Abstract
The mechanisms underlying tumoral secretion of signaling molecules into the microenvironment, which modulates tumor cell fate, angiogenesis, invasion, and metastasis, are not well understood. Aberrant expression of transcription factors, which has been implicated in the tumorigenesis of several types of cancers, may provide a mechanism that induces the expression of growth and angiogenic factors in tumors, leading to their local increase in the tumor microenvironment, favoring tumor progression. In this report, we demonstrate that the transcription factor HOXB9 is overexpressed in breast carcinoma, where elevated expression correlates with high tumor grade. HOXB9 induces the expression of several angiogenic factors (VEGF, bFGF, IL-8, and ANGPTL-2), as well as ErbB (amphiregulin, epiregulin, and neuregulins) and TGF-ß, which activate their respective pathways, leading to increased cell motility and acquisition of mesenchymal phenotypes. In vivo, HOXB9 promotes the formation of large, well-vascularized tumors that metastasize to the lung. Thus, deregulated expression of HOXB9 contributes to breast cancer progression and lung metastasis by inducing several growth factors that alter tumor-specific cell fates and the tumor stromal microenvironment.
Keywords: breast cancer, ErbB, TGF-β, angiogenesis, epithelial to mesenchymal transition
Multifunctional cytokines, such as TGF-β and ErbB, and angiogenic factors secreted by the tumor and stroma initiate a dynamic interaction between the tumor and its microenvironment that modulates tumor growth and cell fates, angiogenesis, invasion, and distal metastasis—processes critical for disease progression. Little is known about the mechanisms underlying tumoral secretion of these signaling molecules. Aberrantly expressed transcription factors, implicated in the tumorigenesis of several types of cancers, may provide a mechanism to induce the expression of growth and angiogenic factors in tumors, leading to their local increase in the tumor microenvironment.
The class I HOX gene family comprises 39 members with a shared, highly conserved 61–amino acid homeodomain motif. These genes are important regulators of development, and their role in neoplastic transformation and tumor progression is being increasingly recognized (1). A number of HOX genes are expressed in the normal mammary gland. Mouse knockouts suggest that the ninth paralogous HOX genes play a role in mammary gland development (2). Mice homozygous for loss of HOXB9 exhibit developmental defects and a decline in newborn survival (3); loss of HOXA9, HOXB9, and HOXD9 impairs branching of the breast epithelium and lobuloalveolar development, leading to a failure to nurse pups (2). Although aberrant expression of some HOX members has been demonstrated in breast tumors (4–13), the functional consequence of deregulated HOX expression in cancer progression is not well understood.
HOX genes regulate several cellular processes, including angiogenesis and maintenance of cell fate (14–16). Epithelial-to-mesenchymal transition (EMT) is an embryonic morphogenetic conversion that is recapitulated during tumor progression. During EMT, destabilization of the epithelium increases cell motility, suggesting that EMT promotes invasion and metastasis. Several pathways, including those involving receptor tyrosine kinases, and TGF-β induce EMT-associated tumorigenic processes (17–21); thus, an interaction between the tumor and a microenvironment enriched with growth factors may facilitate tumor progression.
Our examination of the transcription factor HOXB9 was stimulated by its interaction with BTG2, a cell cycle regulator induced by the Mullerian inhibiting substance (MIS), which inhibits breast cancer cell growth (22–24). In this paper, we demonstrate that HOXB9 is overexpressed in 42% of breast cancers, specifically those of high histological grade, and we define the functional consequences of elevated HOXB9 expression in both breast cancer and nontransformed mammary epithelial cells. HOXB9 expression leads to the production of TGF-β, ErbB ligands, and several angiogenic factors, resulting in the induction of mesenchymal cell fate, invasion, and angiogenesis. In mouse tumor xenograft models, HOXB9 expression promotes increased neovascularization and tumor metastasis to the lung. These findings suggest that HOXB9 overexpression in breast tumors, by altering the microenvironment, induces several tumorigenic phenotypes and promotes disease progression.
Results
HOXB9 Expression in Breast Carcinoma.
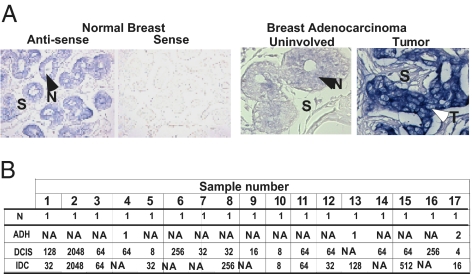
RNA in situ hybridization of a panel of 20 paraffin-embedded breast carcinoma samples demonstrated that HOXB9 mRNA is expressed within the normal breast epithelium; expression is restricted to the basal and luminal cells, with no stromal expression (Fig. 1A). In breast cancer, HOXB9 expression was higher in a fraction of tumors compared with adjacent normal glands (Fig. 1A).
Fig. 1.
Expression of HOXB9 in breast cancer. (A) (Left) Normal human breast tissues derived from reduction mammoplasty were hybridized in situ with antisense and sense-HOXB9 probes. (Right) HOXB9 mRNA expression in breast carcinoma and matched normal glands. The normal tissue and tumor are indicated by closed and open arrows, respectively. S, stroma; T, tumor; N normal. (Original magnification 400×) (B) qPCR analysis of HOXB9 expression in microdissected breast carcinoma samples. HOXB9 mRNA was elevated in 17 patients of a 40-breast cancer patient cohort. The table shows the tissues analyzed for each of the 17 patients (1–17) with elevated HOXB9 expression. The level of HOXB9 mRNA in each tissue compartment within a given patient is shown. The HOXB9 expression level in the matched normal gland was set at 1. NA, tissue not available; N, normal.
To further quantify HOXB9 expression in breast cancer, we analyzed cDNAs generated from laser-captured, purified populations of tumor cells and adjacent normal mammary epithelial cells from 40 clinically and pathologically annotated cases of breast cancer. The 40-patient cohort used in this study has been described previously (25, 26), and the clinicopathologic features of the tissue samples are shown in Table S1. Normal mammary epithelial cells and tumor cells from ductal carcinoma in situ (DCIS) were available for 40 and 39 patients, respectively; tumor cells from invasive ductal carcinoma (IDC) were available for 26 of these patients. Qualitative RT-PCR analysis demonstrated HOXB9 overexpression in 41% (16/39) of DCIS and in 42% (11/26) of IDC. The fold increase in HOXB9 ranged from 4 to 2,048 (mean, 198; median, 64) in DCIS and from 8 to 2,048 (mean, 290; median, 64) in IDC. RNA from atypical ductal hyperplasia (ADH)-derived cells was available from three patients, one of whom demonstrated a two-fold increase in HOXB9 compared with matched normal controls (Fig. 1B). Elevated HOXB9 expression in breast carcinoma correlated with tumor grade (P = 0.03, χ2 test), suggesting an association between HOXB9 overexpression and progression of breast cancer. Th Cochran-Armitage trend test confirmed a significantly increasing trend for HOXB9 overexpression in tumors of increasing grade (P = 0.02). Given the overexpression of HOXB9 in invasive breast cancer, we sought to define its functional properties using both breast cancer and nontransformed breast epithelial cells.
HOXB9 Induces EMT, Cell Motility, and Angiogenesis.
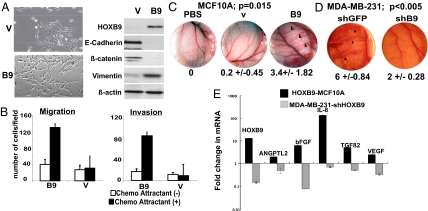
To test the functional consequence of HOXB9 overexpression in breast cancer, we introduced a myc-tagged HOXB9 construct into MCF10A immortalized mammary epithelial cells. Multiple clones were generated to avoid selection bias. Whereas vector-transfected MCF10A cells retained their epithelial characteristics, those expressing HOXB9 (HOXB9-MCF10A) exhibited a spindle-shaped morphology, loss of cell–cell contact, and formation of actin fibers (Fig. 2A, Fig. S1A) reminiscent of EMT. Cells demonstrated the loss of E-cadherin and β-catenin, and increased expression of vimentin (Fig. 2A), N-cadherin, Snail, Twist, Zeb1, and Zeb2 (Fig. S1A). Similar results were obtained with human mammary epithelial cells (HMECs) infected with a HOXB9-expressing lentivirus (Fig. S1B). HOXB9 expression also was associated with increased invasion and migration (Fig. 2B). Thus, ectopic expression of HOXB9 in mammary epithelial cells induces characteristic features of EMT.
Fig. 2.
HOXB9 induces EMT, cell motility, and angiogenesis. (A) (Left) Morphology of MCF10A cells expressing HOXB9 protein. (Right) Expression of HOXB9, E-cadherin, β-catenin, and vimentin in vector (V) and HOXB9 (B9)-MCF10A cells. (B) HOXB9 expression increases cell migration and invasion. Chemomigration and chemoinvasion of vector (V) and HOXB9 (B9)-MCF10A cells were assayed in the presence and absence of chemoattractant. The mean was derived from cell counts of nine fields. (C) HOXB9 induces angiogenesis in vivo. The dorsal air sac assay chambers contained PBS alone, vector-MCF10A (V), or HOXB9-MCF10A (B9) cells. Arrowheads highlight newly formed vasculature with a characteristic zigzag pattern in the HOXB9-MCF10A sample. (Original magnification 40×) The numbers under the images specify the mean number of newly formed vessels larger than 3 mm counted in each animal per experimental group (n = 5 mice per group). (D) Knockdown of HOXB9 suppresses angiogenesis in vivo. The dorsal air sac assay chambers contained MDA-MB-231 cells infected with shGFP or shHOXB9 (shB9). Arrowheads highlight newly formed vasculature with the characteristic zigzag pattern in the MDA-MB-231-shGFP samples. (Original magnification 40×) The numbers specify the mean number of newly formed vessels larger than 3 mm counted in each animal per experimental group (n = 5 mice per group). (E) RNA from vector and HOXB9-MCF10A cells and from MDA-MB-231 cells infected with shGFP and shHOXB9 was analyzed by qPCR to determine the expression of angiogenic factors shown in the figure. The fold change in expression relative to GAPDH is shown. The level of expression for each gene in vector-MCF10A and shGFP-MDA-MB-231 cells was set at 1.
We then tested the effect of HOXB9 expression on tumor-induced angiogenesis, another parameter associated with tumor progression. We used the dorsal air sac assay in mice and measured newly formed zigzag-patterned blood vessels >3 mm in length, a well-characterized measure of tumor-derived angiogenesis (27–29). Each experimental group consisted of five mice. HOXB9-MCF10A cells displayed a dramatic induction of new blood vessel formation, with a mean of 3.4 ± 1.82 vessels formed/chamber/mouse, compared with 0.2 ± 0.45 for vector-transfected MCF10A cells and none for PBS alone (Fig. 2C and Fig. S1C).
To determine whether the loss of HOXB9 is sufficient to suppress these phenotypes in invasive breast cancer cells, we selected MDA-MB-231 for further study, given its high level of HOXB9 expression (Fig. S1D) and well-characterized invasive properties. Two short hairpin (sh) HOXB9 lentiviral constructs capable of reducing endogenous HOBX9 levels by ≈85% were identified (Fig. S1E, Left). MDA-MB-231 cells infected with shHOXB9 exhibited decreased migration and invasion compared with those infected with shGFP (Fig. S1E). Moreover, shHOXB9-mediated knockdown of endogenous HOXB9 in MDA-MB-231 cells suppressed the formation of new blood vessels in vivo (Fig. 2D, Fig. S1C).
Consistent with the induction of angiogenesis, the expression of several genes involved in regulation of blood vessel formation (30), including ANGPTL2, b-fibroblast growth factor (bFGF), IL-8, TGF-β, and VEGF, was elevated in HOXB9-MCF10A. The expression of these genes was reduced in MDA-MB-231 cells in which HOXB9 expression was knocked down with shHOXB9 (Fig. 2E). Taken together, the combination of gain of function and loss of function model systems suggest that HOXB9 is a potent inducer of angiogenic factors and angiogenesis.
ErbB Receptor Activation Is Required for HOXB9-Induced Cell Motility.
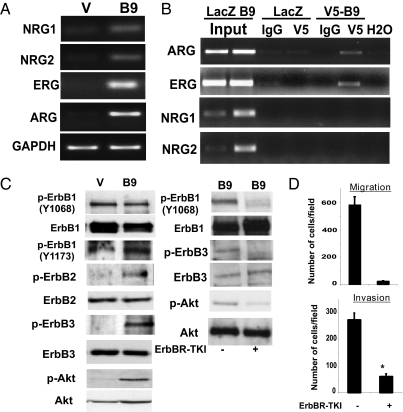
We next set out to identify the modulators and mechanisms responsible for the tumor-associated phenotypes observed in HOXB9-expressing cells. Consistent with the involvement of activated ErbB receptors in EMT and invasion (31–33), the expression of several ErbB ligands was elevated in HOXB9-MCF10A cells. Semiquantitative RT-PCR analysis demonstrated an increase in neuregulin (NRG)-1 and -2, epiregulin (ERG), and amphiregulin (ARG) mRNA in HOXB9-MCF10A cells (Fig. 3A). To determine whether these ligands are transcriptional targets of HOXB9, ChIP assays were performed on MCF10A cells infected with either LacZ- or V5-tagged HOXB9-expressing lentivirus. These ChIP assays identified HOXB9 protein binding to a stretch of DNA on the ARG and ERG promoters (Fig. 3B), which contain putative HOX-binding sites (Fig. S2A), suggesting that these differentially induced ligands are indeed transcriptional targets of HOXB9. Although NRG-1 and -2 promoters also contain putative HOX sites (Fig. S2A), binding of HOXB9 to the sites shown (Fig. 3B) could not be detected.
Fig. 3.
Suppression of ErbB and Akt activation inhibits HOXB9 enhanced cell motility. (A) Expression of NRGs, ERG, and ARG is increased in HOXB9-MCF10A cells. GAPDH is shown to control for equal loading. (B) ErbB ligands are transcriptional targets of HOXB9. MCF10A cells infected with LacZ and V5-tagged HOXB9 lentiviruses were analyzed by ChIP with anti-V5 and control IgG antibodies. Total lysates were used as controls for input. Precipitated DNA was subjected to PCR using primers spanning the promoter region containing the putative HOX-binding sites. (C) (Left) HOXB9 expression is associated with increased ErbB receptor phosphorylation. Proteins from vector and HOXB9-expressing cells were analyzed for activation of ErbB1, ErbB2, and ErB3, and Akt by Western blot. The lysates were also probed for total protein. (Right) Inhibition of ErbB receptor phosphorylation abolishes Akt phosphorylation. HOXB9-MCF10A cells were treated with 10 μM ErbB receptor tyrosine kinase inhibitor (ErbBR-TKI) for 2 h, and proteins were analyzed by Western blot to monitor ErbB receptor and Akt phosphorylation and total ErbB receptor and Akt levels. (D) Suppression of ErbB receptor and Akt phosphorylation is associated with abrogation of cell migration. Migration of ErbBR-TKI–treated HOXB9-expressing MCF10A cells was assayed. The mean was derived from cell counts of nine fields.
Because ErbB ligands are elevated in HOXB9-MCF10A cells, we tested the activation state of the ErbB receptor tyrosine kinases (RTKs) using phosphospecific antibodies against the respective subunits. ErbB2 and ErbB3 phosphorylation was markedly elevated in HOXB9-MCF10A cells, wheereas the phosphorylation of ErbB1 was not significantly altered (Fig. 3C, Left). Consistent with activation of the ErbB pathway, downstream Akt phosphorylation also was elevated in HOXB9-MCF10A cells (Fig. 3C, Left).
Treatment of cells with an ErbB kinase inhibitor (34, 35) effectively suppressed ErbB receptor and Akt phosphorylation (Fig. 3C, Right), suggesting that Akt phosphorylation is driven by activated ErbB receptors. Moreover, the ErbB inhibitor effectively suppressed the migration and invasion of cells (Fig. 3D), supporting an important role for this pathway in HOXB9-mediated motility. The PI3 kinase inhibitor, LY29004, exhibited a similar effect, consistent with Akt phosphorylation being the key intermediate in this RTK-dependent pathway (Fig. S2B); however, the morphological and molecular features of HOXB9-induced EMT were not reversed by inhibition of the RTK pathway (Fig. S2C). Thus, increased signaling through the ErbB–Akt axis induced by HOXB9 promotes cell motility, but not EMT.
Activation of TGF-β Contributes to Both HOXB9-Induced EMT and Cell Motility.
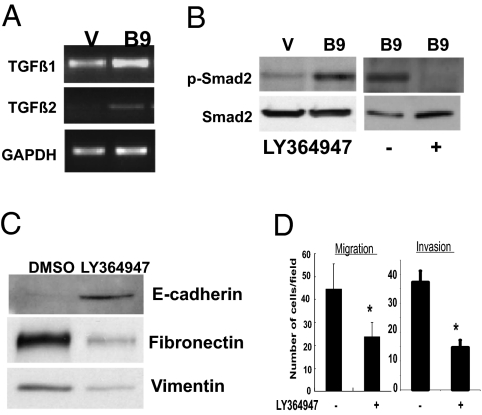
Along with the ErbB ligands, TGF-β, a well-known regulator of EMT (36, 37), is also induced in HOXB9-expressing cells. Thus, we explored whether TGF-β activation influences the EMT phenotype, complementing the effect of ErbB signaling observed in HOXB9-MCF10A cells. Expression of TGF-β1 and TGF-β2 was elevated in HOXB9-MCF10A cells (Fig. 4A). The increase in TGF-β2 was coincident with increased HOXB9 binding to the promoter regions harboring HOX-binding sites (Fig. S2D). Because no consensus HOX-binding sites could be identified in a region 2 kB upstream of the TGF-β1 promoter, ChIP assays were not performed.
Fig. 4.
TGF-β activation contributes to EMT and increased motility of HOXB9-MCF10A cells. (A) Expression of TGF-β1 and TGF-β2 is increased in HOXB9-MCF10A cells. GAPDH is shown to control for equal loading. (B) (Left) HOXB9 expression is associated with increased Smad2 phosphorylation. Proteins from vector (V) and HOXB9 (B9)-MCF10A cells were analyzed for phospho-Smad2 and total Smad2 protein. (Right) HOXB9-MCF10A cells were treated with 10 μM LY364947 for 24 h. Proteins were analyzed for phospho-Smad2 and total Smad2 levels. (C) HOXB9-MCF10A cells were treated with 10 μM LY364947, and total protein was analyzed for E-cadherin, fibronectin, and vimentin expression. (D) Migration and invasion of LY364947-treated HOXB9-MCF10A cells was assayed.
Consistent with TGF-β activation, phosho-Smad2 expression was elevated in HOXB9-MCF10A cells (Fig. 4B). Suppression of TGF-β receptor signaling using the inhibitor LY364947 suppressed Smad2 phosphorylation (Fig. 4B), without affecting the expression of TGF-β1 and TGF-β2 (Fig. S2E). Treatment with LY364947, an inhibitor of TGF-β receptor signaling, induced epithelial morphological changes (Fig. S2E), along with reexpression of E-cadherin and suppression of fibronectin and vimentin (Fig. 4C). Like ErbB inhibitors, suppression of TGF-β signaling also reduced the migration and invasiveness of HOXB9-MCF10A cells (Fig. 4D). This indicates that the induction of ErbB ligands and TGF-β expression by HOXB9 activates two critical signaling pathways implicated in transformation to mesenchymal cell fate: cellular motility and invasive properties of human cancer.
HOXB9 Expression Promotes Tumor Growth and Distal Metastasis.
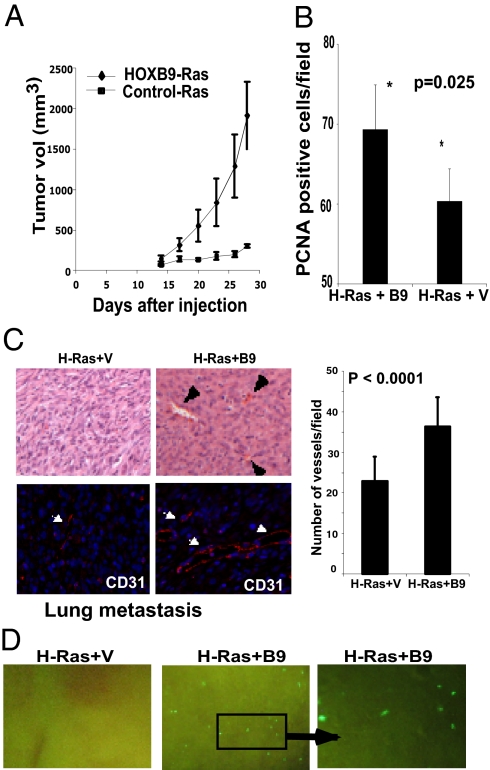
Although HOXB9 was able to induce phenotypes associated with tumor progression, MCF10A cells expressing HOXB9 were unable to grow on soft agar or form tumors when injected into nude mice, suggesting that HOXB9 itself lacks the ability to induce neoplastic transformation. To investigate whether HOXB9 enhances tumorigenesis, we stably introduced the activated G12V H-Ras allele into HOXB9-MCF10A cells and investigated their ability to form tumors upon s.c. inoculation into mice. H-Ras mutations are rare in human breast cancer, but activation of the Ras pathway is an essential characteristic of RTK activation and as such is a valuable model for growth factor receptor–mediated oncogenesis. As expected, vector-MCF10A and HOXB9-MCF10A cells were not tumorigenic. MCF10A cells expressing activated H-Ras alone gave rise to small palpable tumors. In contrast, animals inoculated with HOXB9 + H-Ras-MCF10A cells rapidly developed very large tumors (n = 8 mice per group; Fig. 5A).
Fig. 5.
HOXB9 expression promotes tumor growth, angiogenesis, and distal metastasis to the lung. (A) MCF10A cells expressing activated H-Ras alone or activated H-Ras + HOXB9 were inoculated s.c. into mice; the mean change in tumor volume ± SD for each group is shown (n = 8 mice per group). (B) HOXB9-expressing tumors exhibit a higher proliferation index. Tumors from the two groups were stained with antibody to the proliferation marker PCNA. The mean number of PCNA-positive cells ± SD per field is shown (n = 10 fields). (C) HOXB9+activated-Ras tumors exhibit increased vascularization. Vessels are indicted with arrowheads. Quantification of vessels by CD31 staining is shown below. (Original magnification 200×) (D) HOXB9 expression promotes lung metastasis. Lungs of activated H-Ras+HOXB9 tumor-bearing mice (50%) demonstrate micrometastases, whereas none of the animals (0%) bearing activated H-Ras tumors show signs of lung metastasis. The GFP-expressing cell clusters in the lung were visualized under a dissecting microscope. The right panel shows a higher-magnification image of the inset. (Original magnification 40×).
Proliferating cell nuclear antigen (PCNA) staining of tumors demonstrated a significantly higher proliferation rate in H-Ras+HOXB9 tumors compared with tumors expressing H-Ras alone (Fig. 5B). The HOXB9 + H-Ras tumors were more vascularized than tumors generated from H-Ras-MCF10A cells (Fig. 5C). Moreover, 50% of animals harboring HOXB9 + H-Ras-MCF10A tumors demonstrated metastatic nodules in the lungs, whereas none of the H-Ras-MCF10A tumors metastasized (P = 0.038; Fig. 5D, Fig. S3).
We further explored the tumorigenic potential of HOXB9 using GFP-expressing MDA-MB-231 cells in which endogenous HOXB9 was knocked down with ShHOXB9. Knockdown of HOXB9 led to decreased tumor size (Fig. S4A), a significant decrease in proliferation (Fig. S4B), reduced tumor vascularity (Fig. S4C), and metastasis to lung (Fig. S4D). Thus, HOXB9 expression is a potent enhancer of tumorigenesis and plays a role in the formation of large, vascularized, invasive tumors, capable of metastatic spread to the lung.
Discussion
We have demonstrated frequent HOXB9 overexpression in invasive human breast cancer and have dissected its effect using gain of function studies in nontransformed mammary epithelial cells, as well as loss of function analyses in breast cancer cells expressing endogenous HOXB9. HOXB9 induces cell fate alteration, cellular motility, angiogenesis, and lung metastasis.
Our observation that HOXB9 is overexpressed in 42% of human breast tumors is consistent with the deregulation of other HOX genes (4–13), although only limited insight is available into the functional and molecular consequences of HOX gene alterations in cancer. Analysis of HOXB9-dependent phenotypes suggests that deregulated HOXB genes may be involved in reprogramming cancer cells toward a more mesenchymal and potentially more invasive state by tumoral production and secretion of several growth factors that alter the microenvironment so as to favor tumor progression (Fig. S5).
In addition to cell autonomous changes, such as EMT and motility, HOXB9 enhanced angiogenic recruitment by tumor cells, a key component of tumor–stromal interactions associated with invasiveness. The degree of angiogenesis induced by HOXB9, as assessed by the dorsal air sac assay, is comparable to that reported in other studies (27, 28). HOXB9-mediated angiogenesis is correlated with the induction of bona fide angiogenic factors VEGF, bFGF, TGF-β, ANGPTL2 and IL-8, which are involved in proliferation and differentiation of endothelial cells, smooth muscle cells and fibroblasts, integration of survival signals, regulation of vascular permeability, and cell–matrix interactions (30). Multiple HOX-binding sites are present in the promoters of ANGPTL2, IL-8, VEGF and bFGF, suggesting that these genes are likely targets of HOX proteins; whether they are directly influenced by HOXB9 itself remains to be tested. Nonetheless, our findings support the conclusion that HOXB9 overexpression enriches the microenvironment with angiogenic factors that initiate a broad angiogenic program, enabling tumor vascularization and distal metastasis (Fig. S5). Our results also identify HOXB9 as an effector of breast cancer metastasis to the lung, an observation consistent with a recent report of HOXB9 promoting metastasis of lung adenocarcinoma (38).
The induction of ErbB ligands and TGF-β by HOXB9 (Fig. S5) points to additional pathways that are critical to both cell autonomous growth and tumor–stromal interactions. Among the ErbB receptors, ErbB2 and ErbB3 are highly phosphorylated in HOXB9-MCF10A cells. ErbB3 is the predominant activator of PI3 kinase and Akt signaling (39, 40). ErbB receptors regulate the proliferation and migration of several types of epithelial cells including those of the mammary gland, and ErbB2 and ErbB3 heterodimers have been implicated in enhanced cell migration and invasiveness (41, 42). The ability of an ErbB receptor inhibitor to suppress endogenous baseline phosphorylation of ErbB receptors and Akt in HOXB9-MCF10A cells, and also to abrogate their invasive phenotype, strongly supports the importance of ErbB activation by HOXB9 for this phenotype. TGF-β appears to be involved in both cell migration and EMT induction by HOXB9, as demonstrated by the use of TGF-β inhibitors. Our results thus identify distinct components of the HOXB9 phenotype resulting from the induction of ligands that activate well-defined signaling pathways with diverse cellular consequences.
Although HOXB9 induces several tumor-associated phenotypes, it does not transform mammary epithelial cells. Thus, aberrant expression of HOXB9 in breast cancer is likely to be a tumor progression factor, rather than a tumor-initiating event. This is consistent with the association of HOXB9 overexpression in human breast cancer with increasing tumor grade. Supporting this model, MCF10A cells coexpressing activated H-Ras and HOXB9 form large, invasive tumors, unlike those expressing mutant H-Ras alone. Ras mutations are infrequent in breast cancer (<5%), yet pathologic activation of Ras in breast cancer can be achieved by overexpression of growth factor receptors that signal through Ras (43, 44). Thus, aberrant HOXB9 in human breast cancer may enhance the oncogenic effects of activated ErbB2, EGFR (ErbB1), and other RTKs implicated in breast tumorigenesis. Therapeutic agents targeting ErbB2 alone have a dramatic effect on breast cancers harboring amplification of the ErB2/HER2 gene, but their benefit in tumors lacking such specific oncogene lesions is less evident. Indeed, for breast cancers overexpressing HOXB9 (and possibly other breast cancer–associated HOX genes as well), a more complex but coordinated pattern of growth factor signaling dependencies may be activated. Combined suppression of ErbB, TGF-β, and possibly additional signaling pathways to target this coordinated program may be required for effective tumor inhibition.
Materials and Methods
Cell Culture.
Culture conditions used for the maintenance of cell lines and generation of HOXB9-MCF10A clones are described in SI Materials and Methods.
Western Blot Analysis.
The antibodies used for detection are described in SI Materials and Methods.
RNA Analysis.
PCR conditions and primer sequences used for detection of transcripts are described in SI Materials and Methods.
Cell Migration and Invasion Assays.
In vitro chemomigration and chemoinvasion assays are described in SI Materials and Methods.
In Situ Hybridization.
The HOXB9 probe used for in situ hybridization (ISH) and the protocols used for detection are described in SI Materials and Methods.
Patients, Breast Carcinoma Samples and HOXB9 Expression Analysis.
All human breast cancer samples were collected in accordance with protocols approved by the Massachusetts General Hospital Human Research Committee. The breast tumor cohort used to quantify HOXB9 expression in laser capture microdissected cells, and the tumor samples used for analyzing HOXB9 expression by ISH, are described in SI Materials and Methods.
Mouse Dorsal Air Sac Assay.
The mouse dorsal air sac assay was performed as described in SI Materials and Methods.
Chromatin Immunoprecipitation Assay.
The protocol used for ChIP is described in SI Materials and Methods.
Tumorigenicity in Mice.
All animals were cared for and experiments performed in this facility in accordance with American Association for Laboratory Animal Science guidelines following protocols approved by Massachusetts General Hospital’s Institutional Animal Care and Use Committee. MCF10A xenografts were established as described in SI Materials and Methods. Characterization of the tumors on resection is described in SI Materials and Methods.
Knockdown of HOXB9 in MBA-MB-231 Cells.
Knockdown of HOXB9 expression in MDA-MB-231 cells was done as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Kurt J. Isselbacher, Daniel A. Haber, and Jeffrey Settleman for critically reading this manuscript. This work was supported by National Institutes of Health/National Cancer Institute Grant CA89138 and Susan G. Komen for the Cure Grants PDF0600282 and KG090412 (to S.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912710107/DCSupplemental.
References
- 1.Abate-Shen C. Deregulated homeobox gene expression in cancer: Cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Capecchi MR. Paralogous mouse Hox genes, Hoxa9, Hoxb9, and Hoxd9, function together to control development of the mammary gland in response to pregnancy. Proc Natl Acad Sci USA. 1999;96:541–546. doi: 10.1073/pnas.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, Capecchi MR. Targeted mutations in hoxa-9 and hoxb-9 reveal synergistic interactions. Dev Biol. 1997;181:186–196. doi: 10.1006/dbio.1996.8440. [DOI] [PubMed] [Google Scholar]
- 4.Bodey B, Bodey B. Siegel SE, Kaiser HE., Jr. Immunocytochemical detection of the homeobox B3, B4, and C6 gene products in breast carcinomas. Anticancer Res. 20(5A):3281–3286. [PubMed] [Google Scholar]
- 5.Cantile M, et al. In vivo expression of the whole HOX gene network in human breast cancer. Eur J Cancer. 2003;39:257–264. doi: 10.1016/s0959-8049(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 6.Carrio M, Arderiu G, Myers C, Boudreau NJ. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer Res. 2005;65:7177–7185. doi: 10.1158/0008-5472.CAN-04-1717. [DOI] [PubMed] [Google Scholar]
- 7.Chariot A, Castronovo V. Detection of HOXA1 expression in human breast cancer. Biochem Biophys Res Commun. 1996;222:292–297. doi: 10.1006/bbrc.1996.0737. [DOI] [PubMed] [Google Scholar]
- 8.Jansen MP, et al. HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: A retrospective study. J Clin Oncol. 2007;25:662–668. doi: 10.1200/JCO.2006.07.3676. [DOI] [PubMed] [Google Scholar]
- 9.Ma XJ, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Makiyama K, et al. Aberrant expression of HOX genes in human invasive breast carcinoma. Oncol Rep. 2005;13:673–679. [PubMed] [Google Scholar]
- 11.Raman V, et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000;405:974–978. doi: 10.1038/35016125. [DOI] [PubMed] [Google Scholar]
- 12.Rubin E, et al. A role for the HOXB7 homeodomain protein in DNA repair. Cancer Res. 2007;67:1527–1535. doi: 10.1158/0008-5472.CAN-06-4283. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, et al. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial–mesenchymal transition. Cancer Res. 2006;66:9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- 14.Kapur RP, Gershon MD, Milla PJ, Pachnis V. The influence of Hox genes and three intercellular signalling pathways on enteric neuromuscular development. Neurogastroenterol Motil. 2004;16(Suppl 1):8–13. doi: 10.1111/j.1743-3150.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 15.Cantile M, Schiavo G, Terracciano L, Cillo C. Homeobox genes in normal and abnormal vasculogenesis. Nutr Metab Cardiovasc Dis. 2008;18:651–658. doi: 10.1016/j.numecd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- 17.Cheng GZ, et al. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr Cancer Drug Targets. 2008;8:2–6. [PubMed] [Google Scholar]
- 18.Wu Y, Zhou BP. New insights of epithelial–mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2008;40:643–650. doi: 10.1111/j.1745-7270.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klymkowsky MW, Savagner P. Epithelial–mesenchymal transition: A cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr S, et al. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis. 2008;25:685–693. doi: 10.1007/s10585-007-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 22.Kawakubo H, et al. Loss of B-cell translocation gene-2 in estrogen receptor–positive breast carcinoma is associated with tumor grade and overexpression of cyclin d1 protein. Cancer Res. 2006;66:7075–7082. doi: 10.1158/0008-5472.CAN-06-0379. [DOI] [PubMed] [Google Scholar]
- 23.Segev DL, et al. Mullerian inhibiting substance regulates NFkappaB signaling and growth of mammary epithelial cells in vivo. J Biol Chem. 2001;276:26799–26806. doi: 10.1074/jbc.M103092200. [DOI] [PubMed] [Google Scholar]
- 24.Prévôt D, et al. The leukemia-associated protein Btg1 and the p53-regulated protein Btg2 interact with the homeoprotein Hoxb9 and enhance its transcriptional activation. J Biol Chem. 2000;275:147–153. doi: 10.1074/jbc.275.1.147. [DOI] [PubMed] [Google Scholar]
- 25.Ma XJ, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci USA. 2003;100:5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartwell KA, et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci USA. 2006;103:18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita S, et al. The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumors. Cancer Res. 1999;59:1911–1916. [PubMed] [Google Scholar]
- 28.Yonekura K, et al. UFT and its metabolites inhibit the angiogenesis induced by murine renal cell carcinoma, as determined by a dorsal air sac assay in mice. Clin Cancer Res. 1999;5:2185–2191. [PubMed] [Google Scholar]
- 29.Kohno T, et al. Interleukin-10–mediated inhibition of angiogenesis and tumor growth in mice bearing VEGF-producing ovarian cancer. Cancer Res. 2003;63:5091–5094. [PubMed] [Google Scholar]
- 30.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 31.Lo HW, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial–mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabbah M, et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Hiscox S, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 34.Godin-Heymann N, et al. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther. 2008;7:874–879. doi: 10.1158/1535-7163.MCT-07-2387. [DOI] [PubMed] [Google Scholar]
- 35.Rabindran SK, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 36.Muraoka-Cook RS, Dumont N, Arteaga CL. Dual role of transforming growth factor beta in mammary tumorigenesis and metastatic progression. Clin Cancer Res. 2005;11:937s–943s. [PubMed] [Google Scholar]
- 37.Moses HL, Serra R. Regulation of differentiation by TGF-beta. Curr Opin Genet Dev. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen DX, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HH, Sierke SL, Koland JG. Epidermal growth factor–dependent association of phosphatidylinositol 3-kinase with the erbB3 gene product. J Biol Chem. 1994;269:24747–24755. [PubMed] [Google Scholar]
- 40.Soltoff SP, Carraway KL, 3rd, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Mol Cell Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sithanandam G, Fornwald LW, Fields J, Anderson LM. Inactivation of ErbB3 by siRNA promotes apoptosis and attenuates growth and invasiveness of human lung adenocarcinoma cell line A549. Oncogene. 2005;24:1847–1859. doi: 10.1038/sj.onc.1208381. [DOI] [PubMed] [Google Scholar]
- 42.Xue C, et al. ErbB3-dependent motility and intravasation in breast cancer metastasis. Cancer Res. 2006;66:1418–1426. doi: 10.1158/0008-5472.CAN-05-0550. [DOI] [PubMed] [Google Scholar]
- 43.Clark GJ, Der CJ. Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat. 1995;35:133–144. doi: 10.1007/BF00694753. [DOI] [PubMed] [Google Scholar]
- 44.Malaney S, Daly RJ. The ras signaling pathway in mammary tumorigenesis and metastasis. J Mammary Gland Biol Neoplasia. 2001;6:101–113. doi: 10.1023/a:1009572700317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.