Abstract
Neuregulin 1 (NRG1) is a trophic factor thought to play a role in neural development. Recent studies suggest that it may regulate neurotransmission, mechanisms of which remain elusive. Here we show that NRG1, via stimulating GABA release from interneurons, inhibits pyramidal neurons in the prefrontal cortex (PFC). Ablation of the NRG1 receptor ErbB4 in parvalbumin (PV)-positive interneurons prevented NRG1 from stimulating GABA release and from inhibiting pyramidal neurons. PV-ErbB4−/− mice exhibited schizophrenia-relevant phenotypes similar to those observed in NRG1 or ErbB4 null mutant mice, including hyperactivity, impaired working memory, and deficit in prepulse inhibition (PPI) that was ameliorated by diazepam, a GABA enhancer. These results indicate that NRG1 regulates the activity of pyramidal neurons by promoting GABA release from PV-positive interneurons, identifying a critical function of NRG1 in balancing brain activity. Because both NRG1 and ErbB4 are susceptibility genes of schizophrenia, our study provides insight into potential pathogenic mechanisms of schizophrenia and suggests that PV-ErbB4−/− mice may serve as a model in the study of this and relevant brain disorders.
Keywords: neurotransmission, schizophrenia, GABA, epilepsy
Neuregulin 1 (NRG1) is a family of trophic factors with an epidermal growth factor (EGF)-like domain (1, 2). It acts by stimulating the ErbB family of receptor tyrosine kinases ErbB2, -3, and -4. NRG1 binds only to ErbB3 or ErbB4, but not to ErbB2. On the other hand, ErbB2 and ErbB4 are most active in response to NRG1 stimulation whereas the kinase activity of ErbB3 is impaired. Thus, ErbB2 and ErbB3 function by forming heterodimers with each other or with ErbB4, but an ErbB4 homodimer is functional by itself (2). NRG1 has been implicated in many aspects of neural development including neuron migration, axon projection, myelination, synapse formation, and up-regulation of neurotransmitter receptor expression (2). Recently, CNS-specific mutation of ErbB2 and ErbB4 seemed to have no effect on layered structures of the cerebral cortex, hippocampus, and cerebellum or expression of NMDA receptor subtypes (3, 4), challenging existing views of NRG1 function.
Both NRG1 and its receptors are distributed throughout brain regions critical for higher function in adult animals (5–8), suggesting a role of NRG1 in the brain after neural development is complete. In support of this hypothesis were observations that ErbB4 is present at the postsynaptic density of excitatory synapses presumably via interaction with PSD-95 (9–11). Moreover, ErbB4 mRNA is enriched in regions where interneurons are clustered (5) and GAD-positive neurons of the hippocampus express high levels of ErbB4 (10), suggesting that ErbB4 is enriched in GABAergic neurons. Immunohistochemical analysis indicates that ErbB4 is expressed in most if not all parvalbumin (PV)-positive interneurons in addition to glutamatergic neurons (10, 12). Intriguingly, exogenous NRG1 suppresses the induction of LTP at Schaffer collateral-CA1 synapses in the hippocampus (10, 11, 13) or reverses it (14, 15). These observations suggest that NRG1 may regulate neurotransmission and synaptic plasticity although the underlying mechanisms remain elusive. NRG1 depotentiation of LTP was shown to depend on stimulated internalization of surface glutamate receptor 1-containing AMPA receptors (14) and activation of dopamine D4 receptors (15). In vitro studies showed that NRG1 was able to reduce whole-cell NMDA receptor currents in cultured neurons of the prefrontal cortex (PFC) and to decrease NMDA receptor-mediated excitatory postsynaptic currents (EPSCs) in PFC (16). On the other hand, overexpressed ErbB4 enhances AMPA synaptic currents and increases dendritic spine size, whereas preventing NRG1/ErbB4 signaling destabilizes synaptic AMPA receptors and leads to loss of synaptic NMDA currents and spines (17). Moreover, we demonstrated that ErbB4 is present in terminals of GABAergic neurons in the PFC and NRG1 facilitates activity-dependent release of GABA in a manner dependent on ErbB4 (18). Which of these mechanisms is involved in NRG1 regulation of synaptic plasticity remains unclear.
Recently, studies of NRG1 have gained much attention because both NRG1 and its receptor ErbB4 are susceptibility genes of schizophrenia (2, 19–23). Expression of NRG1 and ErbB4 appeared to be altered in the brains of schizophrenic patients (2). Moreover, null mutation of the NRG1 gene that disrupts expression of various isoforms and the ErbB4 gene causes a spectrum of abnormal behaviors in mice including hyperactivity, disrupted prepulse inhibition (PPI), and spatial learning and memory deficits (3, 19, 24–30), which are thought to be associated with schizophrenia (31–33).
In this study, we investigated whether NRG1 regulates the activity of pyramidal neurons by loose-patch and whole-cell recordings. Both spontaneous spikes and evoked firing activity of pyramidal neurons were inhibited by NRG1, but increased by the neutralizing peptide ecto-ErbB4, suggesting a role of NRG1 in controlling pyramidal neuron activity. To test this hypothesis further, ErbB4 was ablated specifically in PV-positive neurons in PV-Cre;ErbB4−/− mice where expression of Cre is not active until postnatal day 10 (34, 35), a time when the cortical lamination is nearly achieved (35, 36). This mutation prevented NRG1 from promoting activity-dependent GABA release and from suppressing the activity of pyramidal neurons. Furthermore, the mutant mice demonstrated schizophrenia-associated phenotypes that are manifested in NRG1 or ErbB4 null mutant mice. Moreover, treatment with diazepam ameliorated the PPI deficit in PV-Cre;ErbB4−/− mice. These observations reveal a critical function of NRG1 in balancing brain activity, identify PV-positive neurons as a cellular target of NRG1/ErbB4 signaling in synaptic plasticity and complex behaviors relevant to schizophrenia, and suggest that PV-ErbB4−/− mice may serve as a model in the study of this and relevant brain disorders.
Results
NRG1 Inhibition of Firing Rate of Pyramidal Neurons in the PFC.
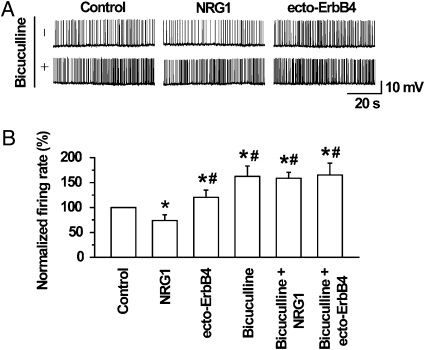
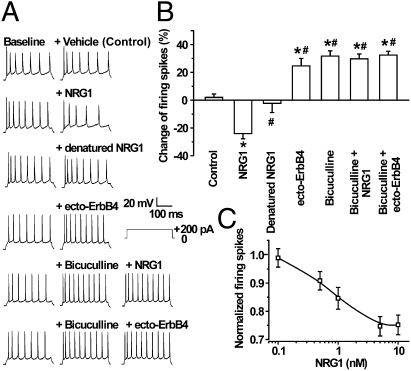
To determine whether NRG1 regulates the activity of PFC pyramidal neurons, spontaneous firing rates were recorded extracellularly in a loose-patch cell-attached configuration. Pyramidal-like neurons with triangular-shaped soma and prominent apical dendrites in layers II–V of coronal PFC sections were visually identified by infrared-differential interference contrast optics. The spontaneous firing rates were 55.9 ± 7.8/min (n = 7). Bath application of 5 nM NRG1 reduced the spontaneous firing rates within 5 min of application (P < 0.05, n = 7; Fig. 1 A and B), suggesting that NRG1 could regulate PFC pyramidal neuron activity. This effect was blocked by 1 μg/mL ecto-ErbB4, a NRG1-neutralizing peptide (18) (Fig. S1 A and B). Interestingly, ecto-ErbB4 alone increased firing rates of PFC pyramidal neurons in the absence of exogenous NRG1 (P < 0.05) (Fig. 1 A and B), suggesting a necessary role of endogenous NRG1 in maintaining pyramidal neuron activity. To test this idea further, we recorded action potentials of PFC layers II–V pyramidal neurons that were generated by a 200-pA suprathreshold somatic current injection in a whole-cell patch-clamping configuration. Pyramidal neurons exhibited a characteristic spiking adaptation (Fig. S2 A) and could be co-stained with calcium/calmodulin-dependent protein kinase II (CaMKII), a marker of pyramidal neurons. The evoked firings of pyramidal neurons also differed from those of interneurons in the after-hyperpolarization amplitude and spike width at half amplitude (Fig. S2 A and B). In agreement with the results of loose-patch recording, bath application of NRG1 decreased the number of action potentials of PFC layers II–V pyramidal neurons (Fig. 2 A and B). During 300 ms of current injection, action potential numbers were reduced from 8.3 ± 0.58 in control to 6.2 ± 0.32 in slices treated with 5 nM NRG1 (n = 9, P < 0.05). The inhibitory effect was evident within 5 min of application and disappeared ∼5 min after removal of NRG1. This effect did not appear to be nonspecific because it was abolished by heat inactivation of NRG1 (Fig. 2 A and B) and was blocked by ecto-ErbB4 (Fig. S1 C and D). Moreover, the effect was concentration dependent with an IC50 value similar to that on GABA release (Fig. 2C) (18). Spike generation was inhibited by 24.8 ± 3.5% at maximal concentrations. These results demonstrate that NRG1 was able to inhibit the activity of pyramidal neurons in the PFC. Treatment with 1 μg/mL ecto-ErbB4 alone increased the number of action potentials (from 7.8 ± 0.72 in control to 9.4 ± 0.93 in slices treated with ecto-ErbB4, n = 9, P < 0.05) (Fig. 2 A and B), suggesting that the evoked spike frequency of pyramidal neurons is regulated by endogenous NRG.
Fig. 1.
NRG1 inhibition of spontaneous spikes of PFC pyramidal neurons. (A) NRG1 reduced whereas ecto-ErbB4 increased spontaneous spike activities of layers II–V pyramidal neurons. PFC slices were treated with vehicle (control), 5 nM NRG1, or 1 μg/mL ecto-ErbB4 in the absence (Upper) or presence (Lower) of 20 μM bicuculline. Pyramidal neuron firings were recorded under loose patch-clamp configuration. (B) Quantitative analysis of spontaneous firing rates. n = 7, *P < 0.05 in comparison with control; #P < 0.05 in comparison with NRG1. There was no significant difference in firing rates of the three groups: bicuculline alone, bicuculline/NRG1, and bicuculline/ecto-ErbB4 (P > 0.05).
Fig. 2.
NRG1 inhibition of evoked firing of PFC pyramidal neurons. (A) NRG1 reduced whereas ecto-ErbB4 enhanced action potential frequency of pyramidal neurons in a whole-cell patch-clamping configuration. Representative action potentials are shown of a single neuron before (baseline) and after bath application of vehicle (control), 5 nM NRG1, 5 nM denatured NRG1, or 1 μg/mL ecto-ErbB4, or 20 μM bicuculline and 5 nM NRG1, or 20 μM bicuculline and 1 μg/mL ecto-ErbB4. (B) Quantitative analysis of data in A. n = 9, *P < 0.05, compared with control; #P < 0.05, compared with NRG1. (C) Dose-dependent inhibition of NRG1 on evoked spikes of pyramidal neurons (n = 11).
We have previously shown that NRG1 enhances activity-dependent release of GABA (18). To investigate if the NRG1 regulation of pyramidal neuron firing requires GABA release, we studied the effects of NRG1 on pyramidal neuron firing under bicuculline, a selective antagonist of GABAA receptors. The firing rate of pyramidal neurons as measured by both loose-patch and whole-cell patch techniques was increased by 20 μM bicuculline (Figs. 1 A and B and 2 A and B). Remarkably, in the presence of bicuculline, NRG1 was no longer able to suppress pyramidal neuron activity, suggesting a requirement for GABAA receptor activation in this event (Figs. 1 A and B and 2 A and B). Furthermore, bicuculline blocked the effect of ecto-ErbB4 (Figs. 1 A and B and 2 A and B). Together, these results support the concept that NRG1, via increasing GABA release, regulates pyramidal neuron activity.
Critical Role of ErbB4 in PV-Positive Interneurons in NRG1 Potentiation of GABA Release and Suppression of Pyramidal Neuron Activity.
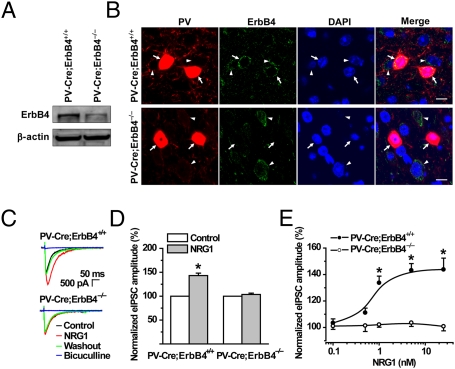
We have shown that NRG1 regulation of evoked GABA release is abolished in PFC slices from ErbB4−/− mice, suggesting that it requires ErbB4 (18). ErbB4 is highly expressed in PV-positive interneurons (12, 37–39), which have been implicated in controlling the output of pyramidal neurons (40, 41). If the NRG1-mediated reduction of the firing rate of pyramidal neurons results from an increase in GABAergic transmission, the effect should require ErbB4 in PV-positive interneurons. To test this idea, we sought to ablate ErbB4 expression specifically in PV-positive interneurons by crossing ErbB4loxP/loxP mice (42) with PV-Cre mice (43, 44). Western blotting analysis indicated that ErbB4 was reduced but not abolished in the PFC of PV-Cre;ErbB4−/− mice (Fig. 3A). This result was not unexpected because ErbB4 has been shown to be expressed by other neurons including glutamatergic neurons (9–11). To determine the extent of ErbB4 deletion in PV-positive neurons, PFC sections were costained with antibodies against PV and ErbB4. As shown in Fig. 3B, ErbB4 was detectable in almost all PV-positive neurons and in neurons that were not positive for PV in control littermates, in agreement with previous studies (12, 37–39). In contrast, ErbB4 immunoreactivity was abolished in PV-positive, but not in PV-negative neurons in PV-Cre;ErbB4−/− slices (Fig. 3B). These results demonstrated the specific loss of ErbB4 in PV-positive neurons.
Fig. 3.
Ablation of ErbB4 in PV-positive neurons prevented NRG1 from increasing GABAergic transmission. (A) Reduced levels of ErbB4 in PV-Cre;ErbB4−/− PFC. PFC was isolated from PV-Cre;ErbB4−/− and control littlemates (PV-Cre;ErbB4+/+) and homogenized. Resulting homogenates (40 μg of protein) were subjected to Western blotting analysis with the indicated antibodies. Equal loading was shown by immunoblotting for β-actin. (B) Specific ablation of ErbB4 in PV-positive neurons. PFC slices of PV-Cre;ErbB4−/− and PV-Cre;ErbB4+/+ were stained with anti-ErbB4 and PV antibody. Immunoactivity was visualized by Alexa 488- and Alexa 594-conjugated secondary antibodies, respectively. Slices were also stained with DAPI to indicate nuclei. Arrows, PV-positive neurons; arrowheads, PV-negative neurons. (Scale bar, 10 μm.) (C) NRG1 increased eIPSCs in PFC slices of control, but not PV-Cre;ErbB4−/−, mice. Concentrations were 5 nM for NRG1 and 40 μM for bicuculline. (D) Quantitative analysis of data in C (n = 8, *P < 0.01, compared with control). Control eIPSC amplitudes were 2,150 ± 128 and 1,650 ± 153 pA for PV-Cre;ErbB4+/+ and PV-Cre;ErbB4−/− mice, respectively (n = 8, P < 0.05). (E) No effect of NRG1 on eIPSCs at higher concentrations in PV-Cre;ErbB4−/− PFC. Dose–response curves are shown for both control and mutant PFC (n = ∼5–8; *P < 0.05, compared with mutant PFC).
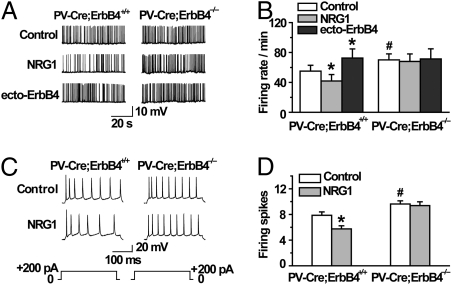
We investigated the effect of the PV-specific ErbB4 knockout on evoked inhibitory postsynaptic current (eIPSC) amplitudes. As observed previously (18), 5 nM NRG1 increased eIPSC amplitudes (by 43.2 ± 5.1%) in PV-Cre;ErbB4+/+ PFC slices within 5 min (Fig. 3 C and D). Remarkably, this effect was abolished in PFC slices from age-matched PV-Cre;ErbB4−/− mice. NRG1 showed little, if any, effect on eIPSC amplitudes in mutant slices even at a concentration 5-fold higher than that eliciting a maximal response in control slices (Fig. 3E). These results indicate a critical role for NRG1/ErbB4 signaling in PV-positive interneurons to regulate GABAergic transmission. In addition, both the spontaneous firing rate in loose-patch recordings (Fig. 4 A and B; n = 12 for both genotypes) and the spikes evoked by a 200-pA current injection in whole-cell recordings (Fig. 4 C and D; n = 9 for both genotypes) were increased in PV-Cre;ErbB4−/− slices in comparison with those from PV-Cre;ErbB4+/+ mice (P < 0.05), indicating that the loss of ErbB4 in PV-positive neurons increases the activity of pyramidal neurons. Importantly, unlike control slices where NRG1 had an inhibitory effect, NRG1 was unable to decrease the firing rate of pyramidal neurons in PV-Cre;ErbB4−/− slices (Fig. 4 A–D). Similarly, the ability of ecto-ErbB4 to increase the rate of firing was also lost in PV-Cre;ErbB4−/− mice (Fig. 4 A and B). These results suggest that regulation of GABAergic transmission by both exogenous and endogenous NRG1 and the subsequent inhibition of pyramidal neuron firing require ErbB4 in PV-positive inhibitory interneurons.
Fig. 4.
NRG1 was unable to inhibit pyramidal neuron firing in PV-Cre;ErbB4−/− mice. (A and B) No effect of NRG1 or ecto-ErbB4 on pyramidal neuron firing in PV-Cre;ErbB4−/− PFC. (A) Representative spontaneous firing patterns of pyramidal neurons of control or treated slices (5 nM NRG1 or 1 μg/mL ecto-ErbB4) of PV-Cre;ErbB4+/+ and PV-Cre;ErbB4−/− mice. (B) Quantitative analysis of data in A (n > 6 for both PV-Cre;ErbB4+/+ and PV-Cre;ErbB4−/− mice, *P < 0.01, compared with control; #P < 0.05, compared with PV-Cre;ErbB4+/+ samples). (C and D) Inability of NRG1 to inhibit evoked action potential frequency of pyramidal neurons in mutant PFC. (C) Representative action potentials produced by a 200-pA current before (Upper) and after (Lower) bath application of 5 nM NRG1 in PFC slices from PV-Cre;ErbB4+/+ and PV-Cre;ErbB4−/− mice. (D) Quantitative analysis of evoked spike frequency of pyramidal neurons (n = 9; *P < 0.05, compared with control; #P < 0.05, compared with PV-Cre;ErbB4+/+ samples).
Hyperactivity in PV-Cre;ErbB4−/− Mice.
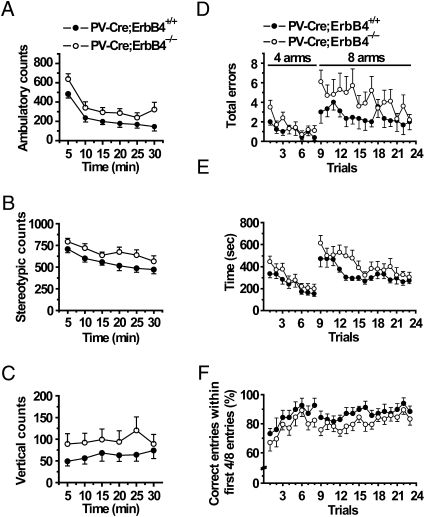
To gain insight into the physiological function of ErbB4 in PV-positive interneurons, PV-Cre;ErbB4−/− mice were subjected to a series of behavioral tests in a blind manner. PV-Cre;ErbB4−/− mutant mice did not exhibit differences in weight, whisker number, and rectal temperature in comparison with wild-type littermates, and there were no significant differences in motor coordination. First we investigated if PV-Cre;ErbB4−/− mice exhibited hyperactivity, a characteristic rodent phenotype that is thought to correspond to the psychomotor agitation of schizophrenic patients (31–33). Hyperactivity has been reported in mice heterozygous for NRG1 or ErbB4 (19, 24, 25, 28–30). Strikingly, PV-Cre;ErbB4−/− mice showed consistent hyperactivity in the open field test in comparison with wild-type controls. They traveled a significantly greater distance (3982.8 ± 501.3 cm) than wild-type controls (2552.7 ± 193.9 cm) during a 30-min period [n = 7 and 6 for control and mutant mice, respectively; F(1, 11) = 3.735, P = 0.017]. Ambulatory counts revealed a significant genotype effect [Fig. 5A; repeated measures, genotype F(1, 11) = 5.096, P = 0.045], suggesting abnormally higher horizontal or locomotory activity of the mutant mice. In addition, PV-Cre;ErbB4−/− mice showed higher stereotypic activity [Fig. 5B; genotype F(1, 11) = 5.237, P = 0.043]. Note that both mutant and control mice exhibited habituation of locomotory and stereotypic activity with time and the rate of habituation was not different between mutant and control [for locomotory activity, time F(5, 55) = 81.073, P < 0.001, genotype × time interaction F(5, 55) = 1.746, P = 0.139; for stereotypic activity, time F(5, 55) = 16.542, P < 0.001, genotype × time interaction F(5, 55) = 1.566, P = 0.185], indicating that both wild-type and mutant mice were able to adapt to a novel environment. No difference was observed in vertical or rearing activity between control and mutant mice [Fig. 5C; genotype F(1, 11) = 1.844, P = 0.202; time F(5,55) = 1.553, P = 0.189; genotype × time interaction F(5,55) = 1.084, P = 0.380]. Together these results are consistent with the idea that specific ablation of ErbB4 in PV-positive interneurons increased locomotor activity and stereotypical activity.
Fig. 5.
Hyperactivity and impaired working memory in PV-Cre;ErbB4−/− mice. (A–C) Mice were placed in a chamber and movements were monitored for 30 min in the open field test. (A and B) Enhanced ambulatory and stereotypic activity in PV-Cre;ErbB4−/− mice (repeated measures for genotype, P = 0.045 for ambulatory activity and P = 0.043 for stereotypic activity). Ambulatory activity was measured as the total horizontal photobeam breaks, and stereotypy was quantified in terms of repetitive breaks of a given beam with intervals of <1 s. Activity was summated at 5-min intervals over a 30-min period. (C) Similar vertical activity between PV-Cre;ErbB4−/− and PV-Cre;ErbB4+/+ mice (repeated measures for genotype, P = 0.202). Vertical activity (rearing) was evaluated as the total number of vertical beam breaks at 5-min intervals over a 30-min period. n = 7 for PV-Cre;ErbB4+/+ mice; n = 6 for PV-Cre;ErbB4−/− mice. (D–F) Working memory of food-restricted mutant (n = 10) and control (n = 9) mice in four-arm or eight-arm radial maze tests. (D) Total number of errors (revisits and omission) were significantly higher in PV-Cre;ErbB4−/− mice in both four-arm and eight-arm tests (repeated measures, P = 0.002 and P = 0.021, respectively). A significant trial effect was observed in the four-arm test (P < 0.001), but not in the eight-arm test (P = 0.290). (E) Time spent by mutant and control mice to retrieve all food pellets was similar (P = 0.096 for four-arm and P = 0.085 for eight-arm test). A significant trial effect was observed for both four-arm and eight-arm tests (P < 0.001). (F) Percentage of correct entries within the first four and eight entries was significantly lower in mutant mice in the four-arm test (P = 0.044) and the eight-arm test (P = 0.040), respectively. A significant trial effect was observed in both tests (P < 0.001 and P = 0.002, respectively).
Impaired Working Memory in PV-Cre;ErbB4−/− Mice.
Working memory deficits are thought to be central to poor cognitive performance in schizophrenia and to result from GABAergic dysfunction (45, 46). To examine whether the loss of ErbB4 in PV-positive interneurons resulted in cognitive deficits, PV-Cre;ErbB4−/− mice and control littermates were evaluated for their performance on an automated radial arm maze to assess changes in working memory. Food-restricted mice were trained to retrieve food pellets from the end of each arm. After the initial shaping, mice were allowed free access to either four arms (less difficult condition) or eight arms (more difficult condition) where all arms were baited. The number of errors (repeated entries into a previously visited arm or omission of an arm) and the total time to retrieve all pellets were scored. When mice were analyzed in the four-arm test, a significant trial effect was observed in total number of errors and the total time to retrieve all pellets [n = 9 and 10 for control and mutant mice, respectively; repeated measures for total errors, trial F(7, 119) = 4.532, P < 0.001; for total time, trial F(7, 119) = 10.532, P < 0.001], but there was no significant genotype × trial interaction [for total errors, F(7, 119) = 0.880, P = 0.525; for total time, F(7, 119) = 0.470, P = 0.855] (Fig. 5 D and E). These results indicated that both control and PV-Cre;ErbB4−/− mice were able to learn to retrieve food pellets by reducing wrong entries and exploration time. Although there was no significant difference between total times mutant and control mice spent to retrieve all food pellets [repeated measures, genotype F(1, 17) = 3.097, P = 0.096; Fig. 5E], PV-Cre;ErbB4−/− mice showed a significantly increased number of total errors [repeated measures, genotype F(1, 17) = 14.158, P = 0.002; Fig. 5D], suggesting possible deficits in working memory.
Because PV-Cre;ErbB4−/− mice were hyperactive (Fig. 5 A–C), the increase in total errors may have resulted from random hyperactivity. To exclude this possibility, we monitored correct entries during the first four entries, eliminating effects of total travel distance and time (24). Intriguingly, the percentage of correct entries within the first four entries was significantly lower in mutant mice in comparison with controls [repeated measures, genotype F(1, 17) = 4.729, P = 0.044, trial F(7, 119) = 5.173, P < 0.001, genotype × trial interaction F(7,119) = 0.121, P = 0.997; Fig. 5F]. These results are in agreement with the idea of impaired working memory in PV-Cre;ErbB4−/− mice. To test this hypothesis further, we increased the difficulty in the working memory test by using an eight-arm radial maze. The PV-Cre;ErbB4−/− mice had an increase in wrong entries and a decrease in the percentage of correct entries [repeated measure, F(1, 17) = 6.436, P = 0.021 for total wrong entries; F(1, 17) = 4.952, P = 0.040 for percentage of correct entries within the first eight entries; Fig. 5 D and F]. These results demonstrated that ErbB4 in PV-positive interneurons is critical for working memory, suggesting that the alteration of NRG1/ErbB4 signaling in PV-positive interneurons might contribute to the cognitive deficits in schizophrenia.
Attenuated PPI in PV-Cre;ErbB4−/− Mice.
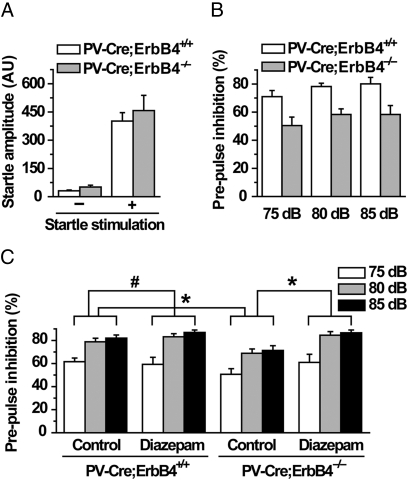
Patients with schizophrenia often show deficits in PPI, a common test of sensory gating that can also be performed in rodents (31–33). Reduced PPI ability is thought to contribute to schizophrenic conditions including inattention, distractibility, and cognitive deficits. Single-nucleotide polymorphisms (SNPs) in the nrg-1 gene are associated with PPI deficits in schizophrenic patients (2) and mutation of the mouse homolog leads to reduced PPI in mice (3, 19). We used a combination of auditory-evoked startle (120 dB) and three levels of prepulses (75, 80, and 85 dB). Mutant and wild-type mice produced similar startle responses to 120-dB stimuli [n = 7 and 6 for control and mutant mice, respectively; F(1, 11) = 1.925, P = 0.539; Fig. 6A]; however, PPI was significantly lower in PV-Cre;ErbB4−/− mice in comparison with controls [repeated measures, genotype F(1, 11) = 13.684, P = 0.004; Fig. 6B]. It should be noted that significant prepulse intensity effects were also observed [repeated measures, prepulse intensity F(2, 22) = 10.615, P = 0.001; genotype × prepulse intensity F(2, 22) = 0.113, P = 0.894; Fig. 6B]. These results indicated that both mutant and control mice could distinguish among each prepulse intensity; however, PV-Cre;ErbB4−/− mice are impaired in PPI.
Fig. 6.
PPI deficit in PV-Cre;ErbB4−/− mice and its amelioration by diazepam. (A) Similar mean baseline startle response in PV-Cre;ErbB4−/− mice and control littermates. Response to background white noise (no stimulus, 70 dB) and auditory-evoked startle stimulus (120 dB) was measured. n = 7 and 6 for PV-Cre;ErbB4+/+ and PV-Cre;ErbB4−/− mice, respectively; P > 0.05. AU, arbitrary units. (B) Reduced PPI in PV-Cre;ErbB4−/− mice. PPI (%) was calculated according to the formula: [100 − (startle amplitude on prepulse-pulse trials/startle amplitude on pulse alone trials) × 100]. n = 7 and 6 for PV-Cre;ErbB4+/+ and PV-Cre;ErbB4−/− mice, respectively; repeated measures, P = 0.004. (C) Diazepam attenuated PPI deficit in PV-Cre;ErbB4−/− mice. Diazepam (3 mg/kg) or vehicle was injected intraperitoneally 30 min before PPI test. Repeated measures, *P < 0.05, #P = 0.573. For vehicle treatment, n = 10 and 12 for PV-Cre;ErbB4−/− mice and control littermates, respectively; for diazepam treatment, n = 9 and 11 for PV-Cre;ErbB4−/− and controls, respectively.
To investigate whether abnormal GABAergic transmission may be a cause of behavioral deficits, PV-Cre;ErbB4−/− mice were treated with diazepam, a GABA enhancer. PPI remained deficient in the mutant mice treated with vehicle [repeated measures, genotype F(1, 20) = 5.302, P = 0.032; n = 10 and 12 for PV-Cre;ErbB4−/− and control littermates, respectively; Fig. 6C]. Three milligrams per kilogram diazepam seemed to have no effect on PPI after 30-min treatment in control mice [repeated measures, genotype F(1, 17) = 0.330, P = 0.573; n = 10 and 9 for vehicle and diazepam treatment, respectively; Fig. 6C], consistent with a previous report (47). In contrast, diazepam significantly enhanced PPI in PV-Cre;ErbB4−/− mice [repeated measures, genotype F(1, 21) = 6.157, P = 0.022; n = 12 and 11 for vehicle and diazepam treatment, respectively; Fig. 6C]. These results indicated that acute administration of low-dose diazepam was able to ameliorate disrupted PPI in PV-Cre;ErbB4−/− mice, providing evidence for impaired GABAergic transmission.
Discussion
Major findings of this paper are as follows. First, NRG1 inhibits the activity of pyramidal neurons in the PFC. Both spontaneous firing rates and the frequency of evoked action potentials in pyramidal neurons were reduced by NRG1, but increased by the neutralizing peptide ecto-ErbB4. These effects were blocked by bicuculline, an antagonist of GABAA receptor, indicating GABA dependence. Second, ablation of ErbB4 in PV-positive neurons blocked NRG1 potentiation of GABAergic transmission and prevented NRG1 from inhibiting pyramidal neuron firing. Finally, PV-Cre;ErbB4−/− mice showed schizophrenic-like phenotypes including hyperactivity, impaired working memory, and PPI deficit that could be ameliorated by diazepam. These observations indicate that NRG1 plays a critical role in balancing brain activity and identify PV-positive neurons as a major cellular target of NRG1/ErbB4 signaling in regulating synaptic plasticity. Our study provides insight into potential pathogenic mechanisms of schizophrenia and suggests that PV-ErbB4−/− mice may serve as a model in the study of this and relevant brain disorders.
The inhibitory effect of NRG1 on the activity of pyramidal neurons could be mediated by decreased excitatory synaptic input and/or increased inhibitory synaptic activity. As noted in SI Materials and Methods, evoked action potentials were recorded under a condition where most, if not all, glutamatergic transmission is blocked, making it unlikely to involve excitatory synaptic activity or input. In contrast, the inhibitory effect of NRG1 on spontaneous and evoked spike generation was blocked by bicuculline (Figs. 1 and 2), indicating the involvement of GABA transmission. This idea was supported by the studies of ecto-ErbB4, a neutralizing peptide that reduces activity-dependent GABA release (18). As shown in Figs. 1 and 2, ecto-ErbB4 increased both spontaneous firing rates and the frequency of evoked action potentials in pyramidal neurons. Remarkably, blockade of GABAA receptors occluded the effect of ecto-ErbB4. These results suggest that the inhibitory effect of NRG1 on pyramidal neuron activity is likely to be mediated by increased GABAergic synaptic transmission.
GABAergic interneurons are a heterogeneous group of neuronal cells with distinct functions (40). Basket cells synapse onto the somata and proximal dendrites of pyramidal neurons whereas chandelier cells preferentially target their axon initial segments. These interneurons regulate the output of pyramidal neurons by affecting the generation and timing of action potentials (40, 41). Both basket and chandelier cells express the calcium binding protein PV (40, 45). Interestingly, the potentiation effect of NRG1 on GABAergic transmission was abolished in PV-Cre;ErbB4−/− mice (Fig. 3 C–E) and NRG1 was no longer able to inhibit spontaneous firing rates and the frequency of evoked action potentials in pyramidal neurons (Fig. 4). These observations provide convincing evidence that PV-positive interneurons are a major cellular target of NRG1/ErbB4 signaling in regulating GABAergic transmission and pyramidal neuron activity.
NRG1 hypomorphic mice including Nrg1(ΔEGF)+/− and Nrg1(ΔTM)+/− mice were hyperactive in novel open-field and alternating-Y maze tests (19, 24, 28–30). Nestin-Cre;ErbB4−/− mice were more active than controls at the initial stage of behavioral evaluation (25). Nrg1 and ErbB4 mutant mice are impaired in PPI (3, 19). Interestingly, these schizophrenia-relevant behaviors of NRG1 and ErbB4 null mutation were also observable in PV-Cre;ErbB4−/− mice where ErbB4 is specifically knocked out in PV-positive interneurons (Figs. 5 A–C and 6). In addition, PV-Cre;ErbB4−/− mice are impaired in working memory (Fig. 5 D–F), in support of the ideas that PV-positive interneurons are important in modulating cognitive processes and that disturbance in GABAergic neurotransmission could be a pathologic mechanism of schizophrenia (40, 41, 45, 46). These observations are consistent with the notion that pharmacological alteration of GABA inhibition onto pyramidal neurons may be beneficial for cognition deficits in schizophrenia (48). In agreement, we show that diazepam could attenuate the PPI disruption in PV-Cre;ErbB4−/− mice (Fig. 6C), indicating that altered GABAergic neurotransmission may account for at least in part behavioral deficits in the mutant mice. It is worth pointing out that mechanisms for the behavioral deficits in PV-Cre;ErbB4−/− mice could be complex and may likely involve other neurotransmitter systems including dopamine, acetylcholine, and glutamate (30–33, 49).
Our results could be interpreted as evidence that PV-positive interneurons are a major cellular target of abnormal NRG1/ErbB4 signaling in schizophrenia. They are in line with the idea that disrupted NRG1 signaling may cause imbalance in neuronal activity in the brain, providing insight into possible pathogenic mechanisms of schizophrenia. Finally, unlike NRG1 and ErbB4 null mutant mice that often die prematurely, PV-ErbB4−/− mice survive into adulthood and thus could serve as a valuable model to study schizophrenia and relevant brain disorders.
Materials and Methods
ErbB4loxP/loxP and PV-Cre mice were described previously (42–44). PV-Cre;ErbB4−/− and control mice were housed in a room with a 12-h light/dark cycle with access to food and water ad libitum. Experiments with animals were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia. PFC slices were prepared as described previously (18) and recorded for eIPSCs and pyramidal neuron activity as described in SI Materials and Methods.
Immunofluorescence staining and Western blot analysis were performed as previously described (18). Behavioral analysis was carried out with 8- to 12-week-old mice by investigators unaware of their genotype. Detailed procedures are described in SI Materials and Methods. Data were analyzed by paired or unpaired t test, one-way ANOVA followed by Dunnett’s test, or repeated measures ANOVA. Data were expressed as mean ± SEM, and statistical significance was considered when P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Mark Slikowski for NRG1 and Dr. Silvia Arber for PV-Cre mice. We also thank the Small Animal Behavior Core at Medical College of Georgia for the help in behavioral analysis. This work was supported in part by grants from the National Institutes of Health (to L.M. and W.-C.X.). L.M. is a National Alliance for Research on Schizophrenia and Depression Distinguished Investigator and 2008 National Alliance for Research on Schizophrenia and Depression Stone Investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910302107/DCSupplemental.
References
- 1.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 2.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros CS, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci USA. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajendran N, et al. Neuregulin signaling is dispensable for NMDA- and GABA(A)-receptor expression in the cerebellum in vivo. J Neurosci. 2009;29:2404–2413. doi: 10.1523/JNEUROSCI.4303-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, et al. Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol. 1994;349:389–400. doi: 10.1002/cne.903490306. [DOI] [PubMed] [Google Scholar]
- 7.Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol. 1999;159:494–503. doi: 10.1006/exnr.1999.7163. [DOI] [PubMed] [Google Scholar]
- 8.Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- 9.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci USA. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YZ, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 11.Ma L, et al. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23:3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: Preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 13.Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon OB, et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci USA. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo RS, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JZ, et al. Association study of neuregulin 1 gene with schizophrenia. Mol Psychiatry. 2003;8:706–709. doi: 10.1038/sj.mp.4001377. [DOI] [PubMed] [Google Scholar]
- 21.Norton N, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 22.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: Association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 23.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 24.Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- 25.Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Thuret S, et al. The neuregulin receptor, ErbB4, is not required for normal development and adult maintenance of the substantia nigra pars compacta. J Neurochem. 2004;91:1302–1311. doi: 10.1111/j.1471-4159.2004.02809.x. [DOI] [PubMed] [Google Scholar]
- 27.Rimer M, Barrett DW, Maldonado MA, Vock VM, Gonzalez-Lima F. Neuregulin-1 immunoglobulin-like domain mutant mice: Clozapine sensitivity and impaired latent inhibition. Neuroreport. 2005;16:271–275. doi: 10.1097/00001756-200502280-00014. [DOI] [PubMed] [Google Scholar]
- 28.O’Tuathaigh CM, et al. Sexually dimorphic changes in the exploratory and habituation profiles of heterozygous neuregulin-1 knockout mice. Neuroreport. 2006;17:79–83. doi: 10.1097/01.wnr.0000192738.31029.0a. [DOI] [PubMed] [Google Scholar]
- 29.O’Tuathaigh CM, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 30.O’Tuathaigh CM, et al. Susceptibility genes for schizophrenia: Characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev. 2007;31:60–78. doi: 10.1016/j.neubiorev.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Gainetdinov RR, Mohn AR, Caron MG. Genetic animal models: Focus on schizophrenia. Trends Neurosci. 2001;24:527–533. doi: 10.1016/s0166-2236(00)01886-5. [DOI] [PubMed] [Google Scholar]
- 32.Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Arguello PA, Gogos JA. Modeling madness in mice: One piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 34.del Río JA, de Lecea L, Ferrer I, Soriano E. The development of parvalbumin-immunoreactivity in the neocortex of the mouse. Brain Res Dev Brain Res. 1994;81:247–259. doi: 10.1016/0165-3806(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 35.Hof PR, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: Phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 36.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 37.Vullhorst D, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: Implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2009 doi: 10.1002/hipo.20675. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 41.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 42.García-Rivello H, et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–H1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 43.Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 44.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 46.Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 47.Ouagazzal AM, Jenck F, Moreau JL. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: A model for detecting antipsychotic activity? Psychopharmacology (Berl) 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- 48.Lewis DA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viggiano D. The hyperactive syndrome: Metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav Brain Res. 2008;194:1–14. doi: 10.1016/j.bbr.2008.06.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.