Abstract
For many applications it would be desirable to be able to control the activity of proteins by using an external signal. In the present study, we have explored the possibility of modulating the activity of a restriction enzyme with light. By cross-linking two suitably located cysteine residues with a bifunctional azobenzene derivative, which can adopt a cis- or trans-configuration when illuminated by UV or blue light, respectively, enzymatic activity can be controlled in a reversible manner. To determine which residues when cross-linked show the largest “photoswitch effect,” i.e., difference in activity when illuminated with UV vs. blue light, > 30 variants of a single-chain version of the restriction endonuclease PvuII were produced, modified with azobenzene, and tested for DNA cleavage activity. In general, introducing single cross-links in the enzyme leads to only small effects, whereas with multiple cross-links and additional mutations larger effects are observed. Some of the modified variants, which carry the cross-links close to the catalytic center, can be modulated in their DNA cleavage activity by a factor of up to 16 by illumination with UV (azobenzene in cis) and blue light (azobenzene in trans), respectively. The change in activity is achieved in seconds, is fully reversible, and, in the case analyzed, is due to a change in V max rather than K m.
Keywords: azobenzene, DNA cleavage, endonuclease, photoswitch, PvuII
Proteins exist in nature whose activity can be controlled by light; perhaps one of the best known examples is rhodopsin, which is regulated by the cis/trans isomerization of its cofactor retinal. For many biological applications it would be desirable to selectively switch the activity of a protein on and off by light in a similar manner (1). This could be accomplished by the introduction of a photosensitive compound into the protein of interest. Recent developments in photosensitive compounds such as the azobenzene derivatives have made the scenario a reality. Azobenzene can be reversibly isomerized between the extended trans- and the more compact cis-configuration by illumination with UV (trans → cis) or blue-light (cis → trans) as well as by thermal relaxation (cis → trans) (2 –4). Four generally applicable approaches have been used to introduce azobenzene groups into peptides or proteins: (i) incorporation during peptide synthesis (5 –8), (ii) incorporation during in vitro translation (9, 10), (iii) incorporation in vivo by using an orthogonal tRNA/aminoacyl tRNA synthetase pair specific for phenylalanine-4′-azobenzene (11), and (iv) chemical modification of peptides and proteins (3, 12, 13). Another more specific approach is to use azobenzene-modified ligands (e.g., inhibitors) for proteins (14, 15). Chemical modification, the most widely used of these approaches, can be done with mono- or bifunctional azobenzene derivatives. Modification with monofunctional azobenzene derivatives relies on steric effects (e.g., interference with ligand binding), whereas modification with bifunctional azobenzene derivatives usually is intended to change the conformation of the target molecule, thereby changing its activity. Site-specific modification is achieved by introducing amino residue-specific reacting groups. For example, bifunctional cross-linkers with a central azobenzene moiety have been synthesized that contain thiol-specific coupling groups to cross-link cysteine residues in a highly specific manner (2, 16, 17). It has been shown that peptides and proteins can be modified with such bifunctional azobenzene derivatives and that these chemically modified peptides and proteins can be induced to change their conformation and/or their activity in a reversible manner by illumination with light (16, 18 –25). To date, this is the most promising generally applicable method to produce photo-switchable proteins, in particular when structural information is available (26). Monofunctional azobenzene derivatives attached to proteins have also been used to regulate protein function by illumination in vitro (27 –30) and in vivo (31 –35). Whereas few examples are known in which enzymes have been modified with monofunctional azobenzene derivatives or with an azobenzene-bearing amino acid such that their catalytic activity could be modulated by light (9, 10, 36, 37), none so far, to our knowledge, have been controlled by bifunctional azobenzene cross-linkers.
There are a number of applications where the temporal control of an enzyme activity is desirable. Reengineered site-specific endonucleases, such as homing endonucleases, zinc finger nucleases, and restriction endonucleases, are being used for genome engineering (38). Although highly specific, they exhibit off-site cleavage over time. Presumably, this phenomenon could be minimized by temporal control of their activity, which could be achieved by introducing a “photoswitch.” Here, we report that we have succeeded in using the cross-linker 4,4′-bis(maleimido)azobenzene to modify a restriction enzyme in order to control its DNA cleavage activity by photo-induced isomerization of the azobenzene moiety. Our best success relies on a combination of chemical modification and protein mutagenesis to achieve a relative 16-fold change in activity.
Results
We have chosen the single-chain variant of the naturally homodimeric restriction enzyme PvuII (scPvuII) for our study (39) because it allows introducing unique amino acid substitutions in the N- and C-terminal “halves” of the protein. The N- and C-terminal halves of the protein correspond to the identical subunits of the homodimeric PvuII, from which scPvuII was derived by joining the C-terminus of one subunit with the N-terminus of the other with a short four-amino-acid linker.
Selection of Azobenzene Attachment (Cross-Link) Sites in Single-Chain PvuII.
In order to generate a photoswitchable variant of scPvuII, several surface-exposed residue pairs were chosen [based on the structure of scPvuII (40) and by using the program sGAL (41)] for cross-linking with the bifunctional azobenzene derivative 4,4′-bis(maleimido)azobenzene (acronym: azomal) (Fig. 1). The Cγ-Cγ-distances of these residue pairs (∼8 ± 3 Å) approximate the reported range of ∼5–12 Å for azobenzene in the cis-configuration, compared to approximately 17 Å in the trans-configuration (17). Residue pairs (Fig. 2) located in α-helices flanking the DNA binding site (V89/Q96, R129/S133, K147/E151) or loops and β-strands next to the active site (T49/N62, E66/Q96, I74/E110) were selected, in addition to residue pairs connecting the linker region (L1–L4, see Materials and Methods) between the two halves of scPvuII and residues next to the active site (L/D61 and L/N62). The residues chosen were exchanged for cysteine, resulting in scPvuII variants with one azobenzene cross-link site (double-cysteine variants have one cross-link site) in the N-terminal half of scPvuII and in variants with “equivalent” azobenzene cross-link sites in the N- and C-terminal halves of scPvuII (quadruple-cysteine variants have two cross-link sites). Altogether, 11 variants with double-cysteine substitutions and 17 with quadruple-cysteine substitutions were produced (Table S1) and modified with azomal. In eight of these, additional amino acid substitutions were introduced that lower the activity by interfering with DNA binding (H83A) (42) and catalysis (Y94F) (43).
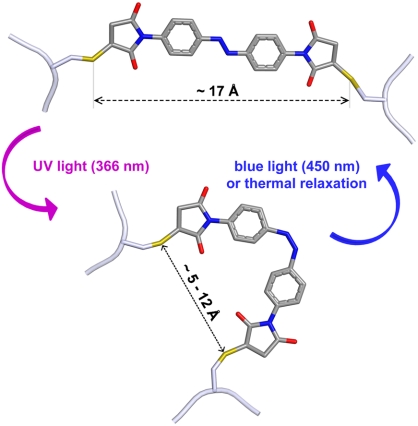
Fig. 1.
A scheme of the trans → cis isomerization of azomal attached to cysteine residues induced by illumination with UV light and cis → trans isomerization with blue light. It is apparent that the sulfur–sulfur distance is considerably larger in the trans-state than in the cis-state.
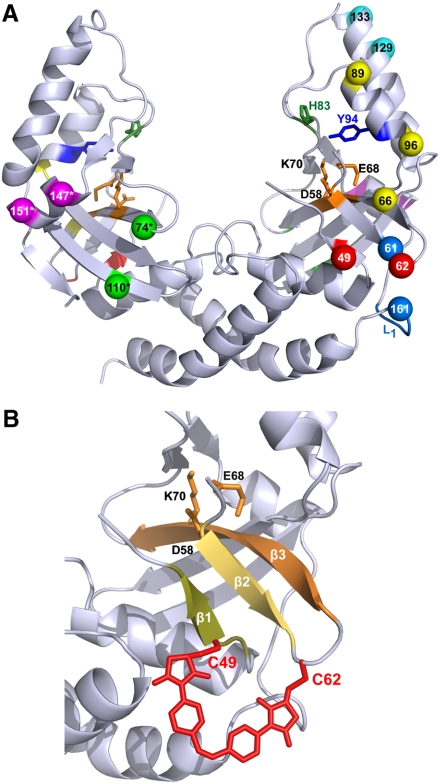
Fig. 2.
(A) The crystal structure of scPvuII (40) with the residue pairs chosen for cross-linking with azomal: (C49 C62), (C61 L1[-GSG C161-]), (C66 C96), (C74* C110*), (C89 C96), (C129 C133), and (C147* C151*). Amino acid residues of these residue pairs are depicted in identical colors. For clarity, residue pairs are shown mostly on the N-terminal half (Right), only those that are on the “backside” are shown in the C-terminal half (Left), denoted with an asterisk (*). Note that amino acid pairs were cross-linked in the N-terminal half, when only one cross-link was introduced into scPvuII, or in equivalent positions of the N- and C-terminal halves, when two cross-links were introduced (Table 1). Amino acids of residue pairs chosen for cross-linking were substituted by cysteines via site-specific mutagenesis. The amino acid residues of the active site (D58, E68, and K70) are indicated in orange. H83 (green) and Y94 (blue), which in some variants were substituted by alanine and phenylalanine, respectively, are also indicated. (B) A blow up of the region comprising β-strands β1, β2, and β3 and the catalytic center is shown, together with a model of azomal cross-linking C49 and C62.
Azomal Modification and Purification of scPvuII Variants.
The modification of the scPvuII variants was performed with azomal in the cis-state (i.e., under illumination with UV light), with an average yield of 50–80% modified protein. The crude modified protein preparation contained, in addition to the desired cross-linked species, side products in which only one cysteine residue was modified by a single azomal molecule (not cross-linked) and in which two cysteine residues were modified with one azomal molecule each (not cross-linked). Enrichment of the desired cross-linked species is essential to detect the magnitude of the photoswitch effect because other species can distort the results (Table S2). The reaction mixture was therefore subjected to a series of chromatographic steps as described in Materials and Methods. It was demonstrated by a mass spectroscopic analysis that cross-linking by the bifunctional sulfhydryl-reactive azobenzene derivative has been achieved (Fig. S1).
Activity of Azomal-Modified scPvuII-Variants in the cis- and trans-States of Azobenzene.
The azomal-modified scPvuII variants were tested for a light-inducible change in activity via the photoswitch. Table 1 shows the ratio of the DNA cleavage activity of all azomal-modified variants measured under UV light (cis) and under blue light (trans). As expected, the unmodified scPvuII and sc(C49 C62)2 (i.e., the scPvuII variant carrying the T49C and N62C substitution in both halves of the protein, see Table 1 for naming convention and Fig. 2 for the position of these residues in the structure of scPvuII) and sc(C49 C62)2(F94)2 have the same activity under UV and blue-light illumination. On the other hand, several of the azomal-modified variants show a higher activity when the azobenzene moiety adopts the cis-configuration as compared to the trans-configuration. In general, larger effects are seen when the azomal modification is in both halves of the scPvuII protein. For example, sc(C49 C62)2Azo has an approximately 7-fold higher activity in the cis-state than in the trans-state; in contrast, the corresponding variant with only one half modified [sc(C49 C62)Azo] showed only an approximately 2-fold increase (Table 1). It is also noteworthy that the azomal modifications close to the active site have a larger impact on the cis/trans activity ratio than azomal modifications in α-helices flanking the DNA binding site. In an effort to increase the cis/trans activity ratio, additional amino acid substitutions were introduced independently: H83A and Y94F, which lower the cleavage activity of wtPvuII (42, 43). The sc(C49 C62)2(F94)2Azo (i.e., the sc(C49 C62)2Azo variant with the additional Y94F substitution in both halves of the protein) has an approximately 16-fold higher activity in the cis- than in the trans-state of the azobenzene moiety, the largest effect that we observed so far for a photoswitchable scPvuII variant (Table 1). A similar photoswitching effect was observed for sc(C61 L4)(C49∗ C62∗)(F94)2Azo (i.e., the scPvuII variant with a cross-link between C61 and a cysteine in the linker L4, and an additional C49* C62* cross-link in the C-terminal half, together with Y94F substitutions in both halves of the protein).
Table 1.
Photoswitching effect of different scPvuII variants cross-linked with 4,4′-bis(maleimido)azobenzene
| Type of modification | Variant | Relativeactivitycis/trans |
| Unmodified | sc | 1.0 ± 0.1 |
| sc(C49 C62)2 | 1.0 ± 0.1 | |
| sc(C49 C62)2(F94)2 | 1.0 ± 0.0 | |
| One azomal modification | sc(C49 C62)Azo | 2.2 ± 0.0 |
| sc(C74 C110)Azo | 1.1 ± 0.2 | |
| sc(C66 C96)Azo | 2.3 ± 0.4 | |
| sc(C147 C151)Azo | 1.5 ± 0.0 | |
| sc(C89 C96)Azo | 1.1 ± 0.1 | |
| sc(C129 C133)Azo | 1.1 ± 0.0 | |
| Two azomal modifications | sc(C49 C62)2Azo | 7.0 ± 0.3 |
| sc(C74 C110)2Azo | 3.0 ± 1.0 | |
| sc(C66 C96)2Azo | 3.4 ± 1.0 | |
| sc(C147 C151)2Azo | 1.1 ± 0.2 | |
| Two azomal modifications and additional mutation(s) | sc(C49 C62)2(A83)Azo | 6.2 ± 0.5 |
| sc(C49 C62)2(A83)2Azo | 10.0 ± 2.9 | |
| sc(C49 C62)2(F94)Azo | 15.9 ± 2.3 | |
| sc(C49 C62)2(F94)2Azo | 15.5 ± 2.4 | |
| sc(C49 C62)2(A83)2(F94)Azo | 5.2 ± 1.1 | |
| sc(C49 C62)2(A83)2(F94)2Azo | 2.6 ± 0.2 | |
| One azomal modification involving the linker | sc(C61 L1)Azo | 3.2 ± 0.3 |
| sc(C62 L1)Azo | 1.0 ± 0.1 | |
| sc(C61 L2)Azo | 0.8 ± 0.2 | |
| sc(C61 L3)Azo | 1.9 ± 0.1 | |
| sc(C61 L4)Azo | 2.2 ± 0.4 | |
| Two azomal modifications, one involving the linker | sc(C61 L1)(C49∗ C62∗)Azo | 3.3 ± 0.4 |
| sc(C62 L1)(C49∗ C62∗)Azo | 3.6 ± 0.5 | |
| sc(C61 L2)(C49∗ C62∗)Azo | 1.8 ± 0.9 | |
| sc(C61 L3)(C49∗ C62∗)Azo | 4.0 ± 0.2 | |
| sc(C61 L4)(C49∗ C62∗)Azo | 6.3 ± 0.9 | |
| sc(C61 L4)(C49∗ C62∗)(F94)Azo | 15.7 ± 2.0 | |
| sc(C61 L4)(C49∗ C62∗)(F94)2Azo | 16.3 ± 1.3 | |
| Two modifications in the homodimer | wt(C49 C62)2Azo | 5.2 ± 0.5 |
| wt(C49 C62)2(F94)2Azo | 6.3 ± 0.2 |
All variants produced for this study are indicated with their cysteine substitutions (see Table S1 for further information). The ratio of activities of the azomal-modified proteins in the trans- and cis-state (± standard deviation) are given, as derived from plasmid cleavage assays after blue-light and UV-light preillumination, respectively, under otherwise identical conditions. The nomenclature of the variants is as follows: sc(C49 C62) carries cysteine residues in positions 49 and 62 in the N-terminal half of scPvuII, sc(C49* C62*) carries cysteine residues in the equivalent positions 49 and 62 in the C-terminal half of scPvuII, sc(C49 C62)2 carries cysteine residues in positions 49 and 62 in the N- and C-terminal halves, and sc(C49 C62)Azo is the sc(C49 C62) variant with an azomal cross-link between the two cysteine residues.
Activity of Azomal-Modified Homodimeric PvuII Variants in the cis- and trans-States of Azobenzene.
Given that the modified scPvuII variants with identical modifications in both the N- and C-terminal halves showed larger photoswitch effects than the variants that were only modified in one half, we wondered whether analogous homodimeric PvuII variants when modified in both subunits would show a similar photoswitch effect. We have analyzed this for two variants and found that the homodimeric PvuII variants showed a lower photoswitch effect (Table 1). Most likely the homodimeric variant is more flexible than the sc variant and can adapt better to conformational strains imposed by the cis vs. trans configurations of the azobenzene moiety.
Reversibility of Photoswitching.
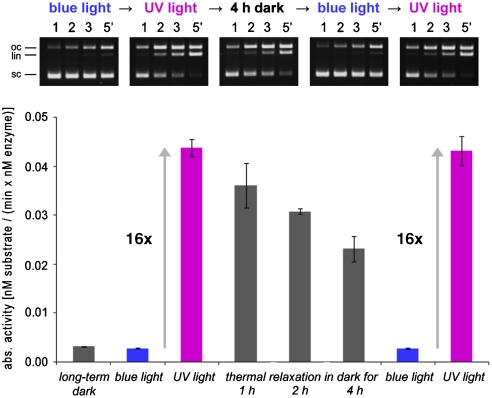
To demonstrate that photoswitching is reversible, the sc(C49 C62)2Azo variant, which shows a cis/trans activity ratio of approximately 7, was subjected to a blue → UV → blue → UV light illumination protocol, and the activity was measured under these conditions. As shown in Figs. 3 A and Fig. S2, the activity changes are fully reversible. The reversibility was also observed during the cleavage reaction by using alternating illumination of the reaction mixture with blue and UV light (Fig. 3 B). Experiments similar to those shown in Fig. 3 B were also carried out with the sc(C49 C62)2(F94)2Azo variant, which shows a cis/trans activity ratio of approximately 16 (Fig. S3). For this variant the thermal relaxation rate was measured by using a DNA cleavage experiment. Incubation in the dark after a period of UV-light illumination leads to the thermal relaxation of the enzyme-attached azobenzene moiety from the cis- to the trans-state with a half-life of approximately 6 h at 4 °C (Fig. 4). It is noteworthy that the relaxation from the more active cis state to the less active trans state suppresses off-site cleavage (Fig. S4).
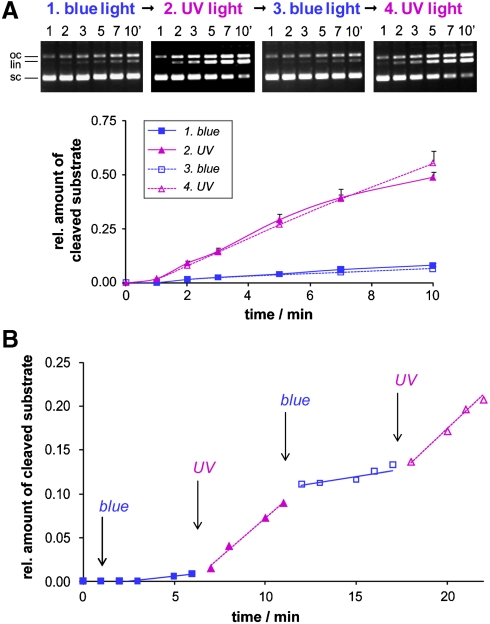
Fig. 3.
(A) Activity changes of sc(C49 C62)2Azo induced by preillumination with blue light → UV light → blue light → UV light as determined by a subsequent DNA cleavage assay (4 nM DNA, 0.5 nM enzyme) under illumination with blue or UV light, respectively. On the Top the gel electrophoretic analysis of samples withdrawn from the incubation mixture at defined time interval is shown, on the Bottom the corresponding activity vs. time of illumination profile. According to the quantitative analysis, sc(C49 C62)2Azo is seven times more active with the azobenzene moiety in the cis- than in the trans-configuration. (B) Reversible photoswitching of the DNA cleavage activity of sc(C49 C62)2Azo observed in situ. After preilluminating the enzyme with blue light, DNA cleavage (4 nM DNA, 0.24 nM enzyme) was followed in situ with variation of the illumination during cleavage (blue light 0–6 min, UV light 6–11 min, blue light 11–17 min, UV light 17–22 min). sc, oc, and li denote the supercoiled, open circular, and linear forms, respectively, of the plasmid DNA substrate.
Fig. 4.
Activity changes of sc(C49 C62)2(F94)2Azo. sc(C49 C62)2(F94)2Azo was illuminated with blue light → UV light → blue light → UV light, interrupted by a long incubation in the dark. DNA cleavage activity was determined by a DNA cleavage assay (4 nM DNA, 12 nM enzyme) under illumination with blue or UV light and during incubation in the dark, respectively. On the Top the gel electrophoretic analysis is shown, and on the Bottom the plot of the absolute activities as determined in the respective illumination states is shown.
Mechanistic Analysis of the Photoswitching Effect.
Since the largest photoswitching effect was observed with variants that had the modification close to the active site, we assumed that the effect was due to differences in V max rather than in K m. The Michaelis–Menten analysis showed that the sc(C49 C62)2Azo variant has a V max of 10.3 ± 0.9 nM min-1 and a K m of 25.4 ± 2.9 nM in the cis-state, and a V max of 2.2 ± 0.1 nM min-1 and a K m of 16.7 ± 1.3 nM in the trans-state, meaning that the effect observed is mainly due to differences in V max (Fig. 5).
Fig. 5.
Steady-state kinetic analysis of DNA cleavage by sc(C49 C62)2Azo. The Upper curve represents the initial rates of DNA cleavage measured under UV-light illumination, and the Lower curve represents the initial rates measured under blue-light illumination. The drawn-out curve is the result of a nonlinear regression analysis: Whereas sc(C49 C62)2Azo (cis) has a K m of 25.4 ± 2.9 nM and a V max of 10.3 ± 0.9 nM min-1, sc(C49 C62)2Azo (trans) has a K m of 16.7 ± 1.3 nM and a V max of 2.2 ± 0.1 nM min-1 (each with ± standard deviation).
Discussion
We have been interested in being able to control the activity of restriction and homing endonucleases for gene targeting in vivo to prevent off-site cleavage before or after the specific DNA cleavage event has taken place. We focused our studies on the restriction enzyme PvuII, for which we had shown previously that targeting to specific sites in genomic DNA in vitro can be achieved by fusing it to a triple-helix forming oligonucleotide (TFO) and thereby extending its six-base-pair recognition by 16 base pairs (44). However, for in vivo purposes it is necessary to control the nuclease activity such that the scPvuII-TFO fusion can be activated only after a stable triple helix has been formed and inactivated after double-strand cleavage has taken place. Temporal control of nuclease activity would be of interest also for other types of meganucleases, i.e., engineered homing endonucleases and zinc finger nucleases, to minimize the problem of off-site cleavage after long exposure of cells to these enzymes.
Azobenzene has been widely used for reversible switching by light between alternative conformations of peptides and proteins. Different approaches have been used to incorporate azobenzene into peptides and proteins. We have chosen the chemical modification approach using bifunctional azobenzene derivatives to cross-link selected amino acid residues (16).
If one wants to modulate the activity of an enzyme by attaching an azobenzene moiety to two positions and thereby cross-linking them, sites must be selected that are either close to the substrate binding site or the catalytic center, or are located at critical regions in the protein, which are likely to react in a sensitive manner when modified. Previous studies with azobenzene derivatives have shown that cross-linking two residues within an α-helix leads to a light-induced change in helical content (3, 13, 16, 18, 20, 25). Following this strategy, we introduced cross-linking sites in three α-helices, two of them located in the DNA binding subdomain (C89-C96 and C129-C133, respectively) and one of them in the catalytic subdomain (C147-C151) (Fig. 2 A). A similar strategy was followed by choosing azobenzene cross-linking sites that involve β-sheets and loop regions adjacent to the active site (D58, E68, and K70; see Fig. 2 A). Residue pairs C49-C62, C74-C110, and C66-C96 were chosen to test this strategy. Cysteines were also introduced in the linker between the two halves of scPvuII at different positions as anchor points for azobenzene attachment to be cross-linked with C61 and C62, located close to the active site residue D58. Cross-linking these sites with azomal could support catalysis with azobenzene in the cis-state as suggested by inspection of the scPvuII structure, whereas isomerization to the trans-state could “push” the β-strands harboring the catalytic center apart, which may result in disruption of the integrity of the catalytic center and cause inactivation of the enzymatic function.
The DNA cleavage activity of the azobenzene-modified scPvuII variants was measured under UV- and blue light to evaluate the photoswitch effect (Table 1). Variants with modification of α-helices close to the DNA binding site showed no or only small changes in activity upon specific illumination (cis/trans activity ratio < 1.5). Compared to small helical proteins (19, 20, 23), the “mechanical stress” due to the azobenzene isomerization in a large protein may not be sufficient to distort an α-helix embedded in a complex tertiary structure, or the conformational change in the protein induced by the azobenzene isomerization is small and compatible with preserving the activity of the enzyme. In contrast, the variants that are supposed to influence catalytic site formation show a larger effect (up to approximately 2-fold) in cleavage activity upon specific illumination, which could be increased (up to approximately 7-fold) by cross-linking also the equivalent positions in the C-terminal half of the protein (Table 1). We conclude from these results that regions close to the active site are sensitive to slight conformational distortions induced by the azobenzene isomerization.
It could be shown that additional amino acid substitutions that lower the activity of PvuII can increase the photoswitch effect (up to approximately 16-fold). Amino acid substitutions that affect the enzyme activity in binding (H83A) or catalysis (Y94F) seem to influence the cleavage rate in the cis- and the trans-state of the azobenzene moiety differently, i.e., the activity in the trans-state is more affected than the activity in the cis-state, thus the overall photoswitching effect is increased.
Introducing symmetric substitutions in both halves of the enzyme seem to give the best results, as in sc(C49 C62)2Azo and sc(C49 C62)2(F94)2Azo. The photoswitching effects in the corresponding homodimeric wtPvuII variants (wt(C49 C62)2Azo and wt(C49 C62)2(F94)2Azo) are not as pronounced as in the single-chain variant; presumably the homodimer might escape the catalytic site disturbance of the tethered azobenzene moiety more easily due to higher flexibility.
With two of the variants we demonstrated complete reversibility of the photoswitching: it could be shown that with a blue → UV → blue → UV, etc. light illumination protocol the increase in activity could be repeatedly obtained. Photoswitching is very fast, as can be seen when the change in the wavelength of illumination is carried out in the presence of DNA (i.e., during catalysis) and the DNA cleavage activity monitored in situ.
We wondered, whether the cis/trans effect is due to a change in K m or V max: Steady-state kinetic experiments demonstrate that the K m value in contrast to the V max value is largely unchanged upon switching from the cis- to the trans-state. This was expected for a variant that carries the cross-link close to the catalytic center.
In conclusion, we have succeeded in producing a photoswitchable restriction enzyme, which upon illumination exhibits an up to 16-fold increase in DNA cleavage activity by switching the configuration of the incorporated azobenzene moieties from trans to cis. The fact that we were able to increase the cis/trans effect by combination of several cross-links and introduction of additional amino acid substitutions suggests that it could be possible to eventually obtain a restriction enzyme that can be controlled by light, within the limits imposed by the photochemistry of azobenzene derivatives (45), which should make conjugates of restriction enzymes with additional DNA recognition modules including zinc fingers more suitable for in vivo gene targeting.
Materials and Methods
Protein Expression and Purification.
The mutated scPvuII-gene with the coding sequence for a C-terminal His6-tag was cloned into the expression vector pRIZ′ (39). pRIZ′ was used to transform the Escherichia coli strain XL10-Gold, which was previously transformed with the pLGM plasmid containing the gene coding for the PvuII methyltransferase. All genetic constructs were confirmed by DNA sequencing over the entire coding region. Cultures were grown at 37 °C to OD600 ≈ 0.7 and protein expression was induced by the addition of 1 mM IPTG. After 3 h of induction, cells were harvested by centrifugation, and the pellet was stored at -20 °C. The cell pellet was resuspended in 30 mM K-phosphate pH 7.4, 500 mM NaCl, 0.01% wt/vol Lubrol, 20 mM imidazol, 1 mM PMSF and lysed by sonication. Cell debris was removed by centrifugation (> 17,000 g) for 30 min at 4 °C. The recombinant His-tagged proteins were purified by affinity chromatography over Ni-NTA agarose (Qiagen) using 30 mM K-phosphate pH 7.4, 500 mM NaCl, 0.01% wt/vol Lubrol, 5 mM EDTA, 200 mM imidazol, 5 mM DTT. Fractions containing pure protein were dialyzed overnight at 4 °C against 20 mM K-phosphate buffer pH 7.4, containing 150 mM KCl, 50% vol/vol glycerol and stored at -20 °C. Protein purification was monitored by SDS-PAGE analysis, and protein concentration was determined by absorbance measurements at 280 nm. The accessibility of the introduced cysteines was checked by modification of the scPvuII variants with PEG-maleimide (Pierce), which results in a 2.3-kDa mass shift in SDS-PAGE analysis. DNA cleavage activity was analyzed by using supercoiled pAT153 tri plasmid DNA as substrate, which has a single PvuII site.
Linker Variations.
The four-amino-acid linker sequence (-GSGG-) in the original scPvuII was exchanged for another four-amino-acid linker (L1 ≡ -GSGC-) or for different eight-amino-acid linkers (L2 ≡ -GSGCGTGG-, L3 ≡ -GSGTGCGG-, and L4 ≡ -GSGTGSGC-), each with a cysteine residue for azomal attachment.
Protein Labeling with Azomal and Purification of Modified scPvuII Variants.
The scPvuII variants, diluted to 5 µM protein concentration with 50 mM K-phosphate pH 7.4, 500 mM NaCl, were incubated with a 2-fold molar excess of azomal (dissolved in DMSO and kept in the cis-configuration by illumination with UV light) over cysteine residues for 10 min at ambient temperature. Cross-linking yield was monitored by additional modification with a 50-fold molar excess (over cysteines) of PEG-maleimide to an aliquot of the reaction mixture. Purification of protein species with two cysteine residues cross-linked with azomal from protein species in which two neighboring cysteine residues each carry an azomal group was performed by adding a 125-fold molar excess (over cysteine residues) of thiolactic acid (which adds extra negative charges and thereby allows removing scPvuII variants with unreacted maleimide groups by anion exchange chromatography), incubation for 10 min at ambient temperature and subsequent centrifugation (5 min, 20,000 g, 4 °C), desalting [HiTrap Desalting 5-mL column (GE Healthcare)], and anion exchange chromatography [ResourceQ 1-mL column (GE Healthcare)]. The first azobenzene-containing peak was collected, dialyzed against dialysis buffer (see above), and analyzed for photoswitching effects by an activity assay under specific illumination. Protein concentration was estimated by SDS-PAGE analysis with silver staining using unlabeled protein as reference.
Photoisomerization of Azobenzene.
Photoisomerization of azobenzene to the trans-state was achieved by illumination with a blue-light source based on a light-emitting diode (470 nm ± 10 nm; 3-µmol photons m-2 s-1) for 2 min at a distance of 15 cm. For isomerization to the cis-state, the sample was illuminated for 4 min with a UV lamp (365 nm, 6 W) at a distance of 9 cm.
Activity Assay Under Specific Illumination.
Azomal-modified protein preparations were incubated with 4 nM supercoiled pAT153 tri plasmid DNA in cleavage buffer (10 mM Tris-HCl pH 7.2, 50 mM NaCl, 10 mM DTT, 0.1 mg/mL bovine serum albumin, 1 mM MgCl2) at 37 °C. Specific illumination was performed either by preillumination of the azomal-modified protein preparation followed by a cleavage reaction under specific illumination or by variation of blue- or UV-light illumination directly during the cleavage reaction. The linearized cleavage product was analyzed by agarose gel electrophoresis; gels were stained with ethidium bromide, and the fluorescence was measured with a BioDocAnalyze system (Biometra) and quantitated.
Steady-State Kinetic Analysis.
Michaelis–Menten analysis was performed by using a [α-32P]-labeled 229-base-pair PCR product as substrate. Substrate DNA (1.8 to 121.8 nM) was incubated with a 2.4-nM concentration of azomal-modified scPvuII-variants in cleavage buffer containing 10 mM MgCl2 under UV-light and blue-light illumination, respectively, at 37 °C. The reaction progress was analyzed after 0, 0.5, 1, 1.5, 2, 2.5, 3, 5, and 8 min by electrophoresis on 10% polyacrylamide gels; substrate and product bands were visualized with an InstantImager system (Packard) and quantified by using the InstantImager software. Initial rates were plotted vs. substrate concentration and analyzed in terms of K m and V max by nonlinear regression analysis.
Supplementary Material
Acknowledgments.
This work has been supported by the German Research Foundation and the Russian Foundation for Basic Research by the DFG-RFBR program “International Research Training Groups” (Grants GRK 1384, 08-04-91974) and the European Union (FP6-2006-15509-NEST). We thank Dr. Woolley for fruitful discussions, Dr. Silva and Dr. Friedhoff for critical reading of the manuscript, and Chun Mei Li for technical assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 1259.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909444107/DCSupplemental.
References
- 1.Gorostiza P, Isacoff EY. Optical switches for remote and noninvasive control of cell signaling. Science. 2008;322(5900):395–399. doi: 10.1126/science.1166022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pozhidaeva N, Cormier ME, Chaudhari A, Woolley GA. Reversible photocontrol of peptide helix content: Adjusting thermal stability of the cis state. Bioconjugate Chem. 2004;15(6):1297–1303. doi: 10.1021/bc049855h. [DOI] [PubMed] [Google Scholar]
- 3.Renner C, Moroder L. Azobenzene as conformational switch in model peptides. ChemBioChem. 2006;7(6):868–878. doi: 10.1002/cbic.200500531. [DOI] [PubMed] [Google Scholar]
- 4.Sadovski O, Beharry AA, Zhang F, Woolley GA. Spectral tuning of azobenzene photoswitches for biological applications. Angew Chem Int Ed Engl. 2009;48(8):1484–1486. doi: 10.1002/anie.200805013. [DOI] [PubMed] [Google Scholar]
- 5.Dong SL, et al. A photocontrolled beta-hairpin peptide. Chemistry. 2006;12(4):1114–1120. doi: 10.1002/chem.200500986. [DOI] [PubMed] [Google Scholar]
- 6.James DA, Burns DC, Woolley GA. Kinetic characterization of ribonuclease S mutants containing photoisomerizable phenylazophenylalanine residues. Protein Eng. 2001;14(12):983–991. doi: 10.1093/protein/14.12.983. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Karanicolas J, Yu C, Zhang Z, Woolley GA. Site-specific incorporation of photoisomerizable azobenzene groups into ribonuclease S. Bioorg Med Chem Lett. 1997;7(20):2677–2680. [Google Scholar]
- 8.Ueda T, Murayama K, Yamamoto T, Kimura S, Imanishi Y. Photo-regulation of hydrolysis activity of semisynthetic mutant phospholipases A2 replaced by non-natural aromatic amino acids. J Chem Soc Perk T. 1994;1(2):225–230. [Google Scholar]
- 9.Muranaka N, Hohsaka T, Sisido M. Photoswitching of peroxidase activity by position-specific incorporation of a photoisomerizable non-natural amino acid into horseradish peroxidase. FEBS Lett. 2002;510(1-2):10–12. doi: 10.1016/s0014-5793(01)03211-2. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama K, Endo M, Majima T. Photochemical regulation of the activity of an endonuclease BamHI using an azobenzene moiety incorporated site-selectively into the dimer interface. Chem Commun (Cambridge, UK) 2004;(21):2386–2387. doi: 10.1039/b409844g. [DOI] [PubMed] [Google Scholar]
- 11.Bose M, Groff D, Xie J, Brustad E, Schultz PG. The incorporation of a photoisomerizable amino acid into proteins in E. coli. J Am Chem Soc. 2006;128(2):388–389. doi: 10.1021/ja055467u. [DOI] [PubMed] [Google Scholar]
- 12.Mayer G, Heckel A. Biologically active molecules with a “light switch”. Angew Chem Int Ed Engl. 2006;45(30):4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 13.Woolley GA. Photocontrolling peptide alpha helices. Acc Chem Res. 2005;38(6):486–493. doi: 10.1021/ar040091v. [DOI] [PubMed] [Google Scholar]
- 14.Pearson D, Alexander N, Abell AD. Improved Photocontrol of alpha-Chymotrypsin Activity: Peptidomimetic Trifluoromethylketone Photoswitch Enzyme Inhibitors. Chemistry. 2008;14(24):7358–7365. doi: 10.1002/chem.200800082. [DOI] [PubMed] [Google Scholar]
- 15.Westmark P, Kelly J, Smith B. Photoregulation of enzyme activity. Photochromic, transition-state-analog inhibitors of cysteine and serine proteases. J Am Chem Soc. 1993;115(9):3416–3419. [Google Scholar]
- 16.Kumita JR, Smart OS, Woolley GA. Photo-control of helix content in a short peptide. Proc Natl Acad Sci USA. 2000;97(8):3803–3808. doi: 10.1073/pnas.97.8.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umeki N, et al. Incorporation of an azobenzene derivative into the energy transducing site of skeletal muscle myosin results in photo-induced conformational changes. J Biochem. 2004;136(6):839–846. doi: 10.1093/jb/mvh194. [DOI] [PubMed] [Google Scholar]
- 18.Flint DG, Kumita JR, Smart OS, Woolley GA. Using an azobenzene cross-linker to either increase or decrease peptide helix content upon trans-to-cis photoisomerization. Chem Biol. 2002;9(3):391–397. doi: 10.1016/s1074-5521(02)00109-6. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero L, et al. Photochemical regulation of DNA-binding specificity of MyoD. Angew Chem Int Ed Engl. 2005;44(47):7778–7782. doi: 10.1002/anie.200502666. [DOI] [PubMed] [Google Scholar]
- 20.Kumita JR, Flint DG, Woolley GA, Smart OS. Achieving photo-control of protein conformation and activity: Producing a photo-controlled leucine zipper. Faraday Discuss. 2002;122:89–103. doi: 10.1039/b200897a. discussion 171–190. [DOI] [PubMed] [Google Scholar]
- 21.Kusebauch U, et al. Photocontrolled folding and unfolding of a collagen triple helix. Angew Chem Int Ed Engl. 2006;45(42):7015–7018. doi: 10.1002/anie.200601432. [DOI] [PubMed] [Google Scholar]
- 22.Kusebauch U, Cadamuro SA, Musiol HJ, Moroder L, Renner C. Photocontrol of the collagen triple helix: Synthesis and conformational characterization of bis-cysteinyl collagenous peptides with an azobenzene clamp. Chemistry. 2007;13(10):2966–2973. doi: 10.1002/chem.200601162. [DOI] [PubMed] [Google Scholar]
- 23.Woolley GA, et al. Reversible photocontrol of DNA binding by a designed GCN4-bZIP protein. Biochemistry. 2006;45(19):6075–6084. doi: 10.1021/bi060142r. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Burns DC, Kumita JR, Smart OS, Woolley GA. A water-soluble azobenzene cross-linker for photocontrol of peptide conformation. Bioconjugate Chem. 2003;14(4):824–829. doi: 10.1021/bc0340161. [DOI] [PubMed] [Google Scholar]
- 25.Kumita JR, Flint DG, Smart OS, Woolley GA. Photo-control of peptide helix content by an azobenzene cross-linker: Steric interactions with underlying residues are not critical. Protein Eng. 2002;15(7):561–569. doi: 10.1093/protein/15.7.561. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, et al. Structure-based approach to the photocontrol of protein folding. J Am Chem Soc. 2009;131(6):2283–2289. doi: 10.1021/ja807938v. [DOI] [PubMed] [Google Scholar]
- 27.Jog PV, Gin MS. A light-gated synthetic ion channel. Org Lett. 2008;10(17):3693–3696. doi: 10.1021/ol8013045. [DOI] [PubMed] [Google Scholar]
- 28.Loudwig S, Bayley H. Photoisomerization of an individual azobenzene molecule in water: An on-off switch triggered by light at a fixed wavelength. J Am Chem Soc. 2006;128(38):12404–12405. doi: 10.1021/ja0642818. [DOI] [PubMed] [Google Scholar]
- 29.Muramatsu S, Kinbara K, Taguchi H, Ishii N, Aida T. Semibiological molecular machine with an implemented “AND” logic gate for regulation of protein folding. J Am Chem Soc. 2006;128(11):3764–3769. doi: 10.1021/ja057604t. [DOI] [PubMed] [Google Scholar]
- 30.Shishido H, Yamada MD, Kondo K, Maruta S. Photocontrol of calmodulin interaction with target peptides using azobenzene derivative. J Biochem. 2009;146(4):581–590. doi: 10.1093/jb/mvp107. [DOI] [PubMed] [Google Scholar]
- 31.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7(12):1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorostiza P, et al. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci USA. 2007;104(26):10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szobota S, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54(4):535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Volgraf M, et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2(1):47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Numano R, et al. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc Natl Acad Sci USA. 2009;106(16):6814–6819. doi: 10.1073/pnas.0811899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada MD, Nakajima Y, Maeda H, Maruta S. Photocontrol of kinesin ATPase activity using an azobenzene derivative. J Biochem. 2007;142(6):691–698. doi: 10.1093/jb/mvm183. [DOI] [PubMed] [Google Scholar]
- 37.Willner I, Rubin S, Riklin A. Photoregulation of papain activity through anchoring photochromic azo groups to the enzyme backbone. J Am Chem Soc. 1991;113(9):3321–3325. [Google Scholar]
- 38.Pingoud A, Silva GH. Precision genome surgery. Nat Biotechnol. 2007;25(7):743–744. doi: 10.1038/nbt0707-743. [DOI] [PubMed] [Google Scholar]
- 39.Simoncsits A, Tjornhammar ML, Rasko T, Kiss A, Pongor S. Covalent joining of the subunits of a homodimeric type II restriction endonuclease: Single-chain PvuII endonuclease. J Mol Biol. 2001;309(1):89–97. doi: 10.1006/jmbi.2001.4651. [DOI] [PubMed] [Google Scholar]
- 40.Meramveliotaki C, et al. Purification, crystallization, x-ray diffraction analysis and phasing of an engineered single-chain PvuII restriction endonuclease. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63(10):836–838. doi: 10.1107/S1744309107040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolley GA, Lee ES, Zhang F. sGAL: A computational method for finding surface exposed sites in proteins suitable for Cys-mediated cross-linking. Bioinformatics. 2006;22(24):3101–3102. doi: 10.1093/bioinformatics/btl530. [DOI] [PubMed] [Google Scholar]
- 42.Nastri HG, Evans PD, Walker IH, Riggs PD. Catalytic and DNA binding properties of PvuII restriction endonuclease mutants. J Biol Chem. 1997;272(41):25761–25767. doi: 10.1074/jbc.272.41.25761. [DOI] [PubMed] [Google Scholar]
- 43.Spyridaki A, et al. Structural and biochemical characterization of a new Mg(2 + ) binding site near Tyr94 in the restriction endonuclease PvuII. J Mol Biol. 2003;331(2):395–406. doi: 10.1016/s0022-2836(03)00692-2. [DOI] [PubMed] [Google Scholar]
- 44.Eisenschmidt K, et al. Developing a programmed restriction endonuclease for highly specific DNA cleavage. Nucleic Acids Res. 2005;33(22):7039–7047. doi: 10.1093/nar/gki1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borisenko V, Woolley GA. Reversibility of conformational switching in light-sensitive peptides. J Photochem Photobiol A. 2005;173(1):21–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.