Abstract
Cell cycle progression requires changes in the activity or levels of a variety of key signaling proteins. G protein–coupled receptor kinase 2 (GRK2) plays a central role in G protein–coupled receptor regulation. Recent research is uncovering its involvement in additional cellular functions, but the potential role of GRK2 in the cell cycle has not been addressed. We report that GRK2 protein levels are transiently down-regulated during the G2/M transition by a mechanism involving CDK2-mediated phosphorylation of GRK2 at Serine670, which triggers binding to the prolyl-isomerase Pin1 and subsequent degradation. Prevention of GRK2 phosphorylation at S670 impedes normal GRK2 down-regulation and markedly delays cell cycle progression. Interestingly, we find that endogenous GRK2 down-regulation is prevented on activation of the G2/M checkpoint by doxorubicin and that stabilized GRK2 levels in such conditions inversely correlate with the p53 response and the induction of apoptosis, suggesting that GRK2 participates in the regulatory network controlling cell cycle arrest and survival in such conditions.
Keywords: cyclin-dependent kinase 2, G2/M checkpoint, Pin1, degradation, p53
Cellular proliferation is regulated by specific membrane receptors, including receptor-tyrosine kinases and G protein-coupled receptors (GPCRs), among others. GPCRs initiate a variety of intracellular signaling cascades that can modulate cell division (1) involving both G protein-dependent and independent mechanisms. Regarding the latter, G protein-coupled receptor kinases (GRKs) and β-arrestins, first identified as key molecules involved in the agonist-induced desensitization of multiple GPCRs, are emerging as alternative signal transducers with a direct or potential impact on cell growth and proliferation. Whereas β-arrestins can bring different signaling molecules into the receptor complex, (reviewed in ref. 2) a growing number of non-GPCR substrates and interacting proteins are being identified for GRKs, particularly for the ubiquitous GRK2 isoform. These include PDGF and EGF receptors, and a variety of proteins involved in pathways controlling cell migration and proliferation, such as p110-PI3K, p38Mapk, GIT, MEK, or AKT (3). Consistently, GRK2 expression has been reported to have distinct impacts on cell proliferation, depending on both the cell type and the mitogenic stimuli analyzed. GRK2 inhibits TGF-mediated cell growth arrest and apoptosis in human hepatocarcinoma cells (4). On the other hand, GRK2 attenuates thyroid stimulating hormone- and PDGF-dependent proliferation of thyroid cancer cell lines (5) and smooth muscle cells (6), respectively, whereas it increases mitogenic signaling pathways in response to EGF in osteoblasts (7) or upon activation of the Smoothened receptor in fibroblasts (8). Increased GRK2 levels also potentiate migration of epithelial cells toward fibronectin and sphingosine-1-phosphate (9).
Interestingly, in addition to its reported alteration in cardiovascular and inflammatory pathologies (3, 10), emerging data indicate changes in GRK2 expression in certain tumors (11). The fact that general but not cardiac-specific GRK2 knockout mice are embryonic lethal (12) further supports the notion that this protein could play a central, general role in key cellular processes as proliferation or migration. However, the potential involvement of this kinase in the cell cycle has not been addressed. Given that the control of the turnover of key kinases throughout the cell cycle represents a major regulatory mechanism in cell proliferation (13), we have explored whether GRK2 expression was modulated during this process. We report that phosphorylation of GRK2 by cyclin-dependent kinase 2 (CDK2) and subsequent interaction with the Pin1 prolyl-isomerase promotes its transient down-regulation in the G2 phase, and that this event is critical for adequate cell cycle progression and control.
Results
GRK2 Levels Are Dynamically Regulated Through the Cell Cycle.
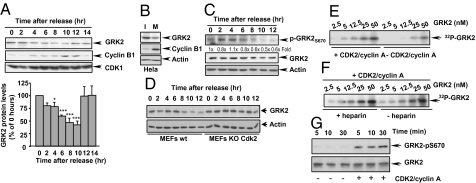
GRK2 protein levels were analyzed in HeLa cells arrested at G1/S and released synchronously into the cell cycle (Fig. S1A). GRK2 levels gradually declined over time during G2 progression, attaining a maximal reduction of 58 ± 7% 10 h after release from the block (Fig. 1A). GRK2 down-regulation was coincident with cyclin B1 accumulation, which peaks at mitosis (10–12 h after release). Consistently, GRK2 expression was also markedly reduced in HeLa cells upon nocodazol treatment (Fig. 1B). GRK2 protein levels were rapidly restored when cells progressed through mitosis and entered into G1, whereas cyclin B1 protein was actively degraded (Fig. 1A). Importantly, a similar pattern of GRK2 expression changes during the cell cycle was observed in primary human endothelial cells (HUVEC) (Fig. S1B), thus stressing the notion that the transient down-regulation of GRK2 levels at G2/M is a general physiological process. Translational shut-off experiments indicated that GRK2 down-regulation was reflecting changes in its degradation rate during cell cycle progression (Fig. S2A).
Fig. 1.
Transient GRK2 down-regulation during G2 progression. HeLa cells were either synchronized at the G1/S boundary and released into the cell cycle for the indicated times (A) or incubated in the absence (I) or presence (M) of nocodazol to block cells in mitosis (B). Cell lysates were subjected to immunoblot analysis to determine GRK2 and cyclin B1 levels, and either CDK1 or actin expression as a control of equal protein loading. In A, GRK2 levels were normalized for CDK1 expression and depicted as percentage of expression at 0 h. Data are mean ± SEM of five independent experiments. *, P < 0.05; ***, P < 0.001 when compared to values at 0 h. (C) Analysis of the phosphorylation status of GRK2 at S670 in the G2 phase. Cell lysates from HeLa cells progressing through the cell cycle as in A were analyzed using a phospho-specific anti-pS670 GRK2 antibody followed by a polyclonal anti-GRK2 antibody after membrane stripping, and actin expression used as loading control. Normalized total GRK2 levels (fold vs. 0 h) are shown above the gel. (D) CDK2 mediates GRK2 down-regulation. Cellular lysates of wild-type and CDK2-deficient mouse embryonic fibroblasts (MEFs) synchronously cycling after G1/S arrest were analyzed for GRK2 and pY15-CDK1 levels or actin as loading control. (E and F) GRK2 is phosphorylated by CDK2. Recombinant GRK2 was incubated for 15 min in the absence (autophosphorylation control) or presence of purified CDK2-cyclin A with or without heparin under phosphorylation conditions as described in SI Materials and Methods, followed by SDS/PAGE and autoradiography. (G) GRK2 is phosphorylated on serine 670 by CDK2-cyclin A. Recombinant GRK2 was incubated with CDK2 or vehicle as in E, and subjected to immunoblot analysis with a phospho-specific anti-pS670 GRK2 antibody. Blots were then stripped and probed with a polyclonal anti-GRK2 antibody. Gels representative of three to four independent experiments are shown in all panels.
Proline-directed phosphorylation plays a key role in the regulation of the stability and activity of several cell-cycle regulators (14, 15). We have previously shown that phosphorylation of GRK2 at residue S670 by MAPK enhances its proteasome-dependent degradation (16). Phosphorylation of GRK2 at S670 was clearly detected throughout the S phase and G2, followed by a sharp decrease at G2/M transition and during mitosis (Fig. 1C and Fig. S2B). A similar apparent inverse correlation between GRK2 levels and phospho-S670 status was observed in primary HUVEC cells (Fig. S1B). However, the pattern of MAPK activation during the cell cycle does not correlate well with that of GRK2-S670 phosphorylation (Fig. S2C) (17, 18). Furthermore, the fact that GRK2 down-regulation in G2/M occurs normally in the absence of β-arrestins (Fig. S3A), the scaffold function of which is instrumental for the MAPK-dependent degradation of GRK2 (16), strongly suggested the involvement of a different proline-directed kinase. Interestingly, GRK2 down-regulation was abrogated in the presence of GW8510 (Fig. S3B), a specific inhibitor of CDK2, the more active cyclin-dependent kinase in S and G2 phases (14). Consistently, CDK2-deficient mouse embryonic fibroblasts (MEFs) displayed an increased GRK2 stability compared to control cells (Fig. S3C) and normal GRK2 down-regulation during the cell cycle was blocked (Fig. 1D), further pointing to a role for CDK2 in such process.
CDK2-Cyclin A Phosphorylates GRK2 at Residue S670.
In vitro kinase assays carried out with purified proteins revealed that GRK2 is clearly phosphorylated over its basal autophosphorylation levels in the presence of CDK2-cyclinA (Fig. 1E), and robust phosphorylation was observed also after addition of the GRK2 inhibitor heparin (Fig. 1F and Fig. S4A), thus ruling out an allosteric effect of CDK2/cyclinA on GRK2 autophosphorylation. A detailed kinetic analysis indicated that GRK2 was readily phosphorylated by CDK2 with an apparent Km of ∼8 nM (Fig. S4B).
Sequence analysis and in vitro phosphorylation experiments with GST-GRK2 fusion proteins pointed to S670 as the main phospho-acceptor residue (Fig. S4C). To further confirm this point, GRK2 phosphorylated by CDK2 was analyzed with the phosphoS670-specific antibody. The presence of CDK2 clearly enhances the extent of recognition by such antibody in an in vitro kinase assay (Fig. 1G) and upon cellular coexpression of GRK2 and CDK2 (Fig. S4D), whereas GRK2-S670 phosphorylation was markedly reduced in CDK2-deficient MEFs during cell cycle progression (Fig. S4E). Taken together, our data demonstrate that CDK2 directly phosphorylates GRK2 on residue S670 in vitro and in a cellular context.
Binding of Pin1 to GRK2 Phosphorylated at Ser670 Regulates Kinase Turnover.
Phosphorylation by proline-directed kinases has been shown to create an optimal binding site for Pin1, a prolyl-isomerase that specifically catalyzes the cis-trans conformational conversion of phosphorylated S/T-proline bonds in many phospho-proteins (15). Pin1 is known to interact with cellular phospho-proteins in several phases of the cell cycle (17), including G2 and the G2/M transition, when GRK2 is actively phosphorylated and down-regulated. We found (Fig. S5 A and B) that GRK2 directly interacted, in a phospho-dependent manner, with the Pin1 family domain responsible for the phosphorylation-dependent recognition of its targets (15).
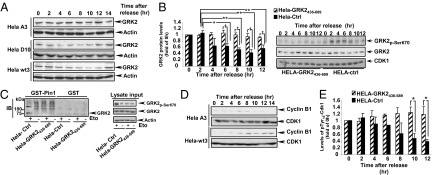
Interestingly, pulse-chase assays in cells growing asynchronously indicated (Fig.2A) that Pin1 overexpression markedly enhanced degradation of wild-type GRK2 (33 ± 4% of GRK2 protein remaining at 1 h of chase versus 52 ± 4% in the absence of Pin1 overexpression, P < 0,001) by a proteasome-dependent pathway (Fig. S5C). Inhibition of either MAPK or CDK pathways attenuated Pin1-enhanced GRK2 degradation, whereas the combined inhibition of both kinases completely abrogated the Pin1 effect. Moreover, the turnover of a kinase mutant unable to be phosphorylated at S670 (GRK2-S670A) was unaffected by Pin1 (44 ± 4% and 51 ± 6% of protein remaining at 1 h of chase with and without extra Pin1, respectively) (Fig. 2A). To further confirm the specificity of the Pin1-dependent modulation of GRK2 stability, we examined the effect on GRK2 turnover of Pin1 mutants (18) that either fail to catalyze isomerization of the pSer/Thr-Pro bond (Pin1-C109A) or to recognize pSer/Thr-Pro motifs (Pin1-Y23A), as both activities are normally required for Pin1 functionality (19). In contrast to wild-type Pin1, neither Pin1 mutant was able to increase GRK2 degradation (Fig. 2B).
Fig. 2.
Pin1 modulates GRK2 stability. (A) Pin1 overexpression promotes degradation of GRK2 in a phosphorylation-dependent manner. HEK-293 cells were transiently cotransfected with either wt-GRK2 or GRK2-S670A and Pin1 or empty vector, in the absence or presence of MEK1/ERK (PD98059) and CDK (olomoucine) inhibitors as indicated in the figure. The turnover of GRK2 proteins was assessed by pulse-chase experiments as described in SI Materials and Methods. (B) Both the ability of Pin1 to interact with phosphorylated proteins and the prolyl-isomerase activity are required for modulating GRK2 stability. GRK2 turnover was analyzed in HEK-293 cells cotransfected with Pin1-wt or mutants displaying impaired phospho-protein binding (Pin1-Y23A) or catalytic function (Pin1-C109A). In A and B, comparable expression levels of GRK2 and Pin1 constructs was assessed in cell lysates by immunoblotting. (C) Pin1 levels correlate with endogenous GRK2 degradation rate. Turnover of endogenous GRK2 was assessed in transformed (MCF7 and MDA-MB468) and nontransformed (MCF10A and 184B5) mammary epithelial cells. Levels of Pin1, total ERK1/2, and of activated phospho-ERK1/2 were determined in cellular lysates by immunoblotting, while the relative extent of phospho-S670-GRK2 was analyzed in GRK2 immunoprecipitates. (D) GRK2 down-regulation in the G2 phase is prevented in cells lacking Pin1 expression. Cell lysates from synchronized wild-type and Pin1-deficient MEFs were subjected to immunoblot analysis with anti-GRK2, anti-actin and anti-pY15-CDK1 antibodies. Data in all panels are the mean ± SEM of three to four independent experiments. *, P < 0.05; **, P < 0.01 for the indicated comparisons. Representative gels are shown.
Interestingly, the rate of endogenous GRK2 degradation correlated with the cellular content of Pin1. Pin1 is overexpressed in human breast cancer and in malignant mammary epithelial cells (20). GRK2 protein decay rate was high in transformed mammary cell lines compared with nontransformed cells (Fig. 2C). Such accelerated GRK2 turnover in MCF7 and MB-MDA-468 cells correlates with elevated levels of Pin1 and a higher steady-state phosphorylation of GRK2 at residue S670 (Fig. 2C). In contrast, the half-life and the steady-state expression of GRK2 were increased in MEFs lacking Pin1 compared to control cells (Fig. S5 D and E). In a cell-cycle context, absence of Pin1 expression (Fig. 2D and Fig. S5F) or pharmacological inhibition of Pin1 activity (Fig. S5G) prevented the normal down-regulation of endogenous GRK2 during G2 progression. Overall, our results strongly suggested that Pin1 was a physiological, phosphorylation-dependent modulator of GRK2 stability.
GRK2 Phosphorylation at Ser670 Is Critical for GRK2 Down-Regulation During G2 Phase and for Adequate Cell Cycle Progression.
To specifically explore whether CDK2-induced, Pin1-mediated down-regulation of GRK2 during the cell cycle was a functionally relevant regulatory event, we generated different HeLa cells transfectants (Fig. S6A) stably overexpressing wild-type GRK2, the GRK2 mutant unable to be phosphorylated at S670 (GRK2-S670A) or a mutant mimicking such modification (GRK2-S670D). In cells overexpressing similar levels (3-fold over endogenous protein) of wild-type kinase (HeLa-wt3 line) or the S670D mutant (HeLa-D10 line), GRK2 levels fluctuate during the cell cycle (Fig. 3A), as does the endogenous kinase in parental HeLa cells. In contrast, the levels of the GRK2-S670A mutant, again expressed at comparable amounts (HeLa-A3 line) remained essentially constant through the cell cycle. As an additional approach, HeLa cells were stably transfected with a C-terminal construct of GRK2 (aa 436–689) to compete the interaction of CDK2 and Pin1 with the endogenous GRK2. Expression of this construct inhibits GRK2-S670 phosphorylation and also prevents down-regulation of the protein during cell cycle progression compared to mock-transfected cells (Fig. 3B). Interestingly, the interaction of endogenous GRK2 with GST-Pin1 detected upon G2 arrest with etoposide is blocked in this condition (Fig. 3C and Fig. S6B), as is also the case for the GRK2-S670A mutant (Fig. S6C). A similar defective GRK2/Pin1 interaction was also observed in MEFs lacking CDK2 upon G2 blockade (Fig. S6D). Overall, these results indicated that phosphorylation of GRK2 at residue S670 is critical to drive Pin1 binding in situ and subsequent down-regulation.
Fig. 3.
Phosphorylation at Ser670 is required for GRK2 down-regulation and for proper cyclin B induction and CDK1 activation during G2/M progression. (A) GRK2 protein decay in G2 is absent in cells expressing GRK2-S670A. Cells expressing similar levels of extra GRK2-wt, GRK2-S670A, or GRK2-S670D were synchronized at G1/S, released into the cell cycle and lysates collected at the indicated times to analyze GRK2 and actin protein levels. (B) The C-terminal domain of GRK2 hampers S670 phosphorylation and down-regulation of endogenous GRK2. Cells stably expressing GRK2(436–689) or an empty vector were synchronized as above and lysates analyzed with the indicated antibodies. GRK2 levels were normalized to the CDK1 loading control. *, P < 0.05 and **, P < 0.01, when compared to values at 0 h in each cell type; †, P < 0. 05, when compared to control HeLa cells. (C) Interference of S670 phosphorylation by overexpression of the competitor construct GRK2436–689 abrogates Pin1 binding to endogenous GRK2. HeLa cells stably transfected with GRK2436–689 or an empty vector (HeLa-Ctrl) were arrested in G2 phase with etoposide as described in SI Materials and Methods or maintained in exponential growth. Lysates were incubated with either GST or GST-Pin1. Precipitated proteins were subjected to SDS/PAGE and analyzed by immnublotting with an anti-GRK2 antibody. pS670-GRK2, GRK2, and actin protein levels were assessed in a 2% volume of input lysates. Equal loading of GST fusion proteins was confirmed by Ponceau staining of the blots (Fig. S6B). (D and E) Impairment of S670 phosphorylation prevents both cyclin B up-regulation and CDK1 activation. Cell lines expressing GRK2-wt (HeLa-wt3), GRK2-S670A (HeLa-A3), the competitor construct GRK2436–689, or an empty vector (HeLa-Ctrl) were synchronized and cell lysates analyzed by immunoblotting with anti-cyclin B or anti-pY15-CDK1 antibodies. After stripping, blots were probed with either anti-CDK1 or anti-actin antibodies as loading controls. Activation of CDK1 was monitored as a decrease in the extent of the inhibitory Y15 phosphorylation. In all panels, data are the mean ± SEM of three to four independent experiments and gels are representative of al least three independent experiments.
Importantly, GRK2-S670A stabilization during cell cycle was accompanied by the absence of cyclin B1 accumulation at G2/M transition, while levels of this cyclin were normally enhanced in HeLa-wt3 (Fig. 3D). Similar defects in cyclin B1, as well as in the activation of the mitotic CDK1, were observed in HeLa-A3 cells arrested at mitotic prometaphase (Fig. S6E). Stabilization of endogenous GRK2 triggered by the GRK2-436–689 construct also delays CDK1 activation (Fig. 3E).
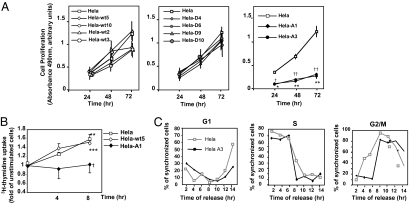
Overall, these results strongly suggested that CDK2 and Pin-mediated GRK2 down-regulation would be needed for the adequate progression of the cell cycle. We therefore compared the proliferation rate of parental HeLa cells with that of transfectants expressing wild-type GRK2, GRK2-S670A, or GRK2-S670D at different levels. Expression of extra wild-type GRK2 or GRK2-S670D did not significantly alter cell proliferation, whereas GRK2-S670A had a marked inhibitory effect (Fig. 4A). The impaired proliferation of these cells was also confirmed by a lower 3H-thymidine uptake through the S phase (Fig. 4B), which indicates a deficit in cell cycle progression. It is worth noting that a similar trend in cell proliferation was observed in HeLa cells stably expressing comparable levels of GRK2-K220R, a catalytically inactive GRK2 mutant (Fig. S7 A–C). Interestingly, this mutant, previously reported to display an intrinsically retarded degradation compared to the wild-type kinase and a deficient S670 phosphorylation-dependent turnover (16, 21), also fails to be down-regulated in G2 (Fig. S7D). In contrast, knock-down of endogenous GRK2 levels in HeLa cells leads to an early cyclin B expression and CDK1 activation (Fig. S8 A and B). Thus, timely attenuation of GRK2 levels/functionality below a certain threshold is mandatory for an efficient cell cycle progression in HeLa cells.
Fig. 4.
Effect of GRK2 phosphorylation on cell cycle progression and cell proliferation. (A and B) Expression of the phosphorylation-defective mutant GRK2-S670A reduces cellular proliferation. Proliferative capacity of HeLa cells with different levels of extra GRK2wt, GRK2-S670A, or GRK2-S670D was analyzed by using either a nonradioactive proliferation assay (A) or a H3-thymidine uptake assay (B). Data are mean ± SEM of three to four independent experiments. **, P < 0.01; ***, P < 0.001 when compared to unstimulated serum-starved cells; †, P < 0.05 comparison between control and HeLa-A1 cells. (C) The presence of GRK2-S670A causes a delay in G2/M progression. Control and HeLa cells stably expressing GRK2-S670A (HeLa A3) synchronized as in Fig. 1 were analyzed by flow cytometry. Cells in G1, S, and G2/M were quantified by curve-fitting FACScan profiles and reported as percentage of total cells.
We next assessed the kinetics of cell cycle progression by flow cytometry analysis of DNA content. HeLa-A3 cells displayed a markedly delayed G2/M transition and a postponed G1 re-entry, as compared to control HeLa cells without signs of enhanced apoptosis, thus leading to a lengthening of the cell cycle (Fig.4C). Consistently, HeLa-A3 cells growing asynchronously displayed a notable accumulation of cells with a 4n DNA content (52% of the cellular population) in contrast to control HeLa or HeLa-wt3 cells (32% and 28%, respectively) (Fig. S8C), which suggests that impairment of GRK2 degradation at G2 phase promotes an inefficient cell cycle progression rather than a cell cycle arrest.
Activation of the G2/M Checkpoint Prevents GRK2 Down-Regulation.
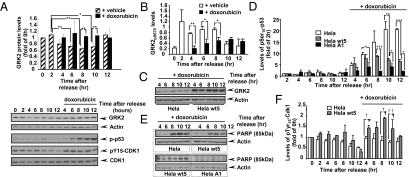
Because activation of CDK2 is physiologically prevented at G2 phase upon DNA damage-induced cell arrest (22), we analyzed GRK2 levels in HeLa cells upon activation of the G2/M checkpoint by the DNA-damaging agent doxorubicin. The down-regulation of endogenous GRK2 protein that takes place in G2 during “normal” cell cycle progression was inhibited in doxorubicin-treated cells (Fig. 5A), consistent with the doxorubicin-induced decrease in GRK2-S670 phosphorylation (Fig. 5B). Such GRK2 stabilization also takes place in HeLa cells stably overexpressing wild-type kinase, leading to higher levels of steady-state GRK2 expression compared to parental HeLa cells upon doxorubicin-triggered arrest in G2 (Fig. 5C). Such pattern of sustained GRK2 levels appears to be inversely related to the ability of genotoxic insults to trigger the p53 response and the induction of apoptosis. Doxorubicin-triggered p53 phosphorylation (Fig. 5D) and up-regulation (Fig. S9A) is lower in wt-GRK2 overexpressing versus parental HeLa cells. In doxorubicin-arrested cells, poly(ADP-ribose) polymerase cleavage (an apoptotic marker) (Fig. 5E) and the expression of the proapoptotic p53-target genes PUMA and Noxa (Fig. S9B) is also strongly attenuated in G2 in wt-GRK2 overexpressing cells compared to control HeLa cells. Moreover, cells expressing GRK2-S670A (intrinsically stabilized during cell cycle) (Fig. 5D) or the competitor GRK2 436–689 construct (leading to stabilization of endogenous GRK2) (Fig.S9 A and C) display even a more reduced p53 induction and a negligible apoptotic response (Fig. 5E). Interestingly, despite a lower expression of p21 in HeLa-wt5 (Fig. S9B), monitoring of CDK1 activation shows that doxorubicin triggers a more potent arrest at G2/M in such cells (Fig. 5F). Overall, these results strongly suggest that stabilization of GRK2 by doxorubicin would potentiate the cell cycle arrest protraction and survival process that operates in the G2/M checkpoint in response to DNA damage.
Fig. 5.
Doxorubicin-triggered GRK2 stabilization correlates with the extent of induction of p53 and apoptosis in the G2 checkpoint response. (A) Degradation of endogenous GRK2 is prevented in cells arrested in G2 upon doxorubicin treatment. Two hours after release from the block at G1/S, synchronized HeLa cells were exposed to doxorubicin (0.8 μM) or vehicle for the indicated times and the protein levels of GRK2, actin, total and phospho-CDK1, and phospho-p53 were analyzed with specific antibodies. (B) Phosphorylation of GRK2 at S670 in the G2 phase decreases upon doxorubicin treatment. Lysates as in A were analyzed by immunoblotting with an anti-pS670 GRK2 antibody as described in Fig 1. In A and B, data are mean ± SEM from three to four independent experiments. *, P < 0.05; **, P < 0.01, when compared to untreated samples. (C) Increased steady-state GRK2 expression upon doxorubicin-triggered arrest in G2 in cells with higher GRK2 levels. Parental and HeLa wt5 cells were synchronized and treated with doxorubicin as in A and GRK2 protein analyzed as above. (D–F) Accumulation of GRK2 in G2 restrains the induction of p53 and the apoptotic response upon DNA damage, but strengthens CDK1 inactivation. Synchronized cells with or without extra wild-type GRK2(Helawt5) or mutant GRK2-S670A (HelaA1) were exposed to doxorubicin or vehicle and levels of total and pS15-p53 (D), the p85 kDa fragment of poly(ADP-ribose) polymerase (E), or of total and pY15-CDK1 (F) were immunodetected with specific antibodies and quantified. p53 densitometry values were corrected for actin expression, while phospho-p53 and phospho-CDK1 band densities were normalized to cognate total p53 and CDK1 values. Data in D and F are mean ± SEM from two to three independent experiments. */†, P < 0.05; **/††, P < 0.01; *** P < 0.001 for the indicated comparisons. Representative gels from three to four independent experiments are shown in all panels.
Discussion
In this article we describe how GRK2, a key GPCR regulatory kinase, establishes a complex network of functional interactions during the cell cycle, leading to a transient down-regulation of its expression levels, which appears critical for adequate cycle progression. GRK2 protein levels progressively decay during G2 as a result of a degradation process triggered by the CDK2-dependent phosphorylation of GRK2 at residue S670. Inhibition of endogenous GRK2 phosphorylation by CDK2 or expression of a mutant unable to be phosphorylated at this residue (GRK2-S670A) completely prevents GRK2 down-regulation and delays cell cycle progression. Importantly, such “default” down-regulation is blocked upon activation of the G2 checkpoint, and the resultant accumulation of GRK2 protein levels inversely correlates with the extent of activation of the p53-dependent apoptotic responses.
We have previously reported that GRK2 is rapidly degraded by the proteasome system in response to GPCR stimulation by means of protein phosphorylation at tyrosine (Y13/86/92) and serine (S670) residues by c-Src and MAPK kinases, respectively (16, 21). Although GRK2-S670 is phosphorylated by MAPK in several cellular conditions (9, 16), we find that CDK2, and not MAPK, is the key enzyme involved in the phosphorylation and subsequent turnover of GRK2 at G2/M transition. Our discovery that Ser670 is also a direct target for CDK2/cyclin A activity indicates that different signaling routes can converge in the phosphorylation of this residue to control GRK2 protein stability, depending on its context-dependent activation state and/or GRK2 localization.
The CDK2-dependent phosphorylation of GRK2 drives the recruitment of the prolyl-isomerase Pin1, an event required for the efficient down-regulation of GRK2 throughout G2/M. Pin1 is a critical cell cycle regulator that modulates the function of many phosphorylated proteins during G2/M transition. Regulation of protein degradation is an important consequence of Pin1 activity, that triggers either enhanced protein turnover as described for cyclin E (23), the transcription factor CF2 (24), and c-myc (25) or protein stabilization as reported for Emi (26) or cyclin D (27). We find that Pin1 over-expression stimulates the proteasome-dependent degradation of GRK2 but not of the GRK2-S670A mutant. Moreover, turnover rate of endogenous GRK2 is inversely correlated with the cellular content of Pin1 in different cell lines, and Pin1 knock-down prevents endogenous GRK2 down-regulation in cells progressing into G2.
Interestingly, the degradation rate of GRK2 in the presence of extra Pin1 resembles that determined for GRK2-S670D, a mutant mimicking permanent S670 phosphorylation (16), which suggests that the role of Pin1 could be to preserve the pS670-GRK2 status, perhaps stabilizing a more suitable prolyl conformer or keeping away phosphatases to allow an efficient degradation of phosphorylated GRK2. It is known that Pin1 modulates the phosphorylation-dependent ubiquitination of several cell-cycle modulators driven by proline-directed kinases via E3-ligases complexes, such as SCF Fbw7, SCFSkp2, or SCFCdc4 (15), thus pointing to a possible participation of such ligases in the degradation of GRK2. Alternatively, Pin1 could stabilize or provide additional protein-protein interaction surfaces in a catalytic-dependent manner, thereby favoring the interaction of GRK2 with other E3 ligases. In this regard, we have recently described that the E3-ligase Mdm2 is involved in GRK2 ubiquitination and degradation (28). Therefore, it is possible that Pin1 binding could induce a conformational change that either unmasks Mdm2 recognition motifs in GRK2 or helps to bring the kinase in close proximity to the ligase, as has been recently suggested for the ERK-triggered degradation of FOXO3a by Mdm2 (29). Future research should address the identity of the E3-ligase involved in the recognition and targeting to degradation of pS670-GRK2 during G2. On the other hand, our data also open the door for investigating a potential modulatory role of Pin1 in other well-established GRK2-dependent cellular processes.
Overall, our data put forward the unique notion that transient changes in GRK2 protein levels and its phosphorylation at serine 670 can influence cell cycle progression by modulating the G2/M transition in a receptor-independent manner, which reveals an unforeseen role for GRK2 in cell growth control. Both cell cycle entry and progression beyond the restriction point in the late G1 phase require the presence of proliferative signals acting through receptor tyrosine kinases and GPCRs that can be regulated by GRK2 in different ways (30). In addition, our data put forward that, when mitogens are no longer essential for cell cycle progression, changes in the phosphorylation status and protein levels of GRK2 may play a central role in G2 in diverse pathophysiological contexts. It has been reported that GRK2 interacts with MEK, p38, Mdm2, PI3K, or c-Src (3), which are known to be implicated in several phases of the cell cycle. It is tempting to suggest that CDK2-triggered GRK2 down-regulation would allow cell cycle progression in normal conditions by preventing untimely functional interactions of GRK2 with pathways that regulate cyclin B1 accumulation and thus CDK1 activation, apparently in a kinase-independent manner, as accumulation of the catalytically inactive GRK2-K220R protein also impairs cell cycle progression. On the other hand, in the presence of DNA, damaging agents that trigger cell cycle arrest in G2, such as doxorubicin, the “default” GRK2 protein decay in G2 is prevented. In cells with higher steady-state levels of the kinase, increased stabilized GRK2 levels in such conditions inversely correlate with the p53 response and the induction of apoptosis, strongly suggesting that GRK2 contributes to orchestrate G2/M checkpoint mechanisms, helping to restrict the apoptotic fate of arrested cells by mechanisms yet to be defined. Because we have reported that GRK2 is up-regulated in the context of oncogenic signaling (28), it is tempting to suggest that inhibition of GRK2 expression might sensitize cells to drug-induced DNA damage. The mechanisms involved in translating GRK2 accumulation during G2 into altered cell cycle progression are being actively investigated by our group.
Materials and Methods
Cell-Cycle Analysis and Cell Synchronization.
HeLa cells and MEFs were synchronized at G1/S transition using a thymidine-aphidicolin double block and primary HUVEC cells arrested in G0 as detailed in the SI Materials and Methods. For metaphase arrest, cells were treated with 5-μM nocodazol for 24 h. To examine the G2 phase, cells were incubated in the presence of 5-μM etoposide for 20 h. Expression profiles of cylin B, cyclin A, and cyclin D were determined in cellular extracts by immunoblotting to confirm cell cycle progression of synchronized cells and cellular quiescence.
Cell Proliferation.
Parental and HeLa cells stably expressing GRK2-WT or the mutant constructs GRK2-S670A and GRK2-S670D were plated in 96-well cell culture plates. Cell proliferation was measured 24, 48, and 72 h after plating using the CellTiter96AQueaous Non-Radioactive Cell Proliferation Assay according to the manufacturer’s protocol (Promega). In parallel, DNA synthesis rates were determined by [3H]thymidine uptake analysis as detailed in the SI Materials and Methods.
Protein Degradation Assays.
Metabolic labeling and pulse-chase experiments were performed as described (21), and detailed in SI Materials and Methods.
Other detailed methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. G. del Sal, J.L. Benovic, M. Barbacid, and M. Sudol for the indicated reagents and tools, Dr. A. Ruiz-Gomez and S. Rojo for recombinant GRK2 and helpful technical assistance, respectively, and Dr. C. Murga for tools and critical reading of the manuscript. Our laboratory is funded by grants from Ministerio de Educación y Ciencia (SAF2008-00552), Fundacion Mutua Madrileña, Fundación Ramón Areces, The Cardiovascular Network of Ministerio Sanidad y Consumo-Instituto Carlos III (RD06-0014/0037), Comunidad de Madrid (S-SAL-0159-2006), and the MAIN European Network Grant LSHG-CT-2003-502935 (to F.M.) and Comunidad de Madrid and Universidad Autónoma de Madrid Grant CCG08-UAM/BIO-4452 (to P.P.). P.P. is the recipient of a “Ramón y Cajal” contract.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905778107/DCSupplemental.
References
- 1.Luttrell LM. Composition and function of g protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J Mol Neurosci. 2005;26:253–264. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- 2.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 3.Ribas C, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Ho J, et al. The G protein-coupled receptor kinase-2 is a TGFbeta-inducible antagonist of TGFbeta signal transduction. EMBO J. 2005;24:3247–3258. doi: 10.1038/sj.emboj.7600794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Métayé T, Levillain P, Kraimps JL, Perdrisot R. Immunohistochemical detection, regulation and antiproliferative function of G-protein-coupled receptor kinase 2 in thyroid carcinomas. J Endocrinol. 2008;198:101–110. doi: 10.1677/JOE-07-0562. [DOI] [PubMed] [Google Scholar]
- 6.Peppel K, et al. Overexpression of G protein-coupled receptor kinase-2 in smooth muscle cells attenuates mitogenic signaling via G protein-coupled and platelet-derived growth factor receptors. Circulation. 2000;102:793–799. doi: 10.1161/01.cir.102.7.793. [DOI] [PubMed] [Google Scholar]
- 7.Bliziotes M, Gunness M, Zhang X, Nissenson R, Wiren K. Reduced G-protein-coupled-receptor kinase 2 activity results in impairment of osteoblast function. Bone. 2000;27:367–373. doi: 10.1016/s8756-3282(00)00338-0. [DOI] [PubMed] [Google Scholar]
- 8.Meloni AR, et al. Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol. 2006;26:7550–7560. doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penela P, et al. G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J. 2008;27:1206–1218. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penela P, Ribas C, Mayor F., Jr Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15:973–981. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 11.Métayé T, Gibelin H, Perdrisot R, Kraimps JL. Pathophysiological roles of G-protein-coupled receptor kinases. Cell Signal. 2005;17:917–928. doi: 10.1016/j.cellsig.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Matkovich SJ, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 13.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 14.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 16.Elorza A, Penela P, Sarnago S, Mayor F., Jr MAPK-dependent degradation of G protein-coupled receptor kinase 2. J Biol Chem. 2003;278:29164–29173. doi: 10.1074/jbc.M304314200. [DOI] [PubMed] [Google Scholar]
- 17.Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP. Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J Biol Chem. 2002;277:47976–47979. doi: 10.1074/jbc.C200538200. [DOI] [PubMed] [Google Scholar]
- 18.Zacchi P, et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature. 2002;419:853–857. doi: 10.1038/nature01120. [DOI] [PubMed] [Google Scholar]
- 19.Lippens G, Landrieu I, Smet C. Molecular mechanisms of the phospho-dependent prolyl cis/trans isomerase Pin1. FEBS J. 2007;274:5211–5222. doi: 10.1111/j.1742-4658.2007.06057.x. [DOI] [PubMed] [Google Scholar]
- 20.Wulf G, Ryo A, Liou YC, Lu KP. The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res. 2003;5:76–82. doi: 10.1186/bcr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penela P, Elorza A, Sarnago S, Mayor F., Jr Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J. 2001;20:5129–5138. doi: 10.1093/emboj/20.18.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstone S, Pavey S, Forrest A, Sinnamon J, Gabrielli B. Cdc25-dependent activation of cyclin A/cdk2 is blocked in G2 phase arrested cells independently of ATM/ATR. Oncogene. 2001;20:921–932. doi: 10.1038/sj.onc.1204177. [DOI] [PubMed] [Google Scholar]
- 23.Yeh ES, Lew BO, Means AR. The loss of PIN1 deregulates cyclin E and sensitizes mouse embryo fibroblasts to genomic instability. J Biol Chem. 2006;281:241–251. doi: 10.1074/jbc.M505770200. [DOI] [PubMed] [Google Scholar]
- 24.Hsu T, McRackan D, Vincent TS, Gert de Couet H. Drosophila Pin1 prolyl isomerase Dodo is a MAP kinase signal responder during oogenesis. Nat Cell Biol. 2001;3:538–543. doi: 10.1038/35078508. [DOI] [PubMed] [Google Scholar]
- 25.Yeh E, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 26.Bernis C, et al. Pin1 stabilizes Emi1 during G2 phase by preventing its association with SCF(betatrcp) EMBO Rep. 2007;8:91–98. doi: 10.1038/sj.embor.7400853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liou YC, et al. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci USA. 2002;99:1335–1340. doi: 10.1073/pnas.032404099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salcedo A, Mayor F, Jr, Penela P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J. 2006;25:4752–4762. doi: 10.1038/sj.emboj.7601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang JY, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penela P, et al. G protein-coupled receptor kinase 2 (GRK2) in migration and inflammation. Arch Physiol Biochem. 2008;114:195–200. doi: 10.1080/13813450802181039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.