Abstract
Objective
Lipopolysaccharide (LPS) pretreatment potentiates HI injury. We hypothesized that docosahexaenoic acid (DHA) pretreatment would improve function and reduce brain damage in this rat model of perinatal brain injury and inflammation.
Study Design
Seven-day-old Wistar rats were divided into 3 groups. One received intraperitoneal (IP) DHA 1 mg/kg and LPS 0.1mg/kg. The second received 25% Albumin and LPS. The third received normal saline (NS). Injections were given 2.5 hours prior to right carotid ligation, followed by 90 minutes 8% O2. Rats underwent sensorimotor testing and brain damage assessment on P14.
Results
DHA pretreatment improved forepaw placing compared to albumin/LPS. (Mean±SD successes/10 trials: 8.57±1.7 DHA/LPS vs 6.72±2.2 Albumin/LPS, p<.0009). There were no significant differences in brain damage among groups.
Conclusions
Inflammatory stimulation before HI resulted in poorer function than HI alone. Although DHA pretreatment had no impact on brain damage, it significantly improved function in neonatal rats exposed to LPS and HI.
Keywords: Docosahexaenoic acid, hypoxia ischemia, neonatal, neuroprotection, inflammation
Introduction
Intrapartum hypoxia-ischemia (HI) may lead to neonatal encephalopathy and to permanent neurologic disability.[1] Electronic fetal monitoring (EFM) was introduced in the 1970s with the expectation that recognition of intrapartum hypoxia and timely intervention would reduce cerebral palsy by as much as 50%.[2] Unfortunately, use of EFM failed to reduce cerebral palsy in large randomized controlled trials,[3] and the widespread use of EFM and expedited delivery in developed countries has not reduced the incidence of cerebral palsy among term infants.[1] One possible reason for the ineffectiveness of EFM in preventing cerebral palsy may be the complex and previously poorly understood etiology of neurologic injury.
Recently, the role of inflammation and the fetal systemic inflammatory response syndrome in the etiology of cerebral palsy has been recognized.[4–6] Using a large California cord blood repository, Nelson demonstrated that increased levels of interleukins (ILs) 1,6,8,9,11,13 and tumor necrosis factor-α, were present in cord blood taken from term infants destined to develop cerebral palsy compared to healthy controls. [4] Similarly, in a prospective cohort study of 123 preterm infants born to mothers who underwent amniocentesis, Yoon demonstrated that elevations of amniotic fluid IL 6 and 8 as well as histologic funisitis were strongly associated with the diagnosis of cerebral palsy at 3 years of age.[5] A population based study demonstrated that the coexistence of a potentially asphyxiating condition, such as tight nuchal cord and maternal infection, conferred a much higher risk of spastic quadriplegic cerebral palsy together than either condition alone. [7] Drawing on these observations, Peebles hypothesized that inflammation lowers the threshold at which intrapartum hypoxia results in neurologic injury.[8] This improved understanding of the interaction between hypoxia and inflammation suggests new approaches to perinatal neuroprotection.
One such novel approach is docosahexaenoic acid, (DHA), a long chain polyunsaturated fatty acid. DHA is an integral component of neuronal cell membranes and synaptic terminals.[9] DHA is readily available in the diet in fish and algae, and epidemiologic observation suggests that maternal diets rich in fish are associated with reduced risk for cerebral palsy.[10] In adult rodent models of brain ischemia-reperfusion and spinal cord injury, DHA has been shown to exert neuroprotective effects and to improve functional outcome.[11, 12] DHA may exert anti-inflammatory effects by altering the presentation of Toll-like receptor 4, the lipopolysaccharide (LPS) receptor, on the microglial cell membrane, thereby modulating the cyclooxygenase-2 signaling pathway, and reducing proinflammatory cytokine production.[13–15] DHA is the metabolic precursor of D-series resolvins and neuroprotectins. Neuroprotectin D1 attenuates NF κB production and COX2 expression, reduces influx of polymorphonucleocytes, and counters apoptosis, thus promoting neural cell survival.[9, 16] D-series resolvins block TNF-α induced IL-1β transcripts in microglial cells and limit PMN infiltration into inflamed brains.[9]
In our earlier experiments using a neonatal (P7) rat model of perinatal hypoxia-ischemia, we have shown that DHA pretreatment with 1 mg/kg, 2.5 mg/kg, and 5 mg/kg doses reduces brain volume loss and improves neurologic functioning as measured by the vibrissae stimulated forepaw placing test.[17] In those dose-finding experiments, we demonstrated that the 1 mg/kg dose was most neuroprotective.[17]
The objective of this second series of experiments is to test DHA pretreatment in a neonatal rat (P7) model of hypoxia-ischemia potentiated by inflammation. We hypothesized that DHA pretreatment would reduce brain volume loss and improve neurologic functioning in an animal model of perinatal HI with inflammation that may more nearly reflect the conditions leading to cerebral palsy than HI alone.
Materials and Methods
Preparation of DHA-Albumin Complex
DHA was delivered as a powder (Sigma, St. Louis, Mo, Cat#: D2534 as cis-4,7,10,13,16,19-DHA. DHA was complexed to human albumin by incubating 4mL of human serum albumin 25% (Baxter, Deerfield, IL) with 4mg of DHA to yield a final concentration of DHA 25mg/25μL. Each vial was aliquoted in 1-mg/mL samples and kept under nitrogen in a −20°C freezer. Nitrogen was reapplied to the vials weekly.
Animals
P7 Wistar rats were obtained in litters adjusted to equal sex distribution (Charles River Laboratories, Portage, MI). Animals were treated in accordance with protocols approved by our University Committee on the Use and Care of Animals in research. Pups were housed with the dam and littermates throughout the duration of the experiments.
To test the effect of DHA pretreatment, we used a modification of the LPS pretreatment model described by Eklind.[18] Rats received intraperitoneal Escherichia coli lipolysaccharide (LPS) 055:B5 (0.1 mg/kg) (Sigma, St. Louis) followed 2.5 hours later by right carotid artery ligation. Ninety minutes after ligation, the animals were then placed in the hypoxia chambers partially submerged in a water bath and then returned to the dams at 36 °C for 90 minutes, In contrast to the Eklind model, we chose the 90 minute duration of hypoxia for consistency with our prior studies with DHA using HI alone. Based on our prior dose finding studies[17], we used the 1 mg/kg dose of DHA for pretreatment in the current experiments. Sample size calculations indicate that 16–17 subjects/group are needed to detect a reduction in percent damage of one standard deviation, with α=0.05, β= 0.8.
Based on these calculations, six litters (N=72) of Wistar pups all underwent both hypoxic ischemia and treatment interventions on P7. Within each litter, pups were divided into three groups. The first group received intraperitoneal (IP) DHA 1mg/kg as DHA-albumin complex immediately followed by IP LPS (0.1mg/kg) [DHA/LPS]. The second group received IP 25% albumin immediately followed by IP LPS [ALB/LPS]. The third group received IP normal saline (NS) immediately followed by IP NS (0.1mg/kg) [NS/NS]. This third group [NS/NS] was used as a control to provide lesioning conditions within the experiments from which we could compare histopathologic lesions in a group of pups undergoing HI alone without an inflammatory stimulus (LPS).
Pups received the initial IP injection (5μL/g body weight) of DHA, 25% albumin or NS followed immediately by their LPS or NS injections. Pups were returned to their dams and allowed to recover. At 2.5 hours after injection, pups were anesthetized with isoflourane (induction at 3.5%, maintenance at 1.5%) and the right common carotid artery was divided after double ligation with surgical silk through a ventral neck incision. Pups then recovered for 1.5 hours (30 minutes in a 37°C incubator then 60 minutes with the dam). After carotid ligation and recovery, HI was induced by placing the pups in 500mL glass jars partially submerged in a water bath at 36°C in 8% oxygen for 90 minutes to induce unilateral cerebral HI. After HI, pups recovered in a 37°C incubator for 15–30 minutes. Once normal activity was resumed, the pups were returned to the dam where they remained until P14.
Animal rectal temperatures were obtained using a 0.6-mm flexible temperature probe (YSI-400 Tele-Thermometer, Yellow Springs Instruments, Yellow Springs, OH) before injection, after injection, before and after carotid ligation, before and after HI, 1 hour after HI and on P14. Pups were weighed before HI on P7 and subsequently on P14. The temperatures and weights were obtained both as representative measures of morbidity and to document that there were no temperature effects of the treatments, i.e. hypothermia that could affect or improve outcomes.
Vibrissae Stimulated Forepaw Placing Test
We chose the Vibrissae-stimulated forepaw placing test as a readily quantifiable functional measure of injury to the sensorimotor cortex or striatum.[19] The vibrissae test was performed on P14 pups in the following manner. Stimulation of the vibrissae (whiskers) on a surface edge results in extension of the forepaw on the same side as the simulated whiskers to reach the stimulating surface. In a partial response, the forepaw is incompletely extended without contacting the stimulating surface. Therefore, stimulation of the left vibrissae can demonstrate left forepaw placement deficits as a consequent of right sided hypoxic ischemic injury. As the right hemisphere controls left sided motor function, stimulation of the left vibrissae tests the right hemisphere. Stimulation of the right vibrissae, contralateral to the intact hemisphere, tests the unaffected cerebral hemisphere. A weighted vibrissae score to incorporate data from both complete and partial responses was calculated using the formula [partial contacts + 2 * (complete contacts)].
Histopathology
P14 rats were deeply anesthetized and euthanized followed by brain removal. Frozen coronal 20μm sections were stained with cresyl violet. Severity of brain injury was evaluated by calculating volume of tissue with intact staining using ImageJ software (U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/). Volumes were calculated from hemispheric and regional (cortex, striatum, hippocampus, and “other”) area measurements in regularly spaced sections. The “other” region included thalamus, septum, fimbria-fornix, corpus callosum, and major white matter tracts. Damage severity (percent tissue loss) of right sided injury was calculated as previously described. [17]
Statistical Analysis
A linear mixed models analysis of variance was used to evaluate differences in percent damage for hemisphere and each brain region among groups. We used litter as a random effect, treatment and sex as fixed effects and evaluated treatment by sex interaction. A similar linear mixed model was used to evaluate forepaw placing successes among treatments. Post hoc comparisons of treatment group means were carried out using the Tukey-Kramer adjustment for multiple comparisons. The effect of LPS on mortality was evaluated by analysis of variance with treatment as a fixed effect and litter as a random effect. NS/NS control pups were excluded from subsequent mortality analysis as there were no deaths in this group. To evaluate an effect of DHA on mortality among all LPS treated pups, we used a generalized mixed model with treatment as a fixed effect and litter as a random effect in order to assess the probability of dying in the ALB/LPS and DHA/LPS treatment groups only.
Results
Survival/Mortality
Of the seventy two rats that underwent the HI procedures, 16 pups (22%) died during the experiments. Of these, 11 died during HI, 2 died by injury by the dam immediately after HI and 3 died in the home cage between days P7 and P14. All of the 16 pups who died had received LPS. LPS treated rats were more likely to die than those who received NS. (p=.0013). Among the LPS treated rats, there was no difference in mortality between those treated with DHA compared to those treated with albumin (DHA/LPS 10/24 vs ALB/LPS 6/24; p=.17, f 1:40).
Body Weights and Temperatures
There were no significant differences between DHA/LPS, ALB/LPS and NS/NS groups with respect to rectal temperatures at any time-point. There was not a significant difference between the groups with respect to pretreatment body weight. Similarly, there were no significant differences in P7 or P14 body weights or in weight change from P7 to P14 among the three treatment groups. In addition, there was no difference in weights between the combined LPS groups and the NS pretreated pups.
Vibrissae Stimulated Forepaw Placing Test
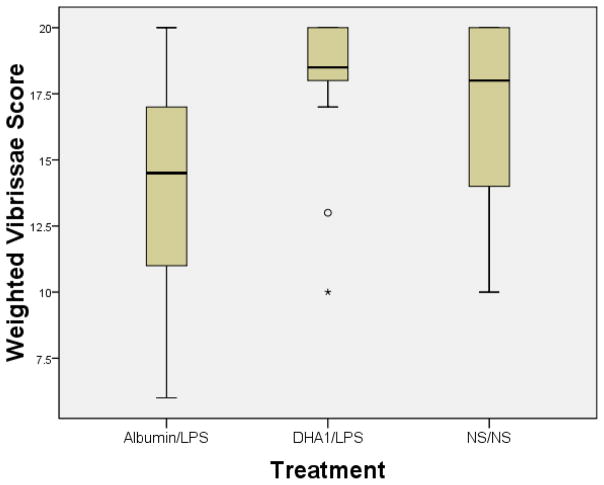
Seven days after injection and HI, the surviving 56 animals underwent the lateral vibrissae-stimulated forepaw placing test. The vibrissae scores of the ALB/LPS rats were significantly worse than those of the NS/NS rats (See Fig A: 13.83 ± 0.82 versus 17.21± 0.0.71; p=.0076), demonstrating that LPS treatment potentiated the functional deficit induced by HI. Among LPS pretreated rats, DHA/LPS rats had significantly better left forepaw placement results when compared to the ALB/LPS group. (See Fig A: 17.72± 0.92 versus 13.83 ± 0.82; p=.007, Tukey adjustment). Furthermore, forepaw placing scores of the DHA/LPS pups were not different than scores of the pups that underwent HI but did not receive LPS (NS/NS). (See Fig A; p=.893, Tukey adjustment). There was a borderline effect of sex on vibrissae response with females performing better than males (17.16 vs. 15.36, p=.059), but there was no treatment by sex interaction. No litter effects on forepaw placing responses were seen. Right vibrissae stimulation consistently elicited correct right paw placement 10 out of 10 trials in all groups. Results of the vibrissae forepaw placing tests are summarized in Figure 1.
Figure 1. DHA pretreatment attenuates forepaw placing deficits among hypoxic ischemic pups exposed to LPS pretreatment.
Vibrissae-stimulated forepaw placing test results in control groups (that received normal saline followed by normal saline) and treatment groups (that received DHA 1mg/kg or Albumin followed by E. coli lipopolysaccharide inflammation) prior to undergoing right (R) carotid ligation followed by 90 minutes of hypoxia at 8% oxygen on postnatal day (P) 7. Testing was performed on P14 (10 trials/side). The y-axis = weighted response score in which complete forepaw placing on stimulus surface received score of 2 and partial response (i.e. forepaw motion without placing on stimulus surface) received score of 1. Best possible weighted score is 20. Boxes= interquartile range; horizontal bars=medians; whiskers=SE. They extend to data points no more than 1.5 width of box. All animals consistently responded to R vibrissae stimulation with appropriate R paw placement. Impairment of left (L) paw placement in response to L vibrissae stimulation was seen in Albumin/LPS rats compared to normal saline/normal saline rats. Among LPS pretreated groups, performance was significantly better in the DHA/LPS treatment group when compared to Albumin/LPS (P=.007; Tukey-Kramer test).
Histopathology
Ninety minutes of HI resulted in moderately severe lesions as demonstrated by the NS/NS group who had striatal and hippocampal atrophy, cortical thinning and/or cystic infarction and an overall 26% reduction in right hemisphere volume compared to intact left. No statistically significant differences are seen when comparing the hemisphere or regional volumes or percent damage between the DHA/LPS group and the ALB/LPS group. Additionally, no statistically significant differences were seen when comparing these same measures in the LPS treated groups (DHA/LPS and ALB/LPS) vs the group that did not receive LPS (NS/NS). Histopathology results are summarized in Table 1.
Table 1.
Effect of Docosahexaenoic acid pretreatment on severity of brain volume loss by region
| Treat | N | Hippocampus | Other* | Cortex | Striatum |
|---|---|---|---|---|---|
| DHA/LPS | 14 | 33.8±23.1 | 14.3±8.5 | 20.4±13.1 | 25.6±10.7 |
| ALB/LPS | 24 | 31.6±29.7 | 14.3±12.8 | 24.8±24.3 | 23.3±15.9 |
| NS/NS | 18 | 38.8±38.2 | 20.9±18.9 | 31.3±27.9 | 29.1±21.3 |
DHA, docosahexaenoic acid; LPS, Escherichia coli lipopolysaccharide; NS, normal saline. Values are means±standard deviation of regional percent damage, calculated from regional volumes using the formula 100*(L-R)/L
other refers to any forebrain tissue other than hippocampus, cortex or striatum
Comment
The objective of our study was to examine whether pretreatment with DHA blocks potentiation of HI injury by Lipopolysaccharide (LPS) induced systemic inflammation in neonatal rats. Our findings are similar to those described by Eklind in that the addition of an inflammatory stimulus (LPS) to the perinatal HI model places neonatal rat pups at significant increased mortality risk.[18] In our study, DHA did not improve the pups’ rates of survival. However, among survivors, function was improved (i.e. impairment was significantly decreased) by DHA-Albumin pretreatment compared to the pups who received albumin alone (p=.007). In fact, DHA treatment before HI/LPS improved the vibrissae-stimulated forepaw placing response to the level observed in neonatal rats exposed to HI alone (NS/NS). That could be interpreted to mean that DHA treatment reversed the potentiation of HI injury by LPS.
Our study used intraperitoneal injection of E. Coli Lipopolysaccharide as a model for intrapartum chorioamnionitis preceding HI conditions producing moderate brain lesion severity. In contrast to the original description of the model by Eklind,[18] the observed percent brain volume loss in rats exposed to HI and LPS in our experiments was not statistically different than that observed in rats exposed to HI alone. This lack of potentiation of damage severity may have resulted from the modification we made to the Eklind model, increasing hypoxia duration to 90 minutes for all animals. Eklind, at al demonstrated that LPS pretreatment changes the injury threshold with short hypoxia durations without significantly changing injury severity at longer hypoxia durations. Our histopathologic findings, with relatively longer hypoxia duration, are consistent with the latter observation. Nonetheless, vibrissae scores for rats receiving LPS were significantly worse than those exposed to HI alone, suggesting potentiation of hypoxic ischemic injury by inflammation.
There are several possible explanations for this disconnect between the effect of LPS on functional outcome and its effect on histopathology in our experiments. In a rat model exploring prenatal risk factors for later development of Parkinson’s Disease, prenatal exposure to LPS alone was demonstrated to significantly reduce dopamine and serotonin in the frontal cortex, striatum, amygdala, hippocampus, nucleus accumbens, and striatum.[20] LPS alone is toxic to dopaminergic neurons in the nigrostriatal pathways involved in regulation of motor function.[20–22] In another rat model, prenatal LPS exposure resulted in alterations in microglial morphology signifying a more advanced stage of microglial activation compared to normal saline exposure.[21] Ling et al demonstrated effects of micoglial activation on motor function that persisted over the lifespan of the rats. These potential mechanisms may explain the significant worsening of vibrissae forepaw placing scores in LPS-exposed animals in our model in the absence of brain volume difference. This is consistent with reported limited utility of brain volume measurements in various regions in predicting clinical outcome in human infants. [23]
Similarly, in our model of HI potentiated by LPS, DHA-Albumin pretreatment significantly improved function as measured by the vibrissae-stimulated forepaw placing test compared to albumin alone, although no reduction in brain volume loss was seen. Potential mechanisms whereby DHA might improve functional outcome independent of observed brain volume include enhancement of synaptic signal transduction, incorporation into neural cell membranes thereby increasing membrane fluidity, and modulation of dopaminergic and seritonergic neurotransmitter function.[24–26] DHA may also have preserved the integrity of essential neural tracts without affecting the overall brain volume loss. In a rodent model of spinal cord injury, DHA was shown to reduce microglial activation and macrophage recruitment, and to preserve myelination.[27]
A limitation of these preliminary experiments is that we have not explored cellular and molecular mechanisms whereby DHA improves functional outcome. Similarly, we have not yet assessed the long-term durability of these beneficial effects. More studies of long-term outcomes after DHA pretreatment are needed before DHA will be ready to be translated into human clinical trials. For the future, an additional important question is whether similar improvements would be observed after maternal dietary DHA supplementation.
Combining the results of the present experiments and our previous studies, we have demonstrated that DHA pretreatment improves functional outcomes after HI alone[17] and after HI potentiated by LPS. There is clearly a discordance between functional and structural evidence of benefit. However, in terms of translation to human studies, the outcome of most clinical interest and significance would be function, not neuropathology.
Acknowledgments
This work was supported through the Rudi Ansbacher Fund at the University of Michigan and the National Institute of Health/National Center for Research Resources Clinical and Translational Science Award Grant UL1RRO24986.
The authors thank Edward D. Rothman, PhD, and Kathleen B. Welch, MPH, MS, at the University of Michigan Center for Statistical Consultation and Research for statistical advice and assistance.
Footnotes
Individual responsible for reprint requests: reprints unavailable
This paper was presented as a poster presentation at The Society for Gynecologic Investigators (SGI) 2009 Annual Meeting in Glasgow, Scotland March 2009 and at Pediatric Academic Societies 2009 Annual Meeting in Baltimore, Maryland May 2–5, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–95. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 2.Quilligan EJ, Paul RH. Fetal monitoring: is it worth it? Obstet Gynecol. 1975;45:96–100. [PubMed] [Google Scholar]
- 3.Thacker SB, Stroup D, Chang M. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD000063. [DOI] [PubMed] [Google Scholar]
- 4.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 5.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:194–202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 7.Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507–13. doi: 10.1016/s0002-9378(98)70387-4. [DOI] [PubMed] [Google Scholar]
- 8.Peebles DM, Wyatt JS. Synergy between antenatal exposure to infection and intrapartum events in causation of perinatal brain injury at term. BJOG. 2002;109:737–739. doi: 10.1111/j.1471-0528.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petridou E, Koussouri M, Toupadaki N, et al. Diet during pregnancy and the risk of cerebral palsy. Br J Nutr. 1998;79:407–12. doi: 10.1079/bjn19980069. [DOI] [PubMed] [Google Scholar]
- 11.Belayev L, Marcheselli VL, Khoutorova L, et al. Docosahexaenoic Acid Complexed to Albumin Elicits High-Grade Ischemic Neuroprotection. Stroke. 2005;36:118–123. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 12.King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 Fatty Acids Improve Recovery, whereas Omega-6 Fatty Acids Worsen Outcome, after Spinal Cord Injury in the Adult Rat. J Neurosci. 2006;26:4672–4680. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through Toll-like receptor 4-derived signaling pathways. FASEB J. 2001;15:2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–86. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.De Smedt-Peyrusse V, Sarqueil F, Moranis A, Harizi H, Mongrand S, Layé S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J Neurochem. 2008;105:296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- 16.Bazan NG. The onset of brain injury and neurodegeneration triggers the synthesis of docosanoid neuroprotective signaling. Cell Mol Neurobiol. 2006;26:901–13. doi: 10.1007/s10571-006-9064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman DR, Mozurkewich E, Liu Y, Barks J. Docosahexaenoic acid pretreatment confers neuroprotection in a rat model of perinatal cerebral hypoxia-ischemia. Am J Obstet Gynecol. 2009;200:e1–6. doi: 10.1016/j.ajog.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 19.Schallert T. Behavioral Tests for Preclinical Intervention Assessment. NeuroRX. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Yan JY, LO YK, Carvey PM, Ling Z. Dopaminergic and serotoninergic deficiencies in young adult rats prenatally exposed to the bacterial lipopolysaccharide. Brain Res. 2009;1265:196–204. doi: 10.1016/j.brainres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Ling Z, Zhu Y, Tong CW, Snyder JA, Lipton JW, Carvey PM. Prenatal lipopolysaccharide does not accelerate progressive dopamine neuron loss in the rat as a result of normal aging. Exp Neurol. 2009;216:312–320. doi: 10.1016/j.expneurol.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Hart AR, Whitby EW, Griffiths PD, Smith MF. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Dev Med Child Neurol. 2008;50:655–663. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 24.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 25.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Physics Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 26.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Michael-Titus AT. Omega-3 fatty acids and neurological injury. Prostaglandins Leukot Essent Fatty Acids. 2007;77:295–300. doi: 10.1016/j.plefa.2007.10.021. [DOI] [PubMed] [Google Scholar]