Abstract
Life-history inference is an important aim of paleoprimatology, but life histories cannot be discerned directly from the fossil record. Among extant primates, the timing of many life-history attributes is correlated with the age at emergence of the first permanent molar (M1), which can therefore serve as a means to directly compare the life histories of fossil and extant species. To date, M1 emergence ages exist for only a small fraction of extant primate species and consist primarily of data from captive individuals, which may show accelerated dental eruption compared with free-living individuals. Data on M1 emergence ages in wild great apes exist for only a single chimpanzee individual, with data for gorillas and orangutans being anecdotal. This paucity of information limits our ability to make life-history inferences using the M1 emergence ages of extinct ape and hominin species. Here we report reliable ages at M1 emergence for the orangutan, Pongo pygmaeus (4.6 y), and the gorilla, Gorilla gorilla (3.8 y), obtained from the dental histology of wild-shot individuals in museum collections. These ages and the one reported age at M1 emergence in a free-living chimpanzee of approximately 4.0 y are highly concordant with the comparative life histories of these great apes. They are also consistent with the average age at M1 emergence in relation to the timing of life-history events in modern humans, thus confirming the utility of M1 emergence ages for life-history inference and providing a basis for making reliable life-history inferences for extinct apes and hominins.
Keywords: dental histology, great apes, tooth eruption, tooth growth
To fully understand the evolution of primate life histories, it is important to be able to evaluate the life histories of fossil species within the wider context of those of extant primates. The correlations between molar eruption schedules and various life-history variables provide a means to do this (1). The age at first molar (M1) emergence in particular provides a reliable proxy from which to infer the overall pace of life history in fossil species (1). This requires accurate knowledge about the relationships between M1 emergence and various life-history attributes in living species. However, there are currently deficiencies in the data on extant primate M1 emergence ages that limit the accuracy and reliability of life-history inference for fossil species.
First, data on age at M1 emergence exist for only 26 primate species (2–4), and there are major impediments to expanding this database by the usual methods, most of which require long-term field or laboratory studies wherein animals are sedated at regular intervals for radiography and/or to monitor tooth emergence. Given the difficulty of collecting dental emergence data from living animals using these techniques, the number of species in the sample is not likely to increase greatly. Apes in particular are poorly represented, and no reliable data exist for any species other than the common chimpanzee, Pan troglodytes (5, 6). Second, the extant primate database has been constructed mostly using captive animals (2). There is now evidence suggesting that captive animals exhibit accelerated development compared with free-living individuals, including earlier emergence of teeth (4, 7–10).
These deficiencies have led some to question the reliability and usefulness of M1 emergence ages for inferring the life histories of fossil species, including fossil hominins (11). Thus, it is critical to obtain M1 emergence data on additional species, especially African and Asian apes, and to obtain these data from noncaptive animals. Here we report reliable ages at M1 emergence for the orangutan (Pongo pygmaeus pygmaeus) and the gorilla (Gorilla gorilla gorilla), obtained from wild-shot individuals in museum osteology collections and calculated entirely from the incremental growth lines preserved in the enamel and dentine of the teeth.
Teeth preserve both short- and long-period growth lines that are visible in histological thin sections. These include, respectively, daily cross striations and Retzius lines in the enamel and the corresponding von Ebner and Andresen lines in dentine (12–15). Although there is intra- and interspecific variation in Retzius/Andresen line periodicity (i.e., the number of daily short-period lines between adjacent long-period lines), within any individual the periodicity is constant (12, 14, 16). Counts of these lines, therefore, reveal the time taken to form a tooth, including the tooth crown and however much root had formed at the time of death (17) (Figs. 1 and 2; see Materials and Methods). The correlations between life-history variables and age at M1 emergence are particularly fortuitous because, in all higher primates, the M1 begins to form at or just before birth. Therefore, in an individual that died when the M1 was emerging, the total formation time of the M1 yields both the age at death as well as the age at M1 emergence.
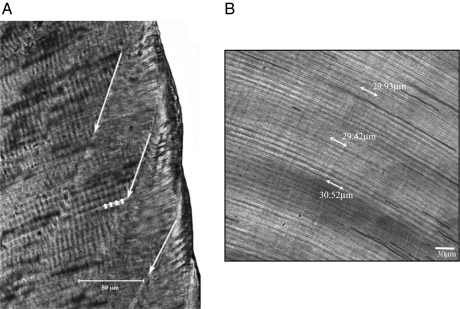
Fig. 1.
Polarized light micrograph of a section through the mesial cusps (protoconid on left) of the Gorilla gorilla M1 (specimen ZSM 1913/1163). (A) Entire section and (B) magnified view of the protoconid cusp showing the neonatal line (red arrows) in the enamel. (C) Magnified view of the lateral enamel illustrates a series of successive striae of Retzius (white arrows) that appear as black lines running from the outer enamel surface (left) toward the dentine (lower right).
Fig. 2.
Close-ups of a section through the mesial cusps of the Pongo pygmaeus M1 (UIC specimen). (A) Striae of Retzius in the lateral enamel (long white arrows) outcropping at the outer enamel surface (right) and daily cross-striations (short white arrows). (B) Close-up of a field of dentine tubules that run obliquely from the upper left to lower right; arrows indicate linear measurements across 10 successive daily von Ebner lines, which can be seen oriented perpendicular to the long axis of the tubules. In this region of dentine, the daily lines are spaced, on average, 2.99 μm apart.
Ground sections were prepared from the M1s (one maxillary, one mandibular) of two Pongo individuals and one Gorilla (mandibular), all of whom died while the M1 was erupting (Fig. 1; see SI Materials and Methods). All could be demonstrated to be just past the stage of initial gingival penetrance—the clinical definition of tooth emergence—despite the lack of gingival remnants in osteological specimens. In one of the Pongo teeth, the cusps were slightly higher than the level typical of gingival emergence, based on comparisons versus other primate individuals for which there is a radiographic record of eruption and direct observations of gingival emergence (18). In the second Pongo M1, and in that of the gorilla, eruption had also proceeded slightly beyond initial gingival emergence, indicated by the presence of food protein stain only at the tips of some cusps and delimited by a distinct gingival line (19).
Total formation time (TFT) for each M1 was calculated from the mesial cusp region (plus one section through the distal cusps in one of the Pongo specimens) using two different methods, one using the incremental lines in both the crown enamel and root dentine, and the other using only the axial dentine extending from the tip of the dentine horn underlying the tooth crown to the last-formed dentine adjacent to the pulp chamber (see Materials and Methods). TFT for one of the Pongo M1s was calculated using both the enamel-plus-dentine and the dentine-only methods to check for consistency of results.
Results
Summary results from each of the histological sections are shown in Table1 (see Tables S1–S3 for full details). For the two Pongo individuals, total M1 formation times, and therefore ages at death, were nearly identical at 4.78 and 4.66 y, respectively. Different age determinations from the first individual were highly consistent regardless of the method used (4.76 vs. 4.79 y) or whether the section was from the mesial or distal cusps (4.79 vs. 4.80 y). As the M1s of both Pongo individuals had undergone gingival emergence, age at M1 emergence in both would have been somewhat earlier than the age at death. Based on the position of the mesial cusps in relation the deciduous premolars and the alveolar margin of the mandible, eruption had proceeded somewhat further in the first individual. Therefore, slightly more time had probably elapsed between M1 emergence and death in this individual than in the second. We estimate this elapsed time at approximately 2 months in the first individual and 1 month in the second, or 0.17 and 0.08 y, respectively (see SI Materials and Methods). This results in an estimated age at M1 gingival emergence of 4.6 y in both individuals. However, we note that, although we did not observe prenatally formed enamel (delimited by an accentuated neonatal growth line [Fig. 1B]) in either Pongo individual, if prenatal enamel was in fact present in these individuals, the ages at death would be reduced by approximately 1 month and ages at gingival emergence would be closer to 4.5 y (see Table S1).
Table 1.
Summary of crown formation time, root formation time, total tooth formation time, and age at death for the two P. pygmaeus M1s and the G. gorilla M1
| Parameter | P. pygmaeus pygmaeus | G. gorilla gorilla | |
| Individual 1 | Individual 2 | ||
| Prenatal formation, d (y) | 0* | 0* | 48 (0.13) |
| CFT , d (y) | 1,079 (2.96)† | 1,152 (3.16) | 1,025 (2.81) |
| RFT , d (y) | 671 (1.84)† | 547 (1.49) | 449 (1.23) |
| TFT (CFT + RFT), d (y) | 1,749 (4.79) | 1,699 (4.66) | 1,474 (4.04) |
| TFT (dentine)‡, d (y) | 1,737 (4.76) | NA | NA |
| Age at death, y | 4.78§ | 4.66 | 3.91|| |
CFT, crown formation time; RFT, root formation time.
*Prenatal enamel was not observed, but some prenatal enamel formation is common in Pongo M1s (see text).
†Average of crown and root formation times from mesial and distal M1 sections (see Table S1 for details).
‡Method II determination for this individual only (see Materials and Methods and Table S1 for details).
§Represents the average TFT derived from both the mesial and distal sections (both determined by method I; see Materials and Methods) and from the axial dentine (method II).
||Age at death = 4.04 − 0.13 y.
Age at death in the gorilla individual was calculated to be 3.91 y. As in the two orangutans, M1 emergence slightly preceded death in this individual, and by approximately the same amount of time judging by the extent of protein stains on the cusps. Therefore, age at M1 emergence is estimated to have been at 3.8 y, or nearly 1 year earlier than in the two orangutans. However, because, in primates, the mandibular M1 nearly always emerges somewhat earlier than the maxillary M1 (ref. 2; see SI Materials and Methods), and because one of our two orangutan specimens is a maxillary tooth, the difference between Pongo and Gorilla might not be quite as great as suggested by these results.
Discussion
The median age at M1 emergence in captive chimpanzees is 3.18 y for the maxillary M1 (range, 2.26–4.38 y) and 3.15 y for the mandibular M1 (range, 2.14–3.99 y) (6). Based on these data, plus questionable estimates of 3.5 y for both Gorilla (20) and two captive Pongo individuals (2), mean ages at M1 emergence for all of the great apes have commonly been reported to be in the range of 3.0 to 3.5 y. Until now, the only reliable estimate for age at M1 emergence in noncaptive apes was for a single individual of Pan troglodytes verus, at approximately 4.1 y for the maxillary M1 (4). The likely age of emergence for the mandibular M1 in this individual was approximately 3.8 to 3.9 y, resulting in a combined age of M1 emergence of approximately 4.0 y (see SI Materials and Methods). The M1 emergence ages for noncaptive animals reported here of 4.6 y in Pongo and 3.8 y in Gorilla (mandibular M1 only) offer support for the likelihood of a later average age at M1 emergence in free-living chimpanzees than in captive animals. Although the M1 emergence age for Pongo is based on only two individuals, the uniformity of the two values suggests that the average age at M1 emergence in the orangutan is likely to be substantially greater than in chimpanzees or gorillas, although data from more individuals will be needed to confirm this. The age at M1 emergence of 3.8 y in the Gorilla mandibular M1 is the same as our estimate of 3.8 to 3.9 y for the mandibular M1 in the noncaptive chimpanzee (4), but, again, more data will be needed to determine if the means for these two great apes are in fact coincident (as discussed further later).
Although limited, the cumulative data therefore suggest that the average age at M1 emergence in noncaptive extant great apes ranges from just younger than 4 y to just older than 4.5 y, or approximately 1 year later than previously supposed.
The ages at M1 emergence reported here, particularly those for Pongo, as well as the later age reported for noncaptive Pan (4), are also consistent with the comparative life histories of these species in relation to one another and in comparison with that of modern humans. Ages at M1 emergence between 3.0 and 3.5 y for great apes represent only approximately 52% to 60% of the average age at M1 emergence of 5.8 y in modern humans, the latter based on an average of various non-European/nonwhite American populations (21, 22) (see SI Materials and Methods). In contrast, the ages for M1 emergence reported here for wild great apes represent 65% to 80% of the modern human value. This range is more concordant with the durations or ages of attainment for many key life-history attributes of great apes, which are generally in the range of 55% to 80% of the modern human values (Table 2) (23–27).
Table 2.
Comparative life-history variables and age at M1 emergence in extant great apes and humans
| Variable | Gorilla | Pan | Pongo | Homo |
| Age at first reproduction, y | 10.1* (51.3%) | 14.3† (72.6%) | 15.7‡ (79.7%) | 19.7 |
| Interbirth interval, y | 4.3 | 5.8† | 6.9‡ | 3.4§ |
| Survivorship, y|| | ? | 29.7 (54.9%) | 43.0 (79.5%) | 54.1 |
| Age at M1 emergence, y | 3.8 (65.5%) | 4.0† (69.0%) | 4.6‡ (79.3%) | 5.8 |
Numbers in parentheses indicate the percentage relative to values in humans. Life history sources: Gorilla (23, 24); Pan (25); Pongo (26); Homo (27); all survivorship data from ref 27. M1 sources: Pan (4); Homo (21, 22); Gorilla and Pongo (present study).
*Value for mountain gorilla, G. g. beringei, which is likely to be earlier than in G. g. gorilla (see text for explanation).
†Values for P. t. verus only (Taï Forest, Ivory Coast).
‡Values for P. p. pygmaeus only (Borneo), omitting a single value for age at first reproduction from Gunung Palung (26), which involves an age estimate.
§Interbirth interval is anomalously low in modern humans compared with other anthropoid species. This life-history variable is included for between-ape comparisons only and percentages of human values were not calculated.
||Expected age at death at age 15 y based on empirically derived survival curves (see Materials and Methods for further discussion).
The relatively late age at M1 emergence for Pongo compared with those of the other great ape species is particularly noteworthy. Recent findings on the life histories of wild orangutans reveal that many life-history milestones occur much later than in chimpanzees or gorillas, and that life stages are consequently of longer duration (26, 28, 29) (Table 2). The late ages at M1 emergence in the two Pongo individuals described here are consistent with this more prolonged life-history profile. The estimated age at M1 emergence in the gorilla, at 3.8 y, is likewise broadly compatible with the comparative life-history data for great apes and humans (Table 2). Based on the even shorter life-history schedule in gorillas than in chimpanzees, we would predict that analyses of larger samples would show a somewhat earlier mean age at M1 emergence in the former. Moreover, the schedule of M1 eruption among Gorilla subspecies might be expected to vary, with the faster-growing and more folivorous mountain gorilla (G. g. beringei) predicted to possess an even earlier eruption age than the more frugivorous western lowland gorilla analyzed here (30, 31).
It has recently been claimed that body mass is a better predictor of great ape life histories than “dental development,” and that the latter is only weakly related to the timing of life-history events (11), but there are several problems with this analysis. First, the authors of this study (11) included data from the entire dentition rather than just the first molar, whereas it is the latter that is most strongly correlated with the timing of life-history events (1). Second, they included data on tooth crown formation as well as crown emergence, despite there being almost no difference in M1 crown formation times among Pongo, Gorilla, and humans (11), resulting in poor correlations with the timing of life-history events. Last, their data on the timing of molar emergence include some unreliable, anecdotal information and also combine data from both wild and captive animals (11). Our results and the life-history data in Table 2 demonstrate that, contrary to the conclusions of Robson and Wood (11) and when the emergence of only the first molar is considered, dental eruption is strongly related to the timing of life-history events in humans and free-living great apes. It follows that age at M1 emergence should provide reliable inferences about the timing of life-history events in fossil members of this clade, including early hominins.
Finally, our results demonstrate the utility of obtaining ages at M1 emergence entirely from the histology of first molars that were in the process of erupting. Whereas previous studies of both extant and extinct primate taxa have demonstrated the feasibility of obtaining ages at death using dental histology (13, 17, 32–39), and these have occasionally included the first molar, we propose that analyses of dental histology be extended specifically to individuals that died while the M1 was erupting in a variety of extant primate species, with the express purpose of providing more reliable ages at M1 emergence and therefore more accurate representation of the correlations between age at M1 emergence and the timing of life-history events for primates as a whole. This is the most practical method for developing an inventory of ages at M1 emergence from noncaptive individuals of many primate species. As we have done here, this can be accomplished by sampling wild-shot individuals from museum collections that died when their first molars were erupting. Using this method, the relationships between various life-history variables and age at M1 emergence in extant primates can be portrayed more accurately than is currently possible with M1 emergence ages that have been obtained mostly from captive animals. This will lead to more accurate interpretations of life history in extinct primates, including early hominins, and a better understanding of the evolution of life-history variation within the primate order.
Materials and Methods
Specimen Preparation.
Erupting M1s were extracted from the skulls of three African and Asian ape osteological specimens and then prepared for histological analysis. Two of the M1s were from Borean orangutans (P. pygmaeus pygmaeus): a maxillary M1 from a skull in the osteology collection of the College of Dentistry, University of Illinois at Chicago, and a mandibular M1 (specimen 1981/233) from a skull in the collections of the Zoologische Staatssammlung, Munich, Germany. The third specimen was a mandibular M1 from the skull of a western lowland gorilla (G. gorilla gorilla, specimen 1913/1163), also from the Zoologicshe Staatssammlung, Munich, Germany.
Following extraction, the teeth were photographed and examined for any wear or staining, either of which would indicate that the molar had pierced the gingiva and/or was in functional occlusion. Each molar was molded, cleaned, and embedded in epoxy to prevent shattering during sectioning. Two sections were prepared from each tooth, one through the apices of the mesial cusps and one through the distal cusps. All sections were initially approximately 500 μm thick and were progressively lapped to a final thickness of approximately 100 μm using a graded series of abrasive discs and then polished with a 3-μm aluminum oxide powder. The sections were then ultrasonicated, rinsed in both 90% and anhydrous alcohol, cleared in CitraSolve, and mounted with DPX medium. The sections were examined using polarized light microscopy and images were captured using a Spot Insight 4-MB digital camera and image analysis system.
TFTs.
To determine TFTs, we employed two different methods as a test of methodological reliability. One method used both crown and root components (method I) and the other used only coronal/axial dentine (method II).
Method I.
TFT equals crown formation time (cuspal enamel formation time plus lateral enamel formation time) plus the time to form however much root was present at the time of death. Cuspal enamel formation time equals the cuspal enamel thickness measured along a prism divided by the average daily secretion rate of ameloblasts, the enamel matrix forming cells. Secretion rate was calculated as the grand mean from >10 measurements of daily cross-striation lengths in each of the inner, middle, and outer regions of the cuspal enamel (secretion rate typically increases from inner to outer enamel; Fig. 2). Lateral enamel formation time equals the total number of lateral enamel Retzius lines multiplied by their periodicity, which is constant in any individual (Figs. 1 and 2). Root formation time was calculated in different ways depending upon the preservation of growth lines in the root dentine (see Fig. S1): (i) by measuring the root cone thickness along an odontoblast path (or dentine tubule) at the tooth cervix (the point of root initiation) and dividing that distance by the average daily secretion rate of odontoblasts (the innermost dentin of the root cone adjacent to the pulp chamber represents the time of death); (ii) by multiplying the number of Andresen lines in the cervical root cone by their periodicity (in d), which is the same as the periodicity of Retzius lines in the enamel; (iii) by measuring the maximum length of root formed (measured along the granular layer of Tomes from the cervical margin to the last formed dentine at the tip of the root cone) and dividing that distance by the average root extension rate (RER), a measure of the rate of root elongation in μm/d, which was determined by averaging extension rates taken from many different regions along the root surface from the cervical margin to the tip of the developing root cone (see Fig. S1 legend for details on calculating regional RERs); or (iv) the same procedure as in i, but with the root segmented into three portions defined by prominent accentuated Andresen lines and then summing the formation times of the three segments.
Method II.
Axial dentine thickness—measured in the coronal dentine along a single dentine tubule from the tip of the dentine horn to the pulp chamber—is divided by the average daily odontoblast secretion rate. This yields an estimate of TFT as it records the time span between the first-formed dentine at the dentine horn (coincident with the initiation of tooth formation) and the last-formed dentine (at the time of death) at the margin of the pulp chamber (see Fig. S1).
Life-History Variables.
For the comparative life-history data, we chose three key variables relating to fecundity or lifetime reproductive success: age at first reproduction, interbirth interval, and survivorship, the latter being an expression of longevity (Table 2). We chose survivorship rather than maximum lifespan as an expression of longevity for several reasons. First, with very small samples, maximum lifespan is not particularly reliable as a species characteristic, and for all the included taxa save modern humans would be based on a single individual. Second, because even the longest periods of continuous observation of great apes in the wild have not been of sufficient duration to record the birth dates of the oldest individuals that have died, ages at death for these individuals are estimates. Finally, and specifically concerning comparisons to modern humans, the ability in human societies for others to partly assume the care of and provide for elderly individuals makes it more likely that some individuals will live nearer to the actual physiological maximum of the species. This combined with samples sizes that are many orders of magnitude larger than those for great apes renders comparisons of maximum lifespan between humans and great apes somewhat questionable. Nevertheless, maximum lifespan data are still useful as supporting documentation of species longevity, especially for comparisons between great ape species (discussed further later).
In contrast to maximum lifespan, survivorship curves incorporate longevity data from the entire sample, which allows for statistically meaningful comparisons between taxa. Among other useful metrics from survivorship curves, in addition to projected age at death for individuals that survive until age 15 y (used in Table 2), is the age at which the probability of survival becomes very low. For example, the ages at which the probability of survival equals 0.1 are approximately 35 y in Pan and 54 y in Pongo (28, 40).
Unfortunately, the only comparison that can be made for gorillas at this time is the estimated age of the longest living individual in the wild. Among mountain gorillas, the longest-lived individual, a female, was estimated to have been somewhat older than 42 y at death. In contrast, the oldest surviving chimpanzee, again a female, was estimated to be 55 y and still alive when this age was reported, whereas the oldest chimpanzee male was estimated to be 46 y at death (40). Among orangutans, the oldest wild female was estimated to be 52 y and still alive, whereas the oldest male was estimated to be 58 y at death (27). Although the longevity records for chimpanzees and orangutans are fairly similar, it has been noted that those for orangutans are based on much smaller samples and suggested that samples equivalent to those of chimpanzees (and with similarly long periods of observation) might be expected to reveal even older orangutan individuals (27). It was also noted that this would be compatible with the consistently higher probabilities of survival at all ages in orangutan survival tables compared with those of chimpanzees (27).
Supplementary Material
Acknowledgments
We thank Dr. Richard Kraft, Director, and the Zoologische Staatssammlung, Munich, Germany, for allowing us to remove and section the molars from specimens housed at their institution. We also thank Kierstin Catlett, Frank Cuozzo, William Hughes, and Don Reid for assistance with specimen preparation and/or analysis, and Chris Dean, Terry Ritzman, Holly Smith, Tanya Smith, and David Watts for helpful conversations or for providing unpublished data. G.S. was supported by the Institute of Human Origins at Arizona State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906206107/DCSupplemental.
References
- 1.Smith BH. Dental development as a measure of life history in primates. Evolution. 1989;43:683–688. doi: 10.1111/j.1558-5646.1989.tb04266.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith BH, Crummet TL, Brandt KL. Age of eruption of primate teeth: a compendium for aging individuals and comparing life histories. Yearb Phys Anthropol. 1994;37:177–231. [Google Scholar]
- 3.Eaglen RH. Behavioral correlates of tooth eruption in Madagascar lemurs. Am J Phys Anthropol. 1985;66:307–315. [Google Scholar]
- 4.Zihlman A, Bolter D, Boesch C. Wild chimpanzee dentition and its implications for assessing life history in immature hominin fossils. Proc Natl Acad Sci USA. 2004;101:10541–10543. doi: 10.1073/pnas.0402635101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissen HW, Riesen AH. The eruption of the permanent dentition of chimpanzees. Am J Phys Anthropol. 1964;22:285–294. doi: 10.1002/ajpa.1330220315. [DOI] [PubMed] [Google Scholar]
- 6.Kuykendall KL, Mahoney CJ, Conroy GC. Probit and survival analysis of tooth emergence ages in a mixed-longitudinal sample of chimpanzees (Pan troglodytes) Am J Phys Anthropol. 1992;89:379–399. doi: 10.1002/ajpa.1330890310. [DOI] [PubMed] [Google Scholar]
- 7.Phillips-Conroy J, Jolly C. Dental eruption schedules of wild and captive baboons. Am J Primatol. 1988;15:17–29. doi: 10.1002/ajp.1350150104. [DOI] [PubMed] [Google Scholar]
- 8.Hamada Y, Udono T, Teramoto M, Sugawara T. The growth pattern of chimpanzees: Somatic growth and reproductive maturation in Pan troglodytes. Primates. 1996;37:279–295. [Google Scholar]
- 9.Kimura T, Hamada Y. Growth of wild and laboratory born chimpanzees. Primates. 1996;37:237–251. [Google Scholar]
- 10.Schwartz GT, Reid DJ, Dean MC, Zihlman AL. A faithful record of stressful life events preserved in the dental developmental record of a juvenile gorilla. Int J Primatol. 2006;27:1201–1219. [Google Scholar]
- 11.Robson SL, Wood B. Hominin life history: reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean MC. Growth layers and incremental markings in hard tissues: a review of the literature and some preliminary observations about enamel structure in Paranthropus boisei. J Hum Evol. 1987;16:157–172. [Google Scholar]
- 13.Dean MC. Tooth microstructure tracks the pace of human life history. Proc R Soc Lond B Biol Sci. 2006;273:2799–2802. doi: 10.1098/rspb.2006.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith TM. Experimental determination of the periodicity of incremental features in enamel. J Anat. 2006;208:99–114. doi: 10.1111/j.1469-7580.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith TM. Incremental dental development: Methods and applications in hominoid evolutionary studies. J Hum Evol. 2008;54:205–224. doi: 10.1016/j.jhevol.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GT, Reid DJ, Dean MC. Developmental aspects of sexual dimorphism in hominoid canines. Int J Primatol. 2001;22:837–860. [Google Scholar]
- 17.Smith TM, Reid DJ, Sirianni JE. The accuracy of histological assessments of dental development and age at death. J Anat. 2006;208:125–138. doi: 10.1111/j.1469-7580.2006.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley J, Smith TM. Age at first molar emergence in early Miocene Afropithecus turkanensis and life-history evolution in the Hominoidea. J Hum Evol. 2003;44:307–329. doi: 10.1016/s0047-2484(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 19.Kelley J, Dean MC, Ross S. In: Interdisciplinary Dental Morphology. Koppe T, Meyer G, Alt KW, editors. Basel: Karger; pp. 128–133. [Google Scholar]
- 20.Willoughby DP. All About Gorillas. South Brunswick, NJ: A.S. Barnes & Company; 1978. [Google Scholar]
- 21.Smith RJ, Gannon PJ, Smith BH. Ontogeny of australopithecines and early Homo: evidence from cranial capacity and dental eruption. J Hum Evol. 1995;29:155–168. [Google Scholar]
- 22.Liversidge H. In: Patterns of Growth and Development in the Genus Homo. Thompson JL, Krovitz GE, Nelson AJ, editors. Cambridge: Cambridge University Press; 2003. pp. 73–113. [Google Scholar]
- 23.Watts DP. Mountain gorilla reproduction and sexual behavior. Am J Primatol. 1991;24:211–226. doi: 10.1002/ajp.1350240307. [DOI] [PubMed] [Google Scholar]
- 24.Yamagiwa J, Kahekwa J. In: Mountain Gorillas: Three Decades of Research at Karisoke. Robbins MM, Sicotte P, Stewart KJ, editors. Cambridge: Cambridge University Press; 2001. pp. 90–122. [Google Scholar]
- 25.Boesch C, Boesch-Achermann H. Oxford: Oxford University Press; 2000. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. [Google Scholar]
- 26.Knott CD, Emery Thompson M, Wich SA. In: Orangutans: Geographic Variation in Behavioral Ecology and Conservation. Wich SA, Utami-Atmoko SS, Mitra Setia T, van Schaik CP, editors. Oxford: Oxford University Press; 2009. pp. 171–188. [Google Scholar]
- 27.Kaplan H, Hill K, Lancaster JA, Hurtado M. A theory of human life history evolution. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- 28.Wich SA, et al. Life history of wild Sumatran orangutans (Pongo abelii) J Hum Evol. 2004;47:385–398. doi: 10.1016/j.jhevol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Shumaker RW, Wich SA, Perkins L. Reproductive life history traits of female orangutans (Pongo spp.) Interdiscip Top Gerontol. 2008;36:147–161. doi: 10.1159/000137705. [DOI] [PubMed] [Google Scholar]
- 30.Breuer T, Hockemba MB, Olejniczak C, Parnell RJ, Stokes EJ. Physical maturation, life-history classes and age estimates of free-ranging western gorillas – insights from Mbeli Bai, Republic of Congo. Am J Primatol. 2008;71:106–119. doi: 10.1002/ajp.20628. [DOI] [PubMed] [Google Scholar]
- 31.McFarlin SC, et al. Recovery and preservation of a mountain gorilla skeletal resource in Rwanda. Am J Phys Anthropol. 2009;S48:187–188. [Google Scholar]
- 32.Beynon AD, Dean MC, Reid DJ. Histological study on the chronology of the developing dentition in gorilla and orangutan. Am J Phys Anthropol. 1991;86:189–203. [Google Scholar]
- 33.Dean CM, Beynon AD, Thackeray JF, Macho GA. Histological reconstruction of dental development and age at death of a juvenile Paranthropus robustus specimen, SK 63, from Swartkrans, South Africa. Am J Phys Anthropol. 1993;91:401–419. doi: 10.1002/ajpa.1330910402. [DOI] [PubMed] [Google Scholar]
- 34.Beynon AD, Dean MC, Leakey MG, Reid DJ, Walker A. Comparative dental development and microstructure of Proconsul teeth from Rusinga Island, Kenya. J Hum Evol. 1998;35:163–209. doi: 10.1006/jhev.1998.0230. [DOI] [PubMed] [Google Scholar]
- 35.Dirks W. Histological reconstruction of dental development and age of death in a juvenile gibbon (Hylobates lar) J Hum Evol. 1998;35:411–425. doi: 10.1006/jhev.1997.0185. [DOI] [PubMed] [Google Scholar]
- 36.Dirks W, Reid DJ, Jolly CJ, Phillips-Conroy JE, Brett FL. Out of the mouths of baboons: Stress, life history, and dental development in the Awash National Park hybrid zone, Ethiopia. Am J Phys Anthropol. 2002;118:239–252. doi: 10.1002/ajpa.10089. [DOI] [PubMed] [Google Scholar]
- 37.Dirks W. Effect of diet on dental development in four species of catarrhine primates. Am J Primatol. 2003;61:29–40. doi: 10.1002/ajp.10106. [DOI] [PubMed] [Google Scholar]
- 38.Smith TM, Toussaint M, Reid DJ, Olejniczak AJ, Hublin JJ. Rapid dental development in a Middle Paleolithic Belgian Neanderthal. Proc Natl Acad Sci USA. 2007;104:20220–20225. doi: 10.1073/pnas.0707051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith TM, et al. Dental development of the Taï Forest chimpanzees revisited. J Hum Evol. in press doi: 10.1016/j.jhevol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Hill K, et al. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.