Abstract
Patients with Duchenne muscular dystrophy (DMD) have a progressive dilated cardiomyopathy associated with fatal cardiac arrhythmias. Electrical and functional abnormalities have been attributed to cardiac fibrosis; however, electrical abnormalities may occur in the absence of overt cardiac histopathology. Here we show that structural and functional remodeling of the cardiac sarcoplasmic reticulum (SR) Ca2+ release channel/ryanodine receptor (RyR2) occurs in the mdx mouse model of DMD. RyR2 from mdx hearts were S-nitrosylated and depleted of calstabin2 (FKBP12.6), resulting in “leaky” RyR2 channels and a diastolic SR Ca2+ leak. Inhibiting the depletion of calstabin2 from the RyR2 complex with the Ca2+ channel stabilizer S107 (“rycal”) inhibited the SR Ca2+ leak, inhibited aberrant depolarization in isolated cardiomyocytes, and prevented arrhythmias in vivo. This suggests that diastolic SR Ca2+ leak via RyR2 due to S-nitrosylation of the channel and calstabin2 depletion from the channel complex likely triggers cardiac arrhythmias. Normalization of the RyR2-mediated diastolic SR Ca2+ leak prevents fatal sudden cardiac arrhythmias in DMD.
Keywords: calcium, excitation-contraction coupling, heart, sudden cardiac death, myopathy
Duchenne muscular dystrophy (DMD), the most common X-linked disorder in males, results from dystrophin deficiency due to nonsense mutations (1 –3). Dystrophin contributes to maintenance of cellular architecture and permits signal transduction between the cytoskeleton and the extracellular matrix. DMD affects 1 in 3,500 newborn males and usually leads to death from respiratory or cardiac failure by age 30 years (1). The severity of cardiomyopathy is not proportional to the severity of the skeletal muscle disorder. Among DMD patients, the cardiac phenotype varies with age, from no discernible cardiac left ventricular enlargement or dysfunction to dilated cardiomyopathy (DCM) with heart failure (4). The incidence of DCM in DMD patients has been estimated to be 25% by age 6 years, 59% at age 10 years, and ∼100% in adults (4). In addition, DMD patients often exhibit electrocardiographic abnormalities and frequent premature ventricular contractions (PVCs) (5). These abnormalities have been found to occur in 85% of the patients before age 10 years and in 91% before age 18 years (6). As cardiomyopathy progresses, ventricular arrhythmias increase, often leading to sudden cardiac death (SCD) (7). Most of the electrical and functional abnormalities have been attributed to cardiac fibrosis; however, electrical abnormalities may occur in the absence of overt cardiac histopathology (5), and ECG changes are similar in patients with DMD regardless of the presence or absence of DCM (8).
Although several cellular mechanisms have been proposed to account for these cardiac abnormalities, the definitive mechanism underlying SCD in DMD has not yet been established. Dystrophin deficiency has been linked to altered Ca2+ homeostasis due to defects in plasma membrane integrity in skeletal muscle (9, 10) and the heart (10 –12). It has been proposed that dystrophin deficiency could increase the activity of stretch-activated channels (SACs), including the TRPC1 channel in the heart (13) and the TRPC3 (14) and TRPV2 (15) channels in skeletal muscle. Jung et al. (11) recently proposed another explanation for the altered Ca2+ homeostasis, based on stretch-induced production of Ca2+-dependent reactive oxygen species (ROS) by mitochondria, which in turn activates sarcoplasmic reticulum (SR) Ca2+ release, resulting in elevated resting Ca2+. Indeed, ROS production and oxidative damage may be involved in the pathogenesis of heart failure in DMD (16). Interestingly, the NO/cGMP signaling pathway also has been reported to exhibit early signs of alteration before any overt evidence of cardiomyopathy (17). Thus, a consensus is emerging that dystrophin deficiency leads to abnormal intracellular Ca2+ homeostasis in cardiac and skeletal muscle, but the mechanisms underlying the altered Ca2+ homeostasis remain to be fully elucidated.
In a recent study, we showed that SR Ca2+ leak via release channel/ryanodine receptor (RyR2) caused by progressive S-nitrosylation of the channel and calstabin1 depletion likely contributes to muscle weakness in DMD (18). Moreover, S-nitrosylation of the channel is associated with a significant up-regulation of inducible nitric oxide synthase (iNOS) in mdx skeletal muscle and its association with the RyR1 macromolecular complex (18). In the heart, decreased neuronal NOS isoform (nNOS) activity and increased iNOS activity have been reported and correlated with abnormal ECG activity (19). We reasoned that early changes in cardiomyocyte Ca2+-dependent signaling could provide particular insight into the critical initiating events in the development of cardiac disease in DMD. Therefore, the goal of this study was to investigate the role of RyR2 in the genesis of cardiac arrhythmias in mdx mice occurring before any sign of overt cardiac contractile dysfunction is apparent.
Results
Cardiac RyR2 Channels Are S-Nitrosylated and Depleted of Calstabin2 in mdx Mice.
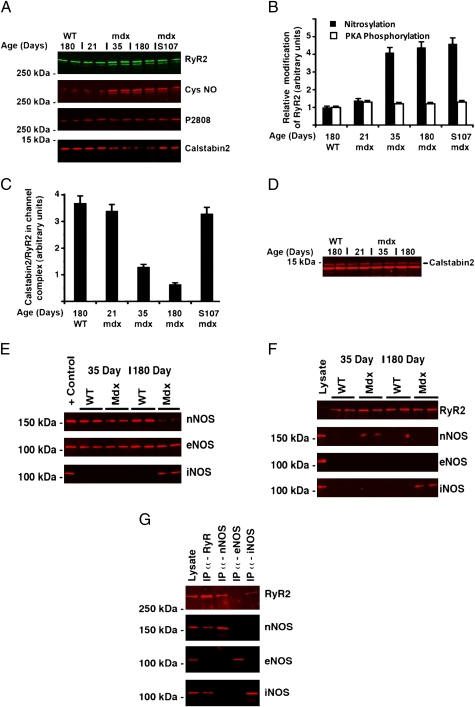
We hypothesized that defects in the RyR2 macromolecular complex might be observed in the heart, and thus assessed the biochemical composition of the RyR2 macromolecular complex in cardiac muscle of mdx mice at age 35 days, a stage at which early signs of muscular dystrophy in skeletal muscle are detectable but no evidence of overt cardiomyopathy is apparent (Table 1). We compared these data with that for cardiac muscle from mdx mice and age-matched WT littermates at age 180 days, at which time characteristic dilation of the myocardium was detected by echocardiography [3.55 ± 0.12 in mdx mice (n = 5) vs. 3.05 ± 0.08 mm in WT (n = 6); P = .015]. We found a significant increase in S-nitrosylation of cysteine in RyR2 from mdx mice compared with that from WT littermates, as well as partial depletion of calstabin2 from the RyR2 complex, but no change in phosphorylation of Ser-2808 (Fig. 1 A–C). Interestingly, RyR2/calstabin2 dissociation increased progressively with age. Nevertheless, the overall expression of calstabin2 was similar in hearts from young and old mice and also similar in WT and mdx mice (Fig. 1D). We have previously reported that in vivo treatment with S107, an orally available, RyR-specific “rycal” (20), inhibited calstabin1 depletion from the RyR1 macromolecular complex in skeletal muscle (18, 20). In mdx hearts, treatment with the rycal S107 inhibited depletion of calstabin2 from the RyR2 complex without affecting the S-nitrosylation state of the channel (Fig. 1 A–C). Interestingly, treatment of the mice with N-acetyl cysteine (NAC) for 2 weeks also prevented RyR S-nitrosylation and calstabin2 depletion (Fig. S1), supporting the idea that S-nitrosylation of RyR2 occurs through the transformation of NO to peroxynitrite in the presence of superoxide anion (O2 .−).
Table 1.
Hemodynamic parameters of WT and mdx hearts in 35-day-old mice, as measured by Doppler echocardiography (SI Materials and Methods)
| WT mice (n = 5) | mdx mice (n = 5) | |
| HW/BW, mg · g−1 | 3.78 ± 0.37 | 4.26 ± 0.32 |
| Fractional shortening, % | 52.0 ± 5.2 | 60.4 ± 2.7 |
| Relative wall thickness | 0.75 ± 0.10 | 0.83 ± 0.06 |
| E wave, m/s | 0.74 ± 0.04 | 0.71 ± 0.05 |
| VTI, cm | 1.43 ± 0.19 | 1.47 ± 0.25 |
HW/BW, heart weight:body weight ratio; VTI, velocity time integral of aortic flow.
Fig. 1.
RyR2 is S-nitrosylated and depleted of calstabin2 in mdx mice hearts. RyR2 was immunoprecipitated from heart homogenate of mdx and WT littermates at 21, 35, and 180 days after birth, as described in SI Materials and Methods. In addition, a group of 5-week-old mdx mice were treated for 2 weeks with S107. (A) Immunoblots prepared for RyR2, S-nitrosylation of cysteine residues on RyR2 (Cys-NO), and calstabin2 bound to RyR2. The blots are representative of three independent experiments. (B) Bar graph depicting the relative amount of RyR2 S-nitrosylation for each group, determined by dividing the Cys-NO signals by the total amount of RyR2 immunoprecipitated. (C) Bar graph depicting the relative amount of calstabin2 associated with the channel complex for each group, determined by dividing the calstabin2 signals by the total amount of RyR2 immunoprecipitated. (D) Immunoblots of heart lysates (100 μg) separated by 15% PAGE, prepared using an anti-calstabin antibody. (E) Immunoblots for nNOS, eNOS, and iNOS in 35- and 180-day-old mdx and WT littermate heart muscle lysate. (F) Coimmunoprecipitation of NOS enzymes with RyR2. RyR2 was immunoprecipitated from 500 μg of WT or mdx heart lysate and probed for RyR2 and the NOS enzymes. (G) Coimmunoprecipitation of RyR2 with NOS enzymes. NOS enzymes were immunoprecipitated separately from 500 μg of mdx heart lysate (35 days for nNOS; 180 days for eNOS and iNOS) and probed for RyR2 and the NOS enzymes. In F and G, positive controls for immunoblotting were 100 μg of 180-day-old WT heart lysate for RyR2, nNOS, and eNOS and 100 μg of 180-day-old mdx heart lysate for iNOS.
In early disease stages, while cardiac pathology is still not readily detectable, mdx mice exhibit abnormal susceptibility to mechanical stress and workload-induced damage (21). Alterations in NO and/or cGMP signaling have been reported despite minimal histological and echocardiographic changes in mdx mice, suggesting that these abnormalities precede overt pathology in the heart (17). In mdx skeletal muscle, RyR1 S-nitrosylation is correlated with an up-regulation of iNOS–RyR2 interaction. Here the total level of NOS isoforms was first determined by immunoblot analysis in hearts of 35-day-old and 180-day-old mice (Fig. 1E). Levels of nNOS were similar and unchanged in young and old WT mice and in young mdx mice; however, in older mdx mice, nNOS was down-regulated and iNOS was up-regulated. Expression of the endothelial isoform (eNOS) was constant in all groups (Fig. 1E). In contrast to the findings in skeletal muscle (18), coimmunoprecipitation analysis of NOS in the RyR2 macromolecular complex showed that nNOS coimmunoprecipitated with RyR2 in the 35-day-old mdx mice, whereas iNOS coimmunoprecipitated with RyR2 in the older mdx mice (Fig. 1 F and G).
Diastolic SR Ca2+ Leak and SR Function in mdx Mice.
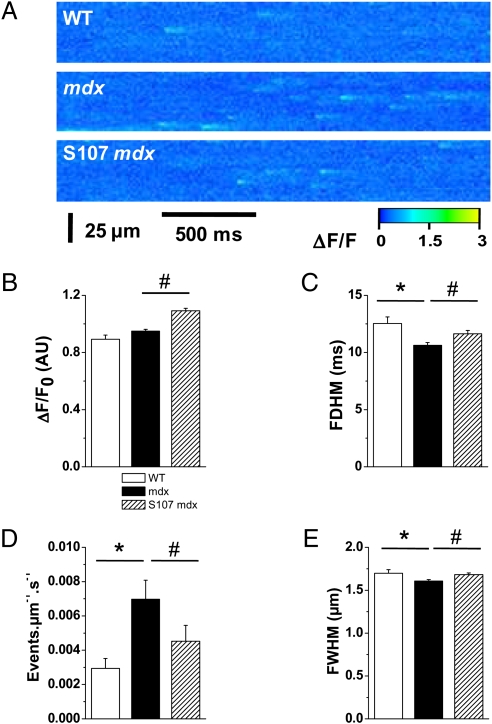
Spontaneous SR Ca2+ release events (Ca2+ sparks) correspond to stochastic release of Ca2+ arising from small clusters of SR Ca2+ RyR release channels (22). The occurrence of Ca2+ sparks reflects the open probability of RyR in situ and can be used as an index of diastolic Ca2+ leak (23). Cardiomyocytes from mdx mice exhibited a significantly higher frequency of spontaneous Ca2+ sparks than those from WT mice (Fig. 2 A and B). The amplitude (Fig. 2C) and spatial spread (Fig. 2E) of Ca2+ sparks were similar in mdx and WT mice, whereas their duration was slightly shorter in mdx mice (Fig. 2D). Treating mdx mice with S107 prevented this increase in the frequency of sparks. Of note, treatment with S107 did increase the amplitude of the sparks, possibly reflecting an increase in SR Ca2+ content. Together, these data indicate that RyR2 were abnormally “leaky” in mdx mice during diastole. This leaky behavior was prevented when isolated cardiomyocytes were preincubated with NAC (Fig. S2), strongly supporting a direct role of ROS/reactive nitrogen species (RNS) on RyR2 function in mdx hearts.
Fig. 2.
SR Ca2+ leak assessed by Ca2+ sparks analysis in mdx mice. Spontaneous SR Ca2+ release events were recorded in fluo-4 AM–loaded intact cardiomyocytes by laser scanning confocal microscopy, as described in SI Materials and Methods. (A) Representative ΔF/F line scan images (1.54 ms/line) were recorded in WT (Top) and mdx mice without (Middle) and with (Bottom) S107 treatment. Diastolic SR Ca2+ leak is estimated by the average sparks frequency (D). Average spatiotemporal properties of Ca2+ sparks, such as amplitude (B), full duration at half maximum (C), and spatial spread (full width at half maximum) (E). Data are expressed as mean ± SEM. *P < 0.05 WT vs. mdx; #P < 0.05 mdx vs. S107-mdx. n = 195 sparks in 20 cells in WT, 1,272 sparks in 58 cells in mdx, and 889 sparks in 60 cells in S107-treated mdx mice.
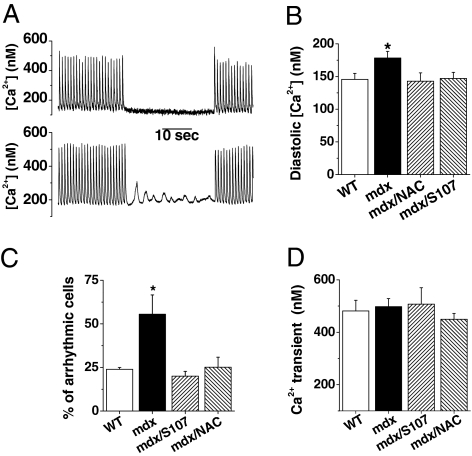
Global Ca2+ signals (i.e., Ca2+ transients) were triggered in isolated ventricular cardiomyocytes loaded with indo-1 AM. Diastolic Ca2+ was significantly higher in the mdx mice; this was prevented by treatment with either S107 or NAC (Fig. 3 A and B). Ca2+ transient amplitude were slightly, but not significantly, reduced in mdx mice (Fig. 3 A and C). Cardiomyocyte contraction was unaffected in the 35-day-old mice, because cell shortening was similar in the mdx and WT mice (reduced cell length of 9.72% ± 0.82% and 9.13% ± 0.70%, respectively).
Fig. 3.
Elevated diastolic Ca2+ concentration in mdx mice. Isolated cardiomyocytes, loaded with indo-1 AM as described in SI Materials and Methods, were paced at 1 Hz. After 1 min, cells were maintained quiescent for 30 s before a second train of stimulation. (A) Global Ca2+ was recorded simultaneously, as illustrated by two typical recordings in WT and in mdx cardiomyocytes. (B) During the stimulation, diastolic Ca2+ was elevated in mdx cardiomyocyte. This was prevented by NAC or S107 treatment. (C) During the 30-s rest period, ∼50% of mdx cardiomyocytes exhibited Ca2+ waves that were not observed in WT or in mdx after NAC or S107 treatment. (D) The peak amplitude of the Ca2+ transients did not differ significantly in all conditions. Data are expressed as mean ± SEM. WT, n = 24; mdx, n = 29; mdx-S107, n = 15; mdx-NAC, n = 28. *P < 0.05.
Initiation of ventricular tachycardia may result from DADs and ectopic Ca2+ transients. To evaluate the propensity of mdx cardiomyocytes to generate DADs, cardiomyocytes were field- stimulated for 1 min at 1 Hz. Diastolic Ca2+ was then recorded during a subsequent rest period of 30 s. Whereas diastolic Ca2+ remained constant in WT cardiomyocytes, Ca2+ oscillations and waves were seen in ∼50% of the mdx cardiomyocytes these were fully prevented by treatment with S107 and NAC (Fig. 3 A and C). DADs are known to be enhanced by β-stimulation; thus, when isoproterenol was applied to cardiomyocytes, spontaneous (i.e., not driven by electrical stimulation) Ca2+ transients due to DADs were observed in 40% of cells from mdx mice, but in <10% of cells from WT mice (Fig. S3). Treatment with S107 reduced the incidence of spontaneous Ca2+ transients in mdx mice (Fig. S4).
ECG Abnormalities and Ventricular Arrhythmias in mdx Mice.
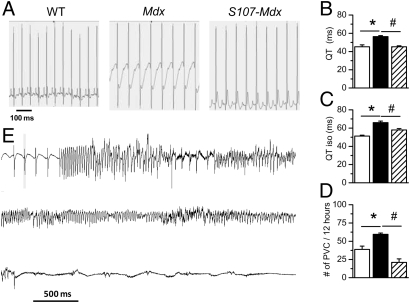
ECG montoring was done in conscious nonanesthetized animals using telemetric recordings in WT, mdx, and S107-treated mdx mice for 24 h and analyzed specifically during the 12-h overnight period (Fig. 4A, Left). ECGs also were recorded after a 2-h isoproterenol challenge performed in each animal at the same time (Fig. 4A, Right). As shown by these representative traces, repolarization of the ventricles was slower in the mdx mice. This property was significantly exacerbated after isoproterenol challenge and prevented by treatment with S107. The heart rate given by the RR interval was similar in WT, mdx, and S107-treated mdx mice (Fig. 4B). Reduced heart rate variability has been demonstrated to be a good predictor of increased mortality (24). Heart rate variability, indicated by the standard deviation of all normal RR intervals (SDNN), was significantly reduced in the mdx mice (Fig. 4C). Treating mdx mice with S107 restored the SDNN to control values (Fig. 4C). PVCs are ectopic contractions that originate from the ventricles. PVC frequency was significantly increased in mdx mice, and was fully reversed by S107 treatment (Fig. 4D). Long QT syndrome has been associated with a high occurrence of PVCs and SCD (25). The QT interval was prolonged in mdx mice and was restored to normal by S107 treatment (Fig. 4F). The ECG abnormalities observed in mdx animals are consistent with prolonged action potential duration (Fig S2), which has been reported to result from diastolic Ca2+ elevation (26, 27). These electrophysiological disturbances were prevented in cardiomyocytes obtained from mdx mice by treatment with S107 and acute application of NAC (Fig S2). The QT interval also was prolonged in mdx mice treated with isoproterenol (Fig. 4G). Furthermore, when challenged with isoproterenol 80% of the mdx mice developed sustained ventricular tachycardia (VT) (Fig. 4H). Importantly, VT was not triggered by isoproterenol stimulation in either WT or S107-treated mdx mice. Together, these findings indicate that even before the development of significant skeletal muscle pathology or cardiac contractility dysfunction, significant and potentially fatal ventricular arrhythmias exist that are exacerbated by sympathetic stimulation, likely triggered by abnormally leaky RyR2 channels. However, ECGs from mdx mice also revealed a significantly higher number of sinus arrests (21.1 ± 2.3 vs. 7.0 ± 2.0 in WT) and atrioventricular blocks (33.2 ± 3.7 vs. 4.0 ± 1.4 in WT) that were not corrected by S107 treatment.
Fig. 4.
ECG recordings in young mdx mice. ECGs were recorded as described in SI Materials and Methods. (A) Typical ECGs acquired by telemetry in 35-day-old vigil mice over 24 h and analyzed specifically during the 12-h overnight period in WT (Top; n = 5), mdx (Middle; n = 5), and S107-mdx treated (Bottom; n = 5) mice in normal condition (Left) and after i.p. injection of isoproterenol (2.5 mg · kg-1) (Right). (B–F) ECG analysis provides various functional parameters, including cardiac frequency given by the RR interval (B), heart rate variability expressed as SDNN (C), PVCs (D), QRS duration (E), and QT interval (F). (G) The QT interval also was measured after isoproterenol challenge. (H) Typical spontaneous sustained VT recorded in mdx mice after isoproterenol challenge. Note that VT was never triggered by isoproterenol treatment in either WT or S107-treated mice. *P < 0.05 control vs. mdx; #P < 0.05 mdx vs. S107-treated mdx.
To explore this finding further, we recorded ECGs in older mdx mice (∼6 months) with early overt signs of DCM. At this age, mdx mice exhibited a slower repolarization phase (Fig. 5A), consistent with QT prolongation (Fig. 5 B and C), as well as increased PVCs (Fig. 5D). When mdx mice were challenged with isoproterenol, they exhibited sustained VT leading to SCD (e.g., Fig. 5E). The mdx mice were extremely sensitive to isoproterenol-induced arrhythmias; 100% exhibited VT after isoproterenol. S107 treatment prevented the development of VT and sudden death in these mdx mice as well.
Fig. 5.
ECG recordings in older mdx mice. (A) Typical ECGs acquired by telemetry in 180-day-old vigil mice over 24 h and analyzed specifically during the 12-h overnight period in WT (Top; n = 5), mdx (Middle; n = 5), and S107-treated mdx (Bottom; n = 5) mice in normal condition. (B and C) As in young mice, QT intervals in baseline condition (B) and after injection of isoproterenol (C) were enlarged in mdx mice (black bars) compared with WT mice (open bars). (D) PVCs also were increased in mdx mice. These electrophysiological abnormalities were fully abolished after S107 treatment (dashed bars). (E) Typical spontaneous sustained VT recorded in mdx mice after isoproterenol challenge. This mouse died from SCD during the acquisition. Note that VT was never triggered under the same treatment in ether WT or S107-treated mdx mice. *P < 0.05 control vs. mdx; #P < 0.05 mdx vs. S107-treated mdx.
Discussion
The present study demonstrates the relationship among S-nitrosylation of RyR2, diastolic SR Ca2+ leak, and the development of fatal cardiac arrhythmias in the dystrophin-deficient heart. Sympathetic stimulation has been shown to exacerbate RyR2-dependent ventricular arrhythmias (28 31). Interestingly, compared with the WT mice, the mdx mice were more sensitive to sympathetic stimulation–induced VT, likely due to the remodeling of the RyR2 channel complex characterized by S-nitrosylation and partial depletion of calstabin2. Indeed, whereas the arrhythmias in the mdx mice were elicited by isoproterenol, the same isoproterenol treatment failed to elicit arrhythmias in age-matched WT mice. Isoproterenol increases the SR Ca2+ load and thus, in the presence of a leaky RyR2 channel, will increase the driving force for a diastolic SR Ca2+ leak that triggers the arrhythmias in mdx mice.
Isoproterenol treatment causes transient Protein Kinase A (PKA) phosphorylation of RyR2 (32). Because the RyR2 channels in WT mice are not leaky, treatment with isoproterenol typically does not elicit arrhythmias. This contrasts with pathological situations such as heart failure, in which the RyR2 channels are chronically PKA-phosphorylated and undergo remodeling such to deplete them of calstabin2, PDE4D3, PP1, and PP2A (32 –34). The role of RyR2 leak in the etiology of VT in mdx mice is similar to that seen in catecholaminergic polymorphic VT (CPVT), in which a single point mutation of RyR2 reduces the affinity of calstabin2 for RyR2, and, under sympathetic stimulation leaky RyR2 channels, provides the trigger for fatal VT (28), which also can be prevented by rycals (29).
The role of the NO pathway in regulating cardiac excitation-contraction coupling remains controversial (35). A direct activation of RyR2 by S-nitrosylation has been reported (36). The effect of NO on the closely related RyR1 channel appears to be biphasic (37). NO also has a biphasic effect on the regulation of cardiac contractility (38). NO is produced in the heart by three different isoforms of NOS: neuronal (nNOS), endothelial (eNOS), and inducible (iNOS). iNOS is expressed in response to cardiac stress, such as ischemia or inflammation, and is thought to remain in the cytoplasm (35). In normal hearts, eNOS is located in membrane caveolae, whereas some nNOS is thought to be located in the RyR2 macromolecular complex (35). Aberrant regulation and relocalization of nNOS may contribute to degeneration of muscle fibers in DMD (39). Thus, NO-mediated signaling depends on both the NOS isoform and compartmentalization (40). For instance, nNOS- deficient mice have decreased cardiac inotropy, whereas eNOS-deficient mice have enhanced contractility, owing to corresponding changes in SR Ca2+ release, potentially indicating opposing effects of these two isoforms on cardiac function. By analogy with our results, increasing NO production by eNOS activation also has been shown to increase spontaneous Ca2+ sparks activity in isolated cardiomyocytes via a cGMP-independent pathway and presumably direct S-nitrosylation of RyR2 (41). This seems to disagree with a recent report that hyponitrosylation of RyR2 mediated a proarrhythmogenic Ca2+ leak (42). This apparent contradiction might be explained by the fact that in the transgenic models used in that previous study, the interaction between RyR2 and calstabin2 was unchanged, whereas in the present study, S-nitrosylated RyR2 was partially depleted of calstabin2. Another possible explanation for this discrepancy is that decreased nNOS and increased iNOS have been observed in the mdx model (19). We also found (as we did previously in mdx skeletal muscle; ref. 18) increased iNOS in the RyR macromolecular complex; however, in contrast to the situation in skeletal muscle, here this occurred at an advanced disease stage (180 days). Thus, the severity of the pathology seems to be linked to the degree of iNOS overexpression in both cardiac and skeletal muscle (18). Because iNOS expression is related to inflammatory processes (35) and based on the differential progression of skeletal and cardiac muscle, it is possible that an inflammatory response contributes to the development of cardiac RyR2 dysfunction via an up-regulation of iNOS. Furthermore, iNOS is significantly increased in the skeletal muscle of both DMD patients and mdx mice, and rescue of the mdx phenotype by adenoviral-mediated dystrophin or utrophin expression has been found to normalize iNOS activity (43). Nevertheless, overexpression of nNOS in mdx mouse heart was found to partially rescue cardiac function in older mice (44). These beneficial effects might, however, be mediated by enhanced cGMP signaling (45) or by a compensatory down-regulation of iNOS, as suggested by diminished inflammatory macrophage infiltration (44).
In addition to gene therapy (46) and the recently reported cardioprotective effect of a membrane sealant (47), which offer promising new therapeutic approaches, we provide here an additional target against sudden fatal arrhythmia in DMD. Normalizing RyR2 function and inhibiting diastolic SR Ca2+ leak by preventing depletion of calstabin2 from the RyR2 complex and stabilizing a closed state of the channel without interfering with normal channel function have antiarrhythmic effects in mdx mice. QT dispersion has been shown to be a good marker of arrhythmias in patients with DMD and represents a major risk factor for the development of ventricular arrhythmias (48). Our results suggest that early antiarrhythmic treatment, through specific modulation of RyR2 properties, could be beneficial in preventing SCD in DMD. Indeed, because multiple therapies have the potential to improve exercise capacity in DMD patients, the risk of cardiovascular pathology and particularly arrhythmias likely will increase. Accordingly, this finding also should be explored in milder muscular dystrophies, such as Becker muscular dystrophy, that are characterized by cardiac manifestations, including arrhythmias and/or SCD (49).
Materials and Methods
For a detailed description of the methods, see SI Materials and Methods.
Animals.
Male mice of strain C57BL/10ScSc-Dmdmdx/J, designated mdx, were obtained from Dr. D. Mornet (Inserm Eri25, Montpellier, France). Male mice of strain C57BL/10J, designated WT, were obtained from Harlan (Gannat, France). Thirty-five–day-old and 180-day-old weight-matched mice were randomized to treatment with either S107 (20) or vehicle (H2O). S107 was diluted in drinking water (25 mg/100 mL). The structure and purity of S107 were confirmed by NMR, MS, and elemental analysis, and the specificity of S107 for RyR channels was assessed by extensive testing against a panel of >250 receptors and kinases (20).
Supplementary Material
Acknowledgments
We thank Dr. D. Mornet for providing the mdx mice, Dr. O. Cazorla for assisting with the calibration of indo-1, and Dr. B. Petrof for providing constructive comments on the manuscript. This work was supported by a grant from the Leducq Foundation.
Footnotes
Conflict of interest statement: A.R.M. is on the scientific advisory board and owns shares in ARMGO Pharma, Inc., a startup company developing RyR-targeted drugs for clinical use in the treatment of heart failure and sudden death.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908540107/DCSupplemental.
References
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- 3.Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: One gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2:731–740. doi: 10.1016/s1474-4422(03)00585-4. [DOI] [PubMed] [Google Scholar]
- 4.Wagner KR, Lechtzin N, Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochim Biophys Acta. 2007;1772:229–237. doi: 10.1016/j.bbadis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Finsterer J, Stöllberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 6.Takami Y, et al. High incidence of electrocardiogram abnormalities in young patients with Duchenne muscular dystrophy. Pediatr Neurol. 2008;39:399–403. doi: 10.1016/j.pediatrneurol.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Chenard AA, Becane HM, Tertrain F, de Kermadec JM, Weiss YA. Ventricular arrhythmia in Duchenne muscular dystrophy: Prevalence, significance and prognosis. Neuromuscul Disord. 1993;3:201–206. doi: 10.1016/0960-8966(93)90060-w. [DOI] [PubMed] [Google Scholar]
- 8.Thrush PT, Allen HD, Viollet L, Mendell JR. Re-examination of the electrocardiogram in boys with Duchenne muscular dystrophy and correlation with its dilated cardiomyopathy. Am J Cardiol. 2009;103:262–265. doi: 10.1016/j.amjcard.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 9.Allen DG, Whitehead NP, Yeung EW. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: Role of ionic changes. J Physiol. 2005;567:723–735. doi: 10.1113/jphysiol.2005.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung EW, Allen DG. Stretch-activated channels in stretch-induced muscle damage: Role in muscular dystrophy. Clin Exp Pharmacol Physiol. 2004;31:551–556. doi: 10.1111/j.1440-1681.2004.04027.x. [DOI] [PubMed] [Google Scholar]
- 11.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: Amplification of cellular damage by Ca2+ signalling and reactive oxygen species–generating pathways. Cardiovasc Res. 2008;77:766–773. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 12.Ward ML, et al. Stretch-activated channels in the heart: Contributions to length dependence and to cardiomyopathy. Prog Biophys Mol Biol. 2008;97:232–249. doi: 10.1016/j.pbiomolbio.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol. 2007;292:H846–H855. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 14.Millay DP, et al. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci USA. 2009;106:19023–19028. doi: 10.1073/pnas.0906591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet. 2009;18:824–834. doi: 10.1093/hmg/ddn408. [DOI] [PubMed] [Google Scholar]
- 16.Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- 17.Khairallah M, et al. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol. 2007;43:119–129. doi: 10.1016/j.yjmcc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger AM, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;3:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bia BL, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31:1857–1862. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 20.Bellinger AM, et al. Remodeling of ryanodine receptor complex causes “leaky” channels: A molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danialou G, et al. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress–induced contractile failure and injury. FASEB J. 2001;15:1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 24.Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: A theoretical and practical guide. Exp Physiol. 2008;93:83–94. doi: 10.1113/expphysiol.2007.040733. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JL, Prystowsky EN. Sotalol: An important new antiarrhythmic. Am Heart J. 1999;137:388–409. doi: 10.1016/s0002-8703(99)70484-9. [DOI] [PubMed] [Google Scholar]
- 26.Fauconnier J, et al. Frequency-dependent and proarrhythmogenic effects of FK-506 in rat ventricular cells. Am J Physiol Heart Circ Physiol. 2005;288:H778–H786. doi: 10.1152/ajpheart.00542.2004. [DOI] [PubMed] [Google Scholar]
- 27.Pogwizd SM, Bers DM. Na/Ca exchange in heart failure: Contractile dysfunction and arrhythmogenesis. Ann N Y Acad Sci. 2002;976:454–465. doi: 10.1111/j.1749-6632.2002.tb04775.x. [DOI] [PubMed] [Google Scholar]
- 28.Wehrens XH, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 29.Lehnart SE, et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci USA. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehrens XH, et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci USA. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehrens XH, et al. Protection from cardiac arrhythmia through ryanodine receptor–stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 32.Reiken S, et al. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts: Role of phosphatases and response to isoproterenol. J Biol Chem. 2003;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- 33.Lehnart SE, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 35.Massion PB, Pelat M, Belge C, Balligand JL. Regulation of the mammalian heart function by nitric oxide. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:144–150. doi: 10.1016/j.cbpb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 37.Hart JD, Dulhunty AF. Nitric oxide activates or inhibits skeletal muscle ryanodine receptors depending on its concentration, membrane potential and ligand binding. J Membr Biol. 2000;173:227–236. doi: 10.1007/s002320001022. [DOI] [PubMed] [Google Scholar]
- 38.González DR, et al. Differential role of S-nitrosylation and the NO-cGMP-PKG pathway in cardiac contractility. Nitric Oxide. 2008;18:157–167. doi: 10.1016/j.niox.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 39.Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 40.Barouch LA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 41.Petroff MG, et al. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci USA. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louboutin JP, Rouger K, Tinsley JM, Halldorson J, Wilson JM. iNOS expression in dystrophinopathies can be reduced by somatic gene transfer of dystrophin or utrophin. Mol Med. 2001;7:355–364. [PMC free article] [PubMed] [Google Scholar]
- 44.Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet. 2005;14:1921–1933. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- 45.Khairallah M, et al. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum Mol Genet. 2006;15(Rev Issue 2):R253–R261. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasuda S, et al. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 48.Yotsukura M, et al. QT dispersion in patients with Duchenne-type progressive muscular dystrophy. Am Heart J. 1999;137:672–677. doi: 10.1016/s0002-8703(99)70221-8. [DOI] [PubMed] [Google Scholar]
- 49.Finsterer J, Stöllberger C. Cardiac involvement in Becker muscular dystrophy. Can J Cardiol. 2008;24:786–792. doi: 10.1016/s0828-282x(08)70686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.