Abstract
Self-renewal and differentiation of embryonic stem cells (ESCs) are controlled by intracellular transcriptional factors and extracellular factor-activated signaling pathways. Transcription factor Oct4 is a key player maintaining ESCs in an undifferentiated state, whereas the Erk/MAPK pathway is known to be important for ESC differentiation. However, the manner in which intracellular pluripotency factors modulate extracellular factor-activated signaling pathways in ESCs is not well understood. Here, we report identification of a target gene of Oct4, serine/threonine kinase 40 (Stk40), which is able to activate the Erk/MAPK pathway and induce extraembryonic–endoderm (ExEn) differentiation in mouse ESCs. Interestingly, cells overexpressing Stk40 exclusively contribute to the ExEn layer of chimeric embryos when injected into host blastocysts. In contrast, deletion of Stk40 in ESCs markedly reduces ExEn differentiation in vitro. Mechanistically, Stk40 interacts with Rcn2, which also activates Erk1/2 to induce ExEn specification in mouse ESCs. Moreover, Rcn2 proteins are specifically located in the cytoplasm of the ExEn layer of early mouse embryos. Importantly, knockdown of Rcn2 blocks Stk40-activated Erk1/2 and ESC differentiation. Therefore, our study establishes a link between the pluripotency factor Oct4 and the Erk/MAPK signaling pathway, and it uncovers cooperating signals in the Erk/MAPK activation that control ExEn differentiation.
Keywords: embryonic stem cells, Rcn2, Ras, Gata6
The mouse embryo at the blastocyst stage contains three cell types, trophectoderm, primitive endoderm, and epiblast, each of which generates distinct cell lineages in the developing embryo. The trophectoderm is the first differentiated cell lineage of embryogenesis, and it generates ectoplacental cone, extraembryonic ectoderm, and trophoblast cells in the fetal placenta. The primitive endoderm, the second differentiated lineage, generates extraembryonic endoderm (ExEn), which contributes to the endoderm layer of the visceral and parietal yolk sacs (1 –3). These ExEn lineage cells in the mammal provide nutritive supports and are crucial for cell-fate specification and axial-pattern initiation (4). Finally, epiblast cells are pluripotent cells that give rise to all of the fetal tissues and other extraembryonic tissues. Embryonic stem cells (ESCs) derived from the epiblast provide an excellent tool for study of the molecular networks that control mammalian development (5 –7). They are capable of differentiating into all cell types of the three germ layers (ectoderm, mesoderm, and endoderm), including germ cells; they can also self-renew indefinitely in vitro. These unique features make ESCs an attractive resource in regenerative medicine (8). However, many obstacles exist on the road to the therapeutic use of ESC derivatives. In particular, our ability to drive ESC differentiation into a chosen fate is limited. Thus, understanding the mechanisms governing self-renewal and differentiation is an important step to overcoming the existing difficulties.
Transcriptional factor Oct4 is a critical maternal and embryonic protein found in the unfertilized egg, zygote, and all blastomeres until the morula stage. When the blastocyst forms, its expression is restricted to the inner cell mass, and it is absent in the trophectoderm (9). Deletion of Oct4 in mice leads to an inability to generate pluripotent populations in the inner cell mass (10). In vitro, Oct4 is also highly expressed in undifferentiated ESCs, and it is down-regulated on differentiation. Knockdown of Oct4 in ESCs triggers primitive endoderm as well as trophectoderm differentiation (11 –13). Of note, Niwa et al.(13) found that up-regulation of Oct4 expression causes differentiation of ESCs into primitive endoderm and mesoderm. These observations suggest that Oct4 at levels either higher or lower than normal could convert ESCs to primitive endoderm lineages. However, it is also possible that ESCs with different epigenetic settings might respond to the same level of Oct4 expression differentially. Although Oct4 is an essential regulator of cell fate, molecular mechanisms underlying its functions are not well-defined. Among the known Oct4 target genes, Cdx2 and Hand1 are transcription factors critical for trophectoderm development (13 –15). However, less is known about the segregation of the epiblast and primitive endoderm within the inner cell mass of the blastocyst. The transcription factor Gata6 is required for the development of primitive endoderm lineages (3), whereas Nanog has been shown to prevent primitive endoderm differentiation by repressing expression of Gata6 (16). However, the reason that Oct4 is implicated in the formation of the primitive endoderm and subsequent ExEn lineages remains elusive.
Here, we identify serine/threonine kinase 40 (Stk40) as a target gene of Oct4 and show that Stk40 is capable of activating the Erk/MAPK signaling pathway. Also, it induces ESC differentiation into ExEn lineages through Rcn2, a Ca2+ binding protein. Our study puts forward the concept that Oct4 suppresses expression of an important component of the Erk/MAPK signaling pathway to protect ESCs from ExEn differentiation.
Results
Oct4 Negatively Regulates Stk40 Expression.
To find new Oct4 target genes, we carried out chromatin immunoprecipitation (ChIP) assays using Oct4 antibody and identified a gene, Stk40. It encodes a protein of 435 amino acid residues with a serine/threonine kinase domain (Fig. 1A). Sequence orthologs of Stk40 were found in a number of other organisms (Fig. S1A). Transcript and protein levels of Stk40 were up-regulated and maintained at high levels during the process of spontaneous embryoid-body (EB) formation (Fig. S1B). Moreover, proteins of Stk40 were found to distribute in both the nucleus and cytoplasm (Fig. S1C).
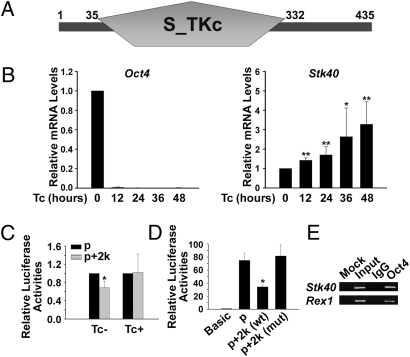
Fig. 1.
Stk40 is a direct target of Oct4 and is negatively regulated by Oct4. (A) The schematic diagram of the Stk40 protein showing a serine/threonine protein kinase domain (S_TKc) in the middle (aa35–332). (B) Analysis of Oct4 and Stk40 dynamic expression patterns in ZHBTc4 mouse ESCs treated with Tc by qPCR. All values are shown as means ± SD of results from three independent experiments. *P < 0.05; **P < 0.01. (C) Luciferase assays of the 2-kb upstream fragment of Stk40 reporter in ZHBTc4 ESCs treated with or without Tc for 72 h. ZHBTc4 cells were pretreated with Tc for 24 h before transfection. p, 0.5 kb of putative promoter; p+2k, 2kb upstream regulatory sequence. *P < 0.05. (D) Luciferase assays of the 2-kb upstream fragment of Stk40 reporter with or without mutations introduced in the octamer motif in CGR8 ESCs. p, promoter; wt, wild type; mut, mutant. Luciferase activities are shown as in C. *P < 0.05. (E) ChIP assays in CGR8 ESCs using rabbit IgG or the specific antibody against Oct4. The representative result of PCR amplification reactions of Stk40 (Upper) or Rex1 (Lower) is shown. Mock, no template; input, 10% of total genomic DNA from the nuclear extract.
To test whether or not Stk40 expression is regulated by Oct4, we used ZHBTc4 mouse ESCs in which Oct4 expression is controlled by tetracycline (Tc) (13). On addition of Tc, Oct4 expression was quickly silenced, and Stk40 expression was substantially elevated but slowly (Fig. 1B). This suggests that Stk40 expression was negatively controlled by Oct4 and that other factors might also be involved in this regulation (Fig. S1D). Moreover, a high level of luciferase activity was observed with an upstream 0.5-kb fragment (the putative Stk40 promoter) in CGR8 mouse ESCs. However, the activity was significantly reduced when either a 2-kb or 4-kb upstream fragment was included (Fig. S1E), suggesting that an inhibitory component or components may exist within the 2-kb fragment. Strikingly, the repressive effect of the 2-kb fragment was completely abrogated by silencing Oct4 expression in the ZHBTc4 cells (Fig. 1C), indicating that Oct4 is a factor that is likely responsible for repression of the reporter activity. Further analysis identified a conserved octamer motif (TTTTGCAT) positioned within the upstream 2-kb region of Stk40. Triple-point mutations in the octamer motif (mut, TTGTTCCT) abolished the inhibition of the 2-kb fragment on the reporter activity (Fig. 1D). Furthermore, we conducted ChIP assays with the Oct4 antibody, which revealed that Oct4 indeed associated with the Stk40 regulatory sequence containing the octamer motif in a physiological context; it also associated with the promoter of Rex1, a known target gene of Oct4 (Fig. 1E). A similar result was obtained with an in vitro DNA-protein binding assay (SI Text). Concisely, Oct4 negatively regulates Stk40 expression through its direct binding to the octamer motif located in the upstream 2-kb regulatory sequence.
Forced Expression of Stk40 Induces ESC Differentiation into ExEn Lineages.
To study the function of Stk40, we overexpressed Stk40 in ESCs using an episomal expression system (17). ESCs overexpressing Stk40 displayed differentiated morphology (Fig. 2A and Fig. S2A) with a few tightly compacted ESC colonies among the differentiated cells. Using colony-forming assays to evaluate the self-renewal ability of ESCs, we found that forced expression of Stk40 resulted in a marked reduction in the number of undifferentiated colonies (Fig. S2B). Quantitative real-time PCR (qPCR) analysis showed that ExEn markers, including Gata6, Gata4, Sox7, Laminin B1, Afp, and Ihh, were substantially activated by Stk40 overexpression (Fig. 2B). A modest increase in the expression of trophectoderm-lineage markers (Cdx2 and Hand1) was also noticed. In contrast, we did not find marked changes in the expression of mesoderm and ectoderm markers. In pluripotency-related genes, transcript levels of Oct4, Nanog, and Rex1 were reduced (Fig. S2C). Furthermore, immunostaining assays revealed evidently increased expression of ExEn markers (Dab2 and Gata4) and decreased expression of Nanog in Stk40 overexpressed cells (Fig. S2D).
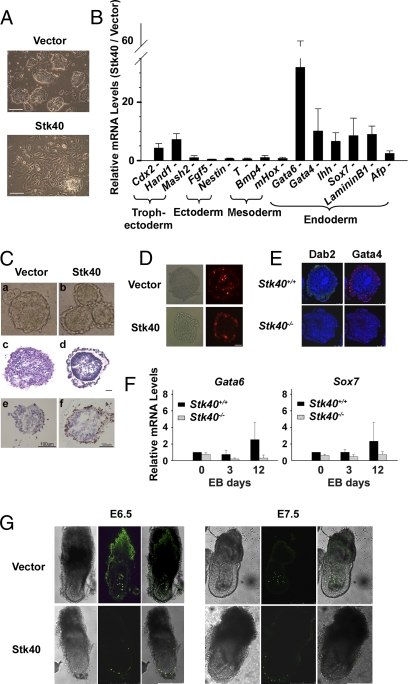
Fig. 2.
Stk40 induces ESCs to differentiate into ExEn lineages. (A) Morphological changes in ESCs induced by Stk40 overexpression. E14T ESCs were transfected with either pPyCAGIP (vector as a control) or pPyCAGIP-Stk40 (Stk40) and cultured for 10 days in the presence of puromycin. (Scale bar: 100 μm.) (B) Effect of Stk40 on expression of differentiation-related markers using qPCR. Data are shown as the ratio of values for Stk40 overexpressing cells over control cells. All values are shown as means ± SD of results from three independent experiments. (C) Representative images showing expansion of ExEn-like cells in EBs aggregated from Stk40 overexpressing ESCs. (a and b) Light microscopic images. (c and d) Hematoxylin/eosin-stained sections of EBs. (e and f) Costained with Dab2 antibody by the DAB (3,3′-Diaminobenzidine) method (brown signal). (Scale bar: 100 μm.) (D) Surface positioning of Stk40-induced differentiated ESCs in the chimeric EB formation assay. (Scale bar: 50 μm.) (E) Immunofluorescence staining of Dab2 and Gata4 in EBs from Stk40+/+ or Stk40−/− ESCs. (Scale bar: 100 μm.) (F) Quantification of ExEn–marker expression by qPCR in EBs aggregated from Stk40+/+ and Stk40−/− ESCs at the indicated time points. All values are shown as means ± SD of results from four independent experiments. (G) Contribution of injected Stk40/E14Tg cells to the ExEn lineage in E6.5 (Left) and E7.5 (Right) chimeric embryos. (Scale bar: 250 μm.)
Wild-type ESCs spontaneously differentiated into cystic EBs containing heterogeneous cell types representing all three germ layers and an outer layer of ExEn cells when cultured in suspension (18). Remarkably, the outer layer of EBs aggregated from Stk40 overexpressing ESCs was more prominent and thicker than that of control EBs (Fig. 2C a, Fig. 2C b, and Fig. S2F). Staining with H&E and the Dab2 antibody confirmed the identity of the cells in the expanded outer layer as ExEn lineages (Fig. 2C c–f). Furthermore, red dye (DiI)-labeled Stk40 overexpressing cells specifically localized at the outer rim of chimeric EBs, whereas the vector-transfected control cells were distributed throughout the EBs (Fig. 2D) in chimeric EB-formation assays. This is consistent with a previously reported phenomenon that ExEn cells have an intrinsic property of surface positioning when mixed with undifferentiated ESCs in the EB-differentiation process (19).
In addition, the role of Stk40 in the ExEn development under physiological conditions was investigated using Stk40+/+ and Stk40−/− ESCs. Notably, Dab2 and Gata4 staining present in the outer layer of EBs of Stk40+/+ ESCs was lost in EBs of Stk40−/− ESCs (Fig. 2E). Moreover, qPCR analysis revealed that induction of ExEn markers, Gata6 and Sox7, but not markers of other germ layers was evidently impaired in EBs of Stk40−/− ESCs (Fig. 2F and Fig. S2J). Deletion of Stk40 was validated by the complete lack of its protein product in Stk40−/− ESCs-derived EBs (Fig. S2K).
Finally, we generated chimeric embryos and compared the developmental potential of Stk40 overexpressing cells (Stk40/E14Tg) with control cells expressing vector only (Vector/E14Tg) at E6.5 (embryo day) and E7.5. Both cell lines expressed a histone2B-GFP fusion protein for visualization. Strikingly, Stk40/E14Tg cells contributed to extra embryonic yolk sacs exclusively (Fig. 2G and Table S1). In contrast, more than 50% of Vector/E14Tg cells contributed to the embryonic portion. In addition, we generated chimeric embryos with E14Tg ESCs where Oct4 expression was knocked down by specific RNAi oligos. Interestingly, cells transfected with Oct4 RNAi oligos predominantly migrated to the ExEn layers, although a minority was found in the epiblast, probably because of the ineffective knockdown of Oct4 in the cells (Fig. S2N, Fig. S2O , and Table S2). The results described above and in the SI Text indicate that Stk40 possesses an ability to induce ESCs to contribute to ExEn lineages.
Stk40 Induces ESC Differentiation Through Activation of the Erk/MAPK Pathway.
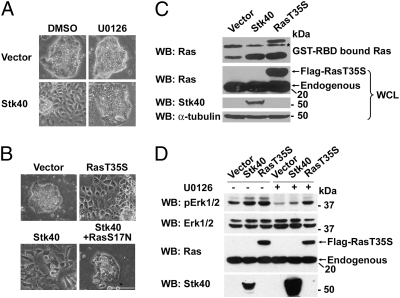
Experimental evidence suggests that multiple signaling pathways including the Erk/MAPK, TGF-β, and PI3K pathways might be involved in ExEn development (4, 20 –22). Using inhibitors of these pathways, we found that only the Mek1/2 inhibitor (U0126) abrogated Stk40-induced ESC differentiation, which was determined by both cell morphology and Sox7 expression (Fig. 3A and Fig. S3A). This finding led to an assumption that forced expression of Stk40 might induce ESC differentiation through activating the Erk/MAPK pathway. Furthermore, overexpression of a constitutively active mutated form of Ras for the Erk/MAPK pathway [H-Ras with G12V and T35S mutations (22) was termed RasT35S in this study] brought about differentiated morphology and high expression of Gata6 in ESCs (Fig. 3B and Fig. S3B); this is consistent with a previously reported role of Ras in ExEn differentiation (22). Notably, coexpression of a dominant negative mutated form of Ras (RasS17N) and Stk40 abolished Stk40-induced differentiation phenotypes (Fig. 3B and Fig. S3B), suggesting that active Ras is indispensable for Stk40 to induce differentiation. In addition, GST-RBD (Ras Binding Domain) pull-down assays showed that activated Ras was dramatically increased in Stk40 overexpressed cells to a level comparable to that in RasT35S-expressed cells (Fig. 3C). Phosphorylation of Erk1/2 (pErk1/2) and Mek1/2 (pMek1/2) was also enhanced when Stk40 or RasT35S was overexpressed, whereas U0126 abolished Stk40-activated pErk1/2 (Fig. 3D and Fig. S3C). Together, these data provide evidence that Stk40 is an activator of the Erk/MAPK pathway through which it induces ESC differentiation into the ExEn lineage.
Fig. 3.
Forced expression of Stk40 induces ESC differentiation through activation of the Erk/MAPK pathway. (A) Phase–contrast images of vector- or Stk40 overexpressed E14T cells treated with DMSO or the Mek1/2 inhibitor, U0126 (Magnification: ×20). (B) Phase–contrast images of E14T cells stably expressing vector, Stk40, or Ras constitutively active form (RasT35S) or coexpressing Stk40 and Ras dominant negative form (RasS17N). (Scale bar: 100 μm.) (C) Western blot analysis of GST-RBD–bound activated Ras in E14T ESCs stably expressing vector, Stk40, or RasT35S. Asterisk, nonspecific bands recognized in GST-RBD–bound groups. (D) Western blot analysis of pErk1/2 in E14T cells stably expressing vector, Stk40, or RasT35S with or without U0126 treatment as indicated. WB, Western blot; WCL, whole cell lysate.
Stk40 Associates with Rcn2 to Activate the Erk/MAPK Pathway.
To understand how Stk40 activates the Erk/MAPK pathway, we searched for proteins associated with Stk40. Among candidate proteins identified by affinity chromatography and mass spectrometric analysis (Fig. S4A, column 2), we were particularly interested in an intracellular Ca2+ binding protein, Rcn2 (23), because it was recently reported to be highly expressed in the mouse ExEn lineage (24). Direct interaction between Stk40 and Rcn2 was verified by GST pull-down assays using bacterially expressed GST-Rcn2 and His-Stk40 in vitro (Fig. 4A Top). Moreover, the formation of protein complexes between Rcn2 and Stk40 in mouse ESCs was shown by coimmunoprecipitation of endogenously expressed Rcn2 with ectopically expressed Flag-tagged Stk40 (Fig. 4B).
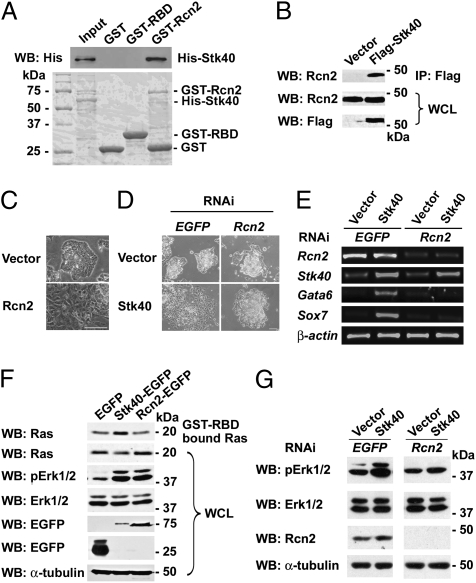
Fig. 4.
Rcn2 is a Stk40-interacting protein implicated in Stk40-induced Erk1/2 activation and ExEn differentiation. (A) Rcn2 associates with Stk40 directly. (Top) Reaction products from GST pull-down assays were analyzed by Western blotting with anti-His antibody. (Bottom) Coomassie blue staining of SDS/PAGE gel containing proteins included in the reaction. The experiment was repeated three times. (B) Flag-tagged Stk40 forms protein complexes with endogenous Rcn2 in E14T ESCs. The representative result of three coimmunoprecipitation experiments is shown. (C) Overexpression of Rcn2 induces ESC differentiation. Phase–contrast images of vector- or Rcn2-expressed E14T ESCs are shown. (Scale bar: 100 μm.) (D) Knockdown of Rcn2 blocks Stk40-induced ESC differentiation. Phase–contrast images of vector- or Stk40 over-expression in the stable EGFP or Rcn2 RNAi E14T ESCs are shown. (Scale bar: 100 μm.) (E) Gene expression levels in the cells described in D were analyzed by RT-PCR. (F) Rcn2 can activate Erk1/2 but not Ras. The representative result of three independent Western blot analyses of GST-RBD–bound activated Ras and pErk1/2 in EGFP-, Stk40-EGFP–, or Rcn2-EGFP–expressed E14T cells is shown. (G) Western blot analysis reveals that knockdown of Rcn2 abrogates Stk40-activated Erk1/2. The representative result of three independent experiments is shown.
Interestingly, ESCs overexpressing Rcn2 displayed morphology indistinguishable from that of Stk40 overexpressed cells (Fig. 4C). Like Stk40-induced differentiation, expression of Gata6 and Sox7 was up-regulated in Rcn2-transfected cells (Fig. S4B). However, knockdown of Rcn2 expression by specific RNAi prevented Stk40 from inducing ESC differentiation and activating Gata6 and Sox7 expression (Fig. 4 D and E). In addition, like Stk40, Rcn2 was able to activate Erk1/2 (Fig. 4F). However, unlike Stk40, Rcn2 could not activate Ras. Nevertheless, knockdown of Rcn2 expression substantially attenuated Stk40-activated pErk1/2 (Fig. 4G). Altogether, our data reveal that Rcn2 is sufficient to activate the Erk/MAPK pathway and is indispensable for Stk40 to induce ESC differentiation into ExEn lineages.
Rcn2 Is a Developmentally Regulated Protein Required for ExEn Differentiation.
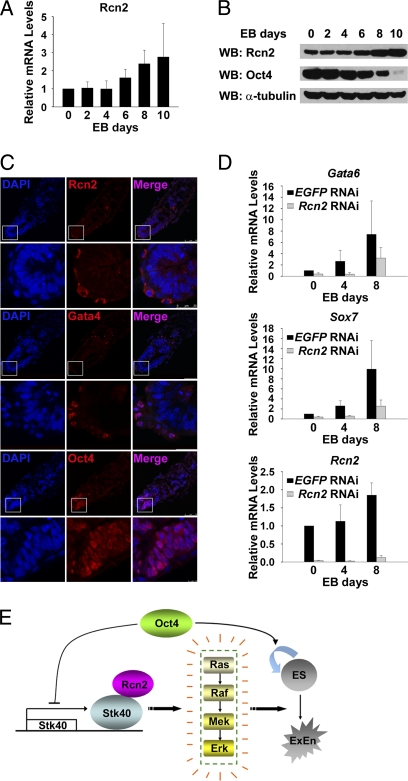
To determine the Rcn2 expression pattern, we first examined its expression levels during ESC differentiation in vitro. Transcript and protein levels of Rcn2 gradually increased along with the differentiation process of EBs (Fig. 5 A and B). Interestingly, Rcn2 was also highly expressed in extraembryonic endoderm cells (Fig. S5A), which are derived from mouse blastocysts and retain properties of the primitive endoderm (25). During early mouse development in vivo, cytoplasmic Rcn2 proteins were readily detected in the endoderm layer of yolk sacs at E6.5 and E7.5 but not in embryos at E3.5 (Fig. 5C, Fig. S5B, and Fig. S5C). In parallel, nuclear Gata4 staining was also found in the ExEn cells. In contrast, Oct4 was exclusively expressed in the nucleus of epiblast cells. The specific expression of Rcn2 in ExEn cells of the embryo suggests its possible role in ExEn lineage specification. Indeed, knockdown of Rcn2 markedly reduced induction of the ExEn markers during EB formation (Fig. 5D). In addition, our immunofluorescence study showed substantial enhancement of Rcn2 staining in Stk40 overexpressing differentiated cells (Fig. S5D). This is consistent with our finding that Stk40 overexpressing cells, which were injected into host blastocysts, expressed cytoplasmic Rcn2 in chimeric embryos (Fig. S5E). Collectively, our data suggest that Rcn2 might be an important protein in ExEn lineage specification in both ESC differentiation in vitro and early development in vivo.
Fig. 5.
Rcn2 is a developmentally regulated protein required for ExEn differentiation. (A) Quantification of dynamic Rcn2 mRNA levels in EBs aggregated from E14T cells for indicated days. All values are shown as means ± SD of results from four independent experiments. (B) Western blot analysis of Rcn2 protein levels in the cells described in (A). A representative result of three independent experiments is shown. (C) Rcn2 proteins are detected in frozen sections of mouse embryos at E6.5. Immunofluorescent images of Rcn2, Gata4, and Oct4 proteins are shown. (Scale bar: 75 μm.) (Inset) Enlarged view at the distal part of ExEn layers covering the epiblast. (Scale bar: 25 μm.) (D) Knockdown of Rcn2 by RNAi in E14T cells reduces induction of ExEn–marker expression during EB formation. Values of qPCR results from three independent experiments are shown as means ± SD. (E) A proposed model for the function of Stk40 in ExEn differentiation.
Discussion
Primitive endoderm is the first cell lineage segregated from the inner cell mass at implantation, and the receptor tyrosine kinases (RTKs)-Ras-MAP kinase pathway is known to be required for this segregation (22, 26). Mutation of Grb2, an adaptor molecule downstream of several RTKs, results in loss of the primitive endoderm lineage in vivo and in vitro (27). Ras has also been shown to play a crucial role in initiation of ExEn differentiation in mouse ESCs (22). However, the manner in which transcription factors such as Oct4 and Nanog are integrated with the Erk/MAPK signaling to control pluripotency and lineage restriction has not been well explored. Our finding provides an example of genetic interaction between core-transcription factors and the Ras-MAPK signaling in ESCs. Suppression of Stk40 expression could be one of the mechanisms through which Oct4 prevents pluripotent cells from differentiating. Moreover, Stk40 could also be a common target of Oct4, Sox2, and Nanog. In fact, the genomic sequence of Stk40 was also reported to associate with Oct4 as well as Sox2 and Nanog in a recent ChIP-chip report (28). The combinational control of Stk40 expression by at least three transcriptional factors could explain the phenomenon that Stk40 expression was gradually up-regulated when Oct4 expression was rapidly silenced, because Sox2 and Nanog expression was also gradually down-regulated during this process. Therefore, ESC differentiation is regulated by a controlled expression of the MAPK signaling-pathway components and is integrated to external cues through an Oct4-coordinated intrinsic transcriptional network.
Another significant advance of this study is the discovery of two proteins capable of activating the Erk/MAPK signaling pathway to induce ESC differentiation into the ExEn lineage. We found that Stk40 can activate the Erk1/2 through Rcn2. A recent microarray-based screening identified Rcn2, together with Gata6 and Dab2, as a potential primitive endoderm-specific gene (24). Supporting this report, we found that cytoplasmic Rcn2 is up-regulated during ESC differentiation in parallel with Stk40, and it is expressed in the ExEn cells of early embryos in vivo. Although both proteins can activate Erk1/2 and induce ESC differentiation when overexpressed in ESCs, only Stk40 was able to activate Ras. This may explain our observation that Stk40 exerts more efficient induction of ESC differentiation than Rcn2. This also implies that other not yet identified molecular mechanisms exist for activation of Ras by Stk40. However, knockdown of Rcn2 was sufficient to block Stk40-induced activation of Erk1/2 and ESC differentiation, suggesting that Rcn2 functions downstream of Stk40. Furthermore, we found that expression of Rcn2 RNAi could partially block RasT35S-mediated induction of Gata6 expression in ESCs (Fig. S6 A and C). However, similarly to Stk40, Rcn2-mediated activation of Gata6 expression was abrogated when RasS17N was coexpressed (Fig. S6 B and D). These observations suggest that Ras and Rcn2 might be functionally dependent on each other in this process. The incomplete blockage of RasT35S-activated Gata6 expression could be explained by the possibility that there are additional factors associated with Ras-induced ExEn differentiation in addition to Rcn2. Currently, we do not know how Rcn2 expression is regulated. However, we speculate that Stk40 regulates the function or protein stability of Rcn2 posttranslationally based on our observations that Stk40 and Rcn2 interacted directly and that Rcn2 protein levels were elevated when Stk40 was overexpressed. However, the exact mechanisms by which Stk40 and Rcn2 stimulate Erk1/2 remain unknown. Other factors in addition to Erk1/2 are most likely important in this process. Further investigation will greatly facilitate our interpretation of early embryogenesis at a molecular level.
In addition to the ExEn lineage, we also detected elevated expression of some trophectoderm marker genes after overexpression of Stk40, which is in agreement with a recent finding that inducible induction of Ras at low levels promoted expression of marker genes for both trophectoderm and primitive endoderm lineages (29). However, they also found that trophectoderm markers predominated at higher levels of Ras (29). Thus, it seems that the effect of Ras activation is dependent on developmental and cellular contexts as well as its activation levels. FGF stimulation of the Erk/MAPK signaling pathway has been considered to be the primary stimulus for undifferentiated ESCs to exit the self-renewal state and enter a state responsive to inductive signals for germ-layer segregation as well as blastocyst-lineage formation in vivo (30, 31). However, pluripotent embryonic cells at various stages of development would respond to the FGF/RAS/Erk1/2 signal differentially and develop into distinct lineages. Toyooka et al. (32) reported that an undifferentiated ESC culture is a heterogeneous population containing subpopulations corresponding to the inner cell mass, epiblast, and primitive ectoderm. Therefore, the lineage to which ESCs would differentiate in response to activation of the FGF/RAS/Erk1/2 cascade may be dependent on the epigenetic setting of ESCs. Furthermore, the activity level of the FGF/RAS/Erk1/2 cascade would also play an important role in cell-fate specification, because the balance among various signaling pathways, such as leukemia-inhibitory factor, TGFβ superfamily members, and FGF signal, would exert a great influence on the fate of cells at a specific developmental stage.
In conclusion, our data reveal that the ESC master regulator Oct4 suppresses expression of Stk40, which plays an active role in induction of ESC differentiation into ExEn cells through the Erk/MAPK signaling pathway, partially through its direct interaction with Rcn2 (Fig. 5E). The study provides insights into molecular mechanisms by which Oct4 maintains ESCs in an undifferentiated state and identifies two proteins important for ExEn development. Further studies will greatly expand our understanding of ESC self-renewal and lineage-specific differentiation.
Materials and Methods
E14T, CGR8, and ZHBTc4 mouse ESCs (Austin Smith) and F9 EC cells (ATCC, Rockville, MD, USA) were grown under feeder-free conditions as previously described (13, 33). ESCs were transiently transfected with LipofectAMINE2000 (Invitrogen) according to the manufacturer’s instructions. For stable cell lines, the cells were transfected by electroporation and selected with antibiotics.
Additional materials and methods as well as PCR primers are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Dr. Randall Mckinnon, Dr. Dangsheng Li, and Dr. Guoliang Xu for their critical reading and suggestions in preparation of this manuscript. The authors also thank Dr. Fengqin Fang for Stk40 orthologs analysis. The study was supported by grants from the National Natural Science Foundation (30570907, 30730051, and 30800660), National High Technology Research and Development Program of China (2006CB94390, 2007CB947904, and 2007CB947101), and Shanghai Science & Technology Developmental Foundations (08JC1413100, 05XD14007, and 0754S21512). The study was also supported by the Shanghai Leading Academic Discipline Project (S30201).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905657107/DCSupplemental.
References
- 1.Rossant J. Stem cells and lineage development in the mammalian blastocyst. Reprod Fertil Dev. 2007;19:111–118. doi: 10.1071/rd06125. [DOI] [PubMed] [Google Scholar]
- 2.Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- 3.Shimosato D, Shiki M, Niwa H. Extra-embryonic endoderm cells derived from ES cells induced by GATA factors acquire the character of XEN cells. BMC Dev Biol. 2007;7:80–91. doi: 10.1186/1471-213X-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43:183–205. [PubMed] [Google Scholar]
- 5.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 6.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 8.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 9.Palmieri SL, Peter W, Hess H, Schöler HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 10.Nichols J, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 11.Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–235. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]
- 12.Zafarana G, Avery SR, Avery K, Moore HD, Andrews PW. Specific knockdown of OCT4 in human embryonic stem cells by inducible short hairpin RNA interference. Stem Cells. 2009;27:776–782. doi: 10.1002/stem.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 14.Strumpf D, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- 15.Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol Cell Biol. 2000;20:530–541. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh AM, Hamazaki T, Hankowski KE, Terada N. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells. 2007;25:2534–2542. doi: 10.1634/stemcells.2007-0126. [DOI] [PubMed] [Google Scholar]
- 17.Chambers I, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 18.Rula ME, et al. Cell autonomous sorting and surface positioning in the formation of primitive endoderm in embryoid bodies. Genesis. 2007;45:327–338. doi: 10.1002/dvg.20298. [DOI] [PubMed] [Google Scholar]
- 19.Yang DH, Cai KQ, Roland IH, Smith ER, Xu XX. Disabled-2 is an epithelial surface positioning gene. J Biol Chem. 2007;282:13114–13122. doi: 10.1074/jbc.M611356200. [DOI] [PubMed] [Google Scholar]
- 20.Afouda BA, Ciau-Uitz A, Patient R. GATA4, 5 and 6 mediate TGFbeta maintenance of endodermal gene expression in Xenopus embryos. Development. 2005;132:763–774. doi: 10.1242/dev.01647. [DOI] [PubMed] [Google Scholar]
- 21.Chen YL, Li XF, Eswarakumar VP, Seger R, Lonai P. Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene. 2000;19:3750–3756. doi: 10.1038/sj.onc.1203726. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida-Koide U, et al. Involvement of Ras in extraembryonic endoderm differentiation of embryonic stem cells. Biochem Biophys Res Commun. 2004;313:475–481. doi: 10.1016/j.bbrc.2003.11.138. [DOI] [PubMed] [Google Scholar]
- 23.Imai T, et al. ERC-55, a binding protein for the papilloma virus E6 oncoprotein, specifically interacts with vitamin D receptor among nuclear receptors. Biochem Biophys Res Commun. 1997;233:765–769. doi: 10.1006/bbrc.1997.6531. [DOI] [PubMed] [Google Scholar]
- 24.Gerbe F, Cox B, Rossant J, Chazaud C. Dynamic expression of Lrp2 pathway members reveals progressive epithelial differentiation of primitive endoderm in mouse blastocyst. Dev Biol. 2008;313:594–602. doi: 10.1016/j.ydbio.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 25.Kunath T, et al. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- 26.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Cheng AM, et al. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu CW, et al. Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet. 2008;40:921–926. doi: 10.1038/ng.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunath T, et al. FGF stimulation of the Erk1/2 signaling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 31.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 32.Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Liao B, Xu M, Jin Y. Post-translational modification of POU domain transcription factor Oct-4 by SUMO-1. FASEB J. 2007;21:3042–3051. doi: 10.1096/fj.06-6914com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.