Abstract
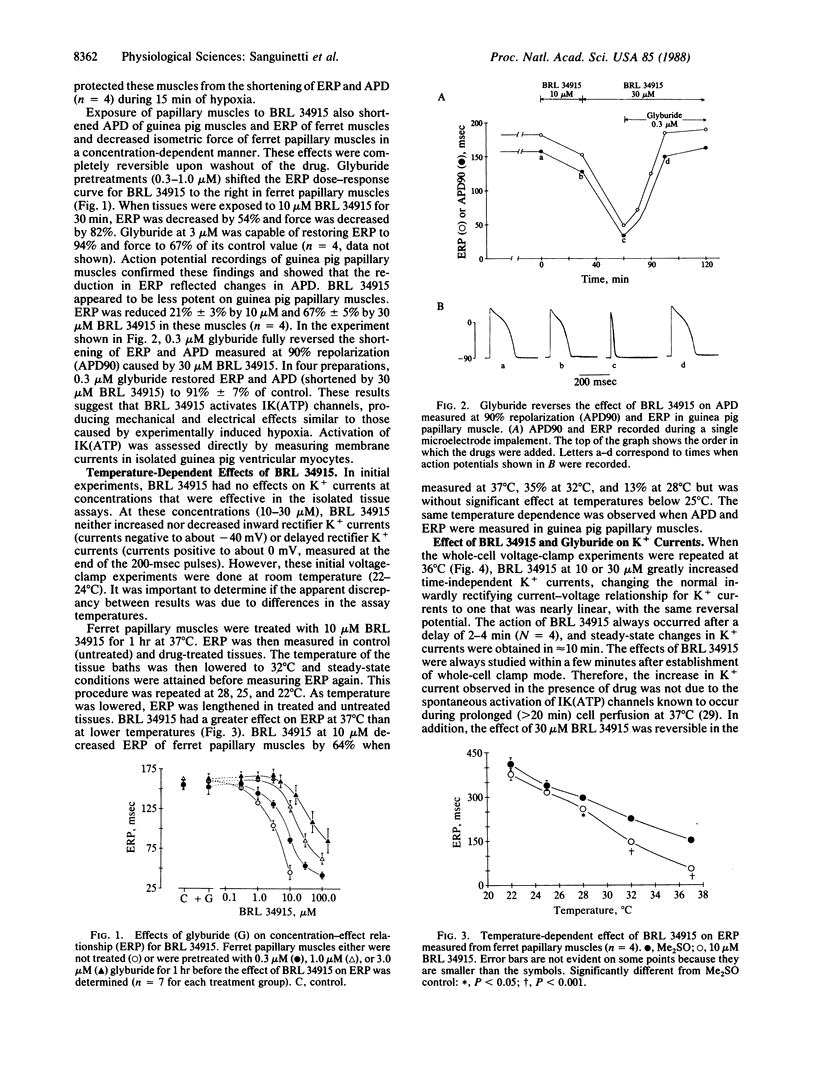
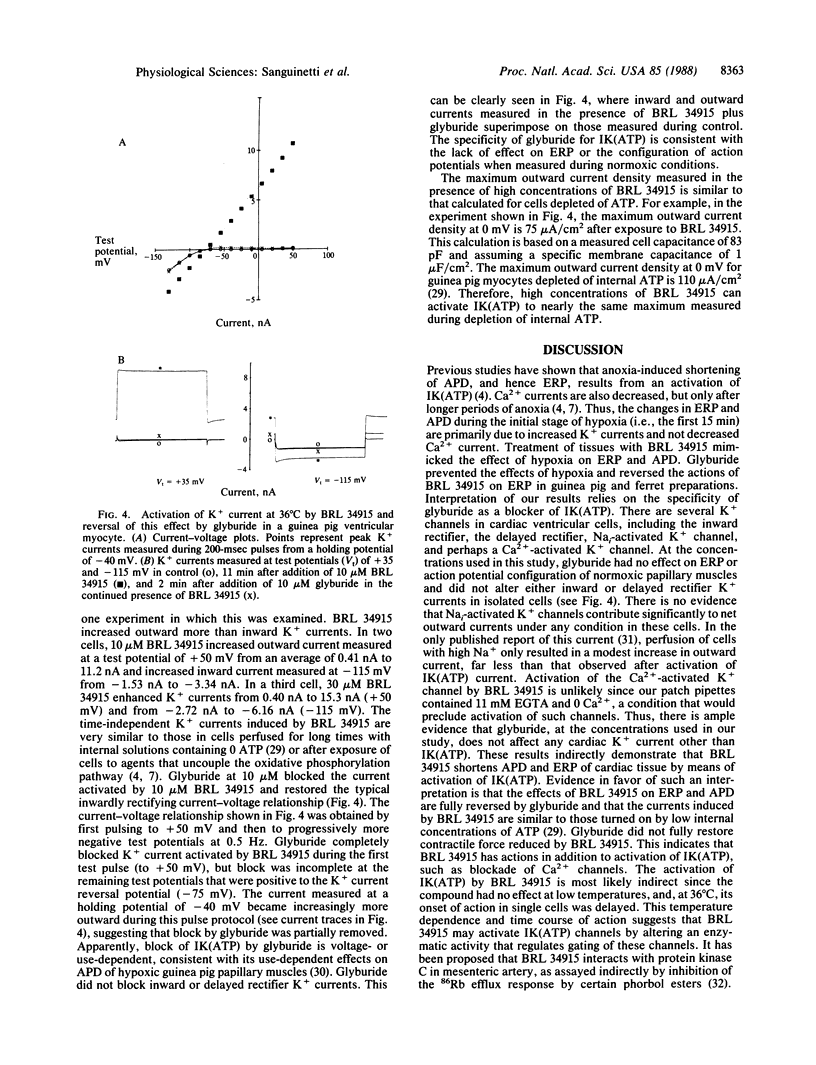
The mechanism by which the antihypertensive agent BRL 34915 (cromakalim) affects action potential duration (APD) and effective refractory period (ERP) in isolated cardiac muscle was investigated. BRL 34915 (greater than or equal to 3 microM) shortened ERP of ferret (Mustela putorius furo) and guinea pig (Cavia porcellus) papillary muscles in a concentration-dependent fashion. The reduction in ERP resulted from a decrease in APD. ERP and APD of papillary muscles were also reduced during hypoxia produced by bubbling the physiological bathing solution with N2 instead of O2. Reduction of APD during hypoxia has previously been attributed to activation of ATP-sensitive K+ channels in heart. Glyburide, an inhibitor of ATP-sensitive K+ channels, prevented or reversed the shortening of ERP and APD produced by hypoxia and BRL 34915, respectively. These results suggest that BRL 34915 acts by opening ATP-sensitive K+ channels in heart. The actions of BRL 34915 were temperature-dependent, decreasing ERP 64% at 37 degrees C, but having no effect at 22 degrees C. The effect of BRL 34915 on K+ currents was tested directly in voltage-clamped guinea pig ventricular myocytes. As observed with the papillary muscles, BRL 34915 was without effect at 22 degrees C. At 36 degrees C, BRL 34915 (after a delay) increased outward currents positive to, and less so at potentials negative to, the K+ current reversal potential. The normal inwardly rectifying current-voltage relationship for peak K+ currents during 200-msec pulses was changed to one that was nearly ohmic. The current activated by BRL 34915 was blocked by glyburide. The data support the hypothesis that BRL 34915, like hypoxia, activates ATP-sensitive K+ channels in the heart. Based upon the profound temperature sensitivity of BRL 34915 action, this activation may be indirect, perhaps by means of modulation of an enzymatic activity that regulates gating of these channels. BRL 34915 and glyburide will be valuable tools for studying the role of ATP-sensitive K+ channels in normal and abnormal cardiac function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belles B., Hescheler J., Trube G. Changes of membrane currents in cardiac cells induced by long whole-cell recordings and tolbutamide. Pflugers Arch. 1987 Aug;409(6):582–588. doi: 10.1007/BF00584657. [DOI] [PubMed] [Google Scholar]
- Borowicz L. E., Schniepp H. C., Sanguinetti M. C. Membrane potential-dependent effects of SC-36602, a new class I antiarrhythmic agent on cardiac action potentials. J Cardiovasc Pharmacol. 1987 Jan;9(1):57–64. [PubMed] [Google Scholar]
- Callewaert G., Vereecke J., Carmeliet E. Existence of a calcium-dependent potassium channel in the membrane of cow cardiac Purkinje cells. Pflugers Arch. 1986 Apr;406(4):424–426. doi: 10.1007/BF00590947. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac transmembrane potentials and metabolism. Circ Res. 1978 May;42(5):577–587. doi: 10.1161/01.res.42.5.577. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Quast U., Hof R. P., Baumlin Y., Pally C. Similarities in the mechanism of action of two new vasodilator drugs: pinacidil and BRL 34915. J Cardiovasc Pharmacol. 1988 Jan;11(1):90–99. doi: 10.1097/00005344-198801000-00014. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Illot M. C., Peterson O. H. Interaction of diazoxide, tolbutamide and ATP4- on nucleotide-dependent K+ channels in an insulin-secreting cell line. J Membr Biol. 1987;99(3):215–224. doi: 10.1007/BF01995702. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Itoh T., Kajiwara M., Kitamura K., Suzuki H., Ito Y., Kuriyama H. Vasodilating actions of 2-nicotinamidoethyl nitrate on porcine and guinea-pig coronary arteries. J Pharmacol Exp Ther. 1981 Jul;218(1):248–259. [PubMed] [Google Scholar]
- Gaines K. L., Hamilton S., Boyd A. E., 3rd Characterization of the sulfonylurea receptor on beta cell membranes. J Biol Chem. 1988 Feb 25;263(6):2589–2592. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Taira N. Pinacidil increases the background potassium current in single ventricular cells. Eur J Pharmacol. 1987 Sep 2;141(1):139–141. doi: 10.1016/0014-2999(87)90421-3. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Vereecke J., van der Heyden G., Carmeliet E. The shortening of the action potential by DNP in guinea-pig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflugers Arch. 1983 Jun 1;397(4):251–259. doi: 10.1007/BF00580257. [DOI] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei M., Yoshinaga M., Saito K., Tanaka H. The potassium current activated by 2-nicotinamidoethyl nitrate (nicorandil) in single ventricular cells of guinea pigs. Proc R Soc Lond B Biol Sci. 1986 Dec 22;229(1256):331–343. doi: 10.1098/rspb.1986.0089. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., MacLeod D. P. Metabolism and the electrical activity of anoxic ventricular muscle. J Physiol. 1973 Mar;229(3):559–582. doi: 10.1113/jphysiol.1973.sp010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisheri K. D., Cipkus L. A., Taylor C. J. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988 Jun;245(3):751–760. [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Noma A., Shibasaki T. Membrane current through adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:463–480. doi: 10.1113/jphysiol.1985.sp015722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder W. Modification of K+ conductance of heart cell membrane by BRL 34915. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jan;337(1):93–97. doi: 10.1007/BF00169483. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Schmid-Antomarchi H., de Weille J., Fosset M., Lazdunski M. The antidiabetic sulfonylurea glibenclamide is a potent blocker of the ATP-modulated K+ channel in insulin secreting cells. Biochem Biophys Res Commun. 1987 Jul 15;146(1):21–25. doi: 10.1016/0006-291x(87)90684-x. [DOI] [PubMed] [Google Scholar]
- Scholtysik G. Evidence for inhibition by ICS 205-930 and stimulation by BRL 34915 of K+ conductance in cardiac muscle. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jun;335(6):692–696. doi: 10.1007/BF00166988. [DOI] [PubMed] [Google Scholar]
- Shetty S. S., Weiss G. B. Dissociation of actions of BRL 34915 in the rat portal vein. Eur J Pharmacol. 1987 Sep 23;141(3):485–488. doi: 10.1016/0014-2999(87)90570-x. [DOI] [PubMed] [Google Scholar]
- Streit J. Effects of hypoxia and glycolytic inhibition on electrical properties of sheep cardiac Purkinje fibres. J Mol Cell Cardiol. 1987 Sep;19(9):875–885. doi: 10.1016/s0022-2828(87)80616-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Noma A., Irisawa H. Modification of the cardiac action potential by intracellular injection of adenosine triphosphate and related substances in guinea pig single ventricular cells. Circ Res. 1983 Aug;53(2):131–139. doi: 10.1161/01.res.53.2.131. [DOI] [PubMed] [Google Scholar]


