Abstract
Maladaptive auditory cortex reorganization may contribute to the generation and maintenance of tinnitus. Because cortical organization can be modified by behavioral training, we attempted to reduce tinnitus loudness by exposing chronic tinnitus patients to self-chosen, enjoyable music, which was modified (“notched”) to contain no energy in the frequency range surrounding the individual tinnitus frequency. After 12 months of regular listening, the target patient group (n = 8) showed significantly reduced subjective tinnitus loudness and concomitantly exhibited reduced evoked activity in auditory cortex areas corresponding to the tinnitus frequency compared to patients who had received an analogous placebo notched music treatment (n = 8). These findings indicate that tinnitus loudness can be significantly diminished by an enjoyable, low-cost, custom-tailored notched music treatment, potentially via reversing maladaptive auditory cortex reorganization.
Keywords: cortical plasticity, human auditory cortex, lateral inhibition , magnetoencephalography, MEG
Subjective tinnitus (1) is among the most prevalent symptoms of hearing disorders in industrialized countries (2, 3). Tinnitus loudness can be considered as the most tangible tinnitus characteristic. In 1–3% of the general population, the tinnitus sensation is loud enough to affect the quality of life (4). Causal treatment strategies for tinnitus are not yet available.
The lack of treatment strategies is due to incomplete knowledge concerning the mechanisms of tinnitus generation and maintenance. However, recent neurophysiological studies have shown that tinnitus is presumably caused by maladaptive auditory cortex reorganization (4–6) (similar phenomena were observed also in somatosensory cortex; refs. 7–9). For instance, magnetoencephalography (MEG) studies have demonstrated that auditory cortical map areas corresponding to the tinnitus frequency were distorted; the amount of distortion correlated positively with perceived tinnitus strength (10). Moreover, auditory cortex activity corresponding to the tinnitus frequency was shown to be enhanced and related to perceived tinnitus intrusiveness (11).
To date, widely used tinnitus treatment strategies (e.g., tinnitus retraining therapy; ref. 12) are merely symptom management approaches. Therefore, there is a great demand for causal treatment approaches targeting the tinnitus percept more directly. Recent neurophysiological studies indicate that behavioral training can be a powerful means to reverse maladaptive cortical reorganization (7, 13).
A previous study (14) demonstrated that listening to spectrally “notched” music can reduce cortical activity corresponding to the notch center frequency, possibly through lateral inhibition. Motivated by this finding, we developed an innovative tinnitus treatment strategy aimed at reducing tinnitus loudness. The treatment regimen consists of regular listening to enjoyable, custom-tailored notched music. Here, we evaluate and report results of the treatment from a longitudinal double-blinded study. Three groups of patients suffering from chronic, tonal tinnitus participated in the study: (i) target notched music treatment (n = 8; Fig. 1 and Movie S1), (ii) placebo notched music treatment (n = 8; Fig. 2 and Movie S2), and (iii) monitoring (n = 7; no treatment). Treatment outcomes were evaluated using both subjective and neurophysiological measurements.
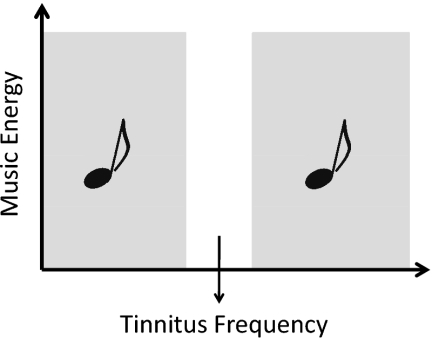
Fig. 1.
Target treatment. A frequency band of one octave width centered at the individual tinnitus frequency was removed from the music energy spectrum via digital notch filter. Exemplary music (MP3 format) is available as Movie S1.
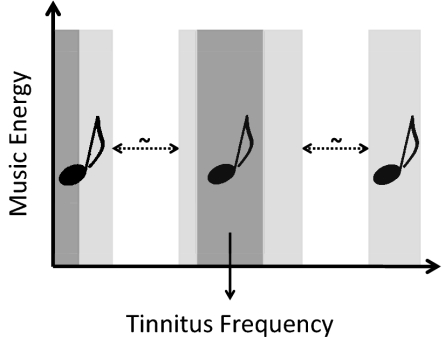
Fig. 2.
Placebo treatment. A moving notch filter (dotted arrow) of one octave width was applied to the music energy spectrum. The energy in the frequency band ranging from 0 to 707 Hz and the energy in the 1-octave frequency band surrounding the individual tinnitus frequency remained strictly unchanged (dark gray areas). The energy in the remaining frequency ranges was subject to filtering (light gray areas). Exemplary music (MP3 format) is available as Movie S2.
Results
The patients who received the music treatment were assigned to the target or placebo group pseudorandomly. The monitoring group consisted of patients who were not able to perform the music training because of lack of time (i.e., these patients were not randomly assigned to this group). On average (mean ± SD), the three groups did not differ significantly in age (40.5 ± 10.8 years; range 18–55 years) or the tinnitus characteristics (i) duration (5.3 ± 5.6 years; range 1.2–24.8 years), (ii) frequency (5,949 ± 1,886 Hz; range 2,375–8,000 Hz), (iii) tinnitus-related distress (15) (18.4 ± 10.8; range 1–38; scale 0–84), and (iv) loudness (49.7 ± 16.9; range 10–78; scale 0–100). Baseline N1m auditory evoked response ratios (16), as well as auditory steady state response (ASSR) (17) ratios as measured by MEG did not differ significantly between groups. Furthermore, retrospective analysis revealed that the target and placebo groups did not differ significantly on measures of average music listening times (12.4 ± 3.5 h per week; range 7–21 h per week) and subjective music enjoyment (67.6 ± 26.9; range 13–100; scale 0–100).
Figs. 3 and 4 demonstrate the results of tinnitus loudness, ASSR, and N1m measurements for all groups. In the target group, tinnitus loudness was significantly reduced after 12 months of treatment compared to baseline (F(1,7) = 26.1, P = 0.001). Moreover, there was a significant interaction between group (target vs. placebo) and time point of measurement [baseline vs. average across months 7–12 (F(1,14) = 5.9, P = 0.030)]. In contrast, for the placebo and monitoring groups significant differences from baseline were not found, indicating that a systematic change in tinnitus loudness was not present in these groups.
Fig. 3.
Normalized tinnitus loudness change after 6 and 12 months of treatment (or monitoring) relative to baseline (0) for the three patient groups (target, placebo, and monitoring). Positive change values reflect impairment, negative change values reflect improvement. The bars indicate group averages, each x indicates an individual data point. The error bars denote confidence intervals. The data were normalized as following: {[(tinnitus loudness_AVG months 1–6 or months 7–12/tinnitus loudness_baseline) − 1] × 100}. As indicated by the confidence interval bars, only the changes in the target group were statistically significant.
Fig. 4.
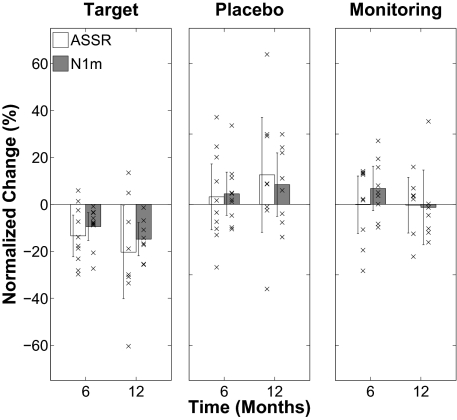
Normalized tinnitus-related auditory cortex evoked activity change after 6 and 12 months of treatment (or monitoring) relative to baseline (0) for the three patient groups (target, placebo, and monitoring). Positive change values reflect increment, negative change values reflect decrement. The bars indicate group averages, each × indicates an individual data point. The error bars denote confidence intervals. ASSR change values are reflected by white bars, N1m change values are reflected by gray bars. The data were normalized as following: {[(ASSR or N1m_tinnitus frequency after 6 or 12 months/ASSR or N1m_control frequency after 6 or 12 months)/(ASSR or N1m_tinnitus frequency baseline/ASSR or N1m_control frequency baseline) − 1] × 100}. As indicated by the confidence interval bars, only the changes in the target group were statistically significant.
In the target group, both ASSR source strength ratios (representing primary auditory cortex evoked activity; ref. 18) and N1m source strength ratios (representing mainly belt auditory cortex evoked activity; ref. 18) were significantly reduced after 12 months of treatment (ASSR: F(1,7) = 5.9, P = 0.045; N1m: F(1,7) = 24.6, P = 0.002). Again, there was a significant interaction between group (target vs. placebo) and time point of measurement (baseline vs. month 12) for both ASSR (F(1,14) = 6.1; P = 0.027) and N1m (F(1,14) = 13.1; P = 0.003). In contrast, for the placebo and monitoring groups no significant differences from baseline were observed in the ASSR or N1m.
All reduction effects observed in the target group (Figs. 3 and 4) were statistically significant already after 6 months of treatment (loudness: F(1,9) = 8.1, P = 0.019; ASSR: F(1,9) = 11.2, P = 0.007; N1m: F(1,9) = 13.2, P = 0.005). Crucially, the correlation between tinnitus loudness change and auditory evoked response ratio change was highly significant for the ASSR (r = 0.69, P = 0.003) but not significant for the N1m (r = 0.17, P = 0.53) after 12 months of treatment. The significant positive correlation indicates a strong correspondence between changes in tinnitus loudness (improvement vs. impairment) and reorganization of neural activity in primary auditory cortex (decrement vs. increment) over time.
Discussion
In the target group we observed significant reductions in both tinnitus loudness and tinnitus-related auditory cortex evoked activity relative to baseline. Crucially, such significant changes were not observed in the placebo or monitoring groups. Moreover, the changes in loudness as well as tinnitus-related auditory cortex evoked activity were significantly different between target and placebo groups. Considering these findings, and taking into account a large epidemiological study (19) demonstrating that there is no general tinnitus loudness reduction trend over time, our findings strongly imply that the improvement in the target group reflects a specific treatment effect of custom-tailored target modification of the music.
It has been clearly demonstrated that tinnitus is generated in the central auditory system, possibly due to maladaptive cortical reorganization (3–6, 20). For instance, auditory cortex neurons that are deprived of normal thalamo-cortical input due to hearing loss do not become inactive, but “rewire” with excitatory inputs from neighboring neurons (21, 22). As a result of bottom-up input deprivation, the neurons are no longer excitable by the frequencies they were originally tuned to, but become sensitive to neighboring frequencies because of the rewiring. In this scenario tonotopic maps can literally, and maladaptively “fuse” (20, 23). Crucially, such fused cortical areas would be characterized by less lateral inhibitory networks (24) and may generate tinnitus by means of synchronized spontaneous neural activity (25). Such pathological spontaneous activity synchronization evidently interacts with other brain regions (26), and has been shown to be closely related to tinnitus loudness (27, 28) and tinnitus duration (29).
Despite the existence of diseases caused by maladaptive cortical reorganization, the consequences of reorganization can be beneficial (7, 30). Here, we used knowledge regarding maladaptive cortical reorganization in tinnitus to design a procedure that appears suited to reduce brain activity corresponding to the tinnitus frequency and thus possibly tinnitus perception. Our target notched music introduced a functional deafferentation of auditory neurons corresponding to the eliminated frequency band, and because this frequency band overlapped the individual tinnitus frequency, the notched music no longer stimulated the cortical area corresponding to the tinnitus frequency, although it still excited surrounding neurons. Thus, the neurons, which were not stimulated due to the notch, were presumably actively suppressed via lateral inhibitory inputs originating from surrounding neurons (14, 31, 32). Alternatively, listening to the target notched music could have induced synaptic and/or cellular plasticity mechanisms (33, 34). For instance, the deprivation from auditory input in the frequency range of the tinnitus frequency could have caused long-term depression of auditory neurons corresponding to the tinnitus frequency.
One might presume that listening to a band-eliminated broadband stimulus like notched music may cause a phantom auditory sensation, the so-called Zwicker tone (35). However, our additional behavioral study (described in SI Text) demonstrated that notched music could not elicit a Zwicker tone, whereas notched broadband noise could. These results support the hypothesis that noise detecting neurons would play an important role in generating the Zwicker tone (36).
The described reversion of maladaptive cortical reorganization by the notched music training would have been initiated by bottom-up neural inputs triggered by the music. However, top-down neural processes also play an essential role in cortical reorganization (37). In the present study, patients were given the opportunity to listen to their most enjoyable music. It is reasonable to assume that enjoyable music strongly engages attention, and evidently it affects brain functioning (38). As such, joyful listening to music activates the reward system of the brain (39) and leads to release of dopamine, which plays an important role in cortical reorganization (40). Thus, a combination of bottom-up and top-down neural processes initiated by the target notched and relished music could provide a basis for the reversion of the putative maladaptive cortical reorganization underlying tinnitus emergence and maintenance in auditory cortex.
Evoked cortical source strength measured by MEG represents the quantity as well as the synchronicity of activated cortical neurons. Therefore, the present MEG results strongly suggest that the number of active neurons and/or the synchrony of these neurons, which correspond to a cortical area that contributes to the tinnitus perception, cumulatively decreased after regular listening to appreciated, target notched music. The decrement of this population-level neural activity likely reflects reduction of pathological auditory neural activity corresponding to the tinnitus frequency and consequently may have resulted in reduced tinnitus loudness.
It is important to note that this interpretation is supported by the correlation between tinnitus loudness change and 40-Hz ASSR ratio change. Given that tinnitus perception arises in auditory cortex, it is possible that the ASSR decrement, which could have resulted from the target notched music induced cortical reorganization, might have resulted in reduced tinnitus loudness. A previous study (28) demonstrated that gamma band (30–45 Hz) oscillations in auditory cortex reflected subjective tinnitus loudness as measured by visual analog scale. This finding might explain why in the present study the 40-Hz ASSR change correlates more strongly with the tinnitus loudness change than does the N1m response change.
In conclusion, our tailor-made notched music treatment strategy is derived from recent neuroscientific findings and targets the reversion of the maladaptive reorganization of a specific cortical area contributing to the perception of tinnitus. The notched music approach can be considered as enjoyable, low cost, and presumably causal treatment that is capable of specifically reducing tinnitus loudness. The notched music training could significantly complement widely used and rather indirect psychological treatment strategies for altering distributed cortical networks (12).
Methods
Patients.
Thirty-nine patients matching the following criteria were recruited: (i) chronic tinnitus (≥12 months), (ii) unilateral/ strongly lateralized tinnitus, (iii) tonal tinnitus (beep- or whistle-like), (iv) tinnitus frequency ≤8 kHz (limit for nonattenuated sound stimulation in our MEG), (v) no severe hearing impairment (41), (vi) no neurological or psychiatric complications. Rather strict criteria were set to maximize potential target notched music induced treatment effects.
Patients willing to participate in the music training were pseudorandomly assigned to one of two groups: (i) target notched music (Fig. 1 and Movie S1), or (ii) placebo notched music (Fig. 2 and Movie S2). The study was run double-blindly. The patients who did not have the time to participate in the treatment constituted a monitoring group. Over the course of the study, few patients dropped out between months 7 and 12 [drop-out rate per group: (i) target 2/13, (ii) placebo 3/13, (iii) monitoring 2/13], or were not included into the analyses due to either (i) unreliable tinnitus frequency, (ii) tinnitus frequency >8 kHz, or (iii) incomplete tinnitus loudness diaries [exclusion rate per group: (i) target 3/13, (ii) placebo 2/13, (iii) monitoring 4/13].
Finally, 23 patients completing the 12-month study were included into data evaluation [(i) target (n = 8), (ii) placebo (n = 8), (iii) monitoring (n = 7)]. Patients were fully informed about execution and goals of the study, and gave written informed consent in accordance with procedures approved by the Ethics Commission of the Medical Faculty, University of Muenster, Muenster, Germany.
Measurement of Subjective Tinnitus Characteristics.
Frequency.
The tonal tinnitus pitch was ipsi-laterally matched to the frequency of a pure tone at least four times on two different days. The median across pitch matches was considered as the tinnitus frequency. The determination of the frequency served as the means to estimate the auditory tonotopic area corresponding to the tinnitus perception and constituted the basis for the music modification [Figs. 1 and 2; Movies S1 (target notched music), S2 (placebo notched music), and S3 (original music)]. Over the course of the study, additional pitch matches were obtained regularly.
Loudness.
Tinnitus loudness was measured weekly on a continuous visual analog scale ranging from 0 (no tinnitus) to 100 (extremely loud tinnitus). Before the study, a baseline period of 4 weeks was surveyed. To compare treatment effects between subjects, we first normalized the tinnitus loudness means across months 1–6 and months 7–12 relative to the baseline period mean, and then calculated the change of the normalized tinnitus loudness {[(tinnitus loudness mean_months 1–6 or 7–12)/(tinnitus loudness mean_baseline) -1] × 100}. Thus, positive or negative change values indicate tinnitus loudness increment or decrement, respectively (Fig. 3). For statistical pre- vs. posttreatment comparison, planned contrasts were calculated. Because the patients had actively decided whether they wanted to participate in the treatment or not, statistics concerning more than one group (i.e., interactions and correlations) involved only target and placebo groups, not the monitoring group.
Auditory-evoked Field Measurements.
Magnetic fields were measured with a 275 channel MEG system in a magnetically shielded silent room. The baseline measurement took place before the study, course measurements were performed every 6 months.
We used two different sound stimuli, which were delivered randomly to either the left or the right ear. The frequency of one stimulus corresponded to a patient’s tinnitus frequency; the other stimulus had a frequency of 500 Hz (control stimulus). The tinnitus frequency stimulus evoked activity from a cortical region contributing to the tinnitus perception, the control stimulus from a cortical area not involved in the tinnitus perception.
Stimuli had a duration of 1 s. The initial 0.3 s were pure tones; the remaining 0.7 s were 40 Hz fully amplitude-modulated. The utilization of these stimuli enabled us to record both clean N1m and ASSR responses simultaneously (42). The loudness of the control stimulus was 45-dB sensation level; the tinnitus frequency stimulus was matched in loudness to the control stimulus before the baseline measurement. The power difference was kept identical across all course measurements. The sound onset asynchrony was randomized between 2 and 3 s.
The contour maps of both ASSR and N1m responses displayed clear dipolar patterns, motivating the use of a single dipole model for source analysis. For ASSR analysis, the grand-averaged magnetic field signals within the time range from 0.5 to 1 s were used for single equivalent current dipole estimations (43), and the maximal source strength for each condition and hemisphere was calculated by using the source space projection technique (44). For the N1m analysis, the grand-averaged magnetic fields were 30 Hz low-pass filtered and baseline corrected (31, 32, 45). Thereafter, the maximal source strength for each condition was calculated in a manner similar to the ASSR source strength calculation.
To control effects of head position differences within subjects between course measurements, we calculated ratios between source strengths evoked by the tinnitus frequency vs. the control frequency. To compare treatment effects on source strength ratios between subjects, we normalized the course measurement data relative to the baseline data, and then calculated the changes of the normalized ratios {[(source strength elicited by tinnitus frequency at month 6 or 12/source strength elicited by control frequency at month 6 or 12)/(source strength elicited by tinnitus frequency at baseline/source strength elicited by control frequency at baseline) − 1] × 100}. As for the tinnitus loudness changes, planned contrasts were also calculated to evaluate the normalized source strength changes.
Music Modification.
The patients from both treatment groups provided their favorite music, which was copied and filtered individually according to one of two protocols: (i) target (fixed) notch (c.f. Fig. 1 and Movie S1) or (ii) placebo (moving) notch (c.f. Fig. 2 and Movie S2). Irrespective of filtering protocol, the frequency bands below 707 Hz and above 15,321 Hz were not filtered. By means of the target notch modification, the frequency band of one octave width centered at the individual tinnitus frequency was removed from the music energy spectrum. In contrast, as a placebo music modification, a moving filter of one octave width, sparing the tinnitus frequency region, was applied. The moving filter randomly chose a frequency band outside the one octave wide tinnitus frequency region. After 5 s of filtering, the center frequency of the filter randomly jumped either 1/18 octave up or down and continued jumping in the same direction every 5 s until its lower or higher edge reached a predefined border, where it changed direction. The music delivered to both ears was filtered identically. Figs. 1 and 2 display the logic of the target and placebo music modifications. The patients listened to their individually modified treatment music daily via supplied closed headphones with convenient loudness over the course of one year. Listening times had to be documented daily.
Supplementary Material
Acknowledgments
We are grateful to Andreas Wollbrink, Karin Berning, Ute Trompeter, and Hildegard Deitermann for technical assistance. This work has been supported by the Deutsche Forschungsgemeinschaft (Pa 392/13-1, Pa 392/10-3) and the Tinnitus Research Initiative.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911268107/DCSupplemental.
References
- 1.McFadden D National Research Council (U.S.), Working Group 89. Tinnitus: Facts, Theories, and Treatments. Washington, D.C: National Academy Press; 1982. pp. xi–150. [Google Scholar]
- 2.Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003;36:239–248. doi: 10.1016/s0030-6665(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 3.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Saunders JC. The role of central nervous system plasticity in tinnitus. J Commun Disord. 2007;40:313–334. doi: 10.1016/j.jcomdis.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggermont JJ. Cortical tonotopic map reorganization and its implications for treatment of tinnitus. Acta Otolaryngol Suppl. 2006;556:9–12. doi: 10.1080/03655230600895259. [DOI] [PubMed] [Google Scholar]
- 7.Elbert T, Rockstroh B. Reorganization of human cerebral cortex: The range of changes following use and injury. Neuroscientist. 2004;10:129–141. doi: 10.1177/1073858403262111. [DOI] [PubMed] [Google Scholar]
- 8.Flor H, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 9.Merzenich MM, et al. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 10.Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diesch E, et al. Enhancement of steady-state auditory evoked magnetic fields in tinnitus. Eur J Neurosci. 2004;19:1093–1104. doi: 10.1111/j.0953-816x.2004.03191.x. [DOI] [PubMed] [Google Scholar]
- 12.Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: clinical implications. Br J Audiol. 1993;27:7–17. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- 13.Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- 14.Pantev C, Wollbrink A, Roberts LE, Engelien A, Lütkenhöner B. Short-term plasticity of the human auditory cortex. Brain Res. 1999;842:192–199. doi: 10.1016/s0006-8993(99)01835-1. [DOI] [PubMed] [Google Scholar]
- 15.Goebel G, Hiller W. The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO. 1994;42:166–172. [PubMed] [Google Scholar]
- 16.Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 17.Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- 18.Eggermont JJ, Ponton CW. The neurophysiology of auditory perception: From single units to evoked potentials. Audiol Neurootol. 2002;7:71–99. doi: 10.1159/000057656. [DOI] [PubMed] [Google Scholar]
- 19.Meikle MB. Electronic access to tinnitus data: the Oregon Tinnitus Data Archive. Otolaryngol Head Neck Surg. 1997;117:698–700. doi: 10.1016/S0194-59989770055-X. [DOI] [PubMed] [Google Scholar]
- 20.Eggermont JJ. Pathophysiology of tinnitus. Prog Brain Res. 2007;166:19–35. doi: 10.1016/S0079-6123(07)66002-6. [DOI] [PubMed] [Google Scholar]
- 21.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 22.Rajan R, Irvine DRF. Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory organ damage. Audiol Neurootol. 1998;3:123–144. doi: 10.1159/000013786. [DOI] [PubMed] [Google Scholar]
- 23.Eggermont JJ. The role of sound in adult and developmental auditory cortical plasticity. Ear Hear. 2008;29:819–829. doi: 10.1097/AUD.0b013e3181853030. [DOI] [PubMed] [Google Scholar]
- 24.Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- 25.Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2:e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlee W, et al. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80. doi: 10.1186/1741-7007-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahlbrock N, Weisz N. Transient reduction of tinnitus intensity is marked by concomitant reductions of delta band power. BMC Biol. 2008;6:4. doi: 10.1186/1741-7007-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Loo E, et al. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009;4:e7396. doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlee W, Hartmann T, Langguth B, Weisz N. Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci. 2009;10:11. doi: 10.1186/1471-2202-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto H, Kakigi R, Gunji A, Pantev C. Asymmetric lateral inhibitory neural activity in the auditory system: A magnetoencephalographic study. BMC Neurosci. 2007;8:33. doi: 10.1186/1471-2202-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pantev C, et al. Lateral inhibition and habituation of the human auditory cortex. Eur J Neurosci. 2004;19:2337–2344. doi: 10.1111/j.0953-816X.2004.03296.x. [DOI] [PubMed] [Google Scholar]
- 33.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turrigiano GG. The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwicker E. Negative afterimage in hearing. J Acoust Soc Am. 1964;36:2413–2415. doi: 10.1121/1.1913052. [DOI] [PubMed] [Google Scholar]
- 36.Franosch JM, Kempter R, Fastl H, van Hemmen JL. Zwicker tone illusion and noise reduction in the auditory system. Phys Rev Lett. 2003;90:178103. doi: 10.1103/PhysRevLett.90.178103. [DOI] [PubMed] [Google Scholar]
- 37.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zatorre R, McGill J. Music, the food of neuroscience? Nature. 2005;434:312–315. doi: 10.1038/434312a. [DOI] [PubMed] [Google Scholar]
- 39.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao SW, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 41.Guide for the evaluation of hearing handicap. JAMA. 1979;241:2055–2059. Anonymous. [PubMed] [Google Scholar]
- 42.Engelien A, Schulz M, Ross B, Arolt V, Pantev C. A combined functional in vivo measure for primary and secondary auditory cortices. Hear Res. 2000;148:153–160. doi: 10.1016/s0378-5955(00)00148-9. [DOI] [PubMed] [Google Scholar]
- 43.Ross B, Herdman AT, Pantev C. Right hemispheric laterality of human 40 Hz auditory steady-state responses. Cereb Cortex. 2005;15:2029–2039. doi: 10.1093/cercor/bhi078. [DOI] [PubMed] [Google Scholar]
- 44.Tesche CD, et al. Signal-space projections of MEG data characterize both distributed and well-localized neuronal sources. Electroencephalogr Clin Neurophysiol. 1995;95:189–200. doi: 10.1016/0013-4694(95)00064-6. [DOI] [PubMed] [Google Scholar]
- 45.Pantev C, et al. Specific tonotopic organizations of different areas of the human auditory cortex revealed by simultaneous magnetic and electric recordings. Electroencephalogr Clin Neurophysiol. 1995;94:26–40. doi: 10.1016/0013-4694(94)00209-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.