Abstract
Metastasis underlies the majority of cancer-related deaths. Thus, furthering our understanding of the molecular mechanisms that enable tumor cell dissemination is a vital health issue. Epithelial-to-mesenchymal transitions (EMTs) endow carcinoma cells with enhanced migratory and survival attributes that facilitate malignant progression. Characterization of EMT effectors is likely to yield new insights into metastasis and novel avenues for treatment. We show that the presence of the receptor tyrosine kinase Axl in primary breast cancers independently predicts strongly reduced overall patient survival, and that matched patient metastatic lesions show enhanced Axl expression. We demonstrate that Axl is strongly induced by EMT in immortalized mammary epithelial cells that establishes an autocrine signaling loop with its ligand, Gas6. Epiallelic RNA interference analysis in metastatic breast cancer cells delineated a distinct threshold of Axl expression for mesenchymal-like in vitro cell invasiveness and formation of tumors in foreign and tissue-engineered microenvironments in vivo. Importantly, in two different optical imaging-based experimental breast cancer models, Axl knockdown completely prevented the spread of highly metastatic breast carcinoma cells from the mammary gland to lymph nodes and several major organs and increased overall survival. These findings suggest that Axl represents a downstream effector of the tumor cell EMT that is required for breast cancer metastasis. Thus, the detection and targeted treatment of Axl-expressing tumors represents an important new therapeutic strategy for breast cancer.
Keywords: carcinoma, receptor tyrosine kinase, breast cancer
Metastasis to distant sites is the most common cause of death from carcinomas (1). Malignant cells discard epithelial restraints and acquire invasive motility that facilitates dissemination to permissive niches (2). This malignant progression is enhanced by tumor cell activation of a epithelial-to-mesenchymal transition (EMT), a developmental program in which epithelial cells assume a mesenchymal phenotype during gastrulation and organogenesis, allowing single cell invasive movement away from the ectodermal layer (3). Early carcinomas harboring oncogenic mutations (e.g., ras) are thought to undergo EMT as the result of contextual cell signaling responses to local tumor stromal signals, such as TGF-β, in the inflammatory and hypoxic tumor microenviroment (4, 5). The EMT program is activated by developmental transcriptional regulators, including Twist, Zeb2 (ZEB2/SIP1), and the Snail family transcription factors Snail (Snail/SNAI1) and Slug (Slug/SNAI2) (6). These EMT transcription factors alter the epithelial gene expression profile, demarcated by the repression of genes encoding epithelial junctional complexes (e.g., E-cadherin, β-catenin) and cytokeratins, and induce the expression of mesenchymal vimentin and N-cadherin. Recent evidence strongly implicates EMT induction in malignant progression (3). EMT regulatory transcription factors are expressed in many malignant cancers and are required for breast cancer metastasis (7). The acquisition of EMT is associated with stem cell features and immunosuppression that collectively promote metastasis (8, 9).
Axl is a member of the TAM (Tyro-Axl-Mer) receptor tyrosine kinases (RTK) that share the vitamin K–dependent ligand Gas6 (growth arrest–specific 6). TAM family RTKs regulate a diverse range of cellular responses including cell survival, proliferation, autophagy, migration, angiogenesis, platelet aggregation, and natural killer cell differentiation (10). Axl is expressed in many embryonic tissues and is thought to be involved in mesenchymal and neural development, with expression in adult tissues largely restricted to smooth muscle cells (MGI Gene Expression Database; www.informatics.jax.org). Axl activation is linked to several signal transduction pathways, including Akt, MAP kinases, NF-κB, STAT, and others (11). Originally identified as a transforming gene from a patient with chronic myelogenous leukemia, Axl has since been associated with various high-grade cancers and correlated with poor prognosis (10). We and others have shown that Axl is required for xenograft growth of breast carcinoma and glioma cells (12, 13).
Here we show that Axl expression in breast cancer independently predicts poor overall patient survival. Axl levels are elevated in metastatic lesions, and expression is required to maintain breast carcinoma cell invasiveness, growth in foreign microenviroments, and metastatic spread. We demonstrate that Axl is a unique EMT effector that is essential for breast cancer progression.
Results
Axl Expression Is a Strong Negaive Prognostic Factor for Survival in Breast Cancer Patients.
To assess the role of Axl in breast cancer pathogenesis and progression, we investigated Axl expression in a panel of 190 human breast cancers comprising tumors diagnosed during screening intervals (n = 95) and a size-matched group of tumors detected by mammography-based screening (n = 95) (14). There were no significant associations between Axl expression (divided into two groups by median staining index; Fig. 1A) and important clinicopathological features, such as tumor diameter, histological grade, expression of estrogen and progesterone receptors, and axillary lymph node status (Fig. S1). Moreover, Axl expression was not significantly associated with Her2 status, E-cadherin expression, markers of basal differentiation (cytokeratin 5/6 or P-cadherin), or tumor cell proliferation by Ki-67 staining.
Fig. 1.
Axl expression is a strong negative prognostic factor for human breast cancer survival. (A) Breast tumors are stratified by weak (Upper, 60% of patients) and strong (Lower, 40% of patients) Axl expression. (B) Univariate survival analysis (Kaplan-Meier method) of breast cancer patients shows a strong negative prognostic value of Axl expression (P = .035, log-rank test). (C) Multivariate survival analysis (proportional hazards method) indicates a negative, independent prognostic importance of Axl expression (P = .021, likelihood ratio test), in addition to the independent prognostic impact of histological grade and axillary lymph node status. HR, hazard ratio. (D) Axl expression was significantly stronger in metastases (P < 0.0005, Wilcoxon rank-sum test) from matched pairs (n = 37) of primary (Left, Upper, Axl-negative; Lower, Axl-positive) and metastatic (Right, Upper, liver; Lower, bone) breast carcinoma.
However, by univariate survival analysis (Kaplan-Meier method, log-rank test), Axl expression was significantly associated with reduced patient survival (P = .035; Fig. 1B). By multivariate analysis (proportional hazards method) and including such basic prognostic factors as tumor diameter, histological grade, and lymph node status in addition to Axl expression (initial regression model), Axl expression status remained an independent negative prognostic factor in the final multivariate model (P = .021, likelihood ratio test) along with histological grade and lymph node status (Fig. 1C), with a hazard ratio of 3.27 between cases with high and low Axl expression.
We then investigated Axl expression in matched pairs of primary breast carcinomas and cognate metastases (liver, bone) from the same patients (n = 37). Axl expression was significantly elevated in metastases compared with the corresponding primary breast carcinoma (P < .0005, Wilcoxon rank test; Fig. 1D). Collectively, these data indicate that Axl expression is closely associated with metastasis and a strong predictor of poor clinical outcome in breast cancer patients.
Axl Is Required for Breast Cancer Cell Invasiveness.
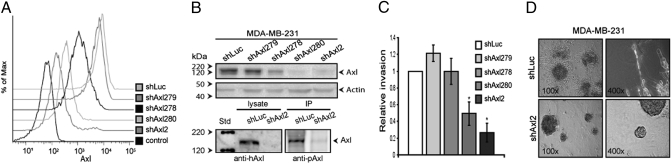
The strong correlation of Axl expression with poor patient survival indicates an important role for Axl in breast cancer pathogenesis. Because breast cancer–related mortality invariably results from complications of metastatic disease, we assessed whether Axl expression was required for breast carcinoma cell invasiveness. Axl is expressed in several highly metastatic human breast carcinoma cell lines, including MDA-MB-231. The Axl ligand Gas6 often is coexpressed, consistent with autocrine activation (12). To effectively relate Axl expression levels to specific cellular behaviors, we developed an epiallelic MDA-MB-231 cell series using a collection of Axl-targeting shRNAs that reduce Axl expression in a graded manner, generated by a recently developed FACS-based RNAi approach (CellSelect RNAi). This Axl epi-allelic MDA-MB-231 cell series has a graded reduction in surface and total Axl protein levels (Fig. 2 A and B). Cell-associated Gas6 and phosporylated Axl levels indicative of autocrine signaling are reduced upon Axl knockdown in MDA-MB-231 cells (Fig. 2B and Fig. S2). Breast carcinoma cells exhibit unicellular (mesenchymal) invasiveness in three-dimensional (3D) extracellular matrix protein gels (Matrigel) that correlates with in vivo metastatic potential (15). Epi-allelic analysis of MDA-MB-231 cell invasion through Matrigel demonstrated a dose dependence on Axl expression in response to serum (Fig. 2C) or the SDF-1 chemokine (Fig. S3A), an important factor in the metastasis of breast carcinoma to the bones and lungs (16). In contrast, Axl knockdown had no effect on MDA-MB-231 cell proliferation (Fig. S4) or matrix metalloproteinase activity (Fig. S5). These findings indicate that Axl is specifically required for 3D growth and invasiveness; thus, we evaluated the effect of Axl knockdown on MDA-MB-231 cells in a 3D model. Normal breast epithelial cells become quiescent and self-organize into polarized, spheroidal acinar structures in 3D Matrigel matrices, whereas malignant cells (e.g., MDA-MB-231) continue to proliferate, forming large, disorganized colonies with stellate invasive cell projections that reflect aggressive tumors (17). Knockdown of Axl expression strongly inhibited the malignant phenotype of MDA-MB-231 cells in 3D Matrigel, generating small, round colonies lacking invasive projections (Fig. 2D and Fig. S3B). Together, these findings suggest that Axl expression is required to maintain the mesenchymal-like invasiveness of metastatic breast carcinoma cells.
Fig. 2.
Axl is required for breast cancer cell invasiveness (A and B) Epiallelic MDA-MB-231 cells comprising Axl-targeting shRNA (shAxl2, shAxl280, shAxl278, and shAxl279) and control shRNA (shLuc) show graded Axl surface expression by flow cytometry (A) and total and phosporylated Axl (pAxl) levels by immunoblotting (B). (C) Invasiveness of epiallelic MDA-MB-231 cells is Axl-dependent. *P < .05, (paired t test). (D) Axl is required for invasive, stellate growth of MDA-MB-231 cells in 3D Matrigel (Lower, shAxl2; Upper, shLuc).
Axl Is Up-Regulated by EMT-Inducing Transcription Factors in Breast Epithelial Cells.
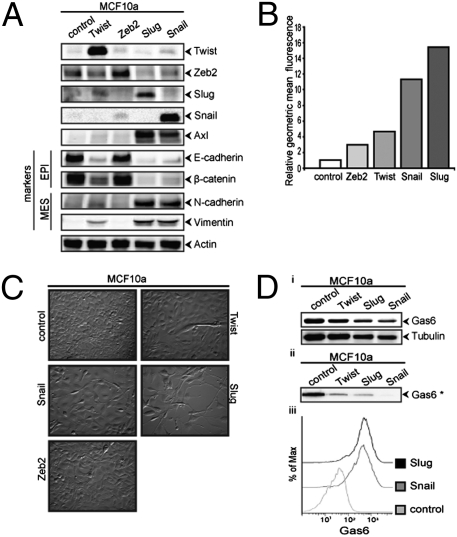
The acquisition of mesenchymal cell characteristics by epithelial cells, in particular the ability to migrate as single cells and invade the extracellular matrix, is the functional hallmark of EMT (3). The EMT-inducing transcription factor Twist is required for metastasis of mouse 4T1 breast carcinoma cells (7); therefore, we investigated whether Twist expression up-regulates Axl in human breast epithelial cells. A nonmalignant breast epithelial cell line (MCF10a) that stably expresses Twist exhibits an EMT phenotype characterized by down-regulation of the epithelial markers E-cadherin and β-catenin and up-regulation of the mesenchymal marker N-cadherin (Fig. S6A). Strikingly, Axl also is strongly up-regulated in these Twist-expressing MCF10A cells (Fig. S6 B and C). To determine whether other EMT program–inducing transcription factors similarly up-regulate Axl expression, we analyzed MCF10A cells transduced with retroviral constructs that express Twist, Zeb2, Snail, or Slug. EMT induction by these transcription factors was accompanied by enhanced Axl expression, with the highest Axl surface levels activated by the Snail family members (Fig. 3 A and B). Axl expression levels correlated with the extent of mesenchymal marker expression and morphology attained by MCF10a cells expressing the different EMT activators (Fig. 3C). Gas6 was constitutively expressed by MCF10A cells and became cell associated on EMT-induced Axl expression (Fig. 3D). These results suggest that EMT program induction leads to Axl expression and can establish autocrine signaling in breast epithelial cells.
Fig. 3.
Axl is up-regulated by EMT inducers in breast epithelial cells. (A) MCF10a cells transduced with retroviral vectors encoding Twist, Zeb2, Slug, and Snail up-regulate Axl, lose epithelial markers (EPI), and increase mesenchymal (Mes) markers relative to vector control (GFP). (B) Axl surface expression is up-regulated in MCF10a cells transduced with Twist, Zeb2, Slug, or Snail retroviral vectors measured by flow cytometry. (C) These cells acquire mesenchymal morphology at 72 h postseeding. (D) MCF10a cells constitutively express (Top, Western blot, total lysate) and secrete (Middle, Western blot, conditioned medium) Gas6 that becomes cell-associated (Bottom, anti-Gas6 flow cytometry analysis) on Slug- and Snail-induced Axl expression.
Axl Expression Is Necessary for Tumor Formation in Experimental Tissue-Engineered Breast Tumors.
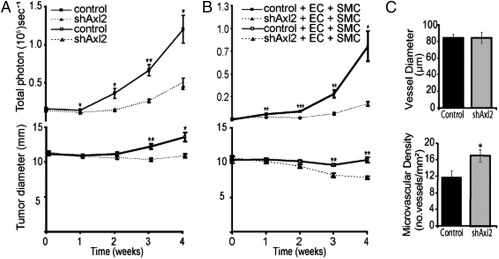
Tumor growth within tissue-engineered 3D biomimetic microenviroments is associated with enhanced tumor cell mesenchymal characteristics (18). To evaluate the requirement for Axl for malignant 3D growth in vivo, we used a tissue engineering approach comprising MDA-MB-231 cells expressing a GFP-luciferase construct (MDA-MB-231/GFP-Luc) for efficient in vivo optical imaging (pCSI; SI Experimental Procedures), seeded with Matrigel into polymeric tissue engineering scaffolds and implanted s.c. into immunocompromised NOD-SCID mice. MDA-MB-231/GFP-Luc cells readily formed tumors in this biomimetic microenviroment, displaying aggressive growth and colonization of the scaffold. In contrast, Axl knockdown strongly inhibited MDA-MB-231/GFP-Luc cell tumor formation, radial spread, and malignant morphology in the tissue engineering scaffold (Fig. 4A and Fig. S7A).
Fig. 4.
Tissue-engineered breast tumors require Axl expression. (A) MDA-MB-231/GFP-Luc tumor growth and colonization within polylactic acid tissue engineering scaffolds in NOD-SCID mice (n = 6 per group) is Axl dependent. (B) Axl is required for the growth of tricellular tissue implants comprising primary human microvascular cells (ECs), vascular smooth muscle cells (SMCs), and MDA-MB-231/GFP-Luc in NOD-SCID mice (n = 7 per group). Temporal in vivo bioluminescence image analysis was used to measure tumor cell number (Upper, total photon) and the extent of radial infiltration (Lower, signal diameter) in controls (solid line) and shAxl2 implants (dashed line). (C) Intrascaffold human vessel diameter (Upper) is unaffected, whereas microvascular density (Lower) is slightly enhanced in tissue-engineered (tricellular) tumors inhibited by shAxl2 expression. *P < .05; **P < .005; ***P < .0005 (paired t test) versus control.
Tissue-engineered microenviroments also increase the angiogenic capacity of tumor cells (19). To explore whether Axl influences the ability of malignant breast cancer cells to attract and co-opt local blood vessels, we developed a novel tricellular tissue engineering approach comprising MDA-MB-231/GFP-Luc cells coseeded with primary human microvascular endothelial cells (ECs) and vascular smooth muscle cells (vSMCs) into polymeric scaffolds to engineer a human tumor vasculature. MDA-MB-231/GFP-Luc cells formed large, highly vascularized tumors in this tricellular implant model (Fig. 4B and Fig. S7B). Axl knockdown blocked tumor formation, without inhibiting blood vessel formation in the scaffold (Fig. 4 B and C). This indicates that Axl is required for tumor formation even in the presence of a local microvasculature that obviates the need for angiogenesis.
A Distinct Axl Expression Level Is Required for Breast Tumor Formation.
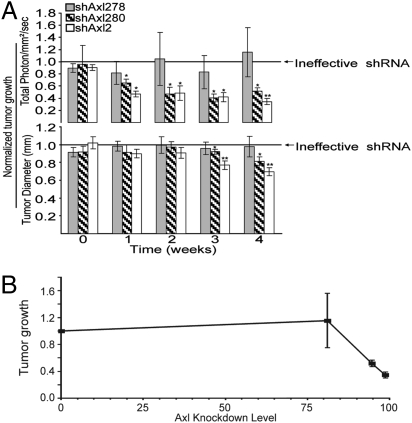
To evaluate the level of Axl expression needed to form a tumor, we conducted an in vivo epiallelic analysis of Axl in s.c. MDA-MB-231 tumors. The epiallelic Axl MDA-MB-231 cell series (Fig. 2A) was injected s.c. and temporally monitored for tumor formation by in vivo bioluminescence imaging (Fig. S8). This approach revealed Axl-dependent tumor growth (Fig. 5A), congruent with the dose-dependent effects of Axl inhibition on invasiveness (Fig. 2C). Correlation of tumor growth with Axl surface levels demarcated a threshold of Axl inhibition (corresponding to 80% reduction of surface expression) necessary to inhibit xenograft tumor growth (Fig. 5B).
Fig. 5.
In vivo epiallelic analysis reveals a distinct Axl expression threshold required for breast tumor formation. (A) Temporal in vivo bioluminescence image analysis of tumor growth (total photon) and radial infiltration (signal diameter) by s.c. Axl epiallelic MDA-MB-231/GFP-Luc xenografts in NOD/SCID mice (n = 6 per group) comprising shAxl278, shAx280, and shAxl2 normalized to shAxl279 (ineffective shRNA). (B) Tumor growth (normalized 28-day total photon measurements) plotted versus Axl knockdown (geometric mean fluorescence) reveal a threshold of 80% reduced surface expression to attain an antitumor effect (therapeutic benefit). *P < .05; **P < .005 (paired t test).
Axl Is Essential for Metastasis of Breast Carcinoma Cells.
To evaluate the requirement of Axl for breast cancer metastasis, we orthotopically injected MDA-MB-231-D3H2LN/GFP-Luc cells into the mammary fat pad of NOD-SCID mice. Using whole-body temporal bioluminescence imaging, we monitored spontaneous metastasis development over a 9-week period. MDA-MB-231-D3H2LN/GFP-Luc cells generated very large primary mammary tumors that became necrosing within 5–6 weeks (Fig. 6A, Top). Axl knockdown (MDA-231-D3H2LN/GFP-Luc-shAxl2) reduced the rate of mammary MDA-MB-231-D3H2LN/GFP-Luc tumor formation but did not inhibit the formation of large primary mammary tumors (Fig. 6 A and B). Spontaneous metastasis was initially detected in the thoracic sentinel lymph nodes of control MDA-MB-231-D3H2LN/GFP-Luc mice at 4 weeks and grew rapidly thereafter (Fig. 6 A and B). Strikingly, no metastases from MDA-231-D3H2LN/GFP-Luc-shAxl2 tumors were detected during the 9-week experiment (Fig. 6 A and B). After the animals were sacrificed at 9 weeks, excised organs were individually scanned for the presence of bioluminescence metastatic cells. All mice with MDA-MB-231-D3H2LN/GFP-Luc tumors displayed extensive spontaneous metastasis to several organs, including prominent spread to lymph nodes, lungs, ovaries, and kidneys; metastasis was not detected in liver, bone, or brain (Fig. 6C and Fig. S9). In contrast, MDA-MB-231-D3H2LN/GFP-Luc-shAxl2 cells did not form detectable metastasis, apart from a single small lesion in the kidney of one mouse that was demonstrated to be derived from a wild-type Axl-expressing cell (Fig. S10). Histological analysis of tissue biopsy specimens confirmed the presence of multiple micrometastases and macrometastases, as predicted by the bioluminescent total photon measurements (Fig. S9). No micrometastases or macrometastases were observed in tissue biopsy specimens from MDA-MB-231-D3H2LN/GFP-Luc-shAxl2 tumor-bearing mice, confirming that the lack of observed bioluminescence was due to inhibited metastasis formation (Fig. S9). These findings demonstate that Axl is essential for breast carcinoma metastasis.
Fig. 6.
Axl is required for human breast carcinoma cells to metastasize from the mammary gland of NOD-SCID mice. (A) Temporal in vivo bioluminescence imaging of mammary fat pad growth of orthotropically injected control and shAxl2-expressing MDA-MB-231-D3H2LN /GFP-Luc cells in NOD/SCID mice (Upper) and Axl-dependent formation of spontaneous thoracic sentinel lymph node metastasis (Lower). (B) Biophotonic quantitation of primary mammary tumor cell number (total photon) and radial infiltration (signal diameter; Upper) and thoracic lymph node metastatic cell number (total photon; Lower) in MDA-MB-231-D3H2LN/GFP-Luc control (solid line) and shAxl2 (dashed line) cell–injected NOD/SCID mice (n = 6 per group). *P < .05; **P < .005; ***P < .0005 compared with control (paired t test). (C) Survey of spontaneous metastasis (at 9 weeks postorthotopic implantation) site monitored by ex vivo bioluminescence detection of MDA-MB-231-D3H2LN/GFP-Luc cells in excised organs shows that tumor cell dissemination is Axl-dependent. (D) Univariate survival analysis (Kaplan-Meier method) between control (vector) and shAxl2 orthographically MDA-MB-231-D3H2LN/GFP-Luc–injected mice demonstrates that overall survival is Axl-dependent (P = .001, log-rank test).
We evaluated the functional contribution of Axl to cancer-related deaths in NOD-SCID mice with orthotopically injected MDA-MB-231-D3H2LN/GFP-Luc control or Axl knockdown cells. Overall survival was significantly increased in MDA-MB-231-D3H2LN/GFP-Luc-shAxl2 tumor-bearing mice (P = .001, log-rank test; Fig. 6D). These results, together with our clinical observations, support the conclusion that Axl is essential for the development of metastatic disease and is a major determinant of overall patient survival.
To validate our results in a different metastatic model, we transduced the highly metastatic mouse breast carcinoma 4T1 cell line with the pCSI construct and selected for GFP-luciferase expression by FACS (4T1-GFP-Luc). The 4T1 cells are dependent on Twist expression for metastasis and exhibit high levels of Axl expression (Fig. S11) (7). We developed a retroviral vector that expresses a distinct mouse Axl-targeting shRNA (shmAxl2) that effectively suppresses mouse Axl surface levels in 4T1 cells. A mismatched human Axl-targeting shRNA (shAxl279) had no effect on mouse Axl expression (Fig. S11). Similar to the results in Twist- knockdown 4T1 cells and MDA-MB-231 cells (Fig. S4), tissue culture expansion of the Axl-knockdown 4T1 cells was not affected.
When introduced into the mammary gland of female normal BALB/c mice, syngenic 4T1 breast tumor cells display a biphasic growth pattern, due to a rigorous immune response associated with leukocyte infiltration and necrosis, which leads to tumor regression (20). After a latency period, regrowth at the primary site coincides with the appearance of metastases in multiple organs (Fig. 7A). We injected 4T1-GFP-Luc cells expressing either a mouse Axl-targeting shRNA (4T1-GFP-Luc-shmAxl2) or negative control human-specific shRNA (4T1-GFP-Luc-shAxl279) into the mammary fat pads of female BALB/c mice and quantified tumor growth and metastasis by temporal whole-body in vivo bioluminescence imaging (Fig. 7A). The control 4T1-GFP-Luc-shmAxl2 cells displayed rapid primary growth, reaching a maximum after 1 week, followed by a precipitous regression over the next 5 weeks (Fig. 7B). At week 6, recurrence at the primary site and multiple distant metastases were observed that subsequently grew rapidly, causing moribundity and lethality in all mice by week 8. The 4T1-GFP-Luc cells expressing the mouse Axl-targeting shRNA (4T1-GFP-Luc-shmAxl2) initially followed a similar course, with rapid primary tumor growth, slightly attenuated by Axl knockdown, followed by tumor regression; however, the subsequent regrowth at the primary tumor site was strongly suppressed, and distant metastases were absent (Fig. 7B and Fig. S12). Indeed, all mice injected with the 4T1-GFP-Luc-shmAxl2 cells were healthy at the time of sacrifice (8 weeks). After sacrifice at 8 weeks, individual excised organs were imaged and total light emission was quantified, confirming the presence of metastases at common dissemination sites in all mice bearing 4T1-GFP-Luc-shAxl279 tumors (Fig. 7C and Fig. S11B). In contrast, no bioluminescent tumor cells were detected in the organs from mice with 4T1-GFP-Luc-shmAxl2 tumors (Fig. 7C and Fig. S11B). These results support the conclusion that Axl is an essential regulator of breast tumor metastasis.
Fig. 7.
Axl is essential for postimmune response recurrence and metastasis of syngeneic breast carcinoma cells in BALB/c mice. (A) Temporal in vivo bioluminescence imaging of orthotropic (mammary fat pad)–injected syngenic 4T1-GFP-Luc cells expressing mouse Axl shRNA (4T1-GFP-Luc-shmAxl2) or negative control human Axl shRNA (4T1-GFP-Luc-shAxl279) into BALB/c mice. (B) Quantitation of whole-body bioluminescence (total photon) in control (4T1-GFP-Luc-shAxl279; solid line) and Axl-knockdown (4T1-GFP-Luc-shmAxl2; gray line)-injected BALB/c mice over an 8-week period reveals that metastasis is Axl-dependent. *P < .05 (t test); n = 7 mice per group. (C) Survey of metastasis sites (at 8 weeks postorthotopic implantation) to monitored by ex vivo bioluminescence detection of 4T1-GFP-Luc cells in excised organs from control or Axl-knockdown tumor-bearing BALB/c mice.
Discussion
We demonstrate here a previously unrecognized role for the RTK Axl as an EMT-induced effector that is required for breast cancer metastasis and progression. Our results reveal that EMT program activation leads to Axl up-regulation, which is essential for invasiveness and spontaneous metastasis. Growth of breast carcinoma cells in foreign (s.c.) and biomimetic microenviroments is Axl-dependent. Axl expression is enhanced in patient-derived metastatic lesions compared with the matched primary tumor and is a strong and independent predictor of breast cancer deaths. Collectively, these results validate Axl as an important target for new therapeutic developments in breast cancer.
Axl is a common downstream effector of the EMT program induced by Twist, Zeb2, and Snail family transcription factors. Expression of these transcription factors in breast epithelial cells elicits to varying degrees the EMT-associated suppression of normal epithelial junctional protein expression (e.g., E-cadherin) while up-regulating mesenchymal adhesion and cytoskeletal proteins (e.g., vimentin) that promote unicellular migration (6, 7, 21). Indeed, the level of Axl induction correlates with the extent of EMT-associated cytoskeletal and morphological changes in MCF10a cells. Twist, Zeb2 and Snail family transcription factors are all associated with poor prognosis in breast cancer (22); however, clinical EMT biomarker levels vary significantly among tumors, and EMT transcription factor expression is not always associated with down-regulation of E-cadherin (23). Notably, Axl is an independent prognosticator of overall survival and does not correlate with the E-cadherin status of patient breast tumors. Thus, Axl expression is an EMT-induced marker that is maintained in metastases and correlates strongly with mortality in breast cancer, suggesting a direct mechanistic link between Axl activation and the development of metastatic disease.
We show here that EMT-associated up-regulation of Axl can drive autocrine interaction with Gas6. Axl, often coexpressed with Gas6, is detected in many metastatic cancers, indicating that autocrine Axl signaling might be a frequent consequence of EMT in many tumor types (12, 24). Interestingly, Axl expression is required to maintain Snail, Slug, and Twist expression in pancreatic adenocarcinoma cells (25). This, together with our findings that these EMT transcription factors potently induce Axl expression, suggests that Axl could participate in a positive feedback loop that sustains the malignant mesenchymal phenotype of tumor cells. This notion is consistent with our observation of elevated Axl expression in metastatic lesions.
Axl expression is associated with the basal-like breast cancer phenotype (basal B) that exhibits invasive mesenchymal cell– and stem cell–like traits (26). We found a close correlation between Axl surface level and cell invasive responses to different inducers, including the chemokine SDF-1, a facilitator of metastatic homing in breast cancer (16). Tumor cell colonization of 3D tissue-engineered microenviroments, which is associated with mesenchymal cell behavior, is Axl-dependent (18). However, we observed that orthotopic breast tumor formation within the mammary gland was less dependent on Axl, consistent with our results from patient samples in which 60% of primary human breast carcinomas did not express detectable Axl levels. Similarly, Twist knockdown in 4T1 cells was shown to not affect primary tumor formation in the mammary fat pad while inhibiting metastasis (7). Thus, Axl-signaling may enhance both invasiveness and subsequent adaptation to the foreign microenvironments encountered by metastatic cells. Indeed, Axl activity is required for angiogenesis, and tumor cell Axl-Gas6 coexpression could promote interaction with the vasculature (12).
We demonstrate that Axl is required for lymphatic and hematogenous metastasis of malignant human and mouse breast tumors from the mammary gland in experimental systems involving both immunocompromised and normal mice. In NOD-SCID mice, the extensive human mammary tumor cell dissemination and growth in lymph nodes and major organs was completely inhibited by Axl knockdown. Quantitation of primary and distant tumor cells was aided by a sensitive temporal bioluminescence imaging approach. The application of biophotonic imaging in the syngenic 4T1 breast cancer model allows evaluation of metastatic capacity in the context of a robust immune response that initially regresses primary mammary tumors, characterized by inflammation and necrosis (20). Tumor inflammation is a major driver of metastasis and promotes EMT in breast cancer (5). Axl suppression in 4T1-GFP-Luc cells did not strongly affect the kinetics of primary tumor growth; however, the lethal resurgence and metastasis was absent. Twist expression also is required for metastasis of 4T1 cells from the mammary fat pad, further emphasizing the requirement of EMT-dependent cell functions for metastasis (7). The Axl requirement for breast cancer metastasis was further verified by the better overall survival of mice bearing Axl-knockdown tumors, validating our correlations of Axl expression with clinical outcome in clinical samples and establishing a critical role for Axl in breast cancer pathogenesis.
Collectively, our results indicate that the detection and targeted treatment of Axl-expressing mammary tumors represents an important avenue for breast cancer therapeutic development. RTKs are a well-established cancer therapeutic class, and small molecule inhibitors of Axl tyrosine kinase activity have been reported (27, 28). Interestingly, Her2 is reported to trigger Axl activation, suggesting crosstalk with this clinically important pathway (29). The strong independent correlation of Axl expression with reduced overall survival and persistent Axl expression in subsequent metastasis suggests that the 40% of patients with Axl-expressing primary tumors could benefit from Axl-targeting therapy.
Materials and Methods
Cell lines, constructs, and antibodies are listed in SI Experimental Procedures. Flow cytometry and Western blot analysis were conducted using standard procedures detailed in SI Experimental Procedures. Collection of clinical samples, immunohistochemical studies, and statistical analysis followed established procedures described in SI Experimental Procedures. Tissue engineering, xenografting, and construction of orthotopic optical imaging–based animal models were performed as described in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Sissel Vik Berge, Marianne Enger, and Paula Ruurs, Gerd Lillian Hallseth and Bendik Nordanger for their excellent technical assistance. This work was supported by University of Bergen predoctoral fellowships (to C. Gjerdrum and C.T.) and by grants from the Norwegian Research Council (183850, 183775), Norwegian Cancer Society (0133/0330), and Helse Vest-Samarbeidsorganet (911352) (to J.B.L.), Norwegian Cancer Society (94070/001) to LAA, and NIH/NCI-CA107245; Jewish Women’s Foundation of Los Angeles, and SG Komen Foundation to C. Glackin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909333107/DCSupplemental.
References
- 1.Gupta PB, Mani S, Yang J, Hartwell K, Weinberg RA. The evolving portrait of cancer metastasis. Cold Spring Harb Symp Quant Biol. 2005;70:291–297. doi: 10.1101/sqb.2005.70.033. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the “pre-metastatic niche”: Within bone and beyond. Cancer Metastasis Rev. 2006;25:521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Mani SA, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafizi S, Dahlbäck B. Signaling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Holland SJ, et al. Multiple roles for the receptor tyrosine kinase Axl in tumor formation. Cancer Res. 2005;65:9294–9303. doi: 10.1158/0008-5472.CAN-05-0993. [DOI] [PubMed] [Google Scholar]
- 13.Vajkoczy P, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci USA. 2006;103:5799–5804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Kåresen R, Hervik A, Thoresen SO. Mammography screening in Norway: Results from the first screening round in four counties and cost-effectiveness of a modeled nationwide screening. Cancer Causes Control. 2001;12:39–45. doi: 10.1023/a:1008999403069. [DOI] [PubMed] [Google Scholar]
- 15.Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 17.Kenny HA, Krausz T, Yamada SD, Lengyel E. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extracellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer. 2007;121:1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 18.Fischbach C, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 19.Fischbach C, et al. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late-stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 22.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 23.Klymkowsky MW, Savagner P. Epithelial–mesenchymal transition: A cancer researcher’s conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutterer M, et al. Axl and growth arrest–specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 25.Koorstra JB, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8:618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang YX, et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008;68:1905–1915. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 28.Mahadevan D, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–3919. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 29.Bose R, et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci USA. 2006;103:9773–9778. doi: 10.1073/pnas.0603948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.