Abstract
Generation of cultured human cells stably expressing one or more recombinant gene sequences is a widely used approach in biomedical research, biotechnology, and drug development. Conventional methods are not efficient and have severe limitations especially when engineering cells to coexpress multiple transgenes or multiprotein complexes. In this report, we harnessed the highly efficient, nonviral, and plasmid-based piggyBac transposon system to enable concurrent genomic integration of multiple independent transposons harboring distinct protein-coding DNA sequences. Flow cytometry of cell clones derived from a single multiplexed transfection demonstrated approximately 60% (three transposons) or approximately 30% (four transposons) stable coexpression of all delivered transgenes with selection for a single marker transposon. We validated multiplexed piggyBac transposon delivery by coexpressing large transgenes encoding a multisubunit neuronal voltage-gated sodium channel (SCN1A) containing a pore-forming subunit and two accessory subunits while using two additional genes for selection. Previously unobtainable robust sodium current was demonstrated through 38 passages, suitable for use on an automated high-throughput electrophysiology platform. Cotransfection of three large (up to 10.8 kb) piggyBac transposons generated a heterozygous SCN1A stable cell line expressing two separate alleles of the pore-forming subunit and two accessory subunits (total of four sodium channel subunits) with robust functional expression. We conclude that the piggyBac transposon system can be used to perform multiplexed stable gene transfer in cultured human cells, and this technology may be valuable for applications requiring concurrent expression of multiprotein complexes.
Keywords: piggyBac, SCN1A, SCN1B, SCN2B, sodium channel
Stable single-gene transfer into cultured cells has revolutionized the study of human disease, provided critical cell models needed for high-throughput screening in drug development, and enabled large-scale production of recombinant proteins and viruses. However, a technology does not currently exist that is capable of simple but efficient engineering of cells to stably coexpress multiple transgenes.
Transposon systems mediate stable integration of exogenous DNA elements into a host cell genome, and have been harnessed as gene transfer vectors for preclinical gene therapy studies and gene discovery applications in mammalian cells (1 –4). The two most commonly used transposon systems for gene transfer into human cells include sleeping beauty (SB) and piggyBac (1, 5, 6). The lepidopteron piggyBac transposable element was initially discovered in mutant Baculovirus strains (7). Subsequent work divided the piggyBac element into the piggyBac transposase that facilitates genomic integration, and 5′ and 3′ inverted repeat elements (5′IR and 3′IR, respectively) delimiting the transposon cassette for integration (8 –10). This arrangement has been harnessed to introduce transgenes between the inverted repeats. Trans introduction of the transposase and transposon results in an efficient “cut and paste” transposition of the transgene into the genome at TTAA nucleotide elements (7, 11).
We and others have previously reported on the high efficiency of the piggyBac system and its ability to mediate genomic integration of transposon cassettes in human and mouse cells (3, 12 –15). Recent investigations have shown the number of piggyBac integrations to be titratable when delivering a single transposon. Transposon elements can allow many (15 or more integrations events) per cell whereas fewer integration events (down to one or two per cell) can be achieved by limiting transposon or transposase availability (13 –15). We hypothesized that the ability of piggyBac to integrate multiple transposon copies per cell could enable the concurrent integration of multiple independent transposons each carrying distinct transgenes as opposed to the typical paradigm of promoting expression of a single transgene.
In this study, we quantified the ability of piggyBac to mediate the stable integration of up to four independent transposons concurrently in human cells following a single transfection. We then harnessed this technology to generate stable cell lines expressing heteromultimeric voltage-gated sodium channels that exhibited robust functional expression of up to four different subunits and were amenable for high-throughput electrophysiological analysis. This technology has potential to enable genetic engineering of cellular models for use in drug discovery applications targeting multiprotein complexes, for simultaneous production of multiple recombinant proteins, and for treatment of genetic disorders that require the stable delivery of multiple genes.
Results
Concurrent Stable Gene Expression from Multiple piggyBac Transposons.
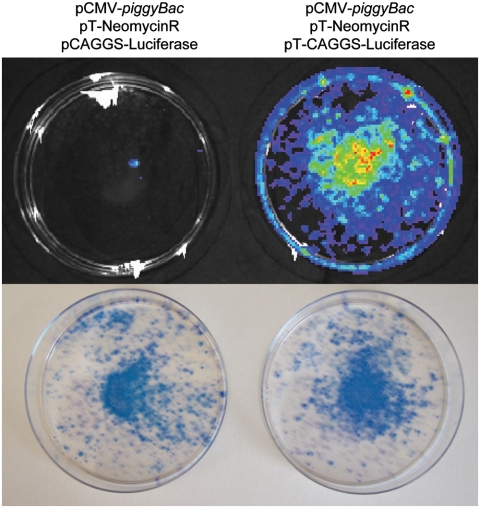
We tested the capability of the piggyBac system to concurrently and stably transfect cells with more than one transposon. HEK-293 cells were transfected with the piggyBac transposase (pCMV-piggyBac), pT-NeomycinR encoding resistance to the antibiotic G418, and either pT-Luciferase that contains the gene for luciferase but does not confer antibiotic resistance or a nontransposon plasmid encoding luciferase (pCAGGS-Luciferase) as a negative control. Transfected cells were selected for three weeks with G418 to isolate cells stably expressing pT-NeomycinR and were then assayed for luciferase-catalyzed light generation. G418-resistant cells that had been cotransfected with pCAGGS-Luciferase produced only two colonies exhibiting luciferase expression, indicating that piggyBac transposition of pT-NeomycinR did not facilitate efficient stable expression from the nontransposon pCAGGS-Luciferase (Fig. 1, Top panel, Left plate). By contrast, most G418-resistant cell colonies cotransfected with pT-Luciferase readily produced light upon luciferin application presumably due to genomic integration of the luciferase transposon (Fig. 1, Top panel, Right plate). We used methylene blue to stain the plates after imaging to demonstrate that similar numbers of G418 resistant cells existed on both plates (Fig. 1, Bottom panel). These findings provide a proof-of-principle that piggyBac can facilitate the concurrent stable expression of more than one separate transposon in the same cells while only selecting for one transposon.
Fig. 1.
Simultaneous stable integration of two piggyBac transposons. Luciferase assay comparing the triple transfection of plasmids encoding the piggyBac transposase (pCMV-piggyBac) and pT-NeomycinR with either a plasmid encoding a luciferase cDNA (pCAGGS-Luciferase, Left) or a luciferase transposon (pT-Luciferase) (Right). Transfected HEK-293 cells were cultured for 2–3 weeks with G418 to select for stable pT-NeomycinR expression and then assayed for luciferase expression (Top). Following imaging, plates were stained with methylene blue to visualize the cells on the plates (Bottom). Shown are images representative of three independent experiments.
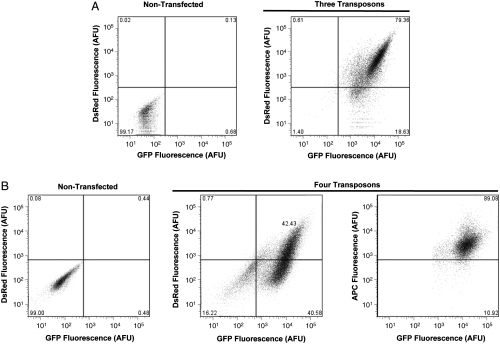
We next evaluated the efficiency of piggyBac-mediated transposition of three to four independent transposons carrying distinct transgenes. For these experiments, we used three different reporter genes including enhanced green fluorescent protein (eGFP), the Discosoma red fluorescent protein (DsRed), and the cluster of differentiation 8 (CD8) cell surface receptor. To quantify efficiency of piggyBac in stably transfecting cells with three independent transposons, HEK-293 cells were cotransfected with pCMV-piggyBac, pT-NeomycinR, pT-eGFP, and pT-DsRed. Importantly, pT-eGFP, pT-DsRed, and pT-CD8 lack neomycin resistance. Transfected cells were selected for 3 weeks with G418 to isolate cells stably expressing pT-NeomycinR, and then flow cytometry was used to quantify eGFP and DsRed expression. As a negative control, nontransfected and nonselected HEK-293 cells were used to establish the thresholds for fluorescent detection. Fig. 2 A Left illustrates that nontransfected HEK-293 cells are detected within the lower left quadrant of the plot corresponding to the absence of measurable eGFP or DsRed fluorescence. By contrast, in Fig. 2 A Right, 79% of G418-resistant cells expressed both eGFP and DsRed (upper right quadrant). For three independent experiments, flow cytometry analysis revealed that 60 ± 8% (mean ± SD) of single G418-resistant cells coexpressed eGFP and DsRed, indicating that piggyBac mediated efficient concurrent expression from three transposons.
Fig. 2.
Concurrent integration of three or four piggyBac transposons. Flow cytometry analysis comparing the delivery and stable coexpression of either three or four piggyBac transposons. (A) HEK-293 cells were cotransfected with pCMV-piggyBac, pT-NeomycinR, pT-GFP, and pT-DsRed. Transfected cells were selected for pT-NeomycinR expression for 3 weeks with G418. The dual expression of pT-GFP and pT-DsRed was detected in approximately 80% of the G418-resistant cells (Right panel, Upper Right quadrant). Nontransfected and nonselected HEK-293 cells were used to determine the thresholds for fluorescent detection (Left panel, Lower Left quadrant). (B) HEK-293 cells were cotransfected with pCMV-piggyBac, pT-NeomycinR, pT-GFP, pT-DsRed, and pT-CD8. Transfected cells were selected for pT-NeomycinR expression for 3 weeks with G418. The dual expression of pT-GFP and pT-DsRed was detected in approximately ∼42% of the G418 resistant cells (center panel, upper right quadrant). Dual GFP/DsRed positive cells were analyzed for CD8 expression using an APC conjugated anti-CD8 antibody. Approximately 90% of the GFP/DsRed positive cells also expressed CD8 (right panel, upper quadarant), indicating that 30% of analyzed G418-resistant cells were positive for GFP/DsRed/CD8. Nontransfected and nonselected HEK-293 cells were used to determine the thresholds for fluorescent detection (Left panel, Lower Left quadrant). Panels A and B illustrate representative experiments (n = 3), and only singlet cells were subjected to analysis.
Similarly, we determined the efficiency of piggyBac in enabling stable expression from four independent transposons. In these experiments, HEK-293 cells were cotransfected with pCMV-piggyBac, pT-NeomycinR, pT-eGFP, pT-DsRed, and pT-CD8 and then selected for 3 weeks with G418 before quantifying eGFP, DsRed, and CD8 expression simultaneously with flow cytometry. Fig. 2 B illustrates that 43% of the cells expressed both eGFP and DsRed (upper right quadrant). Analysis of eGFP/DsRed positive cells revealed that 89% also expressed CD8. For three independent experiments, 31 ± 3% (mean ± SD) of G418-resistant cells coexpressed eGFP, DsRed, and CD8, proving simultaneous expression from four separate transposons in human cells using only a single selectable marker.
We attempted to increase the efficiency of multiple transgene expression by decreasing the amount of the selectable transposon (100 ng), while maintaining 1 μg each of the reporter gene transposons (pT-eGFP, pT-DsRed, and pT-CD8). However, this only resulted in fewer G418-resistant cells, and the percentage of resistant cells stably expressing the three reporter genes (30 ± 6%) was not significantly different.
Transposon-Mediated Stable Expression of a Heteromultimeric Protein Complex.
To evaluate the utility of piggyBac mediated multitransposon delivery, we used this approach to create cell lines stably expressing a human brain voltage-gated sodium channel complex. Sodium channels are heteromultimeric complexes comprised of three component proteins including a pore-forming α-subunit (SCN1A) and two accessory subunits (SCN1B, SCN2B). We constructed two SCN1A transposons in which the sodium channel coding sequence was fused in-frame to either a triple FLAG epitope (pT-SCN1A:3XFLAG) or the fluorescent protein Venus (pT-SCN1A:Venus) and accompanied by a neomycin resistance gene driven by a separate promoter (Fig. S1). We also constructed a separate transposon (pT-SCN1B:cMyc-IRES-SCN2B:HA) containing a bicistronic cassette encoding two epitope tagged accessory subunits and a puromycin resistance gene (Fig. S1).
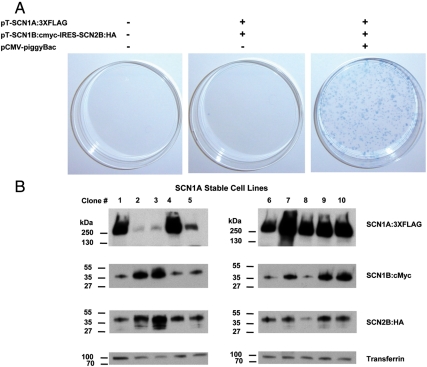
HEK-293 cells were cotransfected with pCMV-piggyBac, pT-SCN1A:3XFLAG, and pT-SCN1B:cMyc-IRES-SCN2B:HA then subjected to dual selection with G418 and puromycin. Only cells transfected with all three plasmids exhibited large numbers of resistant clones (Fig. 3). Omitting the transposase plasmid (middle culture dish in Fig. 3 A) in what would be the traditional method of constructing stable cell lines was substantially less efficient than piggyBac transposition. When plating equivalent numbers of cells (100,000), cotransfection with transposase yielded 650 clones resistant to both G418 and puromycin. In contrast, transfection without transposase yielded a single colony, indicating a > 600-fold increase in generating stable cells expressing the sodium channel and accessory subunits when using piggyBac. These results demonstrate the substantial increase in efficiency realized in generating multiple-subunit stable cell lines using the piggyBac transposon system.
Fig. 3.
Creation of a SCN1A stable cell line by simultaneous genomic integration of two piggyBac transposons. Efficient stable integration of sodium channel transgenes by piggyBac transposition. (A) Methylene blue staining of HEK-293 colonies following transfection with the indicated plasmids and selection with G418/Puromycin for 3 weeks. Colonies stably coexpressing pT-SCN1A:3XFLAG and pT-SCN1B:cMyc-IRES-SCN2B:HA were able to grow in the presence of G418/Puromycin selection. (B) Protein expression from each piggyBac transposon confirmed for ten selected clones by Western blot analysis. Transgene specific proteins were detected by primary antibodies directed against the appropriate epitope tag (FLAG, cMyc, or HA). Detection of the endogenous protein transferrin confirms protein loading.
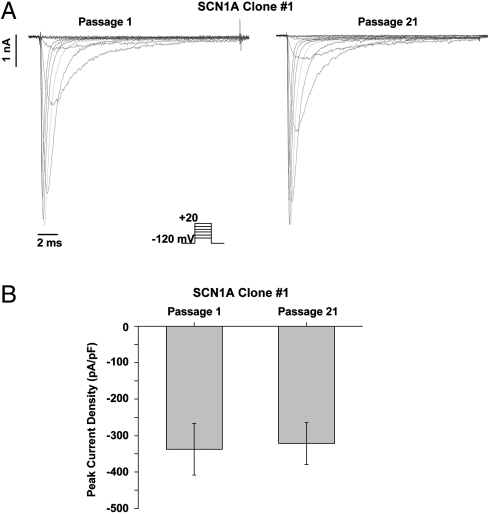
We verified protein expression resulting from piggyBac transposition of pT-SCN1A:3XFLAG and pT-SCN1B:cMyc-IRES-SCN2B:HA by immunoblot analysis using antibodies directed against the tag epitopes linked to each distinct sodium channel subunit (Fig. 3 B). Detection of the endogenous protein transferrin served as a gel loading control. In addition to G418 and puromycin resistance, each of 10 selected clones also expressed SCN1A, SCN1B, and SCN2B proteins, demonstrating simultaneous stable expression of five transgenes using piggyBac. A saturating exposure was used in Fig. 3 B to illustrate the variable levels of protein expression for each clone. Varying levels of protein expression may reflect differences in the number of transposon integration events or positional effects at the site of integration. Protein expression from piggyBac integrated transposons in clone 1 was stable over 38 passages for each of the sodium channel subunits (Fig. S2). Clone 1 was used for subsequent electrophysiology experiments.
Conventional whole-cell patch-clamp recording was used to functionally evaluate the sodium channel stable cell line. Fig. 4 A illustrates representative current recordings in response to step depolarizations from a holding potential of -120 mV to between -80 and +20 mV. Sodium currents were recorded at passages 1 (Left) and 21 (Right), and Fig. 4 B illustrates the average peak current density recorded at -10 mV. This analysis normalizes peak currents by cell capacitance to allow direct comparison of the channel expression level in cells of differing sizes. The current density recorded at passages 1 and 21 were not different (-338 ± 71 pA/pF, n = 5 versus -321 ± 58 pA/pF, n = 5, respectively), reflecting the high temporal stability of functional protein expression. Moreover, the voltage dependence of sodium channel activation was not changed following 20 passages (passage 1 V1/2 = -23.7 ± 1.4 mV, k = 7.3 ± 0.4, n = 5; compared to passage 21 V1/2 = -23.8 ± 0.9 mV, k = 6.3 ± 0.3, n = 5).
Fig. 4.
Functional expression from SCN1A transposons exhibits high temporal stability. Functional analysis of SCN1A clone 1 by conventional whole-cell patch-clamp recording of voltage-gated sodium currents at passage number 1 and 21. (A) Representative voltage-gated sodium currents activated by voltage steps to between -80 and +20 mV from a holding potential of -120 mV (see pulse protocol Inset). The current in response to the -10 mV step is shown in red. (B) Average sodium current density recorded at -10 mV plotted at passage number 1 and 21 (n = 6).
High-Throughput Analysis of Sodium Channel Cell Lines.
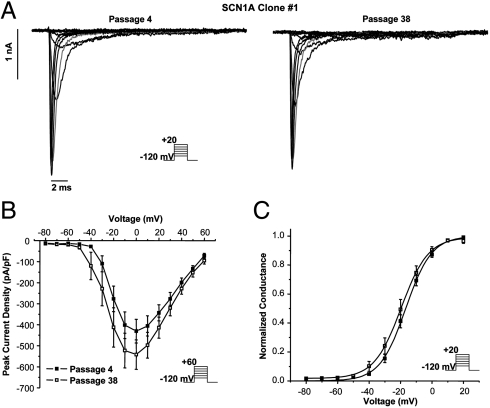
The utility of a sodium channel stable cell line created using transposon technology for high-throughput analysis was investigated using an automated planar patch-clamp system. Fig. 5 A illustrates a representative family of sodium current recordings at passages 4 and 38 in response to step depolarizations from a holding potential of -120 mV to between -80 and +20 mV. The average current density recorded at passage 4 was -402 ± 64 pA/pF (n = 27), and this was not significantly different from that measured by traditional patch clamp. The peak current density-voltage relationship (Fig. 5 B) and activation curve (Fig. 5 C) for the voltage-gated sodium current expressed by stable cells at passage 4 are indistinguishable from data collected using manual patch clamp. The sodium current density measured at passage 38 was 522 ± 82 pA/pF (V1/2 = -20.3 ± 2.2 and k = 6.1 ± 0.5, n = 21), further supporting the high temporal stability of -piggyBac-mediated integration. There were no statistically significant differences between data recorded at passage 4 and passage 38 (current density or activation; Table S1). These data demonstrate that the stable cell line generates voltage-gated sodium currents that are suitable for high-throughput electrophysiology and are potentially useful for drug discovery.
Fig. 5.
High-throughput planar patch-clamp analysis of SCN1A transposon expression. Voltage-gated sodium currents from SCN1A clone 1 were measured at passages 4 and 38 using an automated, high-throughput electrophysiology workstation (Nanion Patchliner). (A) Representative voltage-gated sodium currents activated by voltage steps to between -80 and +20 mV from a holding potential of -120 mV (see pulse protocol Inset). The current in response to the -10 mV step is shown in red. (B) Peak current density elicited by test pulses to various potentials and normalized to cell capacitance. (C) Voltage dependence of channel activation measured during voltage steps to between -80 and +20 mV. There were no statistical differences in current density and activation V1/2 between data recorded at passage 4 and passage 38 (n = 27 and n = 21, respectively).
Construction of a Heterozygous Sodium Channel Cell Line.
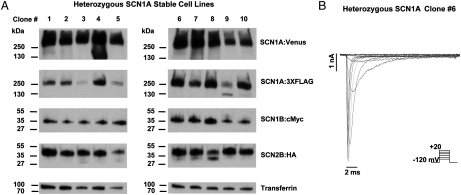
We employed piggyBac transposons to create cell lines stably expressing two alleles of SCN1A by concurrent integration of three separate large transposons. HEK-293 cells were cotransfected with pCMV-piggyBac, pT-SCN1A:Venus, pT-SCN1A:3XFLAG, and pT-SCN1B:cMyc-IRES-SCN2B:HA. Fig. 6 A illustrates immunoblot detection of the four component proteins expressed in G418/puromycin-resistant cells visualized with antibodies directed against the epitopes Venus, FLAG, c-Myc, and HA. Importantly, genomic integration of either pT-SCN1A:Venus or pT-SCN1A:3XFLAG would confer resistance to G418, but due to the efficiency of the piggyBac system, every assayed clone expressed both SCN1A alleles. Electrophysiological recording experiments demonstrated robust sodium currents in the cells (Fig. 6). Moreover, these data demonstrate the stable expression of at least six transgenes from 27 kb of DNA encoded in three separate transposons stably transfected into the genome of human cells. These data demonstrate that piggyBac is capable of stably transfecting cell lines with multiple transgenes, including those encoded by large cDNAs.
Fig. 6.
Creation of a heterozygous SCN1A stable cell line by simultaneous genomic integration of three piggyBac transposons. Efficient stable integration of SCN1A transgenes by piggyBac transposition. Cells stably coexpressing either pT-SCN1A:Venus or pT-SCN1A:3XFLAG with pT-SCN1B:cMyc-IRES-SCN2B:HA transposons are able to grow in the presence of G418/Puromycin selection. (A) Expression from each piggyBac transposon confirmed for ten selected clones by Western blot analysis. Transgene specific proteins were detected by primary antibodies directed against the appropriate epitope tag (Venus, FLAG, cMyc, or HA). Note that every clone expresses both alleles of SCN1A. Detection of the endogenous protein transferrin confirms protein loading. (B) Functional analysis of heterozygous SCN1A clone 6 by conventional whole-cell patch-clamp recording of voltage-gated sodium currents. Representative currents activated by voltage steps to between -80 and +20 mV from a holding potential of -120 mV (see pulse protocol Inset). The current in response to the -10 mV step is shown in red. The average current density for the -10 mV step recorded at passage 1 was -489 ± 53 pA/pF (n = 5). Fitting an activation curve with a Boltzmann equation generated the average fit parameters of V1/2 = -23.6 ± 1.7 and k = 5.7 ± 0.4 (n = 5).
Discussion
In this study, we demonstrated that the plasmid-based piggyBac transposon system can facilitate efficient and concurrent genomic integration of multiple gene segments in human cells. Our results have potential applicability to the creation of cell lines with stable expression of multiprotein complexes useful for drug discovery, developing complex cellular models, and possibly for therapeutic gene transfer when more than one transgene is required.
Specifically, we report on the efficient and stable integration of up to four separate piggyBac transposons in human HEK-293 cells. In experiments with reporter genes, having only one of the transposons (pT-NeomycinR) confer resistance to a selectable marker was sufficient to isolate cells stably expressing the other transposons that lacked a means for selection. Our experiments with three independent transposons demonstrated that after selection, approximately 60% of the cells stably express transgenes encoded by the three transposons. As expected, the efficiency decreases when selecting for stable expression of four independent transposons. However, the ability to efficiently stably integrate four independent DNA segments from a single transfection is unprecedented. Moreover, the vectors employed in this study used bicistronic IRES elements. Therefore, our data suggest the possibility of introducing up to eight transgenes on four independent transposon cassettes. The use of polycistronic transposons for generating induced pluripotent stem cells has recently been demonstrated (2, 4). We anticipate the number of independent transposons might be increased further by implementing innovative cell sorting methodologies to isolate the clonal populations of interest.
Comparisons with Other Gene Transfer Strategies.
In comparison to other available systems such as other transposons and viral vectors, piggyBac may offer distinct advantages. More than 10 years of research has contributed to engineering SB transposase to produce several hyperactive variants including SB100X that in some cases exhibits 100-fold more activity than the original SB (16). However, the capabilities of SB100X to carry large DNA segments and mediate multiple transposon integration are not known. Research efforts are also underway to enhance piggyBac transposase activity and this should further improve efficiency in multigene transfer (17).
Retroviral and lentiviral vectors have also been used for codelivery of multiple genes to cells (18, 19). However, neither of these viral vectors can integrate DNA segments as large as 10 kb, and coinfection with multiple different viruses is required for integration of multiple transgenes. A plasmid-based system has other advantages compared to viral vectors with respect to shorter production time and lower cost.
Applications for Multigene Integration.
Multiplexed gene integration has potential for application in the creation of novel cell-based assays for drug discovery when the target is a multiprotein complex such as a G-protein coupled receptor (GPCR). As a single class of proteins, GPCRs represent targets for approximately 30% of all marketed drugs (20). Further, GPCR signaling is important in all cells, but developing cell type specific pathways within a heterologous system can be complex and difficult. Often, commonly used GPCR cell culture models lack critical components necessary to reconstitute the appropriate signaling pathways. These missing components can compromise the drug response and/or prevent the discovery of novel agonists and antagonists. Therefore, the ability to use a plasmid-based system to create stable cell lines expressing multiple transgenes may improve upon current drug discovery methodology (21).
Multisubunit neurotransmitter receptors (e.g., GABAA receptors) and ion channels (e.g., voltage-gated sodium, potassium, and calcium channels) are also important therapeutic targets. Discovery of novel compounds that target these proteins has been hampered by low-throughput assay methods and difficulty in reconstituting multiprotein complexes. Recent advances in high-throughput electrophysiology are beginning to address the first of these bottlenecks (22). However, traditional transfection methods are unable to efficiently generate stable cell reagents when the target is comprised of multiple protein subunits. The piggyBac transposon system represents a paradigm shift that may overcome this bottleneck in high-throughput drug discovery for ligand and voltage-gated ion channel targets.
In this study, we demonstrated that the piggyBac system was able to stably reconstitute a three subunit sodium channel complex. Creation of this cell line required only a single transfection followed by approximately 5 weeks of colony selection and expansion. In addition, sodium channel activity exhibited by this cell line was more robust than typically observed for transient transfection experiments (23). Moreover, the level of the sodium currents were stable for more than 35 passages and were easily measured using a commercial high-throughput electrophysiology system. Traditional methods of generating this cell line would have required multiple rounds of transfection and selection with a total time commitment of many months. Our strategy employing piggyBac transposon technology may also enable creation of biallelic cell models to mimic certain genetic conditions as we have demonstrated by establishing cells with stable expression of two sodium channels combined with their associated proteins. This cell model would have been difficult, if not impossible, to create with traditional methods. From these illustrations, we conclude that piggyBac offers distinct advantages over traditional methodologies for the delivery and stable genomic integration of multiprotein complexes.
Materials and Methods
For descriptions of the materials and methods used, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
This work was supported by grants from the National Institutes of Health (NS032387 to A.L.G.) and in part by a career development award from the Department of Veterans Affairs to M.H.W.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910383107/DCSupplemental.
References
- 1.Ivics Z, Hackett PB, Plasterk RH, Izsvak Z. Molecular reconstruction of sleeping beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaji K, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cary LC, et al. Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology. 1989;172:156–169. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- 6.Fraser MJ, Cary L, Boonvisudhi K, Wang HG. Assay for movement of Lepidopteran transposon IFP2 in insect cells using a baculovirus genome as a target DNA. Virology. 1995;211:397–407. doi: 10.1006/viro.1995.1422. [DOI] [PubMed] [Google Scholar]
- 7.Fraser MJ, Ciszczon T, Elick T, Bauser C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol Biol. 1996;5:141–151. doi: 10.1111/j.1365-2583.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 8.Elick TA, Lobo N, Fraser MJ., Jr Analysis of the cis-acting DNA elements required for piggyBac transposable element excision. Mol Gen Genet. 1997;255:605–610. doi: 10.1007/s004380050534. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Lobo N, Bauser CA, Fraser MJ., Jr The minimum internal and external sequence requirements for transposition of the eukaryotic transformation vector piggyBac. Mol Genet Genomics. 2001;266:190–198. doi: 10.1007/s004380100525. [DOI] [PubMed] [Google Scholar]
- 10.Li X, et al. piggyBac internal sequences are necessary for efficient transformation of target genomes. Insect Mol Biol. 2005;14:17–30. doi: 10.1111/j.1365-2583.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 11.Bauser CA, Elick TA, Fraser MJ. Proteins from nuclear extracts of two lepidopteran cell lines recognize the ends of TTAA-specific transposons piggyBac and tagalong. Insect Mol Biol. 1999;8:223–230. doi: 10.1046/j.1365-2583.1999.820223.x. [DOI] [PubMed] [Google Scholar]
- 12.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MH, Coates CJ, George AL., Jr PiggyBac Transposon-mediated Gene Transfer in Human Cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Bradley A, Huang Y. A piggyBac transposon-based genome-wide library of insertionally mutated Blm-deficient murine ES cells. Genome Res. 2009;4:667–73. doi: 10.1101/gr.085621.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mates L, et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet. 2009;6:753–61. doi: 10.1038/ng.343. [DOI] [PubMed] [Google Scholar]
- 17.Cadinanos J, Bradley A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 2007;35:e87. doi: 10.1093/nar/gkm446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Yu JY, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Siehler S. Cell-based assays in GPCR drug discovery. Biotechnol J. 2008;3:471–483. doi: 10.1002/biot.200800001. [DOI] [PubMed] [Google Scholar]
- 21.Jones JO, Diamond MI. Design and implementation of cell-based assays to model human disease. ACS Chem Biol. 2007;2:718–724. doi: 10.1021/cb700177u. [DOI] [PubMed] [Google Scholar]
- 22.Milligan CJ, et al. Robotic multiwell planar patch-clamp for native and primary mammalian cells. Nat Protoc. 2009;4:244–255. doi: 10.1038/nprot.2008.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahlig KM, et al. Divergent sodium channel defects in familial hemiplegic migraine. Proc Natl Acad Sci USA. 2008;105:9799–9804. doi: 10.1073/pnas.0711717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.