Abstract
The use of bioengineered human skin as a bioreactor to deliver therapeutic factors has a number of advantages including accessibility that allows manipulation and monitoring of genetically modified cells. We demonstrate a skin gene therapy approach that can regulate blood pressure and treat systemic hypertension by expressing atrial natriuretic peptide (ANP), a hormone able to decrease blood pressure, in bioengineered human skin equivalents (HSE). Additionally, the expression of a selectable marker gene, multidrug resistance (MDR) type 1, is linked to ANP expression on a bicistronic vector and was coexpressed in the human keratinocytes and fibroblasts of the HSE that were grafted onto immunocompromised mice. Topical treatments of grafted HSE with the antimitotic agent colchicine select for keratinocyte progenitors that express both MDR and ANP. Significant plasma levels of human ANP were detected in mice grafted with HSE expressing ANP from either keratinocytes or fibroblasts, and topical selection of grafted HSE resulted in persistent high levels of ANP expression in vivo. Mice with elevated plasma levels of human ANP showed lower renin levels and, correspondingly, had lower systemic blood pressure than controls. Furthermore, mice with HSE grafts expressing human ANP did not develop elevated blood pressure when fed a high-salt diet. These findings illustrate the potential of this human skin gene therapy approach to deliver therapeutic molecules systemically for long-term treatment of diverse diseases.
Keywords: keratinocytes, human engineered skin, hypertension, multi-drug resistance gene, retroviral vectors
Although skin can be easily accessed for manipulation, a major challenge of using skin gene therapy to treat systemic diseases has been the difficulty of achieving sustained expression of desired therapeutic genes in a high percentage of keratinocytes (Kc) (1–8), in part because few good markers of Kc stem cells exist for isolation and gene targeting (9–13). Previously, we described how topical colchicine treatment of human skin equivalents (HSE) containing Kc engineered to express the multidrug resistance (MDR) gene could select and enrich for MDR-expressing human Kc progenitor cells because only MDR-expressing Kc were able to pump-out the antimitotic inhibitor and proliferate (5). However, the therapeutic utility of this gene therapy approach in treating systemic diseases was unproven.
Gene therapy strategies that have been discussed for the treatment of hypertension include overexpression of genes such as atrial natriuretic peptide (ANP) that are relevant in reducing blood pressure (14, 15). ANP is a 126-amino acid peptide hormone synthesized mainly by right atrial cardiomyocytes as an inactive precursor (pro-ANP) (Fig. S1). Pro-ANP is secreted and converted into an active ANP peptide of 28 amino acids (α-ANP) by corin receptors located on the cell surface of cardiomyocytes (16, 17) in response to volume expansion and increases in venous pressure. In target tissues such as kidney and endothelial cells, the active α-ANP peptide signals by binding its specific receptor, NPR-A, which increases intracellular cGMP levels and decreases blood pressure by lowering systemic vascular resistance and increasing renal salt excretion, two effects aided by inhibition of the renin-angiotensin-aldosterone pathway. Infusion of ANP has been demonstrated to decrease blood pressure in hypertensive patients (18, 19) and animal models (20, 21), but long-term systemic expression of ANP would be required to control blood pressure. Although systemic ANP delivery and a decrease in blood pressure has been achieved following i.v. administration of ANP-expressing adenoviral vectors that targeted the liver (22), our goal was to achieve stable, long-term expression of systemic ANP and blood pressure control using an approach that allows regulation of ANP levels without requiring repeated doses of vector and without eliciting unwanted immune responses. We demonstrate a human skin gene therapy approach to express systemically sustained levels of human ANP that are sufficient to normalize blood pressure effectively in hypertensive mice, an approach that could produce and deliver therapeutic molecules for a variety of systemic diseases.
Results
Genetically Modified HSE that Express ANP Exhibit Normal Differentiation and Stratification.
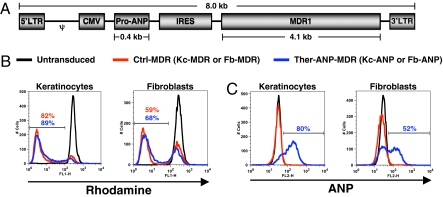
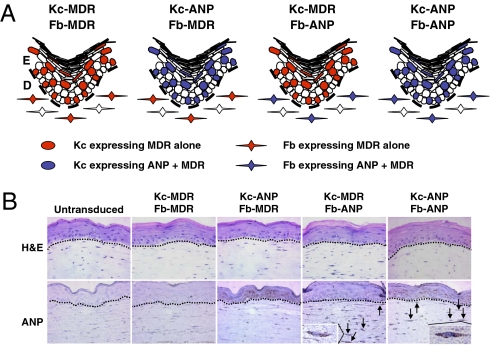
Two bicistronic retroviral vectors were constructed to express ANP and MDR stably in Kc and fibroblasts (Fb): one expressing MDR alone (control vector; Ctrl-MDR), and the other expressing both pro-ANP and MDR (therapeutic vector; Ther-ANP-MDR) (Fig. 1A). High transduction efficiency of human Kc and Fb by both therapeutic and control vectors was demonstrated by flow cytometry using a rhodamine exclusion assay in which the amount of rhodamine pumped out is determined by the cellular MDR level (Kc-MDR, 82%; Fb-MDR, 59%; Kc-ANP, 89%; Fb-ANP, 68%) (Fig. 1B) and by measuring intracellular ANP (Kc-ANP, 80%; Fb-ANP, 2%) (Fig. 1C). HSE that were generated by transducing human Kc or Fb (or both) with Ther-ANP-MDR (50% transduction efficiency) (Fig. 2A) had a normal differentiated and stratified human epidermis (Fig. 2B), similar to the control untransduced HSE. Immunohistochemistry labeling of the genetically modified HSE confirms ANP expression in the epidermis for transduced Kc expressing ANP and MDR/transduced Fb expressing MDR only (Kc-ANP)/(Fb-MDR) and Kc-ANP/ transduced Fb expressing ANP and MDR (Fb-ANP) HSE and in the dermis for transduced Kc expressing MDR only (Kc-MDR)/Fb-ANP and Kc-ANP/Fb-ANP (Fig. 2B). These data show that the expression of two novel proteins in Kc and Fb did not compromise the ability of HSE to form a differentiated and stratified epidermis.
Fig. 1.
Bicistronic retroviral constructs and retroviral transduction efficiencies in primary human Kc and Fb. (A) Schematic of the therapeutic vector, Ther-ANP-MDR, expressing both pro-ANP and MDR, used to generate viral particles. The control vector (Ctl-MDR) lacks the pro-ANP gene but is otherwise identical to Ther-ANP-MDR. Transduction efficiency was determined by the rhodamine exclusion assay that measures MDR functionality (B) and by measuring intracellular pro-ANP (C). Human Kc and Fb were transduced with the therapeutic vector Ther-ANP-MDR (blue line), and control vector, Ctl-MDR (red line). Untransduced Kc and Fb (black line) were used to control for the absence of MDR and ANP expression in skin cells. These data are representative of multiple transduction experiments with similar transduction efficiencies.
Fig. 2.
Human Kc and Fb are genetically modified to express ANP and MDR and are used to generate different HSE combinations. (A) Different combinations of HSE expressing ANP or MDR in Kc (Kc-ANP/Fb-MDR), Fb (Kc-MDR/Fb-ANP) or both (Kc-ANP/Fb-ANP). (B) Representative areas of paraffin-embedded sections of normal HSE generated from untransduced Kc and Fb and different combinations of genetically modified HSE. Arrows indicate Fb expressing ANP.
Colchicine Selection Increases the Amount of ANP that Is Secreted by Genetically Modified HSE.
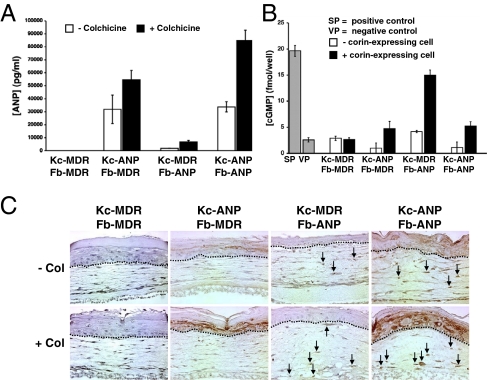
To determine the ability of the different HSE combinations to secrete ANP into medium during in vitro culture and to determine if colchicine selection would increase the amount of ANP secreted, the different HSE combinations were grown at the air–liquid interface for 8 days to ensure differentiation and stratification. HSE were treated with colchicine (10 ng/mL for 10 days), and the levels of human ANP secreted into culture medium were determined by an RIA. HSE that expressed ANP from Kc secreted up to 15 times more ANP (Kc-ANP/Fb-MDR, 3.2 ± 1.1 × 104 pg/mL; Kc-ANP/Fb-ANP, 3.4 ± 0.4 × 104 pg/mL) than did HSE that expressed ANP from Fb alone (Kc-MDR/Fb-ANP, 0.2 × 104 pg/mL) (Fig. 3A). Correspondingly, the contribution of ANP secreted by the dermis in the Kc-ANP/Fb-ANP combination was negligible. Colchicine treatment for 10 days increased the amount of ANP secreted into culture medium by an average of 2.6 ± 0.6-fold (Kc-ANP/Fb-MDR, 5.5 ± 0.7 × 104 pg/mL; Kc-MDR/Fb-ANP, 0.7 ± 0.1 × 104 pg/mL; Kc-ANP/Fb-ANP, 8.5 ± 0.8 × 104 pg/mL) compared with untreated HSE (Fig. 3A). Colchicine enrichment for Kc and Fb expressing increased amounts of ANP was confirmed by immunohistochemistry for ANP expression in treated (+ Col) and untreated (− Col) HSE (Fig. 3C). These data show that HSE epidermal Kc secrete more ANP into culture medium than dermal Fb and that colchicine treatment significantly increased the amount of ANP secreted by both Kc and Fb. Although not directly comparable to in vivo levels, the reported “normal” range of ANP in human plasma is 10–60 pg/mL (23).
Fig. 3.
Colchicine selection increased the number of ANP-positive cells as well as the amount of pro-ANP secreted into culture medium. (A) An ANP RIA was used to measure the amount of human ANP secreted by genetically modified HSE cultured with (solid bar) or without (open bar) colchicine treatment (10 ng/mL). Determinations were made in duplicate and were repeated multiple times with similar results. (B) cGMP assay of pro-ANP levels in culture medium from genetically modified HSE with (solid bar) and without (open bar) preincubation with the corin-expressing NCI-H1688 cell line that can convert pro-ANP into active α-ANP. Ten nM vasopressin (VP) was used as a negative control, and 1 mM sodium nitroprusside (SP) was used as a positive control. (C) ANP localization in genetically modified HSE that were selected with colchicine (+ col) or were not selected (− col) using human anti-ANP antibody. Arrows indicate Fb expressing ANP.
HSE Secretes Functional Pro-ANP that Can Be Converted to Active α-ANP.
To ensure that systemic α-ANP levels are sufficient for decreasing blood pressure, stable pro-ANP secreted by HSE must be converted to the active α-ANP form in the heart atria by corin receptors (Fig. S2). Because the ANP RIA does not distinguish between pro-ANP and active α-ANP, we used a cGMP assay that selectively measures active α-ANP levels. This assay utilizes a target kidney cell line (BHK-21) that increases cGMP levels after active α-ANP selectively binds their NPR-A receptors. Our results demonstrate that the ANP secreted by HSE is intact pro-ANP, because the conditioned medium does not increase cGMP intracellular levels when incubated with BHK-21 cells expressing NPR-A (Fig. 3B). In contrast, preincubation of the HSE medium with cells expressing corin on the cell surface (NCI-H1688) converted pro-ANP to active α-ANP and increased cGMP levels in the target BHK-21 kidney cells (Fig. 3B). These data indicate that human Kc and Fb do not process the secreted human pro-ANP into an active α-ANP, because corin receptors are not expressed by human Kc and Fb (Fig. S3); therefore the presence of Kc and Fb neutral endopeptidase, which could degrade active α-ANP, should not be a problem (Fig. S3). These results suggest that the stable pro-ANP form secreted by the genetically modified HSE should reach the systemic circulation, be converted into active α-ANP by the corin-expressing cardiomyocytes, and bind NPR-A receptors either on blood vessels or in kidneys, decreasing blood pressure (Fig. S2).
Human ANP Is Secreted Systemically Following Engraftment and Is Increased by Topical Colchicine Treatment.
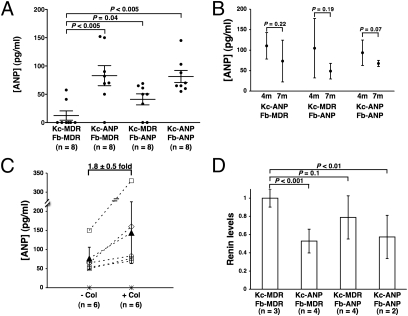
To determine if the genetically modified Kc and Fb in HSE could deliver human ANP systemically and to evaluate their relative contributions, HSE were grafted onto immunocompromised mice (Fig. S4), and plasma levels of human ANP in mice were assessed 2, 4, and 7 months postgrafting. Significantly higher human ANP levels (3.5- to 7-fold) were detected in mice grafted with HSE expressing ANP (Kc-ANP/Fb-MDR, 83 ± 17 pg/mL, n = 8; Kc-MDR/Fb-ANP, 41 ± 9 pg/mL, n = 8; Kc-ANP/Fb-ANP, 81 ± 10 pg/mL, n = 8) compared with the control grafts (Kc-MDR/Fb-MDR, 12 ± 8 pg/mL, n = 8) (Fig. 4A). These levels are within the low-dose ANP infusion ranges (12–180 pg/mL) that previously were reported to produce an effective and prolonged reduction in blood pressure in patients (24) and are within reported ranges (30–100 pg/mL) of murine ANP levels (25–27).
Fig. 4.
Immunocompromised mice grafted with genetically modified HSE secreted biologically active human ANP into their bloodstream. (A) ANP levels in plasma of mice grafted with different HSE combinations at 2, 4, and 7 months postgrafting. (B) Human ANP levels declined over time in the plasma of mice grafted with HSE. (C) Colchicine treatment of HSE grafts increased plasma levels of human ANP. Human ANP levels were determined in 6 individual mice [3 Kc-ANP/Fb-MDR (□); 1 Kc-MDR/Fb-ANP (♢); 1 Kc-ANP/Fb-ANP (○); 1 Kc-MDR/Fb-MDR (*)] before (− Col) and after (+ Col) colchicine selection (45 μg of colchicine applied topically 3 times per week for 4 weeks). The average systemic ANP levels in mice grafted with ANP-expressing HSE before (77 ± 29 pg/mL) and after (144 ± 81 pg/mL) colchicine treatment are represented by ▴. (D) Plasma renin levels correlated inversely with plasma ANP levels in HSE-grafted mice.
Serial measurement of human ANP levels in individual mice, analyzed at 4 and 7 months after grafting of different HSE combinations (Fig. 4B), demonstrated that pro-ANP levels declined 30% or more during this time interval. ANP levels in Kc-ANP/Fb-ANP–grafted mice declined from 94 ± 31 pg/mL (n = 5) at 4 months to 67 ± 7 pg/mL (n = 7) at 7 months (29% decrease; P = 0.07). During the same period, ANP levels declined from 110 ± 32 pg/mL (n = 4) to 73 ± 51 (n = 3), a 33% decrease (P = 0.27), in Kc-ANP/Fb-MDR–grafted mice and from 105 ±73 pg/mL (n = 4) to 49 ±19 pg/mL (n = 3), a 53% decline (P = 0.19), in Kc-MDR/Fb-ANP–grafted mice. Although this consistent downward trend was not statistically significant, these declines in plasma ANP levels over time may reflect inefficient transduction of Kc stem cells and loss of cells containing the ANP transgene and/or the inactivation of the retroviral vectors expressing ANP.
To determine if topical colchicine treatment would reverse this decline or increase the systemic levels of human ANP by selecting for cells expressing both ANP and the MDR selectable marker, HSE grafts in mice more than 7 months postgrafting were treated topically with an optimal dose of colchicine cream (450 μg/g; 0.1g 3 times per week) for 4 weeks using a chamber apparatus (Fig. S5 A–E) .This colchicine dose was determined to inhibit effectively the mitosis of cells lacking the MDR selectable marker while maintaining tissue architecture (Fig. S5 G–K) .Plasma levels of human ANP were measured in 6 individual mice [1 control (Kc-MDR/Fb-MDR) and 5 expressing ANP (3-Kc-ANP/Fb-MDR, 1-Kc-MDR/Fb-ANP, and 1-Kc-ANP/Fb-ANP)] before and after 4 weeks of topical colchicine selection. All mice expressing ANP from Kc, Fb, or both had ANP levels that increased by an average of 1.8 ± 0.5-fold during the selection period (P = 0.018 by paired t-test given the normal distribution; Wilcoxon signed rank test, P = 0.063) (Fig. 4C). Taken together, these in vivo data show that both epidermis (Kc) and dermis (Fb) can deliver significant levels of human ANP systemically and that systemic ANP levels can be increased significantly by topical colchicine selection.
HSE Grafts Expressing Human ANP Lower Renin Levels in Mice.
Because active α-ANP is an inhibitor of the renin-angiotensin-aldosterone pathway (28), we sought to determine if human ANP from grafted HSE could exert a functional effect on plasma renin levels in recipient mice. Renin levels were measured for ANP-expressing mice at 6 months postgrafting (Kc-ANP/Fb-MDR, n = 4; Kc-MDR/Fb-ANP, n = 4; Kc-ANP/Fb-ANP, n = 2) and were compared with levels in control mice (Kc-MDR/Fb-MDR, n = 3). We found that renin levels in mouse plasma were inversely correlated with human ANP levels (Fig. 4 A and D) and were significantly decreased in mice with HSE grafts that contained ANP-secreting Kc (Kc-ANP/Fb-MDR, 0.5 ± 0.1-fold; Kc-ANP/Fb-ANP, 0.6 ± 0.2-fold) compared with the control (Kc-MDR/Fb-MDR, 1 ± 0.1-fold, P < 0.01 for both) (Fig. 4D). In mice grafted with HSE constructed with ANP-expressing dermal Fb, the renin levels were lower than in the controls, but results were not statistically significant (Kc-MDR/Fb-ANP, 0.8 ± 0.2-fold, P = 0.07) (Fig. 4D). Thus, the pro-ANP produced by genetically modified HSE could be processed into an active ANP and exert physiological effects, with renin decreased more by ANP-expressing Kc than by ANP-expressing Fb.
Systemic Human ANP from Grafted HSE Lowers Blood Pressure in Mice.
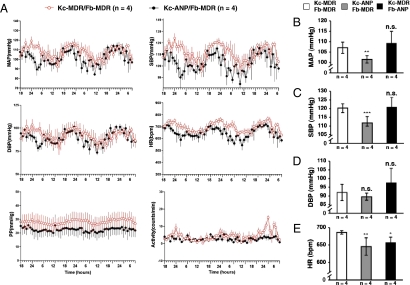
For validation, HSE grafts expressing human ANP should lower blood pressure in the grafted mice and prevent hypertension induced by a high-salt diet in a hypertensive mouse model. Mice grafted with different HSE combinations had blood pressure transducers and transmitters surgically implanted into their carotid arteries to monitor continuously, by telemetry, mean arterial (MAP), systolic (SBP), diastolic (DBP), and pulse pressure (PP) as well as heart rate (HR) and activity under normal diet (n = 12) or high-salt diet (n = 10) (Fig. S6). One week after surgery, blood pressure parameters were recorded for 10 s every 2 min and averaged per hour for a period of 60 h. Fig. 5A depicts pooled data of cardiovascular parameters at different time points over a 3-day period for mice grafted with Kc-ANP/Fb-MDR (n = 4) or control Kc-MDR/Fb-MDR (n = 4) HSE. Mice that had high systemic levels of human ANP and low renin levels (Kc-ANP/Fb-MDR) had a lower MAP (Fig. 5B), lower SBP (Fig. 5C), and lower HR (Fig. 5E) than the control mice. No significant differences were observed for DBP (Fig. 5C) between the two groups. These observations concur with clinical studies in which infusion of low doses of ANP decreased systolic blood pressure in both normotensive patients (29) and patients with uncomplicated essential hypertension (19, 30). Of note, mice grafted with Kc-MDR/Fb-ANP HSE did not have lower MAP, SBP, and DBP than control mice, suggesting that the lower systemic levels of human ANP produced by Fb in these mice were not sufficient to lower blood pressure in normal mice.
Fig. 5.
Determination of cardiovascular parameters, including blood pressure, of mice grafted with HSE secreting human ANP compared with control mice during normal diet consumption. Carotid artery/aortic arch blood pressure transducers with transmitters were implanted in 12 mice, 4 from each group (Kc-MDR/Fb-MDR, Kc-ANP/Fb-MDR, Kc-MDR/Fb-ANP), and different hemodynamic parameters were measured every h for 3 consecutive days. (A) Sample graphs depicting mean values for different cardiovascular parameters (MAP, SBP, DBP, PP, and activity) of mice grafted with HSE expressing ANP from epidermis (Kc-ANP/Fb-MDR,•, n = 4) and mice grafted with control HSE (Kc-MDR/Fb-MDR, ○, n = 4) measured every h for 60 h. The 24-h mean values for MAP (B), SBP (C), DBP (mm Hg) (D), and HR in beats per min (bpm) (E) are shown for control mice (Kc-MDR/Fb-MDR) and mice with ANP-expressing HSE (Kc-ANP/Fb-MDR; Kc-MDR/Fb-ANP). *, P ≤ 0.05; **, P ≤ 0.01; *** , P ≤ 0.005; n.s., not statistically significant.
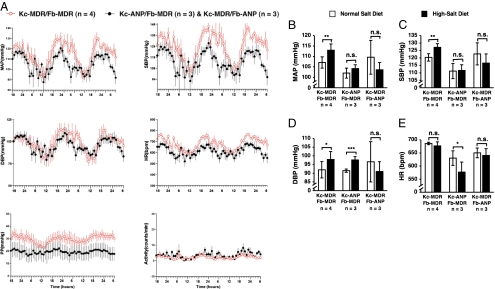
To determine whether HSE grafts expressing human pro-ANP could treat or prevent hypertension in mice fed a high-salt diet, subsets of HSE-grafted mice (three Kc-ANP/Fb-MDR–grafted mice, three Kc-MDR/Fb-ANP–grafted mice, and four Kc-MDR/Fb-MDR–grafted mice) were fed a high-salt diet for 3 weeks to induce hypertension. After 3 weeks, significant increases in MAP, SBP, and DBP were observed by telemetry monitoring in control Kc-MDR/Fb-MDR–grafted mice that were fed a high-salt diet (Fig. 6 A–D), without changes in HR (Fig. 6E). In striking contrast, mice expressing ANP from either Kc (Kc-ANP/Fb-MDR grafts) or Fb (Kc-MDR/Fb-ANP grafts) did not have any significant increase in MAP (Fig. 6B) or SBP (Fig. 6C) after a high-salt diet. Surprisingly, the Kc-ANP/Fb-MDR–grafted mice maintained the decreased MAP and SBP that they had on a normal salt diet (Fig. 5 B and C), even when challenged with a high-salt diet (Fig. 6 B and C). Interestingly, the Kc-ANP/Fb-MDR–grafted mice also had an increase in DBP (Fig. 6D) along with a decreased HR (Fig. 6E) when fed a high-salt diet. These data suggest that both epidermis (Kc) and dermis (Fb) can deliver a therapeutic dose of ANP sufficient to prevent hypertension in mice fed a high-salt diet.
Fig. 6.
HSE grafts that expressed ANP from either epidermis or dermis prevented blood pressure elevation in a hypertensive mouse model. Ten of the 12 mice analyzed under normal diet were fed a high-salt diet for 3 weeks. Averages of different hemodynamic parameters over a period of 3 days were compared before and after high-salt diet for each group of mice. (A) Sample graphs depicting mean values for different cardiovascular parameters (MAP, SBP, DBP, PP, and activity) in mice expressing ANP from epidermis (Kc-ANP/Fb-MDR) and dermis (Kc-MDR/Fb-ANP) pooled together (•, n = 6) and mice grafted with control HSE (Kc-MDR/Fb-MDR, ○, n = 4) measured every h for a period of 60 h after challenge with a high-salt diet. The 24-h mean values for (MAP (B), SBP (C), DBP (mm Hg) (D), and HR (bpm) (E) are shown for control (Kc-MDR/Fb-MDR) and ANP-expressing mice (Kc-ANP/Fb-MDR; Kc-MDR/Fb-ANP). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005; n.s., not statistically significant.
Discussion
By constructing and comparing HSE that secreted ANP from human Kc, human Fb, or both, we found that HSE with human ANP-expressing Kc secreted more human ANP (15-fold higher) during in vitro culture than HSE with ANP-expressing Fb, probably because significantly more human Kc than human Fb were present in the HSE. We did not find a significant difference in the ability of individual Kc and Fb to secrete ANP. In contrast, ANP levels in mice grafted with HSE that contained human ANP-expressing Kc (Kc-ANP/Fb-MDR) are only 2-fold higher than ANP levels in mice grafted with HSE that contained ANP-expressing Fb (Kc-MDR/Fb-ANP). An explanation may be that Fb deliver secreted proteins to the systemic circulation more efficiently than Kc, because Fb are located in close proximity to blood vessels in the dermis, even though fewer Fb are present in grafted HSE. Additionally, factors secreted by Kc in grafted HSE must traverse the epidermal–dermal junction before gaining access to dermal blood vessels, potentially decreasing systemic delivery.
Consistent with the plasma ANP and renin levels, we found that Kc-ANP/Fb-MDR HSE grafts significantly lowered the blood pressure (MAP, SBP, and DBP) in mice that were fed a normal diet and, more importantly, maintained the decreased blood pressure when the Kc-ANP/Fb-MDR–grafted mice were challenged with a high-salt diet. In contrast, Kc-MDR/Fb-ANP grafts did not lower blood pressure in grafted mice that were fed a normal salt diet but did prevent blood pressure increases when these mice were challenged with a high-salt diet. These differences in blood pressure responses correlate with relative ANP levels in mice with Kc-ANP/Fb-MDR and Kc-MDR/Fb-ANP HSE grafts, suggesting that normalization of blood pressure in hypertensive mice may be easier to achieve, at lower plasma ANP levels, than lowering the blood pressure in mice when they are on a normal-salt diet. Importantly, these data demonstrate that modulation of ANP plasma levels can achieve different therapeutic effects. Although none of the Kc-ANP/Fb-ANP–grafted mice survived the postoperative monitoring period of normal and high-salt diets, we would expect equivalent or superior blood pressure control in mice grafted with Kc-ANP/Fb-ANP HSE, based on their plasma ANP and renin levels. Although ANP-secreting Kc can achieve higher plasma ANP levels than Fb, our data suggest that, for clinical applications, it would be beneficial to use HSE grafts in which both Kc and Fb are producing ANP.
The optimal colchicine dose used in this study was determined by finding a dose that provided maximum inhibition of Kc mitosis but did not disrupt tissue architecture (Fig. S5 G–K) .The colchicine-selection approach used here provides some important advantages for ensuring long-term expression of sufficient systemic levels of human ANP. Colchicine treatment could be used to modulate the systemic ANP level. If greater blood pressure control is needed, ANP levels could be raised by colchicine treatment; to lower plasma ANP, colchicine treatments could be stopped or HSE grafts that secrete ANP could be removed. Another advantage is that MDR1 can pump out and protect cells from a large variety of different chemotherapeutic agents, including mitotic inhibitors such as vincristine and taxol, which can select for MDR-expressing Kc progenitor cells.
The human ANP levels in the grafted mice are within the range of ANP levels that have been shown to have a therapeutic effect in patients in clinical studies. Given the relative body surface area for mice (0.007 m2) and humans (1.7 m2), ANP-secreting HSE grafts of ≈200–240 cm2 in total surface area would be calculated to achieve comparable therapeutic ANP levels in human subjects. However, a linear scale-up from the mouse models used in this study to larger animals and humans cannot be assumed, and experimental studies will be needed to determine the graft size required for a therapeutic effect in humans. Safety considerations regarding the use of retroviral vectors also must be addressed, and nonviral methods of gene delivery, such as the use of integrases to integrate desired genes stably into cellular genomes (31), should be considered in the future.
The skin gene therapy approach described here for regulating blood pressure and treating systemic hypertension also could be used to express other endogenous therapeutic factors for treatment of systemic diseases; alternatively, genetically modified skin Kc and Fb could be used as “enzymatic sinks” to process or degrade unwanted systemic factors to achieve a therapeutic effect. This study successfully demonstrates that genetically modified skin can produce and deliver therapeutic proteins and may be a viable alternative approach for treating systemic disease.
Methods
Cell Culture and Human Skin Equivalents.
Primary human Fb and Kc were isolated from human neonatal foreskin as described previously (32) and in SI Text. The acquisition of all human skin specimens was approved by the appropriate institutional review boards.
Retroviral Expression Vectors and Gene Transfer.
The bicistronic retroviral vector pQCXIX (Clontech) was used to generate Ctl-MDR and Ther-ANP-MDR vectors (Fig. 1A). To generate the Ctl-MDR vector, MDR1 cDNA first was retrieved from the pHaMDR1/A vector [kindly provided by Michael Gottesman, National Institutes of Health (NIH)] and cloned in the second position of the pQCXIX. The Ther-ANP-MDR vector was generated by cloning an ANP cDNA into the first position of the Ctl-MDR vector. Retroviral vectors were produced by the Phoenix amphotropic packaging cell line (Clontech), and early-passage Fb and Kc were transduced with Ctl-MDR or Ther-ANP-MDR vectors. Additional details are given in SI Text.
Flow Cytometry Analysis.
Five days posttransduction, Fb and Kc were collected after trypsinization for rhodamine assay or anti-ANP antibody labeling using a primary antibody, mAb ANP (ab20893; Abcam), and secondary antibody, rat anti-mouse IgG1-PE (BD Biosciences), as described in SI Text.
cGMP Assay.
To determine if pro-ANP was secreted into medium during in vitro culture of HSE, the medium first was incubated with a corin-expressing cell line, NCI-H1688, that can convert pro-ANP to active α-ANP. Converted α-ANP can then activate BHK-21 cells that express the α-ANP receptor NPR-A. The amount of cGMP produced in activated BHK-21 cells was assessed by a cGMP assay as described in SI Text.
ANP Measurements in Culture Medium and Mouse Plasma.
The amount of ANP secreted into the medium was measured by RIA (Amersham Biosciences) following the manufacturer’s protocol t. For in vivo studies, human ANP plasma levels were determined at 2 months, 4 months, and 7 months postgrafting, using an RIA (Shionoria ANP; Shionogi & Co) that is specific for human ANP, as previously described (33).
Human Skin Equivalent Grafting.
Four- to 5-week-old immunocompromised NIH male Swiss nu/nu mice (Taconic Farms), housed and used in accordance with NIH institutional guidelines, were grafted with genetically modified HSE as previously described (5, 34). Grafts were placed on the muscle fascia in the correct anatomical orientation (epidermis side up), covered with sterile Vaseline gauze (Sherwood Medical Industries), and secured with a 0.75-inch × 3-inch tape dressing (Johnson & Johnson). The dressing was changed after 2 weeks and was removed after an additional 1 or 2 weeks.
In Vivo Topical Colchicine Treatment.
A chamber was constructed as depicted in Fig. S5. A dose (0.1 g) of colchicine cream (450 μg/g) was applied to human skin grafts for a total of 4 weeks (45 μg colchicine 3 times per week) in mice that were more than 7 months postgrafting. Plasma ANP levels were determined at ≈10 months postgrafting. Colchicine cream was prepared as previously described (5).
Renin Assay.
Plasma renin levels were determined in mice 6 months postgrafting and then were measured in individual grafted mice at 2-week intervals for 4 weeks to control for variability in individual mice; then averaged values for each mouse were calculated. Renin concentration was measured by an RIA (Gammacoat; DiaSorin) that detected the generation of angiotensin-I from an excess of rat substrate angiotensinogen, as described in SI Text.
Blood Pressure Measurement by Telemetry.
Telemetry studies to monitor real-time blood pressure in individual mice were performed 8–10 months after the grafting of ANP-expressing HSE. Telemetric transmitters were inserted into the left carotid artery and advanced to the aortic arch (35, 36). Blood pressure recording began 1 week postsurgery and continued for 1 week while the mice were fed a normal diet. Some mice then were fed a high-salt diet for 3 weeks, during which time blood pressure was recorded.
Supplementary Material
Acknowledgments
We thank Mark Udey, Carole Yee, and Girish Patel of the Dermatology Branch, National Cancer Institute (NCI), National Institutes of Health, for critical input and review of the manuscript (M.U.) and for technical assistance and helpful discussion of experimental design (C.Y. and G.P.). We thank Diane Milenic of the Radiation Oncology Branch, NCI, for assistance with the gamma-counter and Diane Mizel of the Renal Function and Injury Section, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), for assistance with the renin assay. We also thank John Dennis and the animal facility staff for helpful advice regarding the animal studies. This research was supported by the Center for Cancer Research, NCI, and the NIDDK, NIH. J.-P.T. was the recipient of a fellowship from Canadian Institutes of Health Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908882107/DCSupplemental.
References
- 1.Khavari PA, Rollman O, Vahlquist A. Cutaneous gene transfer for skin and systemic diseases. J Intern Med. 2002;252:1–10. doi: 10.1046/j.1365-2796.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 2.Cao T, Wang XJ, Roop DR. Regulated cutaneous gene delivery: The skin as a bioreactor. Hum Gene Ther. 2000;11:2297–2300. doi: 10.1089/104303400750035843. [DOI] [PubMed] [Google Scholar]
- 3.Gerrard AJ, Hudson DL, Brownlee GG, Watt FM. Towards gene therapy for haemophilia B using primary human keratinocytes. Nat Genet. 1993;3:180–183. doi: 10.1038/ng0293-180. [DOI] [PubMed] [Google Scholar]
- 4.Fenjves ES, Smith J, Zaradic S, Taichman LB. Systemic delivery of secreted protein by grafts of epidermal keratinocytes: Prospects for keratinocyte gene therapy. Hum Gene Ther. 1994;5:1241–1248. doi: 10.1089/hum.1994.5.10-1241. [DOI] [PubMed] [Google Scholar]
- 5.Pfutzner W, et al. Topical colchicine selection of keratinocytes transduced with the multidrug resistance gene (MDR1) can sustain and enhance transgene expression in vivo. Proc Natl Acad Sci USA. 2002;99:13096–13101. doi: 10.1073/pnas.192247899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brill-Almon E, et al. Ex vivo transduction of human dermal tissue structures for autologous implantation production and delivery of therapeutic proteins. Mol Ther. 2005;12:274–282. doi: 10.1016/j.ymthe.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Peroni CN, et al. High-level secretion of growth hormone by retrovirally transduced primary human keratinocytes: Prospects for an animal model of cutaneous gene therapy. Mol Biotechnol. 2006;34:239–245. doi: 10.1385/mb:34:2:239. [DOI] [PubMed] [Google Scholar]
- 8.Lei P, Ogunade A, Kirkwood KL, Laychock SG, Andreadis ST. Efficient production of bioactive insulin from human epidermal keratinocytes and tissue-engineered skin substitutes: Implications for treatment of diabetes. Tissue Eng. 2007;13:2119–2131. doi: 10.1089/ten.2006.0210. [DOI] [PubMed] [Google Scholar]
- 9.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 10.Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 12.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohyama M, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raizada MK, Der Sarkissian S. Potential of gene therapy strategy for the treatment of hypertension. Hypertension. 2006;47:6–9. doi: 10.1161/01.HYP.0000196685.91424.01. [DOI] [PubMed] [Google Scholar]
- 15.Phillips MI. Gene therapy for hypertension: The preclinical data. Hypertension. 2001;38([part 2]):543–548. doi: 10.1161/hy09t1.092927. [DOI] [PubMed] [Google Scholar]
- 16.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–16905. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- 18.Cusson JR, Thibault G, Cantin M, Larochelle P. Prolonged low dose infusion of atrial natriuretic factor in essential hypertension. Clin Exp Hypertens A. 1990;12:111–135. doi: 10.3109/10641969009074723. [DOI] [PubMed] [Google Scholar]
- 19.Janssen WM, de Zeeuw D, van der Hem GK, de Jong PE. Antihypertensive effect of a 5-day infusion of atrial natriuretic factor in humans. Hypertension. 1989;13:640–646. doi: 10.1161/01.hyp.13.6.640. [DOI] [PubMed] [Google Scholar]
- 20.Garcia R, et al. Chronic infusion of low doses of atrial natriuretic factor (ANF Arg 101-Tyr 126) reduces blood pressure in conscious SHR without apparent changes in sodium excretion. Proc Soc Exp Biol Med. 1985;179:396–401. doi: 10.3181/00379727-179-rc1aa. [DOI] [PubMed] [Google Scholar]
- 21.Koepke JP, Tyler LD, Blehm DJ, Schuh JR, Blaine EH. Chronic atriopeptin regulation of arterial pressure in conscious hypertensive rats. Hypertension. 1990;16:642–647. doi: 10.1161/01.hyp.16.6.642. [DOI] [PubMed] [Google Scholar]
- 22.Schillinger KJ, et al. Regulatable atrial natriuretic peptide gene therapy for hypertension. Proc Natl Acad Sci USA. 2005;102:13789–13794. doi: 10.1073/pnas.0506807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards AM. Human plasma atrial natriuretic peptide concentrations in health and disease. Curr Opin Cardiol. 1987;2:660–670. [Google Scholar]
- 24.Hollister AS, Inagami T. Atrial natriuretic factor and hypertension. A review and metaanalysis. Am J Hypertens. 1991;4:850–865. doi: 10.1093/ajh/4.10.850. [DOI] [PubMed] [Google Scholar]
- 25.Ellmers LJ, et al. Ventricular expression of natriuretic peptides in Npr1(-/-) mice with cardiac hypertrophy and fibrosis. Am J Physiol Heart Circ Physiol. 2002;283:H707–H714. doi: 10.1152/ajpheart.00677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbee RW, Perry BD, Ré RN, Murgo JP, Field LJ. Hemodynamics in transgenic mice with overexpression of atrial natriuretic factor. Circ Res. 1994;74:747–751. doi: 10.1161/01.res.74.4.747. [DOI] [PubMed] [Google Scholar]
- 27.Dubois SK, Kishimoto I, Lillis TO, Garbers DL. A genetic model defines the importance of the atrial natriuretic peptide receptor (guanylyl cyclase-A) in the regulation of kidney function. Proc Natl Acad Sci USA. 2000;97:4369–4373. doi: 10.1073/pnas.97.8.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoupakis G, Klapholz M. Natriuretic peptides: Biochemistry, physiology, and therapeutic role in heart failure. Heart Dis. 2003;5:215–223. doi: 10.1097/01.HDX.0000074517.30102.64. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls MG, Richards AM. Human studies with atrial natriuretic factor. Endocrinol Metab Clin North Am. 1987;16:199–223. [PubMed] [Google Scholar]
- 30.Singer DR, et al. Prolonged decrease in blood pressure after atrial natriuretic peptide infusion in essential hypertension: A new anti-pressor mechanism? Clin Sci (Lond) 1989;77:253–258. doi: 10.1042/cs0770253. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz-Urda S, et al. Stable nonviral genetic correction of inherited human skin disease. Nat Med. 2002;10:1166–1170. doi: 10.1038/nm766. [DOI] [PubMed] [Google Scholar]
- 32.Pfützner W, Hengge UR, Joari MA, Foster RA, Vogel JC. Selection of keratinocytes transduced with the multidrug resistance gene in an in vitro skin model presents a strategy for enhancing gene expression in vivo. Hum Gene Ther. 1999;10:2811–2821. doi: 10.1089/10430349950016546. [DOI] [PubMed] [Google Scholar]
- 33.Clerico A, et al. Analytical performance and clinical usefulness of a commercially available IRMA kit for measuring atrial natriuretic peptide in patients with heart failure. Clin Chem. 1996;42:1627–1633. [PubMed] [Google Scholar]
- 34.Terunuma A, et al. Stem cell activity of human side population and alpha6 integrin-bright keratinocytes defined by a quantitative in vivo assay. Stem Cells. 2007;25:664–669. doi: 10.1634/stemcells.2006-0434. [DOI] [PubMed] [Google Scholar]
- 35.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: A physiological genomics tool. Physiol Genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- 36.Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension. 2000;35:E1–E5. doi: 10.1161/01.hyp.35.2.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.