Abstract
Membrane binding of Gag, a crucial step in HIV-1 assembly, is facilitated by bipartite signals within the matrix (MA) domain: N-terminal myristoyl moiety and the highly basic region (HBR). We and others have shown that Gag interacts with a plasma-membrane-specific acidic phospholipid, phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2], via the HBR, and that this interaction is important for efficient membrane binding and plasma membrane targeting of Gag. Generally, in protein–PI(4,5)P2 interactions, basic residues promote the interaction as docking sites for the acidic headgroup of the lipid. In this study, toward better understanding of the Gag–PI(4,5)P2 interaction, we sought to determine the roles played by all of the basic residues in the HBR. We identified three basic residues promoting PI(4,5)P2-dependent Gag-membrane binding. Unexpectedly, two other HBR residues, Lys25 and Lys26, suppress membrane binding in the absence of PI(4,5)P2 and prevent promiscuous intracellular localization of Gag. This inhibition of nonspecific membrane binding is likely through suppression of myristate-dependent hydrophobic interaction because mutating Lys25 and Lys26 enhances binding of Gag with neutral-charged liposomes. These residues were reported to bind RNA. Importantly, we found that RNA also negatively regulates Gag membrane binding. In the absence but not presence of PI(4,5)P2, RNA bound to MA HBR abolishes Gag-liposome binding. Altogether, these data indicate that the HBR is unique among basic phosphoinositide-binding domains, because it integrates three regulatory components, PI(4,5)P2, myristate, and RNA, to ensure plasma membrane specificity for particle assembly.
Keywords: basic residues; PI(4,5)P2; phosphoinositide; plasma membrane; retrovirus assembly
HIV-1 assembly is a multistep process mediated primarily by the viral structural protein, Gag. This protein, synthesized as a polyprotein precursor, Pr55Gag, contains four major domains: matrix (MA), capsid (CA), nucleocapsid (NC), and p6. CA and NC contain determinants for Gag multimerization, whereas p6 facilitates release of virus particles (1, 2). In most cell types, Gag assembles on the cytoplasmic leaflet of the plasma membrane (PM). MA is required for this PM-specific targeting as well as the lipid bilayer binding of Gag.
MA has bipartite entities that facilitate membrane binding of Gag: (i) the N-terminal myristoyl moiety and (ii) a highly basic region (HBR) comprising of residues 17–31 (3 –5). The N-terminal myristate is normally sequestered within the globular head domain of MA (6), which also includes the HBR. A structural change, either through Gag multimerization or phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] binding to MA, is required to expose the myristate and enhance membrane binding (6, 7). This mechanism is known as the myristoyl switch (8, 9). Structural and biochemical studies suggest that the HBR of HIV-1 MA and analogous regions of other retroviral MA are apposed to face the cytoplasmic leaflet of membranes and facilitate membrane binding by interacting with acidic lipids (4, 10–14). In addition, MA, especially the HBR, has been shown to interact with RNA (15 –19) although the role of this interaction in virus assembly is less well understood. The HBR is also implicated in other steps of virus replication such as the postentry process (for review, see ref. 20).
Many cellular proteins that interact peripherally with the cytoplasmic leaflet of membranes have basic amino acid-rich domains (21). The subcellular localization of these proteins generally depends on the specific binding of their basic domains with acidic lipids of target membranes. For example, the pleckstrin homology domain of phospholipase C δ1(PHPLCδ1) has basic residues located on the surface of a binding pocket for PI(4,5)P2, a PM-specific acidic lipid. These basic residues specifically interact with the phosphates on the inositol headgroup of PI(4,5)P2 (22). This interaction allows PHPLCδ1 to localize to the PM. Similarly, basic residues of other phosphoinositide-binding domains play crucial roles in specific interactions with target membranes.
Previously, we showed that depleting cellular PI(4,5)P2 reduces virus release of HIV-1 significantly and relocalizes Gag from the PM to cytosol or intracellular compartments (23, 24). Not only HIV-1, but other retroviruses such as HIV-2, murine leukemia virus, and Mason-Pfizer monkey virus also require PI(4,5)P2 or other phosphoinositides for efficient virus release (25 –28). Several studies using various in vitro techniques, including NMR, protein foot printing, liposome binding, and monolayer membrane binding, suggest that MA interacts specifically with PI(4,5)P2 (7, 24, 29, 30). A recent lipid analysis study demonstrated that PI(4,5)P2 is enriched in the HIV envelope in an MA-dependent manner, confirming the MA–PI(4,5)P2 interaction in vivo (26). These studies suggest that MA basic residues, in particular those in the HBR, promote PI(4,5)P2 interaction and membrane binding, as observed for basic residues in other phosphoinositide-binding domains. However, the contribution of each basic residue in the HBR to the interaction between full-length Gag and PI(4,5)P2-containing membrane remains to be examined.
Previous reports showed that mutating lysines 29 and 31 to threonines (29KT/31KT) within the HBR relocalizes Gag from the PM to intracellular compartments, as observed for wild-type (WT) Gag in PI(4,5)P2-depleted cells (31). Consistent with the altered localization, the in vitro liposome binding assay showed that 29KT/31KT Gag binds PI(4,5)P2 less efficiently compared to WT Gag (24). However, the Gag–PI(4,5)P2 interaction was not completely abolished, suggesting a role for other basic amino acids in this interaction.
In this study, toward better understanding of the Gag–PI(4,5)P2 interaction, we sought to identify all of the basic residues in the HBR important for this interaction. We confirmed that the HBR as a whole is indeed essential for PI(4,5)P2 interaction and efficient membrane binding of Gag. Surprisingly, analysis of a panel of Gag mutants revealed that a part of the HBR actually suppresses membrane binding in the absence of PI(4,5)P2. This suppression is likely due to inhibition of the myristate-dependent hydrophobic interaction. Furthermore, RNA bound to the HBR also inhibits Gag-membrane binding through inhibition of acidic lipid binding and to a lesser extent via suppression of myristate exposure. Altogether, these results showed that the HBR regulates membrane binding in both positive and negative manners. Data presented here suggest a unique, RNA-dependent mechanism by which HIV-1 Gag ensures specific binding to the PI(4,5)P2-containing membrane, i.e., the PM.
Results
The HBR of the MA Domain Is Essential for PI(4,5)P2-Dependent Membrane Binding of Gag.
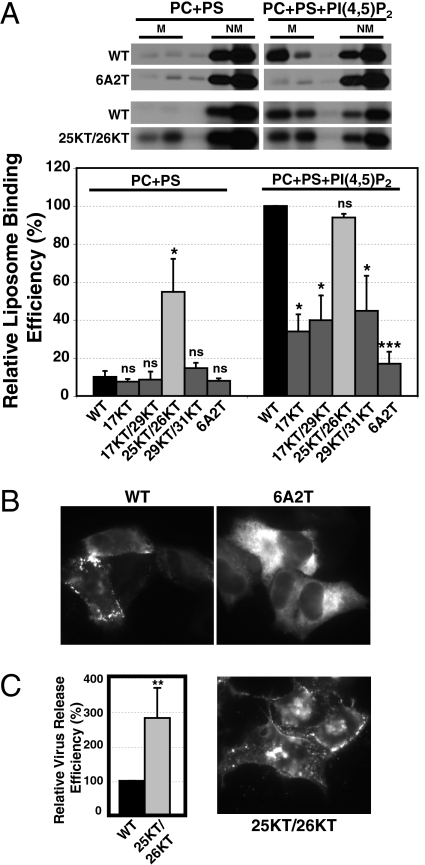
To examine whether the HBR of MA is indeed important for Gag–PI(4,5)P2 interaction, we mutated all of the positively charged residues (6A2T) within the HBR. This mutant was then analyzed for PI(4,5)P2-dependent membrane binding by previously described in vitro liposome binding assay (24). In this assay, myristoylated full-length Gag is synthesized by using rabbit reticulocyte lysates and incubated with liposomes. Liposome-bound and non-liposome-bound Gag proteins are separated by sucrose gradient membrane flotation centrifugation. As shown in Fig. 1A, compared to WT Gag, binding of 6A2T Gag to PI(4,5)P2-containing liposomes was significantly reduced. To examine the effect of the 6A2T change on the localization of Gag in cells, we introduced the same mutations in a Gag derivative tagged with Venus, a variant of yellow fluorescent protein (GagVenus) (24, 32). Consistent with the weak liposome binding, 6A2T GagVenus showed a hazy, cytosolic signal in most cells. Additionally, perinuclear localization of GagVenus was observed in some cells (Fig. 1B). This phenotype is in contrast to that of WT GagVenus that localizes predominantly on the PM. The virus release was undetectable for the 6A2T mutant. Altogether, these results suggest that the HBR likely forms a binding site for PI(4,5)P2 and that this interaction is important for efficient Gag membrane binding and virus release.
Fig. 1.
Lys25 and Lys26 in the HBR inhibit membrane binding of Gag and suppress promiscuous localization. (A) [35S]-labeled WT Gag and Gag mutants with indicated amino acid substitutions were synthesized by the in vitro transcription and translation system by using reticulocyte lysates and incubated with liposomes containing or not containing PI(4,5)P2. The reactions were subjected to membrane flotation centrifugation, and five 1-mL fractions were collected from each tube. Representative gels are shown in Upper. M, liposome-bound; NM, non-liposome-bound. The liposome binding efficiency was calculated as the amount of liposome-bound Gag as a fraction of total Gag. (Lower) The relative liposome binding efficiency was calculated in comparison with the binding efficiency of WT Gag with PI(4,5)P2-containing liposomes for each experiment. Data from three independent experiments are shown as means ± SD. P values were determined by using Student’s t test as a comparison with WT (ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001). (B) HeLa cells expressing WT, 6A2T, and 25KT/26KT GagVenus were analyzed by using Nikon TE2000 microscope. (C) HeLa cells expressing HIV molecular clones encoding WT or 25KT/26KT Gag were metabolically labeled for 2 h. Cell- and virus-associated Gag were recovered by immunoprecipitation. Virus release efficiency was calculated as the amount of virus-associated Gag as a fraction of total Gag synthesized during the labeling period and normalized to the virus release efficiency of WT. The average virus release efficiencies for WT and 25KT/26KT are 7.22% and 17.85%, respectively. Data from five different experiments are shown as means ± SD. **, P < 0.01.
MA Basic Residues Lys25 and Lys26 in the HBR Suppress PI(4,5)P2-Independent Membrane Binding and Inhibit Promiscuous Localization of Gag.
To pinpoint the residues essential for Gag–PI(4,5)P2 interaction, we analyzed liposome binding of Gag mutants with single or double amino acid substitutions of the basic residues in the MA HBR by using in vitro liposome binding assay (Figs. 1, S1, and S2). Several of these mutants have been studied with respect to other proposed MA functions (19, 20, 33, 34). Overall, the mutants could be categorized into three different phenotypes: (i) reduced PI(4,5)P2 binding (17KT, 17KT/29KT, and 29KT/31KT), (ii) increased PI(4,5)P2-independent binding (25KT/26KT), and (iii) no change compared to WT (all of the other mutants). Altogether, these experiments suggest that MA basic residues 17, 29, and 31 promote MA–PI(4,5)P2 interaction.
Among the Gag mutants examined, the 25KT/26KT mutant, where lysines 25 and 26 were changed to threonines, showed no significant change in binding to PI(4,5)P2-containing liposomes. Unexpectedly, however, this mutant had significantly elevated binding to control liposomes that lack PI(4,5)P2 (Fig. 1A). These results suggest that, in addition to facilitating membrane binding through PI(4,5)P2 interaction, some basic residues in the HBR inhibit PI(4,5)P2-independent membrane binding.
To characterize the 25KT/26KT mutant Gag further, we analyzed both virus release and localization by using cell-based assays as described (24). Consistent with the lack of requirement for PI(4,5)P2 observed in the in vitro liposome binding assay (Fig. 1A), the virus release efficiency of 25KT/26KT was substantially augmented compared to WT (Fig. 1C). Notably, 25KT/26KT GagVenus displayed promiscuous membrane binding in cells, localizing both at the PM and intracellular compartments (Fig. 1B). Altogether, these results indicate that MA basic residues 25 and 26 inhibit PI(4,5)P2-independent membrane binding of Gag.
RNA Inhibits Liposome Binding of Gag.
Previous studies showed that the residues Lys25 and Lys26 can interact with RNA (17 –19). Therefore, it is conceivable that the 25KT/26KT mutations abolish RNA binding and allow other MA basic residues to freely interact with acidic lipids, thereby enhancing promiscuous membrane binding. To examine whether RNA does interfere with Gag membrane binding, we first tested the effect of RNase treatment on the liposome binding of WT Gag. Remarkably, treating WT Gag with RNase before incubation with liposomes greatly enhanced interaction of Gag with control liposomes containing phosphatidylcholine (PC) and phosphatidylserine (PS) (PC+PS) (Fig. 2). A similar increase was observed with PI(4,5)P2-containing liposomes [PC+PS+PI(4,5)P2]. Notably, however, as we reported previously (24), a substantial amount of Gag was bound to PI(4,5)P2-containing liposomes even without RNase treatment (Fig. 2). These results indicate that Gag-membrane binding is suppressed almost completely by RNA unless PI(4,5)P2 is present.
Fig. 2.
RNA inhibits membrane binding of Gag. WT and 25KT/26KT Gag were synthesized by using rabbit reticulocyte lysates and either treated or not treated with RNase. Subsequently, liposomes containing indicated lipids were added, and the liposome binding efficiency was calculated as described in Materials and Methods. M, liposome-bound; NM, non-liposome-bound. Data from three (PC+PS) or four [PC+PS+PI(4,5)P2] independent experiments are shown as means ± SD. ns, not significant; **, P < 0.01; ***, P < 0.001. There is a statistically significant difference (*) in binding to PI(4,5)P2-containing liposomes between RNase-treated WT and 25KT/26KT Gag.
To determine whether the phenotype of 25KT/26KT Gag can be explained by the loss of RNA binding, we examined the effect of RNase treatment on liposome binding of this mutant Gag. The enhancement of membrane binding by RNase treatment was smaller than that observed for WT, especially in the absence of PI(4,5)P2 (Fig. 2). These data suggest that the 25KT/26KT mutation and RNase treatment enhance membrane binding by overlapping mechanisms.
The 25KT/26KT Changes Increase the Hydrophobic Interaction with Membranes.
Efficient membrane binding of Gag requires both the myristoyl moiety and the HBR. It is still possible that the 25KT/26KT mutant enhances membrane binding by constitutively exposing the myristoyl moiety. To test this hypothesis, we analyzed the binding of 25KT/26KT Gag to liposomes containing PC alone. Because PC is a neutral lipid, binding of Gag to these liposomes would likely be mediated by hydrophobic interaction through N-terminal myristate moiety. Consistent with the previous findings that the myristate moiety is sequestered inside the globular head of MA, and that PI(4,5)P2 binding is required for facilitating myristate exposure (2, 6, 7), WT Gag was unable to bind well to PC liposomes (Fig. 3). However, the double mutant 25KT/26KT was able to bind efficiently to liposomes containing only PC (Fig. 3). These results suggest that the 25KT/26KT mutant readily exposes the myristate. Therefore, it is likely that lysines 25 and 26 suppress nonspecific membrane binding by regulating hydrophobic interactions dependent on the myristate.
Fig. 3.
25KT/26KT Gag binds efficiently to liposomes lacking acidic phospholipids. Binding of WT and 25KT/26KT Gag to liposomes containing indicated lipids were analyzed as in Fig. 1A. Data from three independent experiments are shown as means ± SD. ns, not significant; *, P < 0.05. There are no statistically significant differences between binding of 25KT/26KT to different liposomes.
RNA Bound to MA but Not NC Inhibits Liposome Binding of Gag.
As described above, RNase treatment enhanced membrane binding of both WT and to a lesser extent 25KT/26KT Gag (Fig. 2). As RNA-mediated inhibition of membrane binding may represent a unique mode of regulation in virus assembly, we sought to understand the mechanism of this inhibition. Coimmunoprecipitation experiments showed that both MA HBR and NC facilitate RNA binding to Gag in reticulocyte lysates (Fig. S3). To test whether RNA bound to NC is involved in the inhibition of membrane binding, we analyzed NC-deleted Gag (delNC) for RNase sensitivity. Liposome binding of delNC Gag was increased upon RNase treatment to a similar extent as WT Gag (Fig. 4A). These results suggest that at least in the liposome binding assay, NC is not required for RNA-mediated inhibition of membrane binding of Gag.
Fig. 4.
MA-bound RNA suppresses membrane binding of Gag by inhibiting interactions between basic residues and acidic lipids and by reducing the myristate-dependent interaction. (A) Liposome binding of WT and delNC Gag was analyzed as in Fig. 2 by using PC+PS liposomes. Data from three independent experiments are shown as means ± SD. **, P < 0.01. There is no statistically significant difference in membrane binding of RNase-treated Gag between WT and delNC. (B and C) Liposome binding of WT and 1GA Gag was analyzed as in Fig. 2 by using liposomes containing either PC+PS or PC+PS+PI(4,5)P2 (B) or only PC (C). Data from three to five independent experiments are shown as means ± SD. ns, not significant; *, P < 0.05; **, P < 0.01. There is a statistically significant difference (*) in binding to PC+PS liposomes but not PI(4,5)P2-containing liposomes between WT and 1GA Gag treated with RNase.
RNase Treatment Increases both the Interaction of MA Basic Residues with Acidic Lipids and the Myristate-Dependent Hydrophobic Interaction.
The results presented above are consistent with the possibility that RNA bound to MA HBR blocks the interaction of the HBR basic residues with acidic lipids and interferes with Gag membrane binding. However, it is also conceivable that RNA binding to the HBR might reduce myristate exposure, thereby suppressing membrane binding of Gag. Both of these possibilities are consistent with the observation that membrane binding of 25KT/26KT Gag that has readily exposed myristate (Fig. 3) is enhanced upon RNase treatment markedly but not as much as WT Gag (Fig. 2). Hence, to further understand the mechanism by which RNA inhibits membrane binding, we analyzed the myristoylation-defective Gag mutant (1GA). Interestingly, although RNase treatment did increase membrane binding of 1GA Gag, the extent of increase depended on the composition of liposomes used in the assay. 1GA Gag bound PI(4,5)P2-containing liposomes as efficiently as WT Gag upon RNase treatment (Fig. 4B). This observation strongly supports the model that interaction of basic residues in the HBR with acidic lipids is increased upon RNase treatment.
In contrast, RNase treatment increased binding of 1GA Gag to PC+PS liposomes only modestly compared to that of WT Gag. These results are consistent with the possibility that RNA also inhibits membrane binding partially by sequestration of myristate. Indeed, WT Gag did show a modest increase in PC-liposome binding upon RNase treatment, whereas 1GA Gag showed no increase (Fig. 4C). These data suggest that removal of RNA has an impact on the myristoyl switch as well. Altogether, these results indicate that RNA inhibits membrane binding both by preventing the electrostatic interaction between basic residues and acidic lipids and by suppressing the myristate-dependent hydrophobic interaction.
Discussion
Phosphoinositide-binding domains found in cellular proteins contain basic residues that are essential for interaction with acidic headgroups of phosphoinositides (21). Similarly, the current study showed that some basic residues within the HBR (Lys17, Lys29, and Lys31) are important for efficient Gag membrane binding mediated by PI(4,5)P2 (Fig. 1A). However, our study also revealed that the HBR contains basic residues (Lys25 and Lys26) that suppress membrane binding of Gag (Fig. 1A). This negative regulation of membrane binding by Lys25 and Lys26 likely occurs via myristate sequestration (Fig. 3). Consistent with the increased PI(4,5)P2-independent liposome binding, 25KT/26KT Gag localizes promiscuously in cells and releases virus particles more efficiently than WT Gag (Fig. 1 B and C), a phenotype reminiscent of Gag derivatives with MA globular head deletions (35).
This work also identified RNA as a negative regulator of Gag membrane binding. Several in vitro studies including this study (Fig. S3) showed that HIV-1 MA interacts with RNA via its HBR (17 –19). Therefore, competition between RNA and acidic lipids for binding to the HBR at least partly accounts for the RNA-mediated suppression of Gag membrane binding. Consistent with this hypothesis, in other in vitro experiments performed in the absence of RNA, binding of MA to PS has been readily observed (36 –38), whereas in our system, Gag binding to the PC+PS liposomes was negligible without RNase treatment. Importantly, in contrast to PC+PS liposomes, a substantial amount of Gag binds to PC+PS+PI(4,5)P2 liposomes even without RNase treatment (Figs. 2 and 4). Consistent with these data, while this manuscript was in revision, Eric Barklis’s group reported that PI(4,5)P2-containing liposomes out-compete oligonucleotides for binding to beads coated with purified myristylated MA domain (39). Thus, it is likely that PI(4,5)P2, but not other acidic lipids such as PS, can bind the HBR in the presence of RNA. In addition, exposure of myristate may also be more dependent on PI(4,5)P2 binding in the presence of RNA, because RNA binding to MA HBR also reduces myristate-dependent hydrophobic interaction (Fig. 4). In any case, our data suggest that RNA binding to MA represents an important regulatory mechanism in Gag membrane binding.
It remains to be determined whether RNA bound to the HBR inhibits unregulated membrane binding in cells. In our in vitro system, the RNA concentration is estimated to be lower than that in the cytoplasm of HeLa cells. Nonetheless, the inhibition of membrane binding by RNA does not require NC, the major RNA binding domain (Fig. 4A). However, in cells, it is likely that the viral genomic RNA bound to NC also binds MA because of their close proximity. This model is also consistent with a recently proposed folded-over conformation of Gag (40, 41) (not depicted in Fig. 5). Notably, two recent reports suggest that RNA export from the nucleus plays a key role in MA-mediated membrane binding of Gag (42, 43). These authors observed that HIV Gag assembly defects linked to specific Gag mRNA export pathways could be rescued by increasing membrane binding ability of Gag, either by MA mutations or replacing MA with heterologous membrane binding domains (42, 43). In light of the results presented here, it is possible that nuclear export pathways used by viral RNAs affect MA–RNA interactions that, in turn, influence Gag membrane binding. This hypothesis is also in agreement with the observed autoinhibition of Gag membrane binding by HIV-1 MA in murine cells (44, 45).
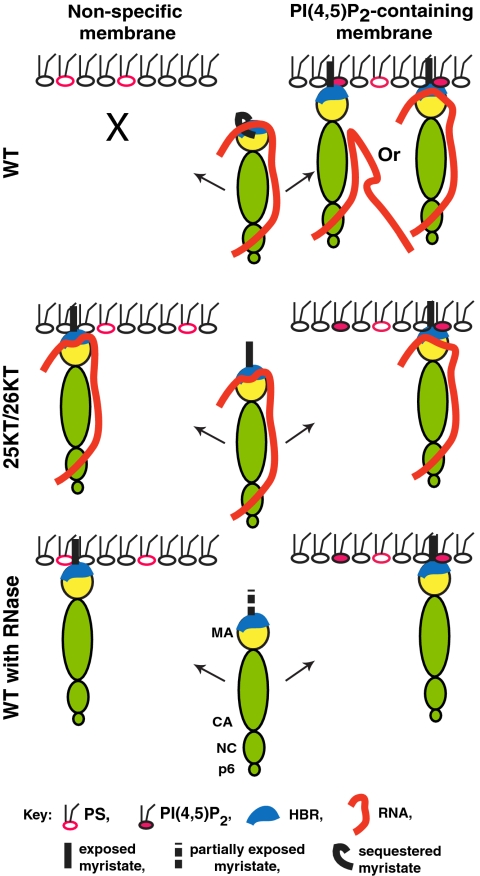
Fig. 5.
A model for the regulation of Gag-membrane binding specificity. PI(4,5)P2-dependent and -independent membrane binding of WT, 25KT/26KT, and RNase-treated Gag are shown. See Discussion for explanation.
It has been long known that retroviral MA interacts with RNA (15–19, 46–50). Although genomic RNA encapsidation does not require MA in the case of HIV-1 (51), a variety of functions have been suggested for the retroviral MA–RNA interaction in the later half of the virus life cycle. These functions include (i) regulation of viral mRNA translation (18, 52), (ii) encapsidation of genomic viral RNA (53), (iii) dimerization of encapsidated RNA (54, 55), and (iv) enhancement of Gag assembly (56, 57). Thus, it is tempting to speculate that binding of the HBR to both PI(4,5)P2 and RNA may play a key role in coordinating the four-way interactions between the PM, viral RNA, MA, and NC during the late phase of the retrovirus life cycle. For example, once Gag reaches the PM, the viral RNA bound to MA might get displaced by the HBR–PI(4,5)P2 interaction. This displaced RNA may then act as a scaffold for NC-dependent Gag multimerization and facilitate efficient particle assembly at the PM. Such a process might also require cellular proteins reported to play a role in Gag multimerization or RNA packaging, including ATP-binding cassette protein in the E subfamily (ABCE1) (58, 59) and Staufen-1 (60). Clearly, further studies are necessary for addressing interrelationships between events during the late phase.
In summary, our data indicate that the HBR within MA modulates membrane binding in three different ways: positively by interaction with PI(4,5)P2, negatively by suppression of myristate-dependent hydrophobic interaction, and negatively by interaction with RNA. RNA binding to MA HBR not only interferes with interactions between MA basic amino acids and acidic lipids, but also appears to reduce myristate exposure. Based on this and previous studies, we put forth the following model (Fig. 5). Both RNA binding and suppression of myristate exposure mediated by the HBR prevent Gag from premature or nonspecific membrane binding. Once Gag reaches PI(4,5)P2-containing membranes such as the PM, PI(4,5)P2 binds the HBR, which then triggers myristate exposure and stabilizes binding to the lipid bilayer. However, if myristate sequestration is blocked (e.g., by the 25KT/26KT mutations) or if RNA detaches from the HBR (e.g., by RNase treatment), Gag binds membrane in a nonspecific, PI(4,5)P2-independent manner (Fig. 5). Cooperation of interactions with multiple membrane factors, which enhances the robustness and specificity of membrane binding, is prevalent in phosphoinositide-binding domains (21). However, MA HBR is unique among these domains for integrating opposing regulatory mechanisms to ensure specificity for the target membrane that contains PI(4,5)P2.
Materials and Methods
Cells and Plasmids.
HeLa cells were cultured and maintained in DMEM supplemented with 5% FBS. HIV-1 molecular clone pNL4-3 (61), its derivative encoding YFP-tagged Gag (pNL4-3/GagVenus) and a Gag expression vector for in vitro translation reaction, pGEMNLNR, were described (24). pNL4-3/1GA, pNL4-3/25KT/26KT, pNL4-3/17KT/19RL, pNL4-3/29KT/31KT, pNL4-3/19RL, and pNL4-3/21RL were kind gifts from E. Freed (HIV Drug Resistance Program, National Cancer Institute, Frederick, MD) (33, 34). Other MA changes were introduced into pNL4-3 by PCR mutagenesis. MA changes in pNL4-3 derivatives were also introduced into pGEMNLNR and pNL4-3/GagVenus by using standard molecular biology techniques. pGEMNLNR/delNC was constructed by replacing BssHII to PflMI fragment (nt 711–5297) with that of pNL4-3/delNC (a kind gift from D. Ott) (AIDS Vaccine Program, SAIC-Frederick, Inc., National Cancer Institute, Frederick, MD) (62).
Liposome Binding Assay.
Liposome binding assay was performed as described (24). Briefly, radiolabeled Gag is synthesized by using in vitro transcription and translation coupled reaction mix containing rabbit reticulocyte lysates (Promega). Liposomes containing corresponding lipids were added after 30 min at 30°C and incubated further for 90 min. Then, the liposome-bound and non-liposome-bound Gag were separated by equilibrium flotation centrifugation.
For RNase treatment experiments, the above protocol was modified as following. Gag synthesis was performed at 30°C for 90 min. Subsequently, 1 μL of RNase, which is a mixture of pyrimidine-specific endoribonucleases from bovine pancreas (Cat. No.: 11119915001, Roche Applied Science), was added to 25 μL of the reaction mix and incubated for 20 min at 37°C. Liposomes were then added and incubated for 15 min at 30°C before equilibrium flotation centrifugation was performed.
Virus Release Assay, Microscopy, and Statistical Analysis.
Virus release assay was performed as described (23). Microscopy and statistical analysis were performed as described (24).
Coimmunoprecipitation of Gag and RNA.
RNA binding to Gag synthesized in in vitro transcription and translation coupled reactions was analyzed as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Mike Summers, Kalola Chancellor, Eric Freed, and the members of our laboratory for helpful discussions and critical review of the manuscript. We would also like to thank Drs. E. Freed and D. Ott for providing plasmids. The following reagent was obtained through AIDS Research and Reference Reagent Program, Division of AIDS, N National Institute of Allergy and Infectious Diseases, National Institutes of Health: HIV-Ig from NABI and National Heart, Lung, and Blood Institute. This work is supported by the National Institutes of Health Grant R01 AI071727 (to A.O.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908661107/DCSupplemental.
References
- 1.Freed EO. HIV-1 gag proteins: Diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 2.Adamson CS, Freed EO. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv Pharmacol. 2007;55:347–387. doi: 10.1016/S1054-3589(07)55010-6. [DOI] [PubMed] [Google Scholar]
- 3.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou W, Parent LJ, Wills JW, Resh MD. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Göttlinger HG, Sodroski JG, Haseltine WA. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang C, et al. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc Natl Acad Sci USA. 2004;101:517–522. doi: 10.1073/pnas.0305665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad JS, et al. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci USA. 2006;103:11364–11369. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W, Resh MD. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte MR, Matthews S. Retroviral matrix proteins: A structural perspective. Virology. 1998;246:191–198. doi: 10.1006/viro.1998.9206. [DOI] [PubMed] [Google Scholar]
- 11.Callahan EM, Wills JW. Repositioning basic residues in the M domain of the Rous sarcoma virus gag protein. J Virol. 2000;74:11222–11229. doi: 10.1128/jvi.74.23.11222-11229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill CP, Worthylake D, Bancroft DP, Christensen AM, Sundquist WI. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: Implications for membrane association and assembly. Proc Natl Acad Sci USA. 1996;93:3099–3104. doi: 10.1073/pnas.93.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton AK, Murray PS, Murray D, Vogt VM. Biochemical characterization of rous sarcoma virus MA protein interaction with membranes. J Virol. 2005;79:6227–6238. doi: 10.1128/JVI.79.10.6227-6238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray PS, et al. Retroviral matrix domains share electrostatic homology: Models for membrane binding function throughout the viral life cycle. Structure. 2005;13:1521–1531. doi: 10.1016/j.str.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Chang CY, et al. HIV-1 matrix protein repositioning in nucleocapsid region fails to confer virus-like particle assembly. Virology. 2008;378:97–104. doi: 10.1016/j.virol.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lochrie MA, et al. In vitro selection of RNAs that bind to the human immunodeficiency virus type-1 gag polyprotein. Nucleic Acids Res. 1997;25:2902–2910. doi: 10.1093/nar/25.14.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purohit P, Dupont S, Stevenson M, Green MR. Sequence-specific interaction between HIV-1 matrix protein and viral genomic RNA revealed by in vitro genetic selection. RNA. 2001;7:576–584. doi: 10.1017/s1355838201002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimarelli A, Luban J. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J Virol. 1999;73:5388–5401. doi: 10.1128/jvi.73.7.5388-5401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearps AC, Wagstaff KM, Piller SC, Jans DA. The N-terminal basic domain of the HIV-1 matrix protein does not contain a conventional nuclear localization sequence but is required for DNA binding and protein self-association. Biochemistry. 2008;47:2199–2210. doi: 10.1021/bi701360j. [DOI] [PubMed] [Google Scholar]
- 20.Hearps AC, Jans DA. Regulating the functions of the HIV-1 matrix protein. AIDS Res Hum Retroviruses. 2007;23:341–346. doi: 10.1089/aid.2006.0108. [DOI] [PubMed] [Google Scholar]
- 21.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 22.Lemmon MA, Ferguson KM, O’Brien R, Sigler PB, Schlessinger J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc Natl Acad Sci USA. 1995;92:10472–10476. doi: 10.1073/pnas.92.23.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:14889–14894. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient gag membrane binding. J Virol. 2008;82:2405–2417. doi: 10.1128/JVI.01614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saad JS, et al. Structure of the myristylated human immunodeficiency virus type 2 matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in membrane targeting. J Mol Biol. 2008;382:434–447. doi: 10.1016/j.jmb.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan R, et al. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol. 2008;82:11228–11238. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jefferies HB, et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9:164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stansell E, et al. Basic residues in the Mason-Pfizer monkey virus gag matrix domain regulate intracellular trafficking and capsid-membrane interactions. J Virol. 2007;81:8977–8988. doi: 10.1128/JVI.00657-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shkriabai N, et al. Interactions of HIV-1 Gag with assembly cofactors. Biochemistry. 2006;45:4077–4083. doi: 10.1021/bi052308e. [DOI] [PubMed] [Google Scholar]
- 30.Alfadhli A, Barklis RL, Barklis E. HIV-1 matrix organizes as a hexamer of trimers on membranes containing phosphatidylinositol-(4,5)-bisphosphate. Virology. 2009;387:466–472. doi: 10.1016/j.virol.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ono A, Orenstein JM, Freed EO. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J Virol. 2000;74:2855–2866. doi: 10.1128/jvi.74.6.2855-2866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagai T, et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 33.Freed EO, Orenstein JM, Buckler-White AJ, Martin MA. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J Virol. 1994;68:5311–5320. doi: 10.1128/jvi.68.8.5311-5320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freed EO, Englund G, Martin MA. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;69:3949–3954. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reil H, Bukovsky AA, Gelderblom HR, Göttlinger HG. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 1998;17:2699–2708. doi: 10.1093/emboj/17.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalton AK, Ako-Adjei D, Murray PS, Murray D, Vogt VM. Electrostatic interactions drive membrane association of the human immunodeficiency virus type 1 Gag MA domain. J Virol. 2007;81:6434–6445. doi: 10.1128/JVI.02757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrlich LS, Fong S, Scarlata S, Zybarth G, Carter C. Partitioning of HIV-1 Gag and Gag-related proteins to membranes. Biochemistry. 1996;35:3933–3943. doi: 10.1021/bi952337x. [DOI] [PubMed] [Google Scholar]
- 38.Alfadhli A, Huseby D, Kapit E, Colman D, Barklis E. Human immunodeficiency virus type 1 matrix protein assembles on membranes as a hexamer. J Virol. 2007;81:1472–1478. doi: 10.1128/JVI.02122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfadhli A, Still A, Barklis E. Analysis of human immunodeficiency virus type 1 matrix binding to membranes and nucleic acids. J Virol. 2009;83:12196–12203. doi: 10.1128/JVI.01197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta SA, et al. Interactions between HIV-1 Gag molecules in solution: An inositol phosphate-mediated switch. J Mol Biol. 2007;365:799–811. doi: 10.1016/j.jmb.2006.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datta SA, et al. Conformation of the HIV-1 Gag protein in solution. J Mol Biol. 2007;365:812–824. doi: 10.1016/j.jmb.2006.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherer NM, Swanson CM, Papaioannou S, Malim MH. Matrix mediates the functional link between human immunodeficiency virus type 1 RNA nuclear export elements and the assembly competency of Gag in murine cells. J Virol. 2009;83:8525–8535. doi: 10.1128/JVI.00699-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J, Sturgeon T, Weisz OA, Mothes W, Montelaro RC. HIV-1 matrix dependent membrane targeting is regulated by Gag mRNA trafficking. PLoS One. 2009;4:e6551. doi: 10.1371/journal.pone.0006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatziioannou T, Martin-Serrano J, Zang T, Bieniasz PD. Matrix-induced inhibition of membrane binding contributes to human immunodeficiency virus type 1 particle assembly defects in murine cells. J Virol. 2005;79:15586–15589. doi: 10.1128/JVI.79.24.15586-15589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hübner W, Chen BK. Inhibition of viral assembly in murine cells by HIV-1 matrix. Virology. 2006;352:27–38. doi: 10.1016/j.virol.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Steeg CM, Vogt VM. RNA-binding properties of the matrix protein (p19gag) of avian sarcoma and leukemia viruses. J Virol. 1990;64:847–855. doi: 10.1128/jvi.64.2.847-855.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sen A, Todaro GJ. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977;10:91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- 48.Leis JP, McGinnis J, Green RW. Rous sarcoma virus p19 binds to specific double-stranded regions of viral RNA: Effect of p19 on cleavage of viral RNA by RNase III. Virology. 1978;84:87–98. doi: 10.1016/0042-6822(78)90220-9. [DOI] [PubMed] [Google Scholar]
- 49.Karpel RL, Henderson LE, Oroszlan S. Interactions of retroviral structural proteins with single-stranded nucleic acids. J Biol Chem. 1987;262:4961–4967. [PubMed] [Google Scholar]
- 50.Darlix JL, Spahr PF. Binding sites of viral protein P19 onto Rous sarcoma virus RNA and possible controls of viral functions. J Mol Biol. 1982;160:147–161. doi: 10.1016/0022-2836(82)90172-3. [DOI] [PubMed] [Google Scholar]
- 51.Poon DT, Li G, Aldovini A. Nucleocapsid and matrix protein contributions to selective human immunodeficiency virus type 1 genomic RNA packaging. J Virol. 1998;72:1983–1993. doi: 10.1128/jvi.72.3.1983-1993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson EC, Lever AM. Human immunodeficiency virus type 1 Gag polyprotein modulates its own translation. J Virol. 2006;80:10478–10486. doi: 10.1128/JVI.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Norris KM, Mansky LM. Involvement of the matrix and nucleocapsid domains of the bovine leukemia virus Gag polyprotein precursor in viral RNA packaging. J Virol. 2003;77:9431–9438. doi: 10.1128/JVI.77.17.9431-9438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parent LJ, et al. RNA dimerization defect in a Rous sarcoma virus matrix mutant. J Virol. 2000;74:164–172. doi: 10.1128/jvi.74.1.164-172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garbitt RA, Albert JA, Kessler MD, Parent LJ. trans-acting inhibition of genomic RNA dimerization by Rous sarcoma virus matrix mutants. J Virol. 2001;75:260–268. doi: 10.1128/JVI.75.1.260-268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ott DE, Coren LV, Gagliardi TD. Redundant roles for nucleocapsid and matrix RNA-binding sequences in human immunodeficiency virus type 1 assembly. J Virol. 2005;79:13839–13847. doi: 10.1128/JVI.79.22.13839-13847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crist RM, et al. Assembly properties of human immunodeficiency virus type 1 Gag-leucine zipper chimeras: Implications for retrovirus assembly. J Virol. 2009;83:2216–2225. doi: 10.1128/JVI.02031-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmerman C, et al. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature. 2002;415:88–92. doi: 10.1038/415088a. [DOI] [PubMed] [Google Scholar]
- 59.Dooher JE, Schneider BL, Reed JC, Lingappa JR. Host ABCE1 is at plasma membrane HIV assembly sites and its dissociation from Gag is linked to subsequent events of virus production. Traffic. 2007;8:195–211. doi: 10.1111/j.1600-0854.2006.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatel-Chaix L, Boulay K, Mouland AJ, Desgroseillers L. The host protein Staufen1 interacts with the Pr55Gag zinc fingers and regulates HIV-1 assembly via its N-terminus. Retrovirology. 2008;5:41. doi: 10.1186/1742-4690-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adachi A, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ott DE, et al. Elimination of protease activity restores efficient virion production to a human immunodeficiency virus type 1 nucleocapsid deletion mutant. J Virol. 2003;77:5547–5556. doi: 10.1128/JVI.77.10.5547-5556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.