Rarely do the two broad motivations propelling molecular biological research—discovery of intricate molecular mechanisms and understanding the underlying causes of human disease—converge in a single system. An example of such convergence is provided by cystic fibrosis (CF), an all-too-common genetic disease characterized by devastating chronic lung infection in children and young adults (1).
The molecular culprit underlying the disease has been known for 20 years: cystic fibrosis transmembrane conductance regulator (CFTR), an epithelial, ATP-gated Cl− channel that normally functions to secrete fluid onto the air-exposed alveolar surface, thus maintaining mucus at a viscosity just right for capture and clearance of inhaled microorganisms and other junk and, according to an appealingly idiosyncratic proposal (2), to deliver to this surface a natural antimicrobial agent, the pseudohalide anion SCN−. The prominence of CF in the human population has provoked much research on the workings of CFTR and, in this issue, Csanády and coworkers (3) nail down a key feature—appealing in its own mechanistic idiosyncrasy—of how this Cl− channel opens and closes. By closely examining single CFTR channels in action, they establish that hydrolysis of ATP, not merely its binding, lies at the heart of the opening-closing process, and identify specific steps and structural features of the protein where ATP hydrolysis drives channel activity.
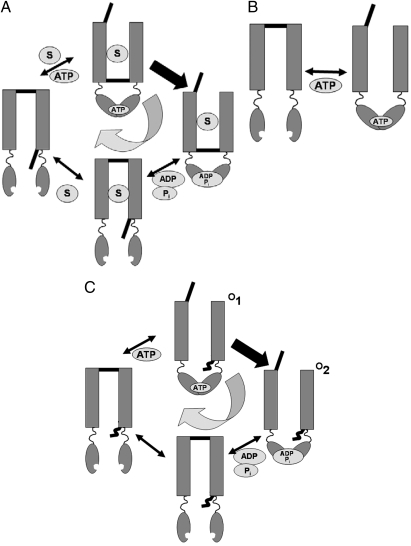
By amino acid sequence and imagined three-dimensional structure, CFTR should not be an ion channel at all, but rather an ATP-powered ‘‘pump.’’ The protein belongs to the large ABC family of membrane transporters whereby cells use ATP hydrolytic energy to push solutes uphill against concentration gradients, accumulating nutrients from the environment, for instance, or expelling xenobiotics such as chemotherapeutic drugs (4). Recent X-ray crystal structures show that ABC transporters couple conformational changes in a pair of cytoplasmic ATP-binding domains, where hydrolysis occurs, to rearrangements in the transmembrane domains, where transported substrates are alternately exposed to the two sides of the membrane. The pump can be crudely viewed as a substrate-binding site sandwiched between two “gates,” each blocking access to the corresponding side of the membrane (Fig. 1A). Energy-dependent pumping relies upon tight coordination of the inner and outer gates, which alternately open and close to bind substrate from one side and release it to the other in a scheme that is kept cycling in one direction by the ATP hydrolysis step, which operates far from equilibrium. For pumps like this to work, the protein must prevent both gates from ever being open simultaneously, lest a leak-channel form that would dissipate the very substrate gradient that the pump labors so assiduously to build up.
Fig. 1.
Skeleton mechanisms of ABC transporters and ligand-gated ion channels. (A) ABC transporter pumping cycle. A grossly oversimplified ABC-type pump is composed of two nucleotide-binding domains (NBD) attached to a membrane-spanning domain, which binds substrate S between two “gates.” Two conformations can exist, outward-facing (upper gate open, NBDs occupied) or inward-facing (lower gate open, NBDs empty). Double-headed arrows represent reversible steps, single-headed arrow an irreversible step. The scheme ignores details crucial to ABC mechanisms, such as the participation of two ATP molecules in the cycle, and intends merely to emphasize net cycling (curved arrow) resulting from including an irreversible step. (B) Equilibrium gating for idealized ATP-gated ion channel. The membrane-embedded part of the channel carries a single gate, open only when ATP is bound. Opening is thus driven merely by ATP binding, closing by dissociation. (C) Hydrolysis-driven CFTR gating. CFTR viewed as adhering to an ABC-like conformational cycle as in A, but with an always-open vestigial inner gate (for which no evidence exists). ATP hydrolysis drives the irreversible transition between the two Cl−-conducting states, O1 and O2.
Ligand-activated ion channels, in contrast, need not work by such elaborate schemes. All that is required is for the binding of a ligand such as ATP to favor an ion-conducting conformation of the protein in a linked set of equilibria (Fig. 1B). Here, there is no cycling; channel opening and closing occur across the same pathway, but in opposite directions. Microscopic reversibility—the strict equality of forward and reverse rates across every step in a scheme, and a fundamental demand of thermodynamic equilibrium—is satisfied. What about CFTR? Its phylogenetic membership in the ABC club provides it with all of the molecular equipment of an ATP-driven pump—nucleotide-binding domains linked by a familiar “coupling loop” to 12 membrane-embedded helices—and yet it appears in electrical recordings as a channel; single CFTR channels open and close stochastically in an ATP-dependent manner, the open state catalyzing exclusively “downhill” Cl− movement at rates of millions of ions per second, orders of magnitude too high for any enzymatic pump cycle to support. So, at first glance, CFTR looks like a familiar ligand-activated ion channel (Fig. 1B), with ATP as ligand.
But work beginning some 15 years ago (5–7) had indicated that CFTR requires hydrolysis of ATP, not just binding, and several schemes to account for this have been under consideration since that time. Conventional equilibrium gating mechanisms also remain in contention (8, 9). The present study nails the coffin closed on such mechanisms by recording Cl− currents through single CFTR channels, documenting in unprecedented detail a property that violates any equilibrium gating scheme. In particular, with goosed-up signal-to-noise recording, the authors quantify the statistical distribution of open-channel dwell times, finding it roughly bell-shaped (“peaked” in the field’s jargon). This observation may seem unremarkable, but to channel kineticists it immediately raises alarm bells, because fundamentals dictate that channel fluctuations at equilibrium must always have
CFTR should not be an ion channel at all, but rather an ATP-powered “pump.”
dwell-time distributions composed of monotonically decaying exponential functions (10). The observed peaked distribution with a clear rising phase at early times definitively signals a nonequilibrium step in CFTR’s gating scheme (Fig. 1C); it reflects the mechanistic property that channels leave the open state by a pathway different from the one by which they enter it—a direct violation of microscopic reversibility. This model-independent inference thus rules out all linked-equilibrium schemes for CFTR gating, as in Fig. 1B. (The complexities of proteins often invite respectable ambiguity in interpretation of experiments, but nobody wants to be caught committing outrages against thermodynamics in broad daylight.) To ice the cake, the authors exploit our developing mechanistic understanding of ABC transporters by mutating away the ATPase activity of the protein’s “catalytic” nucleotide-binding domain, a maneuver that eliminates the rising phase of the open-time distribution and restyles the channel to conventional, albeit crippled, equilibrium gating.
The results further show that CFTR cycles through at least two distinct conducting states (O1 and O2 in Fig. 1C). With this model in hand, the authors identify the irreversible step as ATP hydrolysis—no surprise there—and confirm previous suggestions that this occurs in the transition between O1 and O2 (1, 5, 6). In other words, simple ATP binding initially opens the channel into O1 but, once in O2, the channel, having hydrolyzed ATP, cannot close again to restart the cycle until it sheds its hydrolysis products. Thus, CFTR is a conformationally kosher membrane pump, but with a crucial difference from all other known ABC proteins. This pump has evolved to be leaky: to violate the rule that both gates must never be open simultaneously. Thus, Cl−-conducting conformations—the ragged lineaments of a broken pump—appear during the enzymatic cycle, to bestow on CFTR its physiological purpose. Although we do not yet have a high-resolution structure of CFTR itself, these experiments beautifully illustrate how the awesome power of single-channel analysis can reveal fundamentals as well as subtleties of ABC-family pumps.
Footnotes
The author declares no conflict of interest.
See companion article on page 1241.
References
- 1.Gadsby DC, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y, Szép S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci USA. 2009;106:20515–20519. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csanády, et al. This Issue.
- 4.Rees DC, Johnson E, Lewinson O. ABC transporters: The power to change. Nat Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baukrowitz T, Hwang T-C, Nairn AC, Gadsby DC. Coupling of CFTR Cl− channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: The role ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- 7.Carson MR, Travis SM, Welsh MJ. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem. 1995;270:1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- 8.Aleksandrov AA, Aleksandrov LA, Riordan JR. CFTR (ABCC7) is a hydrolyzable-ligand-gated channel. Pflugers Arch. 2007;453:693–702. doi: 10.1007/s00424-006-0140-z. [DOI] [PubMed] [Google Scholar]
- 9.Aleksandrov AA, Chang X, Aleksandrov L, Riordan JR. The non-hydrolytic pathway of cystic fibrosis transmembrane conductance regulator ion channel gating. J Physiol. 2000;528:259–265. doi: 10.1111/j.1469-7793.2000.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion-channel mechanisms. In: Sakmann B, Neher E, editors. Single-Channel Recording. 2nd Ed. New York: Plenum; 1995. pp. 397–482. [Google Scholar]