rapid calculation on several websites. The MELD's usefulness will especially apply to those individuals with multiple, potentially conflicting, univariable predictors for LVAD mortality, allowing one to gauge mortality odds using a single independent predictor. While dichotomization of MELD scores was undertaken in subsets of this data analysis, the MELD demonstrated independent risk prediction for operative bleeding and death as a continuous variable. As such, the MELD score will function best when used as a continuous variable for risk assessment, making it potentially useful for those subjects with intermediate pretest probabilities. Finally, foresight gained through MELD risk stratification may also provide clinicians with a proactive, rather than reactive, means of managing perioperative complications via targeted improvement in end-organ function through earlier or increased use of preoperative RV and/or LV inotrope or extracorporeal MCS and correction of coagulation abnormalities with aggressive factor or vitamin K repletion. Studies are needed to determine if strategies directed at reducing preoperative MELD scores improve postoperative outcomes.
Limitations
This study has several limitations, many of which are inherent to the nature of cohort studies. As this was a nonblinded evaluation, patient selection and management biases likely exist that may have influenced study endpoints and MELD dichotomization thresholds. This is especially important when pre-, intra-, and post-operative interventions can significantly impact bleeding risk. Intraoperative dose requirements for heparin, protamine, vitamin K and factor VIIa were only available in 52% of UMHS subjects. While individuals without this data did not differ clinically from those with available data, we acknowledge this as a source of information bias. We hoped to validate all of the UMHS findings using a national LVAD database. However, detailed transfusion requirements and pre and postoperative morbidity tallies using the same event definitions were not available in INTERMACS, preventing us from accurately examining events other than 6-month mortality. For these same reasons, we had to use a surrogate endpoint for operative mortality (death at 1 month) in the INTERMACS analysis. Nonetheless, on a continuous basis, the MELD was predictive of operative death and was categorically associated with increased risk for 6-month LVAD mortality in the INTERMACs cohort.
Due to the relative rarity of the LVAD intervention, study power was also limited. While the UMHS cohort size was similar to the 231 patient sample used in the derivation of the original MELD formula, the larger confidence intervals and the reduced power may account for nonsignificant trends noted when analyzing the impact of transfusion requirements on mortality. Power also limited the number of variables able to be examined on the mortality multivariable analysis. Finally, subjects in this cohort did not have liver biopsies to rule-out concomitant cirrhosis as a confounder of results, which is of concern in subjects with a high prevalence of concomitant RV dysfunction and chronic hepatic congestion. No UMHS subject with a MELD ≥17 had preoperative ultrasound evidence of cirrhosis and MELD scores were not associated with lower preoperative albumins (data not shown).
Conclusions
Bleeding in the perioperative LVAD period is associated with increased morbidity and mortality. The preoperative MELD score is a noninvasive, simple means of assessing an LVAD candidate's operative bleeding risk and identifies individuals at increased risk for renal failure, RV failure, device infection and prolonged hospital stays. The MELD score was also a predictor of operative and 6-month mortality in the UMHS and INTERMACS cohorts. Further studies are needed to determine if clinical intervention to improve MELD scores could impact perioperative outcomes.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Matthews's research experience was supported under the NIH T32 HL007853 Multidisciplinary Cardiovascular Research Training Grant.
Footnotes
Clinical Perspective: Bleeding following implantation of a left ventricular assist device (LVAD) is associated with increased mortality and an increased risk for several morbidities which can impact a patient's quality of life on LVAD therapy and candidacy for future cardiac transplant. Causes for perioperative bleeding in LVAD patients are often multifactorial. Identifying patients at high risk for perioperative LVAD bleeding would assist with LVAD candidate risk stratification and may offer the potential for improving LVAD outcomes by triggering clinicians to institute therapies in the preoperative period directed at reducing bleeding risk. The present study employs the model for end-stage liver disease (MELD) in the LVAD preoperative period to assess patient risk for perioperative bleeding, morbidity, and mortality.
Disclosures: Drs. Aaronson and Pagani have received grant support from Terumo and HeartWare not directly related to this study. Dr. Aaronson has a consulting relationship with Thoratec, HeartWare, and Circulite. Dr. Matthews is a paid speaker for Terumo and Thoratec.
References
- 1.Goldstein DJ, Beauford RB. Left ventricular assist devices and bleeding: adding insult to injury. Annals of Thoracic Surgery. 2003;75:S42–7. doi: 10.1016/s0003-4975(03)00478-8. [DOI] [PubMed] [Google Scholar]
- 2.Pavie A, Szefner J, Leger P, Gandjbakhch I. Preventing, minimizing, and managing postoperative bleeding. Annals of Thoracic Surgery. 1999;68:705–10. doi: 10.1016/s0003-4975(99)00628-1. [DOI] [PubMed] [Google Scholar]
- 3.Murphy PJ, Connery C, Hicks GL, Jr, Blumberg N. Homologous blood transfusion as a risk factor for postoperative infection after coronary artery bypass graft operations. Journal of Thoracic & Cardiovascular Surgery. 1992;104:1092–9. [PubMed] [Google Scholar]
- 4.Massad MG, Cook DJ, Schmitt SK, Smedira NG, McCarthy JF, Vargo RL, McCarthy PM. Factors influencing HLA sensitization in implantable LVAD recipients. Annals of Thoracic Surgery. 1997;64:1120–5. doi: 10.1016/s0003-4975(97)00807-2. [DOI] [PubMed] [Google Scholar]
- 5.Moazami N, Itescu S, Williams MR, Argenziano M, Weinberg A, Oz MC. Platelet transfusions are associated with the development of anti-major histocompatibility complex class I antibodies in patients with left ventricular assist support. Journal of Heart & Lung Transplantation. 1998;17:876–80. [PubMed] [Google Scholar]
- 6.McKenna DH, Jr, Eastlund T, Segall M, Noreen HJ, Park S. HLA alloimmunization in patients requiring ventricular assist device support. Journal of Heart & Lung Transplantation. 2002;21:1218–24. doi: 10.1016/s1053-2498(02)00448-5. [DOI] [PubMed] [Google Scholar]
- 7.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71. doi: 10.1053/he.2000.5852. see comment. [DOI] [PubMed] [Google Scholar]
- 8.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. see comment. [DOI] [PubMed] [Google Scholar]
- 9.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R. United Network for Organ Sharing Liver Disease Severity Score C. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 10.Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for End-Stage Liver Disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Annals of Surgery. 2005;242:244–51. doi: 10.1097/01.sla.0000171327.29262.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suman A, Barnes DS, Zein NN, Levinthal GN, Connor JT, Carey WD. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clinical Gastroenterology & Hepatology. 2004;2:719–23. doi: 10.1016/s1542-3565(04)00296-4. [DOI] [PubMed] [Google Scholar]
- 12.Teh SH, Nagorney DM, Stevens SR, Offord KP, Therneau TM, Plevak DJ, Talwalkar JA, Kim WR, Kamath PS. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132:1261–9. doi: 10.1053/j.gastro.2007.01.040. see comment. [DOI] [PubMed] [Google Scholar]
- 13.Befeler AS, Palmer DE, Hoffman M, Longo W, Solomon H, Di Bisceglie AM. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease score is superior to Child-Turcotte-Pugh classification in predicting outcome. Archives of Surgery. 2005;140:650–4. doi: 10.1001/archsurg.140.7.650. discussion 655. [DOI] [PubMed] [Google Scholar]
- 14.Deng MC, Edwards LB, Hertz MI, Rowe AW, Keck BM, Kormos R, Naftel DC, Kirklin JK, Taylor DO. Mechanical Circulatory Support Device Database of the International Society for Heart and Lung Transplantation: Third Annual Report--2005. The Journal of Heart and Lung Transplantation. 2005;24:1182–1187. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Xia VW, Du B, Braunfeld M, Neelakanta G, Hu KQ, Nourmand H, Levin P, Enriquez R, Hiatt JR, Ghobrial RM, Farmer DG, Busuttil RW, Steadman RH. Preoperative characteristics and intraoperative transfusion and vasopressor requirements in patients with low vs. high MELD scores. Liver Transplantation. 2006;12:614–20. doi: 10.1002/lt.20679. [DOI] [PubMed] [Google Scholar]
- 16.Frasco PE, Poterack KA, Hentz JG, Mulligan DC. A comparison of transfusion requirements between living donation and cadaveric donation liver transplantation: relationship to model of end-stage liver disease score and baseline coagulation status. Anesthesia & Analgesia. 2005;101:30–37. doi: 10.1213/01.ANE.0000155288.57914.0D. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein DJ, Seldomridge JA, Chen JM, Catanese KA, DeRosa CM, Weinberg AD, Smith CR, Rose EA, Levin HR, Oz MC. Use of aprotinin in LVAD recipients reduces blood loss, blood use, and perioperative mortality. Annals of Thoracic Surgery. 1995;59:1063–7. doi: 10.1016/0003-4975(95)00086-z. see comment. discussion 1068. [DOI] [PubMed] [Google Scholar]
- 18.Cave AC, Manche A, Derias NW, Hearse DJ. Thromboxane A2 mediates pulmonary hypertension after cardiopulmonary bypass in the rabbit. J Thorac Cardiovasc Surg. 1993;106:959–67. [PubMed] [Google Scholar]
- 19.Foxton MR, Kendrick S, Sizer E, Muiesan P, Rela M, Wendon J, Heaton ND, O'Grady JG, Heneghan MA. Change in model for end-stage liver disease score on the transplant waiting list predicts survival in patients undergoing liver transplantation. Transpl Int. 2006;19:988–94. doi: 10.1111/j.1432-2277.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 20.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 21.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007;84:1556–62. doi: 10.1016/j.athoracsur.2007.05.095. discussion 1562-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


