Abstract
Deep brain stimulation (DBS) is a reversible technique that is currently used for the treatment of Parkinson disease and may be suitable for the treatment of psychiatric disorders. Whether DBS inactivates the target structure is still a matter of debate. Here, from findings obtained in rats, we propose DBS of the subthalamic nucleus (STN) as a possible treatment for cocaine addiction to be further tested in human studies. We show that STN DBS reversibly reduces the motivation to work for an i.v. injection of cocaine, and it increases motivation to work for sucrose pellets. These opposite effects may result from STN DBS effect on the positive affective properties of these rewards. Indeed, we further show that STN DBS reduces the preference for a place previously associated with the rewarding properties of cocaine, and it increases the preference for a place associated with food. Because these findings are consistent with those observed after STN lesions [Baunez C, Dias C, Cador M, Amalric M (2005) Nat Neurosci 8:484–489], they suggest that STN DBS mimics an inactivation of the STN on motivational processes. Furthermore, given that one of the major challenges for cocaine addiction is to find a treatment that reduces the craving for the drug without diminishing the motivation for naturally rewarding activities, our findings validate STN as a good target and DBS as the appropriate technique for a promising therapeutic strategy in the treatment of cocaine addiction.
Keywords: addiction, basal ganglia, motivation, food, rat
Over the past decade, there has been an increasing interest in neurosurgical procedures using deep brain stimulation (DBS) to treat neurological and psychiatric disorders, such as Parkinson disease (1, 2) and Huntington disease (3), as well as depression (4) and obsessive compulsive disorders (5).
A recent study showing that the effects of subthalamic nucleus (STN) DBS mainly result from a stimulation of the cortico-STN fibers questioned the inactivation of STN by DBS (6). However, there was previously a consensus to consider that action of STN DBS as an inactivation of the cell bodies of the targeted structure with an activation of the passing fibers (7). The mechanisms of DBS remain thus a matter of debate.
From the clinical point of view, DBS represents a reversible way to inactivate a particular structure in the brain, a strategy that is preferred to ablative surgery in most cases. The choice of the targeted structure is adapted to the disease.
In the case of drug addiction, a devastating disorder whose hallmark feature is an uncontrolled motivation to take the drug while naturally rewarding activities are forsaken, it is crucial to decrease the compulsive motivation for the drug, at the same time preventing decreased motivation for alternative rewarding activities.
Because cocaine acts by blocking the dopaminergic transporter, and therefore temporarily increases the amount of dopamine (DA) available in the brain, early treatments for addiction targeted DA system to counteract cocaine effects. However, it is now largely accepted that interfering with the DA system affects motivation in general. Other surgical targets such as the nucleus accumbens (8) or the cortex (9) have been tested in animals or in opiates addicts (10) with no convincing results so far.
We have shown that the STN is one of the cerebral structures in which dissociation occurs between natural reward, such as food, and drugs of abuse, such as cocaine (11). Indeed, STN lesions decrease motivation for cocaine while increasing motivation for food. We thus hypothesized that STN may be a possible target for treatment of cocaine addiction. Furthermore, some Parkinsonian patients treated with STN DBS can exhibit hypersexuality and weight gain, resulting in part from binge eating, suggesting increased motivation for natural rewards (12, 13). At the same time, in Parkinsonian patients suffering from dependence toward their L-DOPA treatments (i.e., DA dysregulation syndrome), STN DBS had a beneficial effect, reducing their drug seeking (14).
Drug seeking is one of the criteria for addiction according to the DSM-IV (15). In the present study, we thus used a measure of motivation to take the drug or take some food. We have tested the effects of bilateral STN DBS on motivation for a natural reward (food) and for a drug of abuse (cocaine) to determine whether STN DBS mimics STN inactivation, and therefore can decrease motivation for cocaine without decreasing motivation for food.
Results
We first assessed whether rats subjected to STN DBS (see Fig. 1A for an example of electrode location) would modify their spontaneous food intake over a 24-h period. The application of STN DBS did not affect the quantity of food (i.e., lab chow) eaten (Fig. 1B). Furthermore, STN DBS did not affect the food (i.e., sucrose pellets) intake measured in a continuous schedule of reinforcement (fixed ratio 1; FR1), in which every lever press was followed by the delivery of one food pellet. In a different group of rats trained to self-administer cocaine (250 μg/infusion), STN DBS had no significant effect on cocaine intake in the continuous reinforcement (FR1) task (Fig. 1C), but significantly increased the number of lever presses during the inactive period immediately following an injection (i.e., perseverative lever presses) [F(1,18) = 0.04 (ns) and 5.75, P < 0.05 for infusions and perseverative lever presses, respectively]. Consistent with the effects of bilateral STN lesions on motivation for food, cocaine, or alcohol (11, 17, 18), STN DBS did not affect the consummatory processes for either food or cocaine when the behavioral cost to obtain the reward is low.
Fig. 1.
Histology and consummatory behavior under STN DBS. (A) Photograph of the track of a stimulating electrode implanted at the level of the STN (outlined with dashed lines according to the atlas) (16).The brain slice was stained with cresyl violet, allowing delimitation of the STN because of the density of neurons and their orientation in this structure. (B) Effect of STN DBS on food intake over 24-h periods under various conditions: OFF stimulation with no connection (white bar), OFF stimulation with connection to the stimulation system (gray bar), ON stimulation (black bar), and OFF stimulation with connection to the stimulation system (gray bar). Error bars illustrate standard errors (SEM). (C) Effect of STN DBS on performance under a FR1 schedule of reinforcement for food (Upper Graphs) and for cocaine (Lower Graphs). Results are illustrated in terms of mean number of rewards obtained in nonstimulated control animals (OFF, white bar) and STN DBS animals (ON, black bar) during each session and the mean number of perseverative lever presses (i.e., lever presses during the inactivation period of the lever (before reward collection in the magazine for food experiment and during injection time and 20-s timeout period in the cocaine experiment). Asterisk indicates significantly different from OFF condition.
One way to assess the motivation for a reward is to measure the willingness of the animals to expend effort to obtain the reward using the progressive ratio (PR) schedule of reinforcement (19), a task that allows comparison and classification between different types of rewards and between different drugs of abuse (for review, see ref. 20). In this task, rats are required to progressively increase the amount of work produced on a lever to obtain the next reward. The measure of the last ratio at which the animal stops, also called "breaking point," indicates the level of motivation for the reward. Using this procedure, we found that STN DBS increased the motivation of the rats to work for one sucrose pellet. Indeed, though the control group earned on average 27.8 pellets (±1.7) [a mean ratio of 43.94 (±3.25) and cumulative lever presses of 593.63 (±84.2)], the stimulated animals earned 36.2 (±2.0) [mean breaking point of 58.79 (±3.37) and cumulative lever presses of 1,061 (±124.72)] (ANOVA group effect: F(1,20) = 7.93, 8.43, and 7.47, P < 0.05 and 0.01 for number of pellets obtained, last ratio reached, and cumulative lever presses respectively; Fig. 2A). In contrast, STN DBS produced an opposite effect on the willingness to work for i.v. cocaine infusion (250 μg/infusion). Though the nonstimulated control animals were highly motivated to work for cocaine, earning a mean number of 12.44 (±0.73) injections [mean ratio of 123.84 (±17.07) and cumulative lever presses of 424.64 (±75.39)], rats subjected to STN DBS only earned 10.24 (±0.43) injections [mean ratio of 71.81 (±7.59) and cumulative lever presses of 230.67 (±32.4)] (ANOVA group effect: F(1,18) = 6.03, 6.66, and 5.34, P < 0.05 for number of injections obtained, last ratio reached, and cumulative lever presses respectively; Fig. 2B). A general depressive effect of STN DBS on cocaine-seeking behavior can be ruled out because STN DBS increased perseverative lever presses under the FR1 schedule of reinforcement.
Fig. 2.
Effects of STN DBS on willingness of the animals to produce an effort to obtain food or cocaine. Behavioral performances on the progressive ratio task illustrated as the mean number of rewards obtained (Upper) and the mean breaking point (i.e., last ratio reached) (Lower) for food reward (A) and for i.v. cocaine (B) in rats ON (black bars; n = 14 and 9 for A and B respectively) and OFF STN DBS (white bars; n = 8 and 9 for A and B respectively). Error bars illustrate standard errors (SEM). *P < 0.05 (ANOVA group effect), significant difference between groups STN DBS OFF and STN DBS ON.
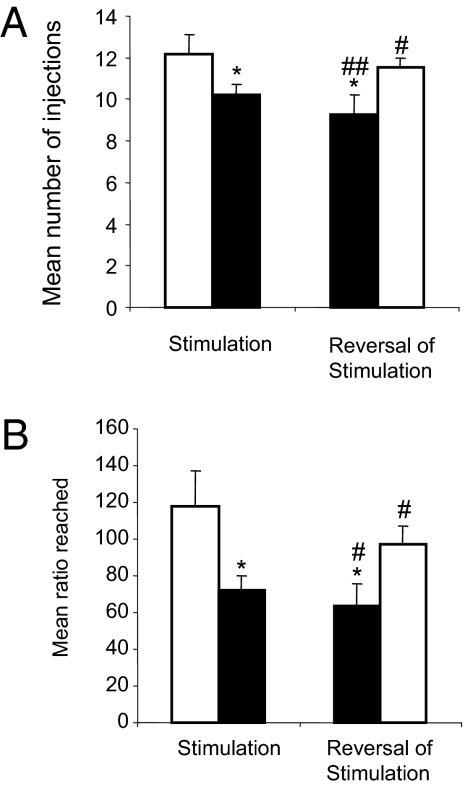
These findings show that, like STN lesions (11), STN DBS reduced the willingness of the animals to work for cocaine, but increased their willingness to work for sucrose. We then tested whether the repeated application of STN DBS during the long sessions of PR testing [mean cocaine PR session duration in the stimulated group: 160 (±6) min] could have damaged the STN and reproduced the behavioral effects of a lesion. We thus tested the reversibility of the effects by activating the stimulation in the animals from the former control group and stopping the stimulation in the former STN DBS group of the cocaine experiment. When the former control animals were stimulated, the mean number of cocaine injections sought in the PR task was significantly diminished, whereas in the former STN DBS animals (which were no longer under stimulation) the number of cocaine injections sought increased, thus proving the reversibility of DBS effects (P < 0.05, paired t test for both groups). The “new control” and “new STN DBS” animals differed significantly in terms of number of injections obtained [11.57 (±0.43) vs. 9.29 (±0.91) injections respectively, ANOVA F(1,14) = 6.95, P < 0.05; Fig. 3A]. In terms of ratio (or breaking point), the now-stimulated animals showed a reduction of 46%, whereas stopping the DBS resulted in a 36% increase of the breaking point, and the two groups remained significantly different [63.4 (±12.16) vs. 97.34 (±9.62), ANOVA F(1,14) = 5.65, P < 0.05; Fig. 3B].
Fig. 3.
STN DBS reversibility on its behavioral effects in the progressive ratio task for i.v. cocaine. Performance is illustrated as the mean number of injections obtained (A) and the mean ratio reached (B) during 10 sessions under the initial conditions of stimulation (Left) (OFF n = 7 (white bar), ON n = 9 (black bar) and after reversal of the stimulation conditions (former ON group was turned OFF, n = 9 (right white bar), and former OFF group was turned ON, n = 7 (Right, black bar). Error bars illustrate standard errors (SEM). *P < 0.05 (ANOVA group effect), significant difference between groups STN DBS OFF and STN DBS ON. #, P < 0.05; ##, P < 0.01; paired t test between the initial condition and its reversal.
To test whether STN DBS might affect the sensitivity to cocaine, we tested the behavioral adaptation of the animals to dose changes in the FR1 schedule of reinforcement. In nonstimulated control animals, decreasing the dose led the rats to increase the number of injections to compensate for the decreased level of cocaine in the blood. In the STN DBS animals, the number of injections also increased, but there was a vertical downshift of the dose–response curve (Fig. S1), suggesting cocaine now had lower reinforcing properties (21).
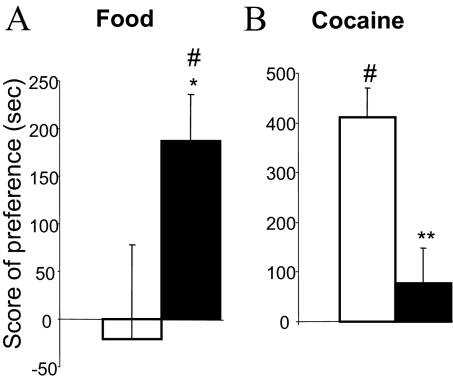
To address further the effects of STN DBS on positive affective properties of both food and cocaine, we used a place conditioning paradigm. In this paradigm, the affective properties of a given reward (food or cocaine) are inferred by measuring the time spent in a specific environment previously paired with the reward, in comparison with another environment never paired with the reward. In a choice situation, and in absence of any reward, measuring the time spent in these environments gives an indication of the positive or negative affective memory that the animal has of its food or cocaine experience. The nonstimulated group did not show any preference for the environment previously associated with food (P > 0.05 sign test), whereas the group subjected to STN DBS showed a significant preference (conditioning effect: P < 0.05 sign test) and therefore a stronger food preference than observed for the nonstimulated animals (P < 0.05, Mann–Whitney test; Fig. 4A). In contrast, after conditioning with cocaine (10 mg/kg), the nonstimulated animals exhibited a clear preference for the compartment previously associated with cocaine (P < 0.05 sign test), but the STN DBS group showed no significant preference for the compartment associated with cocaine (P > 0.05 sign test) and a significantly decreased preference score compared with the nonstimulated group (P < 0.01, Mann–Whitney test; Fig. 4B).
Fig. 4.
Effects of STN DBS on conditioned place preference for food and for cocaine. The bars represent the score of preference for food [A; STN DBS OFF (white bar) n = 7 and STN DBS ON (black bar) n = 9] and for cocaine (10 mg/kg; i.p.) [B; STN DBS OFF (white bar) n = 7 and STN DBS ON (black bar) n = 7]. Error bars illustrate standard errors (SEM). *P < 0.05; **P < 0.01 (Mann–Whitney test), significant difference between groups STN DBS OFF and STN DBS ON. #, P < 0.05, sign test: score of preference significantly different from zero.
Discussion
The present findings show that DBS of the subthalamic nucleus can decrease motivation for cocaine, while increasing the motivation for other “natural” rewards, such as food. This finding concurs strikingly with the previously observed effects following lesions of the STN (11). The mechanism underling STN DBS appears to be more complex than a simple inactivation of the structure (6, 7). In the present study, however, the similarities observed between the behavioral effects of DBS and lesion (11) suggest that, in the case of motivational function, STN DBS mimics an inactivation of the STN. These findings confirm that the STN might represent a therapeutic target for cocaine addiction. Indeed, it is recognized that one of the most important features of drug addiction is the narrowing of the behavioral repertoire toward drugs at the expense of natural rewards (15). This is believed to result from either increased preoccupation/anticipation toward drug- or drug-withdrawal effects (22, 23) or from drug-induced stimulus-response habits (24). The fact that STN DBS, or an STN lesion, preserves or increases food motivation, and that they both decrease cocaine motivation, indicates that the STN is involved in the behavioral bias toward cocaine following repeated cocaine intake.
STN DBS effect was evidenced when rats were switched from an FR1 schedule to the progressive ratio task. The lack of effect of STN DBS under continuous reinforcement (FR1) suggests that STN manipulation does not affect the direct pharmacological effect of the drug which directly controlled the level of responding under a FR1 schedule. STN DBS might rather affect the incentive properties of the drug that motivate cocaine seeking as assessed in the progressive ratio schedule of reinforcement. This is further supported by the decreased preference of STN DBS rats for the environment paired with cocaine in the place preference paradigm.
It can therefore be hypothesized that STN plays a particular role in encoding the incentive value of a given reward, a hypothesis that is consistent with our electrophysiological recordings in the STN (25, 26).
Dissociating motivation for different types of rewards at the level of the STN raises questions about the current view of the reward system. In its current form, the reward system does not account for differential functioning depending on the nature of the reward. So far, no lesion or DBS studies within a single brain structure have reported such an opposite dissociation. Although DBS applied at a fixed intensity for all animals in the nucleus accumbens shell has been recently reported to diminish cocaine-seeking behavior in a reinstatement experiment, there was no increase on food seeking (27). Consistent with a possible dissociation between natural reward and drugs of abuse, specific subpopulations of neurons in the nucleus accumbens have been proposed to encode either cocaine or sucrose reward (28). Supporting these data, we have recently shown that subpopulations of STN neurons also exhibit specific responses to either cocaine or sucrose rewards (26), suggesting that reward-dependent microcircuits may also exist within the STN. Because the nucleus accumbens is connected to the STN, mainly via the ventral pallidum, these microcircuits across connected structures might represent a neurobiological substrate for the behavioral dissociation reported here.
It has also been shown that preferred and less-preferred rewards are encoded differentially in the orbitofrontal cortex (29, 30). The so-called “hyperdirect” pathway linking the orbitofrontal, and more generally the prefrontal cortex (31), to the STN may therefore play a critical role in the present behavioral effects. This hypothesis is further supported by our study showing a differential encoding for preferred and less-preferred reward in the STN (25). A recent study has shown that pretest stimulation (i.e., stimulation only before the behavioral test) of the prefrontal cortex diminished cocaine seeking without diminishing sucrose seeking (9), further supporting the idea that the hyperdirect pathway might be critical for the differential influence of STN on various reward-related behavioral responses. Further studies focusing on the specific contribution of the hyperdirect pathway in reward-related behavior are in due course.
Modification of the activity of mesencephalic dopaminergic (DA) neurons is a potential mechanism through which STN DBS could decrease the response to cocaine. According to current theories of drug addiction, drugs activate and, via neuroadaptive processes, change dopaminergic neurotransmission in the nucleus accumbens and its related circuitry (32, 33) when repeatedly taken. The STN exercises an excitatory influence on the activity of the DA neurons in the ventral midbrain (34). The loss of excitatory input of the STN under DBS may thus lower the basal level of dopaminergic neuron activity in these structures and ultimately alter their reactivity to food and cocaine reward. It is interesting to note however that some studies suggest that STN DBS may result in increased activity of the DA system (35). The dissociation of STN effects, when low workload and high workload were required, such as in the FR1 and PR schedule of reinforcement, has been previously observed after manipulation of the DA system. On one hand, it has been shown that a DA D3 agonist decreased cocaine self-administration when the workload was high but had no effect when the workload was low (i.e., similar effect to STN DBS) (36). Quite contradictory, it has also been shown that (i) an inactivation of the DA system induced the same kind of effect for food reward (i.e., decreased motivation under high workload and no effect under low workload) (37, 38) and (ii) DAT knock-down mice whose characteristic is to have an increased DA transmission showed enhanced motivation for food under high workload (39). To clarify how STN DBS affects DA-related functions, STN and DA systems interactions need to be further investigated.
The possible close relationship between the STN and the DA system may account for a specificity of effect of STN DBS on drugs of abuse acting more specifically on the dopaminergic system, such as cocaine. So far, we have already shown that STN lesions increased the motivation for alcohol in high-drinker rats (17), indicating that STN manipulation consequences might not be identical for all drugs of abuse. Further studies are in progress to study the effects of STN inactivation in rodents on their voluntary intake of other substances.
In summary, application of STN DBS reduced the motivation for cocaine by decreasing its motivational properties, while increasing motivation for food. The increased motivation for food did not appear to elicit binge eating, ruling out the possible undesirable effect of weight gain in the stimulated subjects. Although increased weight is often reported in PD patients with STN DBS, these patients typically have a long history of DA depletion and DA treatments that may interfere with the effects of STN DBS itself; this would be an unlikely condition in cocaine addicts.
Because the major goal for the treatment of cocaine addiction is to decrease the motivation for the drug without decreasing motivation for other naturally rewarding activities, a promising avenue for therapeutic success may be the application of STN DBS to cocaine addicts.
Methods
Subjects.
Male, Long-Evans rats (n = 88; Elevage Janvier) were housed in pairs and maintained on a 12-h light/dark cycle. After surgery, the animals were housed individually. For food experiments, they were kept at 80–85% of their free-feeding weight (15-17 g/rat per day). For cocaine experiments, the animals had no food restriction. This difference does not affect the results because we have also seen increased motivation for sweet food after STN lesions in sated rats. Water was provided ad libitum, except during experimental sessions. All procedures were conducted in accordance with the European Community Council Directive of November 24, 1986 (86/609/EEC), and our national French Agriculture and Forestry Ministry (decree 87–849).
Surgery.
All animals were anesthetized with xylazine (15 mg/kg, i.m.) and ketamine (100 mg/kg, i.m.). The electrodes were implanted at the following coordinates (16) from bregma: AP: 3.7 mm, L: ±2.4 mm, DV: 8.3 mm (from skull), incisor bar set at 3 mm.
Twenty-eight animals were further subjected to implantation of i.v. catheter. The catheter was implanted in the jugular vein and exited dorsally between the scapulae. During the recovery period (10 days), the catheters were flushed with 0.2 mL of a sterile antibiotic solution containing heparinized saline (280 UI/mL) and cefalexine (Rilexine; 8 mg/mL).
Electrodes and Stimulation.
As described previously (40, 41), electrodes made of two Teflon-insulated platinum wires were implanted bilaterally in the STN. Based on that former study, the stimulation parameters were set with frequency at 130 Hz and 60-μs pulse width. The intensity was set for each individual rat on the first day of behavioral testing, just below the threshold of induction of hyperkinetic movements of the contralateral paw (50-130 μA). STN DBS was turned to ON just prior the start of each behavioral session and was applied during the whole session.
Behavioral Procedures.
All of the behavioral procedures for continuous reinforcement, progressive ratio (PR) schedule of reinforcement, and conditioned place preference (CPP) have been described previously (11).
Food and Cocaine Self-Administration.
Before the start of behavioral sessions, STN DBS was either activated (ON) or not (OFF) for the whole session. Cocaine self-administration experiments used a typical dose of 250 μg/90 μL per injection (42).
Acquisition (continuous reinforcement FR1).
Rats were first trained to perform the FR1 for sucrose pellets and were then subjected to surgery. Two weeks after surgery, rats (n = 12 OFF and n = 16 ON) were trained to self-administer cocaine or obtain sucrose pellets (n = 8 OFF and n = 16 ON).
Progressive ratio experiments.
For food, the ratios followed an arithmetically increasing fixed-ratio (FR) schedule in steps of five, with three repetitions of each step (i.e., 1, 1, 1, 5, 5, 5, 10, 10, 10 …). To limit the number of injections, the ratios for cocaine reward followed the modified equation of Roberts used by Depoortere et al. (43) (i.e., 1, 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, etc.). If the rats failed to complete a ratio for 60 min, the session ended.
To test the reversibility of STN DBS, those animals belonging to the OFF group (n = 7 at this stage in the experiment) had their stimulation activated for 10 further daily sessions under PR conditions, whereas the animals of the ON group (n = 9) had their stimulation turned to OFF for 10 sessions under PR conditions for cocaine self-administration.
CPP.
For the CPP experiments, the two compartments differed only in the spatial organization of two vertical columns placed in each compartment (44). Thirty rats [OFF n = 7 (food) + 7 (cocaine); ON n = 13 (food) + 9 (cocaine)] were first exposed to both compartments for 15 min. The time spent in each was measured. From the following day and for 8 consecutive days, all rats were exposed alternatively to sucrose pellets (total 4.5 g; 100 pellets, 45 mg each) or one cocaine injection (10 mg/kg) in one compartment and no food or one injection of NaCl (0.9%) in the other compartment for 30 min. The compartment associated with the reward was counterbalanced across subjects. At the end of this conditioning, the animals were exposed to both compartments for 15 min, and the time spent in each compartment was measured. The score of preference was calculated as the difference between the time spent in the sucrose (or cocaine)-paired compartment on the last day and the time spent in the same compartment on the first day before conditioning. A positive score thus indicates a preference for the compartment associated with the food or cocaine, and a negative score would mean that the animals avoided the compartment associated with food or cocaine (45).
STN DBS was applied at each stage of the procedure for the ON group.
Statistical Analysis.
Findings are expressed as means for each of the variables (i.e., number of pellets or injections, last ratio reached in the PR procedures, etc.) in the different groups of animals.
For each variable, the data were submitted to mixed-design ANOVAs using Statview (Abascus) with group (OFF vs. ON) as the between-subject factors and sessions as the within-subject factor, when appropriate. When significant effects were found, post-hoc comparisons between means were made using simple main effects analysis. Nonparametric Mann–Whitney test was used for the conditioned place preference experiment for group effects, and nonparametric sign test for conditioning effect.
Histology and Dose–Response Study.
See SI Text.
Supplementary Material
Acknowledgments
The authors are grateful to Profs. B. J. Everitt and G. Meredith, and Drs. S. H. Ahmed, M. Amalric, J. Coull, B. Poucet, and V. McGinty for critical comments and proofreading of the manuscript. This research was supported by the French National Centre for Scientific Research (CNRS), the National Agency for Research (ANR) Contract JC05_48262 (to C.B.), the Interministerial Mission for the fight against drugs and drug addiction (MILDT)-National Institute of Health and Medical Research (-INSERM)-Institute of Cancer (InCa) (Contract MIL 0701), and the Foundation for Brain Research (FRC) (C.B. and M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908189107/DCSupplemental.
References
- 1.Limousin P, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Moro E, et al. Bilateral globus pallidus stimulation for Huntington’s disease. Ann Neurol. 2004;56:290–294. doi: 10.1002/ana.20183. [DOI] [PubMed] [Google Scholar]
- 4.Lozano AM, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Mallet L, et al. STOC Study Group. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 6.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degos B, et al. Neuroleptic-induced catalepsy: Electrophysiological mechanisms of functional recovery induced by high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2005;25:7687–7696. doi: 10.1523/JNEUROSCI.1056-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn J, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: Valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78:1152–1153. doi: 10.1136/jnnp.2006.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy D, et al. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao G, et al. Clinical study for alleviating opiate drug psychological dependence by a method of ablating the nucleus accumbens with stereotactic surgery. Stereotact Funct Neurosurg. 2003;81:96–104. doi: 10.1159/000075111. [DOI] [PubMed] [Google Scholar]
- 11.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat Neurosci. 2005;8:484–489. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- 12.Barichella M, et al. Body weight gain rate in patients with Parkinson’s disease and deep brain stimulation. Mov Disord. 2003;18:1337–1340. doi: 10.1002/mds.10543. [DOI] [PubMed] [Google Scholar]
- 13.Romito LM, et al. Bilateral high frequency subthalamic stimulation in Parkinson’s disease: Long-term neurological follow-up. J Neurosurg Sci. 2003;47:119–128. [PubMed] [Google Scholar]
- 14.Witjas T, et al. Addiction in Parkinson’s disease: Impact of subthalamic nucleus deep brain stimulation. Mov Disord. 2005;20:1052–1055. doi: 10.1002/mds.20501. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders . Washington, DC: American Psychiatric Assoc; 1994. 4th Ed. [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. 3rd Ed. [DOI] [PubMed] [Google Scholar]
- 17.Baunez C, Amalric M, Robbins TW. Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J Neurosci. 2002;22:562–568. doi: 10.1523/JNEUROSCI.22-02-00562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lardeux S, Baunez C. Alcohol preference influences the subthalamic nucleus control on motivation for alcohol in rats. Neuropsychopharmacology. 2008;33:634–642. doi: 10.1038/sj.npp.1301432. [DOI] [PubMed] [Google Scholar]
- 19.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 20.Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: A review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 21.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 24.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 25.Lardeux S, Pernaud R, Paleressompoulle D, Baunez C. Beyond the reward pathway: Coding of the reward salience and error in the rat subthalamic nucleus. J Neurophysiol. 2009;102:2526–2537. doi: 10.1152/jn.91009.2008. [DOI] [PubMed] [Google Scholar]
- 26.Lardeux S, Paleressompoulle D, Pernaud R, Cador M, Baunez C. Selective encoding of natural reward versus cocaine by subthalamic nucleus neurons. Soc Neurosci Abs. 2008;34:88.4. [Google Scholar]
- 27.Vassoler FM, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 30.van Duuren E, et al. Neural coding of reward magnitude in the orbitofrontal cortex of the rat during a five-odor olfactory discrimination task. Learn Mem. 2007;14:446–456. doi: 10.1101/lm.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurice N, Deniau JM, Glowinski J, Thierry AM. Relationships between the prefrontal cortex and the basal ganglia in the rat: Physiology of the corticosubthalamic circuits. J Neurosci. 1998;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 33.Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 34.Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- 35.Lee KH, et al. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: Potential mechanism of action in Parkinson’s disease. Eur J Neurosci. 2006;23:1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- 36.Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- 37.Salamone JD, Correa M. Motivational views of reinforcement: Implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 38.Barbano MF, Le Saux M, Cador M. Involvement of dopamine and opioids in the motivation to eat: Influence of palatability, homeostatic state, and behavioral paradigms. Psychopharmacology (Berl) 2009;203:475–487. doi: 10.1007/s00213-008-1390-6. [DOI] [PubMed] [Google Scholar]
- 39.Cagniard B, Balsam PD, Brunner D, Zhuang X, Xiaoxi Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- 40.Darbaky Y, Forni C, Amalric M, Baunez C. High frequency stimulation of the subthalamic nucleus has beneficial antiparkinsonian effects on motor functions in rats, but less efficiency in a choice reaction time task. Eur J Neurosci. 2003;18:951–956. doi: 10.1046/j.1460-9568.2003.02803.x. [DOI] [PubMed] [Google Scholar]
- 41.Baunez C, Christakou A, Chudasama Y, Forni C, Robbins TW. Bilateral high-frequency stimulation of the subthalamic nucleus on attentional performance: Transient deleterious effects and enhanced motivation in both intact and parkinsonian rats. Eur J Neurosci. 2007;25:1187–1194. doi: 10.1111/j.1460-9568.2007.05373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 43.Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW. Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav. 1993;45:539–548. doi: 10.1016/0091-3057(93)90503-l. [DOI] [PubMed] [Google Scholar]
- 44.Cabib S, et al. Dose-dependent aversive and rewarding effects of amphetamine as revealed by a new place conditioning apparatus. Psychopharmacology (Berl) 1996;125:92–96. doi: 10.1007/BF02247398. [DOI] [PubMed] [Google Scholar]
- 45.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.