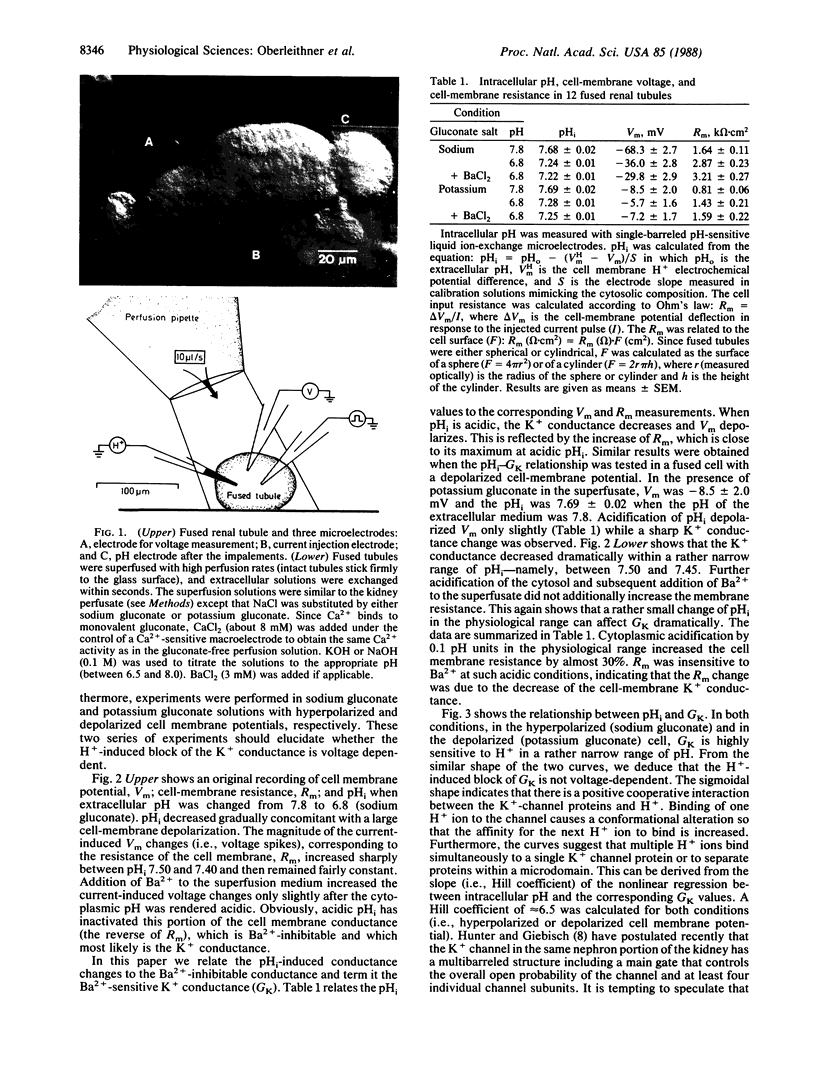
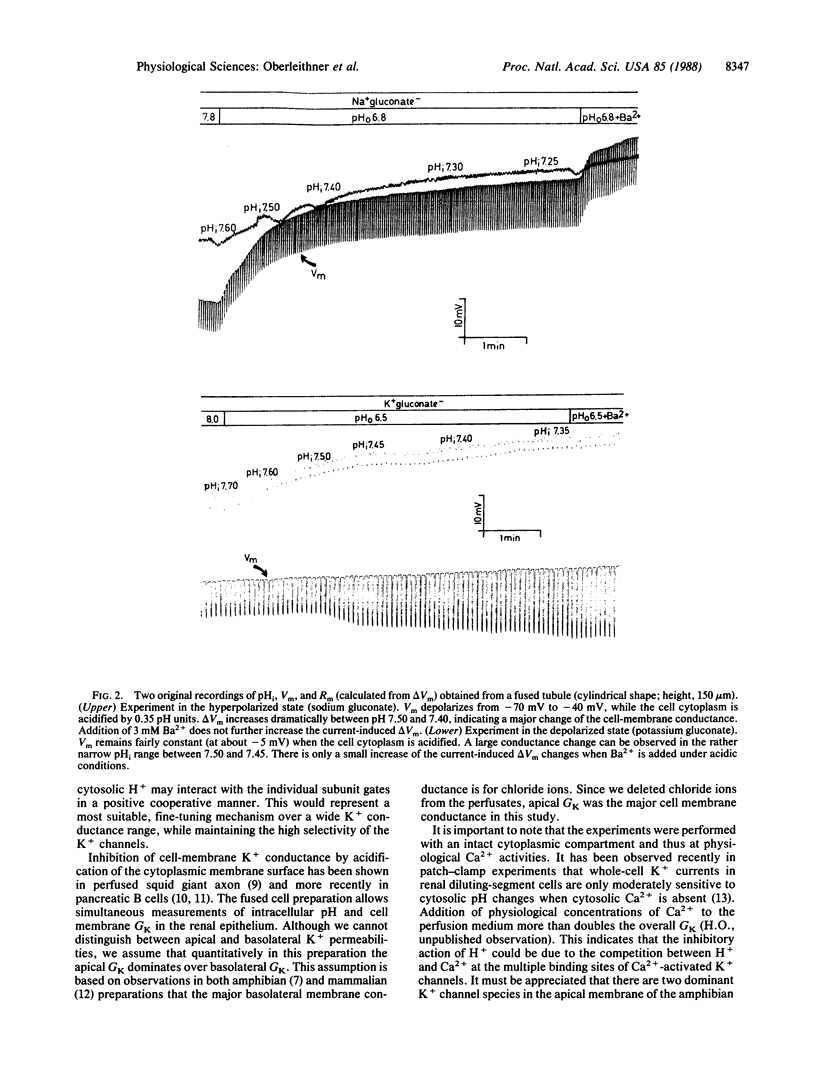
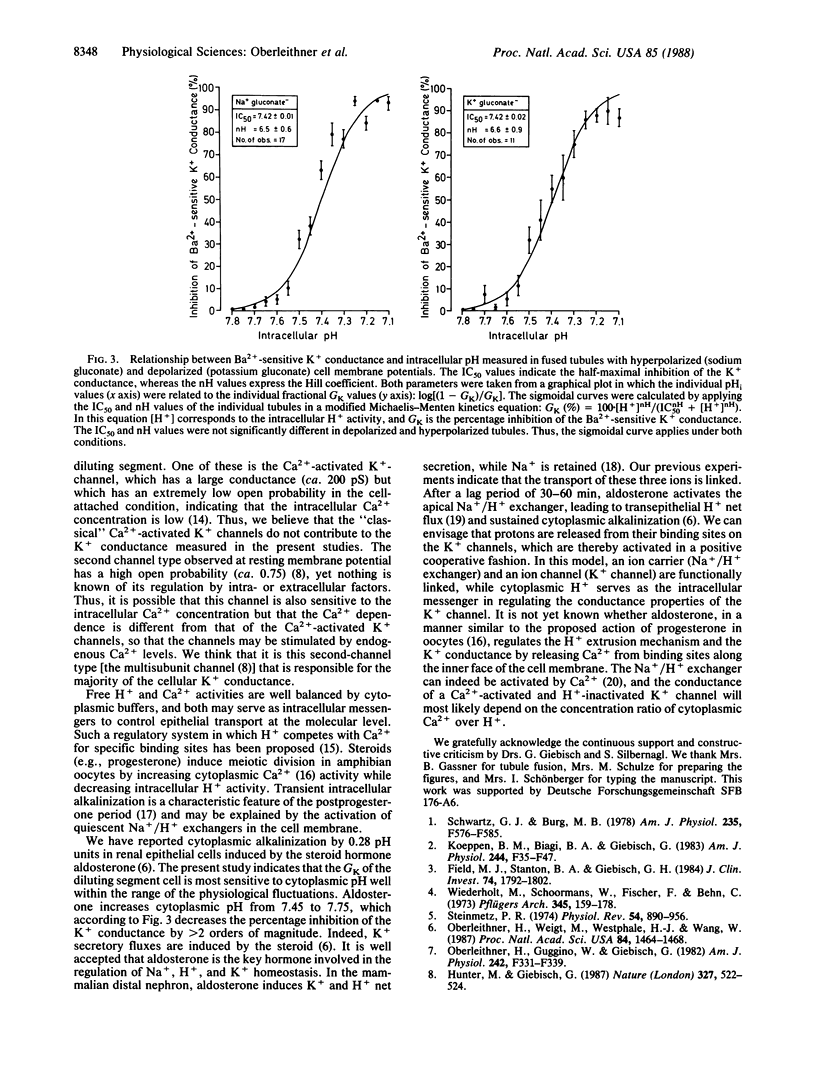
Abstract
The mineralocorticoid hormone aldosterone maintains acid-base balance and K+ homeostasis by regulating H+ and K+ secretory mechanisms in kidney epithelial cells. We have shown recently in the amphibian distal nephron that aldosterone activates a Na+/H+ exchange system in the luminal cell membrane, thus leading to transepithelial H+ secretion and cytoplasmic alkalinization. Since H+ secretory fluxes were paralleled by K+ secretion, it was postulated that the hormone-induced increase of intracellular pH activates the luminally located K+ channels. In "giant" cells fused from individual cells of the distal nephron, we measured simultaneously cytoplasmic pH and cell membrane K+ conductance during acidification of the cell cytoplasm. The experiments show that cell membrane K+ conductance is half-maximal at an intracellular pH of 7.42 and that a positive cooperative interaction exists between K+-channel proteins and H+ (Hill coefficient = 6.5). Moreover, the cellular K+ conductance is most sensitive to cytoplasmic pH in the range modified by aldosterone. This supports the hypothesis that intracellular H+ activity, regulated by the Na+/H+ exchanger, serves as the signal to couple aldosterone-induced K+ secretory flux to H+ secretion in renal tubules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Fanestil D. D., Park C. S. Steroid hormones and the kidney. Annu Rev Physiol. 1981;43:637–649. doi: 10.1146/annurev.ph.43.030181.003225. [DOI] [PubMed] [Google Scholar]
- Field M. J., Stanton B. A., Giebisch G. H. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984 Nov;74(5):1792–1802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H., Asher C., Yeger O. Direct inhibition of epithelial Na+ channels by a pH-dependent interaction with calcium, and by other divalent ions. J Membr Biol. 1987;95(2):151–162. doi: 10.1007/BF01869160. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S. Cytoplasmic [Ca2+] and intracellular pH in lymphocytes. Role of membrane potential and volume-activated Na+/H+ exchange. J Gen Physiol. 1987 Feb;89(2):185–213. doi: 10.1085/jgp.89.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M., Giebisch G. Multi-barrelled K channels in renal tubules. Nature. 1987 Jun 11;327(6122):522–524. doi: 10.1038/327522a0. [DOI] [PubMed] [Google Scholar]
- Hunter M., Kawahara K., Giebisch G. Potassium channels along the nephron. Fed Proc. 1986 Nov;45(12):2723–2726. [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Mechanism of distal tubular chloride transport in Amphiuma kidney. Am J Physiol. 1982 Apr;242(4):F331–F339. doi: 10.1152/ajprenal.1982.242.4.F331. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Weigt M., Westphale H. J., Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1464–1468. doi: 10.1073/pnas.84.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario L. M., Rojas E. Modulation of K+ conductance by intracellular pH in pancreatic beta-cells. FEBS Lett. 1986 May 5;200(1):203–209. doi: 10.1016/0014-5793(86)80539-7. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular mechanisms of urinary acidification. Physiol Rev. 1974 Oct;54(4):890–956. doi: 10.1152/physrev.1974.54.4.890. [DOI] [PubMed] [Google Scholar]
- Wanke E., Carbone E., Testa P. L. K+ conductance modified by a titratable group accessible to protons from the intracellular side of the squid axon membrane. Biophys J. 1979 May;26(2):319–324. doi: 10.1016/S0006-3495(79)85251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigt M., Dietl P., Silbernagl S., Oberleithner H. Activation of luminal Na+/H+ exchange in distal nephron of frog kidney. An early response to aldosterone. Pflugers Arch. 1987 May;408(6):609–614. doi: 10.1007/BF00581163. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Schoormans W., Fischer F., Behn C. Mechanism of action of aldosterone on potassium transfer in the rat kidney. Pflugers Arch. 1973 Dec 12;345(2):159–178. doi: 10.1007/BF00585838. [DOI] [PubMed] [Google Scholar]





