Abstract
The human histone H3 variant, CENP-A, replaces the conventional histone H3 in centromeric chromatin and, together with centromere-specific DNA-binding factors, directs the assembly of the kinetochore. We purified the prenucelosomal e-CENP-A complex. We found that HJURP, a member of the complex, was required for cell cycle specific targeting of CENP-A to centromeres. HJURP facilitated efficient deposition of CENP-A/H4 tetramers to naked DNA in vitro. Bacterially expressed HJURP binds at a stoichiometric ratio to the CENP-A/H4 tetramer but not to the H3/H4 tetramer. The binding occurred through a conserved HJURP short N-terminal domain, termed CBD. The novel characteristic identified in vertebrates that we named TLTY box of CBD, was essential for formation of the HJURP-CENP-A/H4 complex. Our data identified HJURP as a vertebrate CENP-A chaperone and dissected its mode of interactions with CENP-A.
Keywords: histone chaperone, histone variant
The centromere is a specialized region on eukaryotic chromosomes required for the assembly of active kinetochore. The centromere is of vital importance for genetic stability. Defects in meiotic chromosomes segregation may lead to aneuploidy and tumor formation (1).
The structure of the centromeres, despite the many efforts invested, remains elusive (2, 3). CENP-A (termed also CenH3, (2, 3), a centromere-specific histone H3 variant, is found in all eukaryotes (4) and is required for the assembly and the maintenance of active centromeres (5 –8). Human CENP-A shows > 60% sequence identity with the C-terminal histone fold domain of H3, but its N-terminal tail is highly divergent (4, 9, 10). A domain in the histone fold of CENP-A, termed CATD, is required for the targeting of the newly synthesized protein to the centromeres (9, 11 –13). Substitution of the CATD into canonical H3 is sufficient to replace the essential function of CENP-A suggesting that any specific CENP-A chaperone would recognize the (CENP-A/H4)2 tetramer via the CATD and deliver it to centromeric chromatin (14).
A fundamental question in centromere biology is how CENP-A is specifically deposited to and maintained on centromeric DNA. Recent studies have identified several factors that affect CENP-A localization but their precise roles in this process remain to be determined. In Drosophila, p55 (RbAp48) was found to be associated with CenH3/H4 tetramer and to facilitate its deposition to DNA (15). A genome wide RNAi screen for defects in Drosophila CenH3 localization at centromeres identified CAL1 and CENP-C as essential proteins for assembly of newly synthesized CenH3 (16). In S. pombe Mis 6 and Ams2 proteins are involved in CenH3 localization (17, 18), Mis16 and Mis18 are required for CenH3 loading and Sim3 might act to escort CENP-A to centromere (19, 20). The human proteins hMis18 and M18BP1, recruited to centromere at telophase-G1, and RbAp46/RbAp48 may act to prime centromere for CENP-A localization (21). In S. cerivisiae and S. pombe, Scm3 (Suppressor of chromosome mis-segregation 3) protein was shown to specifically bind the CenH3-H4 complex and to be required for its assembly into the centromeric chromatin (22 –26). Despite the identification of CENP-A associated proteins little is known about specific histone chaperones in humans that could bind CENP-A and assist its specific deposition to centromeres.
In this study, we purified the prenucleosomal CENP-A complex from soluble nuclear fraction of HeLa cells. We present evidence that HJURP (Holliday Junction Recognition Protein) (27), a member of the CENP-A prenucleosomal complex (28), is essential for the deposition of CENP-A at the centromeres in cell cycle dependent manner. We further analyzed how HJURP interacts with CENP-A and identified the domains of both proteins involved in this interaction.
Results
Purification of Prenucleosomal CENP-A and H3.1 Complexes.
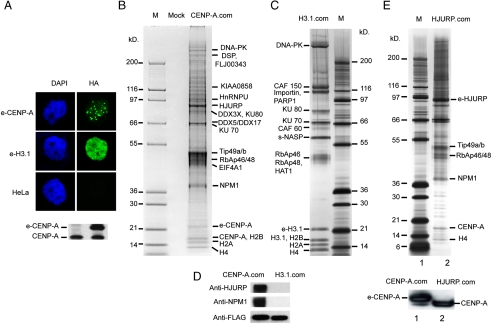
To identify proteins interacting specifically with CENP-A, we established stable HeLa cell lines expressing either a C-terminal FLAG-HA epitope tagged CENP-A (e-CENP-A) or a C-terminal FLAG-HA epitope tagged H3.1 (e-H3.1). The immunofluorescence analysis of e-CENP-A and e-H3.1 in these cells revealed that the tagged histones are found in the nucleus (Fig. 1 A). As expected for a conventional histone, e-H3.1 shows a rather diffuse nuclear staining. In contrast, e-CENP-A was localized in discrete foci, a distribution pattern typical for endogenous CENP-A (7, 10). These data indicate that the presence of the tag epitopes do not interfere with the deposition and association with chromatin of both e-H3.1 and e-CENP-A.
Fig. 1.
Purification of CENP-A preassembly complex. (A) Localization of e-CENP-A and e-H3.1. Stable cell lines expressing either e-CENP-A or e-H3.1 were immunostained with antiHA antibody (Green) to detect the epitope tagged proteins and DAPI staining (Blue). (Lower) Western blotting of total cell extract from control HeLa cells (Lane 1) and stable HeLa cell line (Lane 2) expressing e-CENP-A. An anti-CENP-A antibody was used to reveal the blot. (B) Silver staining of proteins associated with e-CENP-A. The preassembly e-CENP-A complex (CENP-A.com) was purified by tandem immuno-affinity and the associated polypeptides were identified by mass spectrometry. Lane M corresponds to a protein molecular mass marker. Lane Mock, corresponds to a mock purification from a nontagged HeLa cell line. (C) Silver staining of proteins associated with e-H3.1. The prenucelosomal e-H3.1 complex (H3.1.com) was purified by tandem immunoaffinity and the associated polypeptides (Left) were identified by mass spectrometry. Lane M corresponds to a protein molecular mass marker. (D). Western blot detection of HJURP and NPM1 in the e-CENP-A preassembly complex. e-Cenp-A and e-H3.1 complexes were run on 4–12% SDS PAGE and after transfer, the blot was revealed with an antiHJURP, an anti-NPM1, and antiFLAG (to detect e-CENP-A and e-H3.1). (E) Silver staining of proteins associated with e-HJURP. The specific partners of e-HJURP were purified by tandem immunoaffinity and identified by mass spectrometry analyses (Lane 2, Upper). The identified proteins are indicated on the right. Lane M corresponds to a protein molecular mass marker. (Lower) Western blot detection of CENP-A present in the preassembly e-CENP-A complex (Lane 1) and e-HJURP complex (Lane 2). Both complexes were run on 4–12% SDS PAGE and after transfer, the blot was revealed with an anti-CENP-A antibody. The higher molecular mass of e-CENP-A is due to the presence of the HA-FLAG peptide fused to CENP-A.
Cell extracts from the tagged cells were prepared and the e-H3.1 and e-CENP-A prenucleosomal complexes were purified by sequential immunoprecipitations with antiFLAG antibody followed by antiHA antibody (29). The proteins associated with e-CENP-A and e-H3.1 were separated in 4–12% gradient PAGE containing SDS and silver stained (Fig. 1 B and C). Mass spectrometry analysis identifies the following proteins as common components of the e-CENP-A and e-H3.1 complexes: Core histones (H2A, H2B, H4), RbAp46/RbAp48 proteins, Ku proteins (Ku70 and Ku80), and DNA-dependent protein kinase (DNA-PK). Two of the three CAF-1 subunits, CAF 150 and CAF 60, were specific to the e-H3.1 complex, whereas the third CAF-1 subunit RbAp48 and RbAp46 was a common component to e-CENP-A and e-H3.1 complexes (Fig. 1 B and C). RbAp46 and RbAp48 are highly homologous histone chaperones found in many chromatin-related complexes (30) and apparently they interact with H4 (31 –33). These results are in agreement with the reported data, showing that CAF-1 subcomplex is part of e-H3.1 containing-complex (34).
The e-H3.1 prenucleosomal complex contained also importin, s-NASP, and histone acetyl transferase-1 (HAT1) (Fig. 1 C). The prenucleosomal e-CENP-A complex is associated with Tip49a/ Tip49b, DEAD (Asp-Glu-Ala-Asp) box polypeptide (DDX3X, DDX5, and DDX17) and some RNA/DNA binding proteins like HnRNPU (Heterogenous nuclear Ribonucleoprotein U) and EIF4A1 (Fig. 1 B). None of these proteins would be expected to have histone chaperone properties. With this in mind we focused on the two other specific members of the e-CENP-A complex, HJURP, and nucleophosmin (NPM1), two proteins found associated with the CENP-A nucleosome (28). Immunoblotting of the purified complexes evidence additionally that both proteins were present in the e-CENP-A complex, but not in the e-H3.1 complex (Fig. 1 D). In vitro experiments showed that NPM1 was able to bind equally well to the CENP-A/H4 and H3/H4 tetramers (see SI Text), strongly suggesting that it cannot be a bona fide chaperone specific for CENP-A. Consequently, the best candidate for a specific CENP-A chaperone remained HJURP.
If HJURP was a CENP-A chaperone, it should exhibit a cell cycle dependent association with CENP-A chromatin, because the incorporation of CENP-A is cell cycle dependent and its deposition occurs at G1 (35). And indeed, we found that in G1, in contrast to S and M phases, the quasi-totality of HJURP was tightly associated with CENP-A chromatin (SI Text).
We next conducted experiments to further confirm the presence of HJURP in the e-CENP-A prenucleosomal complex. We established a stable HeLa cell line expressing a N-terminal FLAG-HA epitope tagged HJURP. Tandem affinity purification of e-HJURP from soluble HeLa nuclear cell extract followed by mass spectrometry analysis identifies CENP-A, H4, NPM1 Tip49a/Tip49b, and RbAp46/RbAp48 as integral components of the human HJURP complex (Fig. 1 E). Immunoblotting of the purified complex with an anti-CENP-A antibody further confirmed the presence of CENP-A in this complex (Fig. 1 E Lower).
HJURP Is Required for Loading CENP-A to Centromeres.
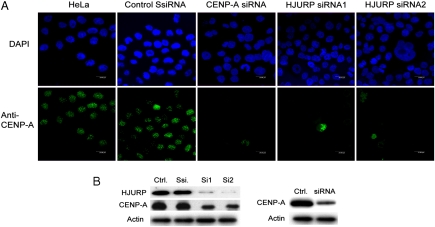
The above described data suggest strongly that HJURP is a specific CENP-A chaperone. If this was the case, its depletion would result in impediment of CENP-A delivery to the centromeres. To test this, we used HeLa cells where HJURP was depleted by siRNA treatment. Two distinct siRNAs against HJURP (Si1 and Si2) were used to suppress its expression in HeLa cells. A scrambled (Ssi) sequence was used as a negative control. The suppression of HJURP expression was confirmed 72 hr posttransfection by a specific antibody (Fig. 2 B). Note that the depletion of HJURP was very efficient, because the cells transfected with either one of the siRNA probes expressed ≤ 5 - 10% of the amount of HJURP in the control, treated with scrambled siRNA cells (Fig. 2 B). Remarkably, immunostaining with anti-CENP-A antibody showed a loss of CENP-A at the centromeres (Fig. 2 A). Essentially identical results were obtained when CENP-A was depleted by siRNA transfection (Fig. 2 A). This suggests that in the HJURP depleted cells either the stability of the already incorporated CENP-A or the provision of new CENP-A at centromeres, or both, are compromised. In addition, immunoblotting shows that the depletion of HJURP resulted in decrease of CENP-A, i.e. the amount of CENP-A present in the depleted cell was reduced to at least 50% of its initial level before siRNA treatment (Fig. 2 B). These data are evidence for a key role of HJURP in the CENP-A loading at the centromeres.
Fig. 2.
HJURP is required for CENP-A localization to centromeres. (A) The centromeric association of CENP-A is lost in cells depleted of HJURP. HeLa cells were transfected with either scrambled siRNA (SsiRNA) or with CENP-A siRNA or with HJURP siRNA1 and siRNA2. Seventy-two hr posttransfection cells were immunostained (Green) with anti-CENP-A antibody and DAPI staining (Blue). (B) Western blot analysis of the depletion of HJURP and CENP-A upon treatment with siRNA. HeLa cells were transfected with the respective siRNA and 72 hr posttransfection they were harvested, total cell extracts were prepared, and the presence of HJURP and CENP-A was detected by Western blotting using antiHJURP and anti-CENP-A antibodies. The blot was also revealed with an antiactin antibody as a control for equal loading. (Left) Depletion of both CENP-A and HJURP upon treatment with siRNA against HJURP. (Right) Depletion of CENP-A upon treatment with siRNA against CENP-A. Ctrl, nonsiRNA treated cells; Ssi, cells treated with scrumble siRNA; Si1 and Si2, cells treated with two distinct (Si1 or Si2) siRNAs against HJURP (Si1 and Si2 siRNA were used to suppress the expression of HJURP in the experiments presented in (A).
HJURP Recognizes the CENP-A/H4 and Specifically Interacts With It.
The unambiguous identification of HJURP within the prenucleosomal CENP-A complex indicates that the two proteins should be closely associated but do not distinguish between direct and indirect binding. If HJURP is a chaperone for CENP-A, a direct interaction between the two proteins should be observed. To address this question, GST-HJURP fusion (GST-HJURP) together with CENP-A/H4 were coexpressed in bacterial cells. Then GST-HJURP, together with the associated proteins, was purified and run on a SDS gel and the gel was stained with coomassie. The data clearly show that GST-HJURP binds stoichiometrically to CENP-A/H4 tetramers (Fig. 3 A, lane 2). Immunoblotting with an anti-CENP-A antibody confirmed these results (Fig. 3 A Lower). Note that the GST-HJURP binding to CENP-A/H4 tetramers does not depend on the presence of either DNA or RNA and thus, it involves protein–protein interactions only (SI Text).
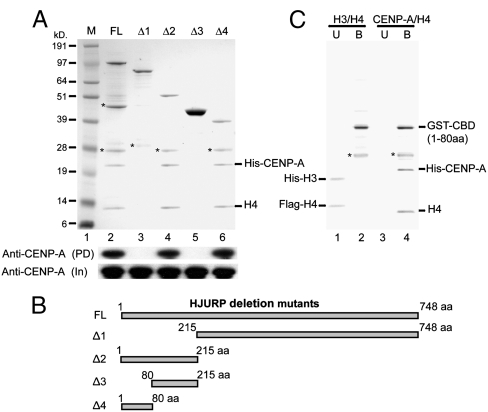
Fig. 3.
Identification of a short N-terminal domain of HJURP required for interaction with CENP-A. (A) Interaction of full-length and deletion mutants of HJURP with CENP-A. Full-length HJURP and its deletion mutants (Δ1–Δ4) fused to GST, together with CENP-A and H4, were coexpressed and purified from bacteria. The purified material was separated on a SDS-PAGE and stained with coomassie. (Lower) Western blot of either the eluted samples (PD) or the input (In) revealed with anti-CENP-A antibody. Note that a short amino acid sequence (1–80 AA) from the N-terminal of the protein recapitulates the main property of the full-length protein and was able to bind stoichiometrically to the CENP-A-H4 tetramer (compare lanes 2, 4, and 6). (B) Schematic representation of the different HJURP deletion mutants used as GST-fusions in (A). (C) The GST-fusion with CBD (CBD of HJURP, 1–80 AA) does not interact with the H3-H4 tetramer. GST-CBD was coexpressed with either H3.1/H4 or with CENP-A/H4. The purified material was run on a SDS-PAGE and stained with coomassie. U, unbound material. B, bound material. The bands designed with stars are degradation products of the fusions.
By using the same assay, we have mapped the specific region of HJURP that interacts with CENP-A. GST fusions of different deletion mutants of HJURP (Fig. 3 B) were coexpressed with CENP-A/H4 in bacteria and tested for their interactions with CENP-A. Interestingly the N-terminal deletion mutants Δ1 (215–748 AA) and Δ3 (80–215 AA) of HJURP did not interact with CENP-A (Fig. 3 A), suggesting that the CENP-A binding domain is a part of the N-terminal domain of HJURP. In agreement with this, the two C-terminal deletion mutants Δ2 (1–215 AA) and Δ4 (1–80 AA) showed essentially the same binding capacity as the full-length HJURP (Fig. 3 A). We conclude that the N-terminal part of the protein corresponding to amino acids 1–80 aa is the CENP-A Binding Domain (CBD) of HJURP.
Using the identified CENP-A binding domain (CBD, 1–80 aa) as a GST-fusion, we next asked whether it interacts also with H3/H4 or it is exclusively specific to CENP-A/H4. Importantly, no binding to histones H3/H4 was detected (Fig. 3 C). These results evidence that the binding of HJURP to CENP-A/H4 is (i) specific, (ii) direct, and (iii) stoichiometric.
HJURP Interacts with the CATD Domain of CENP-A Through a Highly Conserved TLTY Box.
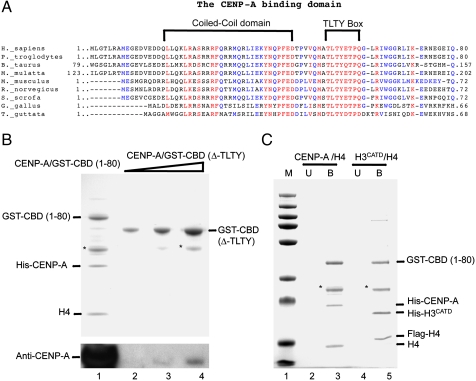
We next aimed to identify the peptide sequence within the CBD of HJURP required for the specific binding to CENP-A. Bioinformatic analysis using SMART (Simple Modular Architecture Research Tool), pBLAST and multiple sequence alignments were conducted. By SMART analysis a coiled-coil (CC) motif consisting of 26 amino acids residues (16–42 AA) was found in the N-terminal part of the protein (Fig. 4 A). Coiled-coil motifs are known to function as oligomerization domains for a wide variety of proteins and are unlikely to be involved in the interaction with CENP-A (36). By multiple sequence alignment analysis of HJURP, we identified a novel box TLTY that it is highly conserved across vertebrate from human (Homo sapiens) to chicken (Gallus gallus) (Fig. 4 A). To explore the importance of this novel TLTY box for CENP-A interaction, we deleted this box from the CENP-A binding domain of HJURP (CBD, 1–80 aa) and coexpressed this mutant with CENP-A/H4 in bacteria. The analysis of the binding was carried out as described above. We could not detect binding of the TLTY deleted mutant CBD (Δ-TLTY) to CENP-A/H4 (Fig. 4 B compare lane 1 with lanes 2–4) whereas the CBD showed a stoichoimetric interaction (Fig. 4 B, lane 1). Immunoblotting analysis with an anti-CENP-A antibody confirmed this result and only detected a trace amount of CENP-A interacting with CBD (Δ-TLTY) (Fig. 4 B, compare lane 1 with lanes 2–4). We concluded that the TLTY box is required for the interaction of HJURP with CENP-A.
Fig. 4.
The identified novel TLTY box within the HJURP vertebrate homologs is essential for the interaction with CENP-A. (A) Identification of a conserved coiled-coil domain and a novel TLTY box in higher-eukaryote HJURP homologs. The sequence alignments for the indicated species are shown. Alignments were generated by MultAlin. The brackets highlight the coiled-coil and the TLTY motifs, which are conserved from birds to human. (B) The TLTY box is essential for the interaction of HJURP with CENP-A. The TLTY box of HJURP was deleted from the minimal CBD and coexpressed as a GST-fusion [GST-CBD (Δ-TLTY)] in bacteria together with CENP-A and H4. Increasing amounts (Lanes 2–4) of the eluted from the gluthatione column GST-CBD (Δ-TLTY) complex was analyzed by SDS PAGE. GST-CBD was used as a positive control (Lane 1). (Lower) Western blot revealed with anti-CENP-A antibody for the respective samples. (C) The CBD of HJURP recognizes and binds to CATD, the CENP-A centromere targeting domain. GST-CBD was coexpressed with either H3CATD/H4 or CENP-A/H4 in bacteria, the GST-CBD complexes were purified as described above, run on SDS PAGE, and stained with coomassie. U, unbound material. B, bound material. M, protein molecular mass markers. (*), a degradation product of GST-CBD.
The next question we addressed was whether the CBD of HJURP, containing the conserved TLTY box, can specifically interact with the previously dentified CENP-A targeting Domain (CATD) (9). The CATD, consisting of the loop1 and helix 2 of the histone fold domain, is required for centromeric loading of CENP-A (9, 11). The substitution of CATD into H3.1 led to a H3CATD chimera that recapitulated the functional properties of CENP-A (9, 11). These findings suggested that any specific histone chaperone for CENP-A deposition should also bind to the CATD of CENP-A. To test this, GST-CBD fusion together with H3CATD/H4 were coexpressed in bacteria and their association analyzed (Fig. 4 C). GST-CBD was found associated in stoichiometric ratio with H3CATD/H4 as it was with CENP-A/H4 (Fig. 4 C). We conclude that HJURP binds to CENP-A through its CATD domain and this interaction is likely to occur via the TLTY box of CBD.
HJURP Stimulates CENP-A Deposition on DNA.
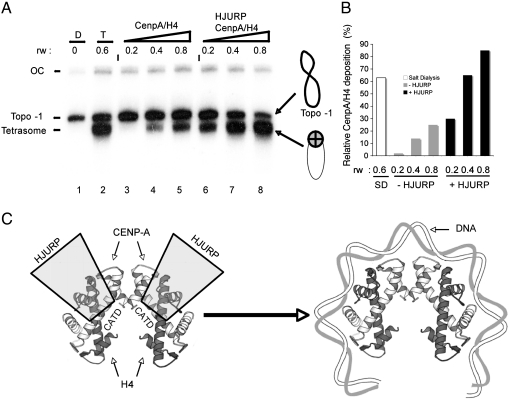
If HJURP is a bona fide CENP-A chaperone it should be able to deposit CENP-A/H4 to DNA and to assemble a CENP-A/H4 tetrasome. We have approached this problem as follows. Labeled 360 bp alpha satellite DNA was circularized under conditions that generated one negative supercoil corresponding to topoisomer -1 (37). Negatively supercoiled DNA was then incubated with an increasing amount of CENP-A/H4 histones in the absence or presence of equimolar amount of HJURP and then the deposition of histones onto DNA was analyzed by EMSA (Fig. 5 A). In the absence of HJURP, very low amount of CENP-A/H4 tetramer deposition was observed (Fig. 5, lanes 3–5). In contrast, the presence of HJURP strongly facilitates the CENP-A/H4 tetramer deposition and enhanced (up to 15-fold at low histone concentration and up to 3-fold at high histone concentration) the assembly of the CENP-A/H4 tetrasome (Fig. 5 A, lanes 6–8 and Fig. 5 B). The HJURP-mediated deposition of CENP-A/H4 tetramers on DNA was also at least as efficient as that obtained by the salt dialysis method (Fig. 5, compare lane 2 with lane 8 and Fig. 5 B). These data illustrate the ability of HJURP to assemble CENP-A variant particles.
Fig. 5.
HJURP is able to deposit efficiently CENP-A/H4 tetramer on DNA. (A) Negatively supercoiled human alpha-satellite DNA corresponding to topoisomer -1 (Lane 1, D) was incubated with increasing amount of CENP-A/H4 (at the indicated histone/DNA ratio, rw) in the absence (Lanes 3–5) or presence (Lanes 6–8) of equimolar (to the tetramers) amount of HJURP. The reaction was carried out for 30 min at 37 °C. The reaction products were then analyzed on native 4.5% polyacrylamide gel. (Lane 1) topoisomer-1 DNA; (Lane 2) reconstituted CENP-A/H4 tetrasomes on topoisomer -1 by salt dialysis using the indicated histone/DNA ratio (rw). (Right) Drawings showing the naked topoisomer -1 DNA and the CENP-A/H4 tetrasome. The positions of the naked topoisomer -1 DNA and the salt dialysis reconstituted tetrasome are also indicated. (B) Quantification of the relative amount of Cenp-A/H4 tetrameres deposited by HJURP in Fig. 5 A. The tetrasome/DNA ratio was quantified using ImageJ software. S.D. indicates tetramers assembled by salt dialysis. (C) Model of CENP-A deposition. Two molecules of HJURP dimerize through their coiled-coil domains and bind, via their TLTY boxes, the CATD of two molecules of CENP-A (Left).
Discussion
In this work we have identified by affinity purification and mass spectrometry HJURP as a major partner in the CENP-A nuclear soluble complex. Depletion of HJURP by siRNA affected the expression of CENP-A and impaired its deposition at centromeres. Immunoprecipitation experiments show that HJURP is associated with CENP-A chromatin in a cell cycle dependent manner, concomitant with the new CENP-A deposition. These results are in complete agreement with the recently reported experiments, where very similar approaches were used (38, 39). All these data strongly suggest that HJURP is a specific CENP-A chaperone, required for the cell cycle deposition of CENP-A in chromatin.
In addition to these in vivo experiments, we have performed a series of in vitro studies. This has allowed the identification and characterization of a conserved HJURP short N-terminal domain, responsible for the specific and stoichiometric binding to the CENP-A/H4 complex. We found that a TLTY box within this domain was required for the binding. Interestingly, the TLTY box was found to bind to the previously identified CENP-A targeting Domain (CATD) (9).
The recently identified yeast CenH3 chaperone Scm3 (22 –26) is likely to be a distant ortholog of HJURP. Scm3 is required for kinetochore assembly, conserved across fungi, and displays a remarkable variation in protein size (40). Though Scm3 has extensively diversified in course of fungal evolution to make different types of potential DNA contacts via its C-terminal regions, it is likely to mediate a conserved interaction with the CenH3-H4 complex via its N-terminal Scm3 domain. Indeed, recent bioinformatics analysis established some similarity between fungal Scm3 domain and mammalian HJURP N-terminal domain (41). This result is in agreement with our data implicating the N-terminal domain of HJURP in CENP-A binding.
Taken together our and the reported data (38, 39) demonstrate that HJURP is a key chaperone responsible for the targeting and deposition of newly synthesized CENP-A at centromeres. Our in vitro experiments suggest a model for HJURP binding to the CENP-A/H4 complex (Fig. 5 C). According to the model, two molecules of HJURP are supposed to dimerize through their coiled-coil domains and to bind, via the TLTY box, two dimers of CENP-A/H4. This would constrain the CENP-A/H4 tetramer in a specific conformation that facilitates its deposition to DNA and allows the assembly of the CENP-A/H4 tetrasome.
Materials and Methods
Purification of e-CENP-A and e-H3.1 Complexes.
Prenucleosomal CENP-A and H3.1 complexes were purified from soluble nuclear extracts prepared from stable HeLa cell lines expressing either CENP-A or H3.1 proteins fused to C-terminal FLAG and HA epitope tags (e-CENP-A/e-H3.1). A tandem affinity purification protocol on antiFlag antibody-conjugated agarose followed by antiHA purification and peptide elution was used (29).
Immunofluorescence.
Immunofluorescence was performed using standard procedures. Anti-CENP-A was used at 1∶200 dilution, the secondary antibody used is a goat antirabbit IgG coupled to Alexa Fluor 488 (Molecular Probes) at 1∶400 dilution. Rat antiHA antibody (Roche) was used at 1∶400 dilution; the secondary antibody used is a goat antirat IgG coupled to Alexa Fluor 488 (Molecular Probes) at 1∶400 dilution.
Supplementary Material
Acknowledgments.
We thank Dr. Yoda for the kind gift of pHCE-CENP-A expression plasmid. This work was supported by grants from the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, ANR (N° NT05-1_41978) (A.H. and S.D.), ANR “EPIVAR” (N° 08-BLAN-0320-02) (A.H and S.D), INCA (A.H), the Association pour la Recherche sur le Cancer (A.H), and La fondation pour la Recherche Medicale (A.H.). La Ligue Nationale contre le Cancer (A.H). S.D. acknowledges the Ligue Nationale contre le cancer (Equipe labellisée “La Ligue).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913709107/DCSupplemental.
References
- 1.Mitelman F. Catalog of Chromosome Aberrations in Cancer. 5th Ed. New York: Wiley; 1994. [Google Scholar]
- 2.Henikoff S, Dalal Y. Centromeric chromatin: What makes it unique? Curr Opin Genet Dev. 2005;15:177–184. doi: 10.1016/j.gde.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JL, Henikoff S. Adaptive evolution of the histone fold domain in centromeric histones. Mol Biol Evol. 2004;21:1712–1718. doi: 10.1093/molbev/msh179. [DOI] [PubMed] [Google Scholar]
- 4.Smith MM. Centromeres and variant histones: What, where, when, and why? Curr Opin Cell Biol. 2002;14:279–285. doi: 10.1016/s0955-0674(02)00331-9. [DOI] [PubMed] [Google Scholar]
- 5.Earnshaw WC, Migeon BR. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92:290–296. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- 6.Palmer DK, O’Day K, Wener MH, Andrews BS, Margolis RL. A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol. 1987;104:805–815. doi: 10.1083/jcb.104.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vafa O, Sullivan KF. Chromatin containing CENP-A and alpha-satellite DNA is a major component of the inner kinetochore plate. Curr Biol. 1997;7:897–900. doi: 10.1016/s0960-9822(06)00381-2. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad K, Henikoff S. Centromeres are specialized replication domains in heterochromatin. J Cell Biol. 2001;153:101–109. doi: 10.1083/jcb.153.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black BE, et al. Structural determinants for generating centromeric chromatin. Nature. 2004;430:578–582. [Google Scholar]
- 10.Sullivan KF, Hechenberger M, Masri K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J Cell Biol. 1994;127:581–592. doi: 10.1083/jcb.127.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black BE, et al. Centromere identity maintained by nucleosomes assembled with histone H3 containing the CENP-A targeting domain. Mol Cell. 2007;25:309–322. doi: 10.1016/j.molcel.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Vermaak D, Hayden HS, Henikoff S. Centromere targeting element within the histone fold domain of Cid. Mol Cell Biol. 2002;22:7553–7561. doi: 10.1128/MCB.22.21.7553-7561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelby RD, Vafa O, Sullivan KF. Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites. J Cell Biol. 1997;136:501–513. doi: 10.1083/jcb.136.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black BE, et al. An epigenetic mark generated by the incorporation of CENP-A into centromeric nucleosomes. Proc Natl Acad Sci USA. 2007;104:5008–5013. doi: 10.1073/pnas.0700390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuyama T, Dalal Y, Henikoff S. Chaperone-mediated assembly of centromeric chromatin in vitro. Proc Natl Acad Sci USA. 2006;103:6172–6177. doi: 10.1073/pnas.0601686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erhardt S, et al. Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J Cell Biol. 2008;183:805–818. doi: 10.1083/jcb.200806038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ES, Saitoh S, Yanagida M, Takahashi K. A cell cycle-regulated GATA factor promotes centromeric localization of CENP-A in fission yeast. Mol Cell. 2003;11:175–187. doi: 10.1016/s1097-2765(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh S, Takahashi K, Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–143. doi: 10.1016/s0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, et al. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118:715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Dunleavy EM, et al. A NASP (N1/N2)-related protein, Sim3, binds CENP-A and is required for its deposition at fission yeast centromeres. Mol Cell. 2007;28:1029–1044. doi: 10.1016/j.molcel.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita Y, et al. Priming of centromere for CENP-A recruitment by human hMis18alpha, hMis18beta, and M18BP1. Dev Cell. 2007;12:17–30. doi: 10.1016/j.devcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Mizuguchi G, Xiao H, Wisniewski J, Smith MM, Wu C. Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell. 2007;129:1153–1164. doi: 10.1016/j.cell.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Stoler S, et al. Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization. Proc Natl Acad Sci USA. 2007;104:10571–10576. doi: 10.1073/pnas.0703178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camahort R, et al. Scm3 is essential to recruit the histone h3 variant cse4 to centromeres and to maintain a functional kinetochore. Mol Cell. 2007;26:853–865. doi: 10.1016/j.molcel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Pidoux AL, et al. Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell. 2009;33:299–311. doi: 10.1016/j.molcel.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams JS, Hayashi T, Yanagida M, Russell P. Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol Cell. 2009;33:287–298. doi: 10.1016/j.molcel.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato T, et al. Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res. 2007;67:8544–8553. doi: 10.1158/0008-5472.CAN-07-1307. [DOI] [PubMed] [Google Scholar]
- 28.Foltz DR, et al. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8:458–469. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- 29.Ouararhni K, et al. The histone variant mH2A11 interferes with transcription by down-regulating PARP-1 enzymatic activity. Genes Dev. 2006;20:3324–3336. doi: 10.1101/gad.396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Koning L, Corpet A, Haber JE, Almouzni G. Histone chaperones: An escort network regulating histone traffic. Nat Struct Mol Biol. 2007;14:997–1007. doi: 10.1038/nsmb1318. [DOI] [PubMed] [Google Scholar]
- 31.Murzina NV, et al. Structural basis for the recognition of histone H4 by the histone-chaperone RbAp46. Structure. 2008;16:1077–1085. doi: 10.1016/j.str.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song JJ, Garlick JD, Kingston RE. Structural basis of histone H4 recognition by p55. Genes Dev. 2008;22:1313–1318. doi: 10.1101/gad.1653308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosomal DNA regulates the core-histone-binding subunit of the human Hat1 acetyltransferase. Curr Biol. 1998;8:96–108. doi: 10.1016/s0960-9822(98)70040-5. [DOI] [PubMed] [Google Scholar]
- 34.Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H31 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 35.Jansen LE, Black BE, Foltz DR, Cleveland DW. Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol. 2007;176:795–805. doi: 10.1083/jcb.200701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigoryan G, Keating AE. Structural specificity in coiled-coil interactions. Curr Opin Struct Biol. 2008;18:477–483. doi: 10.1016/j.sbi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamiche A, et al. Interaction of the histone (H3-H4)2 tetramer of the nucleosome with positively supercoiled DNA minicircles: Potential flipping of the protein from a left- to a right-handed superhelical form. Proc Natl Acad Sci USA. 1996;93:7588–7593. doi: 10.1073/pnas.93.15.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foltz DR, et al. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137:472–484. doi: 10.1016/j.cell.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunleavy EM, et al. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137:485–497. doi: 10.1016/j.cell.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 40.Aravind L, Iyer LM, Wu C. Domain architectures of the Scm3p protein provide insights into centromere function and evolution. Cell Cycle. 2007;6:2511–2515. doi: 10.4161/cc.6.20.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Pulido L, Pidoux AL, Ponting CP, Allshire RC. Common ancestry of the CENP-A chaperones Scm3 and HJURP. Cell. 2009;137:1173–1174. doi: 10.1016/j.cell.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoda K, et al. Human centromere protein A (CENP-A) can replace histone H3 in nucleosome reconstitution in vitro. Proc Natl Acad Sci USA. 2000;97:7266–7271. doi: 10.1073/pnas.130189697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.