Abstract
Nuclear fusion is an essential process in the sexual reproduction of animals and plants. In flowering plants, nuclear fusion occurs three times: once during female gametogenesis, when the two polar nuclei fuse to produce the diploid central cell nucleus, and twice during double fertilization. The yeast Ig binding protein (BiP) is a molecular chaperone Hsp70 in the endoplasmic reticulum that regulates nuclear membrane fusion during mating. Here we report that in Arabidopsis thaliana, BiP is involved in the fusion of polar nuclei during female gametophyte development. BiP-deficient mature female gametophytes contain two unfused polar nuclei, in spite of their close contact. This indicates a surprising conservation of BiP function in nuclear fusion between plants and yeasts. We also found that endosperm nuclear division becomes aberrant after fertilization of the BiP-deficient female gametophytes with wild-type pollen. This is experimental evidence for the importance of fusion of the polar nuclei in the proliferation of endosperm nuclei.
Keywords: endoplasmic reticulum, molecular chaperone, nuclear fusion, fertilization, female gametogenesis
Nuclear fusion is the process by which two nuclei fuse to produce a single nucleus. This process is essential for the sexual reproduction of various organisms including animals and plants. During the life cycle of angiosperms, nuclear fusion occurs three times: twice during double fertilization, when two sperm cells fertilize the egg and the central cell, and once during the development of female gametophytes (1).
The female gametophyte, also referred to as the embryo sac, develops within the ovule. The developmental pattern of female gametophytes in most angiosperm species, including Arabidopsis thaliana, is the Polygonum type: a single megaspore produced by meiosis undergoes three rounds of mitosis to produce an eight-nucleate cell. The subsequent migration of nuclei and cellularizations result in a seven-celled female gametophyte consisting of one egg cell, two synergid cells, three antipodal cells, and one central cell containing two polar nuclei (2). In A. thaliana and other species, the polar nuclei fuse before pollination to form the secondary nucleus in the central cell.
Mutants affecting the fusion of polar nuclei have been isolated; however, little is known about the molecular mechanisms of this fusion process (3 –6). The mating of budding yeast involves one of the best-characterized nuclear fusion processes in eukaryotic cells. In yeast, the Ig binding protein (BiP), which is a molecular chaperone Hsp70 in the endoplasmic reticulum (ER), was shown to play key roles in the nuclear membrane fusion process (7, 8). Furthermore, a nuclear membrane protein that possibly functions in this process was identified (9). This prompted us to assess the effects of mutations in BiP on the fusion of polar nuclei during female gametophyte development in A. thaliana.
A. thaliana contains three BiP genes (10) (BiP1, BiP2, and BiP3/BiP-L), two of which (BiP1 and BiP2) encode proteins that are 99% identical with each other and are expressed ubiquitously. In contrast, BiP3 encodes a less-conserved BiP paralog (80% identical to BiP1 and BiP2) and is expressed only under ER stress conditions, such as those caused by tunicamycin treatment (10). We report here that female gametophytes containing the bip1 bip2 double mutation are specifically defective in the fusion of polar nuclei during their development, indicating a striking conservation of the role of BiP in nuclear fusion between plants and yeasts. We also found that the proliferation of endosperm nuclei became aberrant after fertilization of the BiP-deficient female gametophyte with wild-type pollen, indicating the importance of the fusion of polar nuclei in the proliferation of endosperm nuclei.
Results and Discussion
BiP Is Required for the Fusion of Polar Nuclei During Female Gametogenesis.
We obtained mutant lines, each carrying one of four BiP1 alleles (bip1-1, bip1-2, bip1-3, and bip1-4), two BiP2 alleles (bip2-1 and bip2-2), and one BiP3 allele (bip3-1), from the T-DNA insertion collections (11 –13) (Fig. S1A). None of the single homozygous plants showed any obvious defects during plant growth and development or any notable mutant allele transmission defects. Expression analysis showed that bip1-2, bip1-3, bip1-4, bip2-1, and bip3-1 are null alleles, and bip1-1 is a knock-down allele (Fig. S1B). We thus decided to use bip1-4 (b1), bip2-1 (b2), and bip3-1 (b3) for further analyses of BiP1, BiP2, and BiP3 functions.
To obtain double mutants for the BiP genes, we produced F1 plants by crossing b1 with b2, b1 with b3, and b2 with b3. The F2 siblings produced by self-fertilization of the F1 plants were genotyped using PCR. Although we obtained b1/b1 b3/b3 and b2/b2 b3/b3 double mutant plants, no double homozygous b1/b1 b2/b2 plants were found among the 45 screened F2 lines, indicating that BiP1 and BiP2 share essential but redundant functions. Self-pollinated b1/+ b2/b2 and b1/b1 b2/+ plants produced siliques containing approximately 50% aborted seeds (Fig. 1 A and B; Table S1). In reciprocal crossing experiments, seed abortion was observed only when b1/+ b2/b2 or b1/b1 b2/+ pistils were pollinated with wild-type pollen (Table S1). These results raise the possibility that female gametophytes containing both the b1 and b2 alleles are defective. PCR genotyping confirmed that b1 and b2 could not be cotransmitted through the female (Table S2). Essentially, the same results were obtained when we used plants carrying the bip1-2 or bip1-3 alleles, but not the bip1-1 allele, instead of b1 (Table S1). The bip1-1 bip2-1 double homozygous mutant is viable and grows as well as wild-type plants (Fig. S1 C and D). We did not observe a fertility defect in this double mutant (Table S1).
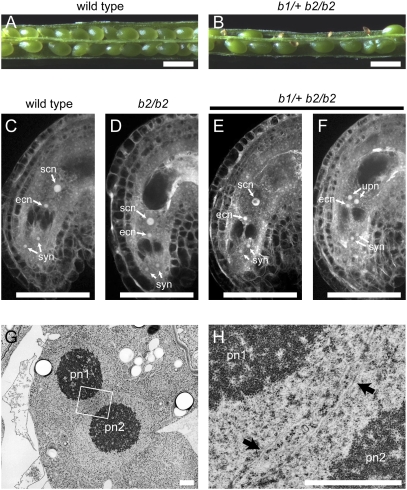
Fig. 1.
The bip1 bip2 double mutation causes defects in the fusion of polar nuclei during female gametophyte development. (A and B) Siliques of wild-type (A) and b1/+ b2/b2 (B) plants at 8 DAP. Half of the seeds in the b1/+ b2/b2 siliques aborted during development. (Scale bars, 0.5 mm.) (C–F) CLSM analysis of ovules in wild-type (C), b2/b2 (D), and b1/+ b2/b2 (E and F) plants. Half of the ovules in b1/+ b2/b2 plants are morphologically normal (E), and in the other half, the polar nuclei remain unfused (F). syn, synergid nuclei; ecn, egg nucleus; scn, secondary nucleus; upn, unfused polar nuclei. (Scale bars, 50 μm.) (G and H) Electron micrographs of the central cell of a b1 b2 female gametophyte containing unfused polar nuclei. The region indicated by a box in G is magnified in H. The arrows indicate the nuclear envelopes of the two polar nuclei. pn1, polar nucleus 1; pn2, polar nucleus 2. (Scale bars, 1 μm.)
The b1/+ b2/b2 qrt1/qrt1 plants produced pollen tetrads consisting of four viable pollen grains containing morphologically normal sperm and vegetative nuclei (Fig. S2 A–I). There were no significant differences in the pollen germination rates of tetrads from wild-type (qrt1/qrt1) and b1/+ b2/b2 qrt1/qrt1 plants (Fig. S2J), indicating that the bip1 bip2 double mutation does not affect the formation of viable pollen. However, we observed significant decreases in the cotransmission of the b1 and b2 alleles through the male (Table S2), indicating that the double mutant pollen is less competitive for fertilization. A detailed analysis of the BiP functions in pollen will be reported elsewhere.
We analyzed the female gametophytes of b1/+ b2/b2 plants and found that the bip1 bip2 double mutation affects the fusion of polar nuclei during female gametophyte development. An analysis of the wild-type A. thaliana mature FG7 stage (14) female gametophyte by confocal laser-scanning microscopy (CLSM) showed four nuclei at the micropylar pole of the embryo sac: one egg nucleus, two synergid nuclei, and one secondary nucleus of the central cell (Fig. 1C) (2, 14). The antipodal cells degenerate by the FG7 stage of female gametophyte development and are not visible here. When we analyzed emasculated b1/+ b2/b2 mutant pistils, we found that 48% (n = 306) of the ovules contained unfused polar nuclei (Fig. 1F) even though the terminal positions of the various nuclei within the embryo sac (and by inference, their migrations during female gametophyte development) were normal. These results indicate that failure of the polar nuclei to fuse is the only visible defect during female gametophyte development. The remainder of the ovules (52%) contained a single secondary nucleus in their central cells (Fig. 1E). Furthermore, the failure of polar nuclei to fuse was not observed in the b2/b2 single mutant female gametophytes (Fig. 1D).
We introduced a construct containing the BiP1 cDNA driven by the BiP1 promoter (pBiP1::BiP1) into b1/+ b2/b2 plants. Four transgenic T1 lines containing the b1 allele were obtained, and all lines showed approximately 20% increases in seed set (approximately 76%, n > 200) when compared with the levels of seed set in the b1/+ b2/b2 plants (56.2%, Table S1). We isolated b1/b1 b2/b2 plants that were also homozygous for pBiP1::BiP1 from the T2 seedlings. In the pistils of these plants, 98.2% of the ovules contained a single secondary nucleus (n = 167), indicating the complete rescue of the mutant phenotype by the BiP1 cDNA. These results indicate that expression of the pBiP1::BiP1 construct complemented the defect in fusion of the polar nuclei, and that this defect arose from the bip1 bip2 double mutation. We produced BiP1 cDNA constructs driven by the promoters of various genes expressed in specific cells of the female gametophyte (15) and used the constructs to transform b1/+ b2/b2 plants. We found that BiP1 expression in the central cell, but not in other female gametophytic cells, suppressed the seed abortion phenotype (Table S3). Consistent with these observations, the expression of BiP1 in the central cell via the DD65 promoter resulted in suppression of the defect in fusion of the polar nuclei. In pistils of the b1/+ b2/b2 plants that were also homozygous for pDD65::BiP1, 98.0% of ovules contained a single secondary nucleus in the central cell (n = 450). Therefore, the loss of BiP1 and BiP2 expression in the central cell before fertilization leads to the defect in fusion of the polar nuclei.
Because BiP functions in the nuclear membrane fusion step during yeast mating (7), we examined whether A. thaliana BiP also functions in the same process in the fusion of polar nuclei. Transmission electron microscopy indicated that the unfused polar nuclei in the b1 b2 female gametophyte were in close contact, but no membrane fusion took place (Fig. 1 G and H). Thus, the loss of BiP function in the A. thaliana female gametophyte caused a defect nuclear membrane fusion. This result suggests that the function of BiP in promoting nuclear membrane fusion is conserved between yeasts and plants.
BiP-Deficient Female Gametophytes Are Not Defective in Fertilization.
Several A. thaliana mutants that are defective in the fusion of polar nuclei were also reported to be defective in other processes before fertilization (3, 4, 6). For example, pollen tube guidance into the ovule was affected in the maa mutants (3), and a role for the central cell in pollen tube guidance has been proposed (16). We therefore analyzed pollen tube guidance in b1/+ b2/b2 plants, using pollen from a transgenic line expressing a β-glucuronidase (GUS) construct driven by the BiP1 promoter, which is expressed in the pollen tubes. However, the bip1 bip2 double mutation did not impair pollen tube guidance (Fig. 2 A and B). The mutant gfa2 is defective in both the fusion of polar nuclei and the synergid degeneration process (4), which takes place after pollen tube arrival but before double fertilization in wild-type plants (2). However, the bip1 bip2 double mutation did not affect synergid cell degeneration (Fig. 2 C–E).
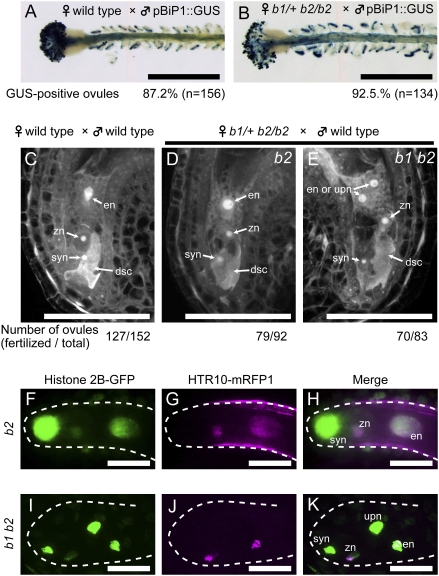
Fig. 2.
The bip1 bip2 female gametophyte is not defective in fertilization. (A and B) The bip1 bip2 double mutation does not affect pollen tube guidance. Wild-type (A) or b1/+ b2/b2 (B) pistils were pollinated using pollen from a pBiP1::GUS transgenic plant. Pollen tube entry into the female gametophytes was visualized by GUS staining at 1 DAP (blue color). The percentages of GUS-positive ovules are shown below the panels. (Scale bars, 0.5 mm.) (C–E) Pistils of wild-type (3 pistils) or b1/+ b2/b2 (4 pistils) plants were pollinated with wild-type pollen. Ovules were fixed at 7 h after pollination, cleared, and observed by CLSM. Representative images of ovules containing degenerated synergid cells are shown. Each wild-type ovule contains a endosperm nucleus in the central cell (C), whereas the b1/+ b2/b2 pistils have two types of ovules, one type (b2) containing a secondary nucleus (D) and the other (b1 b2) containing unfused polar nuclei (E) in the central cell. The numbers of fertilized ovules (containing degenerated synergid cells) and total ovules observed are shown below the panel. (F–K) Pistils of a b1/+ b2/b2 plant expressing histone H2B-GFP from the ACTIN11 promoter were pollinated with wild-type pollen expressing HTR10-mRFP1. Ovules were dissected from the pistils and observed by CLSM at 8 h after pollination. (F–H) Images of a fertilized b2 ovule. (I–K) Images of a b1 b2 ovule. (F and I) GFP fluorescence (green), (G and J) mRFP fluorescence (magenta), and (H and K) merged images. The dashed lines indicate the edges of the embryo sacs. syn, synergid nucleus; dsc, degenerated synergid cell; en, endosperm nucleus; zn, zygote nucleus; upn, unfused polar nucleus. (Scale bars, 50 μm.)
In addition to the fusion of polar nuclei, two distinct nuclear fusion events take place during double fertilization in plants: the two sperm cells released by the pollen tube fertilize the egg and the central cell. The nfd1 mutant is defective in these nuclear fusion processes; sperm nuclei were found to persist within the egg and the central cell when nfd1/+ pistils were pollinated with wild-type pollen (6). To analyze sperm nuclear fusion in the bip mutants, we generated transgenic b1/+ b2/b2 plants producing histone H2B-GFP (17) in their female gametophytes and crossed with wild-type plants producing HTR10-monomeric red fluorescent protein 1 (mRFP1) (18) in their pollen. After fertilization of a b2 ovule (i.e., one with fused polar nuclei) with the wild-type pollen, both the zygote and the endosperm contained single nuclei, which were each labeled with both GFP and monomeric red fluorescent protein (mRFP) (Fig. 2 F–H) (18). Similar results were obtained when b1 b2 ovules producing histone H2B-GFP were fertilized with wild-type pollen producing HTR10-mRFP1. We observed colocalization of the GFP and mRFP signals both in the egg cell and in one of the two unfused polar nuclei (Fig. 2 I–K). Importantly, we did not observe unfused sperm nuclei in any of the b1 b2 female gametophytes with a degenerated synergid (n = 70, Fig. 2E). These results indicate that the sperm nuclei from the wild-type pollen fused with the egg nucleus and one of the unfused polar nuclei in the b1 b2 female gametophyte. Consistent with this, we observed the initiation of embryo and endosperm development in all fertilized ovules of b1/+ b2/b2 pistils after pollination with wild-type pollen (Fig. 3 A–H).
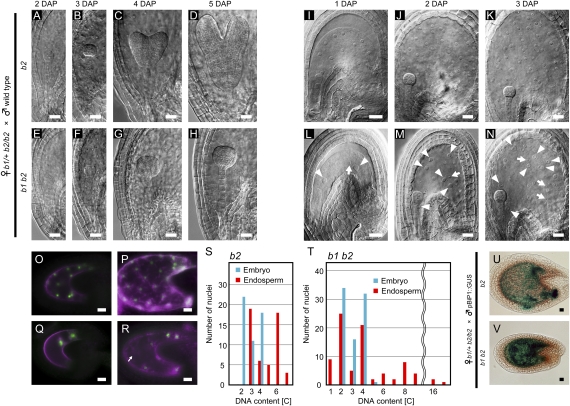
Fig. 3.
Fusion of polar nuclei during female gametogenesis is essential for normal proliferation of endosperm nuclei. (A–H) Embryo development after b2 female gametophytes were fertilized with wild-type pollen (b2 seeds, A–D) and after b1 b2 female gametophytes were fertilized with wild-type pollen (b1 b2 seeds, E–H). (A and E) 2 DAP embryo sacs. (B and F) 3 DAP embryo sacs. (C and G) 4 DAP embryo sacs. (D and H) 5 DAP embryo sacs. (I–N) Endosperm development after b2 female gametophytes were fertilized with wild-type pollen (b2 seeds, I–K), and after b1 b2 female gametophytes were fertilized with wild-type pollen (b1 b2 seeds, L–N). (I) and (L) 1 DAP embryo sacs. (J and M) 2 DAP embryo sacs. (K and M) 3 DAP embryo sacs. Arrowheads and arrows indicate endosperm nuclei that are larger and smaller than the ones observed in wild-type seeds, respectively. (O–R) Expression of paternally derived AGL62-GFP in the b2 (O and P) or b1 b2 seeds (Q and R). Pistils of the b1/+ b2/b2 plants were pollinated with pollen from the AGL62-GFP transgenic plant, and the GFP fluorescence (green) was observed at 1 DAP (O and Q) and 2 DAP (P and R). Chlorophyll autofluorescence appears as a magenta color. The arrow indicates a nuclear cytoplasmic domain without a GFP signal in the nucleus. (S and T) The DNA contents of embryo nuclei (blue bars) and peripheral endosperm nuclei (red bars) in the b2 (S, data from 2 seeds) and b1 b2 seeds (T, data from 3 seeds). The DNA contents of the nuclei were examined in optical sections of the seeds stained with propidium iodide. The DNA contents of at least 17 diploid sporophytic nuclei in the integument cells in the same seeds were used as references for the diploid DNA content. (U and V) Expression of a paternally derived BiP1::GUS transgene in the b2 (U) and b1 b2 (V) seeds at 3 DAP. (Scale bars, 20 μm.)
A simple interpretation of the above results is that the loss of both the BiP1 and BiP2 functions does not affect nuclear fusion during double fertilization. Alternatively, the BiP protein supplied by wild-type pollen could have complemented the nuclear fusion defect during double fertilization of the BiP-deficient female gametophytes. However, we believe that this is most likely not the case. Self-fertilized b1/+ b2/b2 pistils did not have any unfertilized ovules, but contained only fertilized seeds that were either green or aborted (Table S1).In addition, we observed the initiation of embryo and endosperm development in all fertilized ovules in self-pollinated b1/+ b2/b2 pistils. These observations suggest that sperm nuclei from b1 b2 double mutant pollen fused with nuclei in the egg cell and the central cell of b1 b2 double mutant female gametophytes. Taken together, we conclude that the bip1 bip2 double mutation specifically affects polar nuclei fusion. It should be noted that in the experiment shown in Fig. 2 (H–K), we observed an unfused polar nucleus labeled only with histone H2B-GFP in addition to an endosperm nucleus labeled with both histone H2B-GFP and HTR10-mRFP1 (Fig. 2 I–K). This suggests that the unfused polar nucleus does not have the ability to fuse with the endosperm nucleus.
Aberrant Proliferation of Endosperm Nuclei After Fertilization of bip1 bip2 Female Gametophytes with Wild-Type Pollen.
As shown above, genetic analyses revealed that the bip1 bip2 double mutation in female gametophytes caused seed abortion after fertilization by wild-type pollen. Pistils of the b1/+ b2/b2 plants pollinated with wild-type pollen contain approximately 50% aborted seeds (Table S1). The observed seed abortion phenotype was probably due to abnormal development of the embryo and/or the endosperm (2, 19). We therefore analyzed embryos in the seeds produced by the fertilization of double mutant (b1 b2) ovules with wild-type pollen (designated as b1 b2 seeds hereafter) and found that embryonic development was arrested at the globular stage (Fig. 3 A–H). We also observed abnormalities in endosperm development in the b1 b2 seeds. In wild-type seeds, the division of endosperm nuclei is highly synchronized during the early phase of endosperm development (20). In contrast, the b1 b2 endosperm nuclei at 3 days after pollination (DAP) displayed irregularities in size and did not spread uniformly throughout the endosperm (Fig. 3N and Fig. S3). This was observed in all b1 b2 seeds (n = 55). We did not observe cellularization of the endosperm in either wild-type or b1 b2 seeds until 3 DAP.
We also observed significantly fewer endosperm nuclei in the b1 b2 seeds. Although wild-type seeds contained 53.4 ± 12.3 (n = 17) endosperm nuclei, the b1 b2 seeds contained 20.4 ± 7.9 (n = 20) endosperm nuclei at 2 DAP. This was presumably due to a delay in the onset of endosperm nuclear division in b1 b2 seeds because they contained only 3.4 ± 1.2 (n = 28) endosperm nuclei at 1 DAP, whereas wild-type seeds contained 13.8 ± 3.6 (n = 36) endosperm nuclei. It should be noted that the size variation in endosperm nuclei was already observed at this early developmental stage (Fig. 3 L and M). We next examined the proliferation of fertilized endosperm nuclei using paternally derived AGL62-GFP, which is expressed in syncytial nuclei during early endosperm development (21). In pistils of the b1/+ b2/b2 plants fertilized with pollen of the AGL62-GFP transgenic plant, the mean number of GFP-positive endosperm nuclei in wild-type seeds was 12.7 ± 4.1 (n = 9) and 52.2 ± 12.5 (n = 13) at 1 DAP and 2 DAP, respectively, suggesting that all nuclei were GFP positive (Fig. 3 O and P). However, only about half of the b1 b2 endosperm nuclei were GFP positive: 2.2 ± 0.6 (n = 8) and 9.2 ± 3.2 (n = 13) were GFP positive at 1 DAP and 2 DAP, respectively (Fig. 3 Q and R). Furthermore, we observed nuclear cytoplasmic domains that did not contain GFP-positive nuclei at 2 DAP in the b1 b2 endosperms (Fig. 3R). The presence of both GFP-positive and GFP-negative nuclei in the b1 b2 seeds indicates the proliferation of both diploid (fertilized) and haploid (unfertilized) nuclei.
We analyzed the DNA contents of embryo and endosperm nuclei by propidium iodide staining of 3 DAP seeds. In wild-type seeds, the nuclear DNA contents were in the range 2C to 4C in embryos and 3C to 6C, with peaks at 3C and 6C, in endosperms (Fig. 3S). In b1 b2 seeds, the DNA contents of embryo nuclei also ranged between 2C and 4C (Fig. 3T), indicating that the bip1 bip2 double mutation did not affect embryonic cell division. However, we observed significant variations in the DNA contents of endosperm nuclei in the b1 b2 seeds, ranging from 1C to 16C (Fig. 3T). These results also indicate aberrant endosperm nuclear division in the b1 b2 seeds. Because endosperm cellularization was not observed in the b1 b2 seeds, the observed variation in the DNA contents may be caused by premature endoreduplication.
Fusion of Polar Nuclei Is Essential for the Proper Proliferation of Endosperm Nuclei After Fertilization.
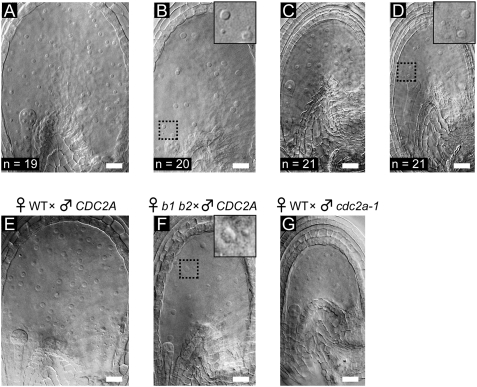
The aberrant proliferation of endosperm nuclei in the b1 b2 seeds may have been caused by the abnormal fusion of polar nuclei. The cdc2a-1 (cdka-1) mutant pollen (referred to here as cdc2a pollen) contains a single sperm-like cell that preferentially fuses with the egg cell during fertilization, leaving an unfertilized central cell (22, 23). Four types of seeds (types A–D) were produced in pistils of b1/+ b2/b2 plants pollinated with pollen from CDC2A/cdc2a-1 plants (Fig. 4). The type A seeds (Fig. 4A) were viable and looked identical to seeds produced from a wild-type cross. The other three types of seeds were small and eventually aborted. The type B seeds contained proliferating endosperm nuclei of variable sizes (Fig. 4B, Inset), which is characteristic of the b1 b2 seeds (Fig. 4F, Inset). The types C and D seeds (Fig. 4 C and D) were smaller than the type B seeds, but the same size as those produced by fertilization of a wild-type female gametophyte with cdc2a pollen (cdc2a seeds, Fig. 4G). Although the type C seeds and the cdc2a seeds contained endosperm nuclei of uniform size (Fig. 4 C and G), we observed size variations in the endosperm nuclei of type D seeds (Fig. 4D, Inset). Because the segregation ratio of these seed types was 1:1:1:1, the type D seeds were probably produced by fertilization of b1 b2 female gametophytes with cdc2a pollen. The above results indicate the importance of fusion of the polar nuclei for proper endosperm nuclear proliferation.
Fig. 4.
Size variation in the endosperm nuclei of the b1 b2 seeds without fertilization of the central cell. (A–D) Endosperm development after b1/+ b2/b2 pistils were pollinated with pollen of the CDC2A/cdc2a-1 plant. Four types of seeds were observed at 2 DAP. (A) Type A seed. (B) Type B seed. (C) Type C seed. (D) Type D seed. The number of each type of seed observed in two siliques is shown in each panel. (E) Wild-type seed produced by fertilization of a wild-type female gametophyte with CDC2A pollen at 2 DAP. (F) The b1 b2 seed produced by fertilization of a b1 b2 female gametophyte with wild-type pollen at 2 DAP. (G) The cdc2a seed produced by fertilization of a wild-type female gametophyte with cdc2a-1 pollen. (B, D and F Insets) Enlarged images of the framed areas. (Scale bars, 20 μm.)
Because BiP functions in essential cellular processes, including protein translocation across the ER (24), the repression of paternally-derived BiP genes could be the primary reason for the abnormal endosperm development in b1 b2 seeds. However, this is not the case, because (i) we observed expression of paternally derived BiP1 and BiP2 genes in both embryos and endosperms (Fig. S4), (ii) transcripts of the paternally derived BiP1 gene were detected as early as 1 DAP (Fig. S4A), and (iii) a paternally derived BiP1 promoter::GUS construct was expressed in the embryos and endosperms of the b1 b2 seeds (Fig. 3 U and V). The above results also indicate that the expression of paternally-derived BiP1 and BiP2 genes in the endosperm does not rescue the lethality of b1 b2 seeds.
In A. thaliana, mutations in FIS class genes, which function in maternal genome imprinting, also cause maternal effects on seed development. Maternal inheritance of fis perturbs endosperm development, and this cannot be rescued by pollination with wild-type pollen (19, 25). However, the maternal effect of b1 b2 on seed development appears to be different from the effects of fis class mutants (26). The fis class mutants are characterized by their defects in repression of PHERES1, which is the only known paternally expressed and maternally silenced plant gene (27). However, we did not observe such up-regulation of PHERES1 in b1 b2 seeds (Fig. S5). We conclude that all of our results together provide direct evidence for the importance of polar nuclear fusion during female gametophyte development for the proper regulation of nuclear division in endosperms after fertilization in A. thaliana.
Conclusions
We have found that the simultaneous mutation of both the BiP1 and BiP2 genes specifically disrupts the fusion of polar nuclei in female gametophytes, but not the fusion of sperm nuclei during double fertilization, in A. thaliana. Our results indicate a striking conservation of BiP function in nuclear fusion in yeasts and plants. By taking advantage of the fact that the b1 b2 ovules are not defective in fertilization with wild-type pollen, we provide evidence that the fusion of polar nuclei during female gametogenesis is essential for proper regulation of endosperm nuclear proliferation. Because the endosperm has essential functions in nurturing the growing embryo, the loss of synchronous nuclear division in the endosperm also causes defects in embryo development in the b1 b2 seeds. Further studies of endosperm development in the b1 b2 seeds will help to uncover molecular mechanisms that link polar nuclear fusion with endosperm nuclear proliferation.
Materials and Methods
Additional methods are given in the SI Text.
Plant Material and Growth Conditions.
A. thaliana qrt1-2 (CS8846; ecotype Columbia) was used as a wild-type strain in this study. Information on the T-DNA insertion mutants CS836493 (bip1-1), CS853146 (bip1-2), CS801763 (bip1-3), CS856879 (bip1-4), CS842467 (bip2-1), SALK_047956 (bip2-2), SALK_024133 (bip3-1), and SALK_106809 (cdc2a-1) can be obtained from the Salk Institute Genomic Analysis Laboratory (SIGnAL) website (http://signal.salk.edu). All of the seeds were provided by the Arabidopsis Biological Resource Center at Ohio State University, Columbus, OH. T-DNA insertions were verified by PCR using flanking region-specific primers (Table S4), which were designed using the SIGnAL iSect tool (http://signal.salk.edu/isects.html). The transgenic line expressing HTR10-mRFP1 from the HTR10 promoter (18) was provided by Frédéric Berger (Temasek Life Sciences Laboratory, Singapore). The AGL62-GFP expressing line (21) was provided by Gary Drews (Universtiy of Utah, Salt Lake City, UT). The b1/+ b2/b2 line expressing histone H2B-GFP from the ACTIN11 promoter was constructed by introducing pACTIN11::HISTONE H2B-GFP (a gift from Tetsuya Higashiyama, Nagoya University, Nagoya, Japan) into A. thaliana by the floral dip method (28). Seeds were surface-sterilized by chlorine gas and then sown on soil or Murashige-Skoog medium (Wako) containing 0.7% agar and 1% sucrose. Plants were grown at 22 °C under continuous light.
Confocal Laser-Scanning Microscopy.
The preparation of ovules or developing seeds for CLSM was performed as described by Christensen et al. (14). The b1/+ b2/b2 line expressing histone H2B-GFP from the ACTIN11 promoter was pollinated with wild-type pollen expressing HTR10-mRFP1 from the HTR10 promoter. Eight hours after pollination, ovules were mounted on glass slides in 80 mM sorbitol. Ovules were observed using a CSU10 confocal laser scanning system (Yokogawa Electric) equipped with a Kr/Ar laser mounted on a BX60 microscope (Olympus). The 488 nm laser line was used for observation of cleared ovules. The 488 nm and 568 nm laser lines were used to observe GFP and mRFP fluorescence, respectively. All images were captured using a Cool SNAP HQ2 cooled CCD camera (Photometrics) in 0.5 μm optical sections, and stacked using the Metamorph Ver. 7.5.2.0 software (Universal Imaging). Propidium iodide staining of seeds was performed as described by Ngo et al. (29). Samples were observed using a FV1000 confocal laser-scanning microscope (Olympus). The DNA contents of nuclei were examined in optical sections of the seeds stained with propidium iodide. The DNA contents of at least 17 diploid sporophytic nuclei in the integument cells in the same seeds were used as references for the diploid DNA content.
Analysis of Seed Development.
Developing seeds in siliques at 8 DAP were observed and photographed using a SteREO Lumar V12 stereomicroscope (Carl Zeiss). Developing embryos in seeds from 2 to 5 DAP were fixed in 10% acetic acid (vol/vol) and 90% ethanol (vol/vol) for at least 3 h and then rehydrated with an ethanol series. Samples were cleared in 70% (wt/wt) chloral hydrate and 8.8% (wt/wt) glycerol, and observed using a BX51 microscope (Olympus) equipped with a DP70 cooled CCD camera (Olympus).
Transmission Electron Microscopy.
Pistils were cut into 1-mm segments and fixed in 2% glutaraldehyde, 2% paraformaldehyde, and 30 mM hepes-KOH, pH 7.4, for 3 days at 4 °C. The tissue segments were washed in buffer and postfixed for 8 h in 2% aqueous osmium tetroxide at 4 °C. The tissue was then dehydrated in a graded ethanol series, transferred into propylene oxide, infiltrated, and embedded in Quetol 651. Thin sections (70 nm) were stained with 2% aqueous uranyl acetate and with lead citrate, and examined in a JEOL JEM 1200 EX electron microscope at 80 kV.
Image Processing.
All images were processed for publication using Adobe Photoshop CS Ver. 8.0.1 (Adobe Systems Inc.).
Supplementary Material
Acknowledgments
We thank F. Berger, G. Drews, T. Higashiyama, and T. Nakagawa for materials; Y. Hamamura for technical assistance; R. Palanivelu and T. Higashiyama for critically reading the manuscript; and members of the Endo Laboratory for useful discussions. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Areas (Grant No. 16085202) from the MEXT Japan (no. 1685101) to S.N, and by Grants-in-Aid for Scientific Research from the MEXT Japan to T.E.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905795107/DCSupplemental.
References
- 1.van Went JL, Willemse MTM. In: Embryology of Angiosperms. Johri B, editor. Berlin: Springer-Verlag; 1984. pp. 273–317. [Google Scholar]
- 2.Yadegari R, Drews GN. Female gametophyte development. Plant Cell. 2004;16:S133–S141. doi: 10.1105/tpc.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu KK, Okada K. Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development. 2000;127:4511–4518. doi: 10.1242/dev.127.20.4511. [DOI] [PubMed] [Google Scholar]
- 4.Christensen CA, et al. Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. Plant Cell. 2002;14:2215–2232. doi: 10.1105/tpc.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagnussat GC, et al. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis . Development. 2005;132:603–614. doi: 10.1242/dev.01595. [DOI] [PubMed] [Google Scholar]
- 6.Portereiko MF, et al. NUCLEAR FUSION DEFECTIVE1 encodes the Arabidopsis RPL21M protein and is required for karyogamy during female gametophyte development and fertilization. Plant Physiol. 2006;141:957–965. doi: 10.1104/pp.106.079319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose MD. Nuclear fusion in the yeast, Saccharomyces cerevisiae . Annu Rev Cell Dev Biol. 1996;12:663–695. doi: 10.1146/annurev.cellbio.12.1.663. [DOI] [PubMed] [Google Scholar]
- 8.Rose MD, Misra LM, Vogel JP. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa S, et al. Nep98p is a component of the yeast spindle pole body and essential for nuclear division and fusion. J Biol Chem. 2003;278:9938–9943. doi: 10.1074/jbc.M210934200. [DOI] [PubMed] [Google Scholar]
- 10.Noh SJ, Kwon CS, Oh D-H, Moon JS, Chung W-I. Expression of an evolutionarily distinct novel BiP gene during the unfolded protein response in Arabidopsis thaliana . Gene. 2003;311:81–91. doi: 10.1016/s0378-1119(03)00559-6. [DOI] [PubMed] [Google Scholar]
- 11.Sessions A, et al. A high-throughput Arabidopsis reverse genetics system. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana . Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, et al. Resources for targeted insertional and deletional mutagenesis in Arabidopsis . Plant Mol Biol. 2003;53:133–150. doi: 10.1023/B:PLAN.0000009271.08420.d9. [DOI] [PubMed] [Google Scholar]
- 14.Christensen CA, King EJ, Jordan JR, Drews GN. Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex Plant Reprod. 1997;10:49–64. [Google Scholar]
- 15.Steffen JG, Kang IJ, Macfarlane J, Drews GN. Identification of genes expressed in the Arabidopsis female gametophyte. Plant J. 2007;51:281–292. doi: 10.1111/j.1365-313X.2007.03137.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y-H, et al. The central cell plays a critical role in pollen tube guidance in Arabidopsis. . Plant Cell. 2007;19:3563–3577. doi: 10.1105/tpc.107.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S, An Y-Q, McDowell JM, McKinney EC, Meagher RB. The Arabidopsis ACT11 actin gene is strongly expressed in tissues of the emerging inflorescence, pollen, and developing ovules. Plant Mol Biol. 1997;33:125–139. doi: 10.1023/a:1005741514764. [DOI] [PubMed] [Google Scholar]
- 18.Ingouff M, Hamamura Y, Gourgues M, Higashiyama T, Berger F. Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol. 2007;17:1032–1037. doi: 10.1016/j.cub.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Berger F, Grini PE, Schnittger A. Endosperm: An integrator of seed growth and development. Curr Opin Plant Biol. 2006;9:664–670. doi: 10.1016/j.pbi.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Dumas C, Rogowsky P. Fertilization and early seed formation. C R Biol. 2008;331:715–725. doi: 10.1016/j.crvi.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Kang I-H, Steffen JG, Portereiko MF, Lloyd A, Drews GN. The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. . Plant Cell. 2008;20:635–647. doi: 10.1105/tpc.107.055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowack MK, et al. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet. 2005;38:63–67. doi: 10.1038/ng1694. [DOI] [PubMed] [Google Scholar]
- 23.Iwakawa H, Shinmyo A, Sekine M. Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 2005;45:819–831. doi: 10.1111/j.1365-313X.2005.02643.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AE, van Waes MA. The translocon: A dynamic gateway at the ER membrane. Annu Rev Cell Dev Biol. 1999;15:799–842. doi: 10.1146/annurev.cellbio.15.1.799. [DOI] [PubMed] [Google Scholar]
- 25.Baroux C, Pien S, Grossniklaus U. Chromatin modification and remodeling during early seed development. Curr Opin Genet Dev. 2007;17:473–479. doi: 10.1016/j.gde.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Köhler C, Makarevich G. Epigenetic mechanisms governing seed development in plants. EMBO Rep. 2006;7:1223–1227. doi: 10.1038/sj.embor.7400854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köhler C, Page DR, Gagliardini V, Grossniklaus U. The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet. 2005;37:28–30. doi: 10.1038/ng1495. [DOI] [PubMed] [Google Scholar]
- 28.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 29.Ngo QA, Moore JM, Baskar R, Grossniklaus U, Sundaresan V. Arabidopsis GLAUCE promotes fertilization-independent endosperm development and expression of paternally inherited alleles. Development. 2007;134:4107–4117. doi: 10.1242/dev.007310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.