Abstract
Most genetically engineered mouse (GEM) models for colon cancer are based on tissuewide or germline gene modification, resulting in tumors predominantly of the small intestine. Several of these models involve modification of the adenomatous polyposis coli (Apc) gene and are excellent models for familial cancer predisposition syndromes. We have developed a stochastic somatic mutation model for sporadic colon cancer that presents with isolated primary tumors in the distal colon and recapitulates the entire adenoma–carcinoma–metastasis axis seen in human colon cancer. Using this model, we have analyzed tumors that are either solely mutant in the Apc gene or in combination with another colon cancer-associated mutant gene, the Kras G12D allele. Because of the restricted location in the distal colon, the natural history of the tumors can be analyzed by serial colonoscopy. As the mammalian target of rapamycin (mTOR) pathway is a critical component of the complex signaling network in colon cancer, we used this model to assess the efficacy of mTOR blockade through rapamycin treatment of mice with established tumors. After treatment, Apc mutant tumors were more than 80% smaller than control tumors. However, tumors that possessed both Apc and Kras mutations did not respond to rapamycin treatment. These studies suggest that mTOR inhibitors should be further explored as potential colorectal cancer therapies in patients whose tumors do not have activating mutations in KRAS.
Keywords: colon cancer, mouse model, adenovirus, colonoscopy, mammalian target of rapamycin
Colon cancer continues to be one of the leading causes of cancer-related deaths. The prognosis for patients with early stage cancers is good, but the majority of cancers are diagnosed at later stages (1). Most drug development strategies use transplantation of human tumor cells into immunocompromised mice. These models are not truly predictive of response in human patients because they are derived from tumor cell lines grown in vitro and are implanted into ectopic sites that bear no resemblance to the colonic microenvironment (2). Furthermore, these xenograft models fail to recapitulate the heterogeneous nature of cancer and the critical endogenous interplay between tumor and supporting stroma. Genetically engineered mouse (GEM) models circumvent these shortcomings, making them an attractive platform for biomarker discovery, study of cancer biology, and preclinical therapeutic trials (2, 3).
Several GEMs that use germline or tissuewide modification of genes known to be mutated in human colon cancer spontaneously develop intestinal tumors (4). Depending on the gene(s) that is modified, these are reasonable models for inherited cancer predisposition syndromes, such as familial adenomatous polyposis (FAP) and hereditary nonpolyposis colon cancer (HNPCC). However, these are not models for sporadic colon cancer, which comprises 75–80% of all cases in humans (5). In these mouse models for FAP and HNPCC, the genetic mutations are present in the germline. Consequently, they are expressed throughout intestinal development. In sporadic colon cancer, the genetic mutations develop somatically. As such, mouse models that somatically modify critical carcinogenic genes in a stochastic fashion are needed to accurately model sporadic colon cancer.
A true sporadic model for colon cancer should have the following features: (i) the model develops one or two tumors in the colon, (ii) the tumors derive from somatic modification of genes known to be involved in human colorectal cancer, (iii) the somatic mutations involve the colonic epithelium, and (iv) the tumors present along the entire adenoma–carcinoma–metastasis axis. Mice with conditional mutations in Apc are an excellent starting point for developing such models.
Delivery of adenovirus expressing cre recombinase (adeno-cre) to conditional knockout mice is an attractive approach, as the spatial and temporal sequence of gene modification(s) can be controlled (6). This approach has been used to focally modify critical carcinogenic genes in lung, liver, ovarian, and other mouse cancer models (7 –12). Colon tumorigenesis using rectal adeno-cre enemas in mice carrying floxed Apc alleles has been described, but we and other groups have found that the incidence, multiplicity, and location of the intestinal tumors can be highly variable in this model (13).
In this report, we describe a unique surgical procedure to limit adeno-cre infection to the most distal colon, resulting in highly penetrant tumor formation (14). These tumors present with the full spectrum of adenomas, invasive carcinoma, and metastases. The restricted location of the primary tumors makes this an ideal model for serial endoscopic assessment in preclinical therapeutic trials. With the increasing interest in mTOR blockade as an anticancer therapy, we used this model to examine the efficacy of rapamycin as a therapeutic agent. We observe that tumors in mice with Apc mutation respond well to treatment with rapamycin, but when the Apc mutation is combined with an activating mutation in Kras, the resulting tumors no longer respond.
Results
Adenovirus Infects Basal Colonic Crypt Cells.
To generate a mouse model for sporadic colon cancer, we hypothesized that adeno-cre could be delivered to stochastically modify floxed genes within the distal colonic epithelium. Because the murine intestinal epithelium renews every 5 days, infection of the functional stem cell compartment in the basal crypt is required for these changes to have a biological impact (15). To assess the infection efficiency in the basal crypt, we administered 109 pfu of adenovirus expressing β-galactosidase (adeno-LacZ) in 100 μL PBS to the distal colon of C57BL/6 mice. To increase the efficiency of adenoviral infection, we employed an approach in which surgical clips, flanking the site of injection, were placed throughout the entire infection period (Fig. S1A). After 48 h, the distal colons were removed, sectioned along the sagittal plane, and stained for β-galactosidase. Histological examination revealed positive blue staining mainly in the superficial epithelium (Fig. S1B). However, occasional staining was noted in basal crypt cells (Fig. S1C). Although the precise nature of all of the cells infected by the adenovirus cannot be accurately determined, the staining of basal crypt cells and the fact that these mice do develop tumors (see below) suggest that at least a few of the critical cells in the functional stem cell compartment are indeed infected by adenovirus.
Adeno-Cre Treatment of Apc CKO Mice Results in Isolated Distal Colonic Tumors.
Apc inactivation is known to be one of the critical initial genetic alterations for entry into the adenoma–carcinoma sequence (5). In many different mouse models, germline or tissuewide inactivation of the Apc gene results in predominantly small intestinal tumor formation (4). To assess whether critical genes involved in colon carcinogenesis could be stochastically modified to produce distal colonic tumors in a highly reproducible fashion, 109 pfu of adeno-cre in 100 μL PBS was introduced into the colons of mice that were homozygous for a floxed exon 14 allele of the Apc gene (Apc CKO) (16). As a control, 109 pfu of adenovirus containing an empty expression cassette (adeno-WT) was administered to Apc CKO mice. In one experiment, of the 66 mice that were infused with adeno-cre, we were able to detect tumors in 47 (71%) of the mice in as little as 6 weeks after viral administration (Fig. 1A). None of the mice injected with adeno-WT ever developed tumors (n = 12). The mean tumor multiplicity was 1.3 per animal, and the mean distance from the anus was 22.5 mm. We examined colonic tumors from 60 different Apc CKO mice ranging from 9 to 35 weeks after adeno-cre injection. Of these, 56 (93%) exhibited uniform cells with minimal pleomorphism that recapitulated glandular structures in an organized fashion and were classified as adenomas (Fig. 1B). As early as 18 weeks after adeno-cre infection, tumor cells in 4 (7%) of the mice showed greater mitotic activity, were more anaplastic, and had very pleomorphic nuclei. The cells were not orderly arranged in glands or crypts, but exhibited finger-like projections of cells, with individual cells breaking off at the deepest parts and some invading the muscularis layer. Furthermore, there was a vigorous stromal reaction to the tumor. On the basis of these findings, these tumors were classified as carcinomas (Fig. 1C).
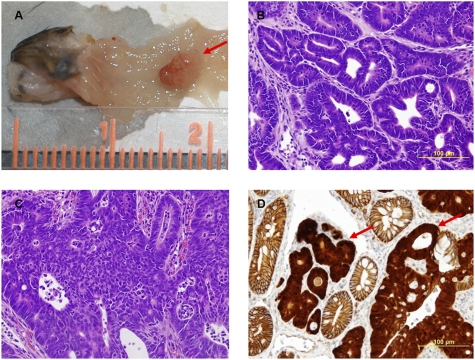
Fig. 1.
Adeno-cre treatment of floxed Apc mice results in distal colonic tumors. (A) Gross picture of solitary tumor (red arrow) after adeno-cre infection of floxed Apc mice in distal colon. Resulting primary tumors range from (B) adenomas to (C) carcinomas. (D) Immunohistochemistry nuclear β-catenin staining (red arrows) in tumors indicating activation of Wnt pathway.
To confirm that the tumors are indeed the result of adeno-cre-mediated recombination, we assessed the status of the Apc gene in several tumors. In all tumors, we detected recombinant Apc alleles that resulted from the homozygous deletion of exon 14, suggesting that tumor initiation occurred after inactivation of the Apc gene. Immunohistochemistry revealed that unlike normal epithelium, the colonic tumors exhibited strong nuclear β-catenin staining, suggesting that tumor progression occurs through the activation of the canonical Wnt signaling pathway (Fig. 1D). These results suggest that adeno-cre can be administered to Apc CKO mice to reliably induce isolated tumor formation in the distal colon that results from Apc inactivation and subsequent activation of Wnt signaling.
Apc Tumors Do Not Develop Spontaneous Kras Mutations.
Thirty to fifty percent of human colonic adenomas and carcinomas contain activating mutations in one of the Ras genes, primarily KRAS (5). It is also known that such mutations are relatively early events during the development of these tumors. As germline Apc mutant mouse models do not develop spontaneous Kras mutations, we hypothesized that the tumors derived from Apc CKO similarly do not develop spontaneous Kras mutations (17). To test this hypothesis, we examined Kras gene transcripts by direct sequencing of RT-PCR products from 20 different colonic tumors at 17–41 weeks following adeno-cre treatment of Apc CKO mice. All of these tumors contained cDNA that was wild type for Kras.
Adeno-Cre Treatment of Apc CKO/LSL-Kras Mice Results in Advanced Primary and Metastatic Colon Tumors.
To assess whether the incorporation of an activated Kras gene would alter tumor progression in our mouse model, we generated mice that were homozygous for the Apc CKO allele and heterozygous for a latent activated allele of Kras (Krastm4tyj/+) (Apc CKO/LSL-Kras) (7). The distal colons of these mice were treated with 109 pfu of adeno-cre in 100 μL PBS. As with the Apc CKO mice, the induced Apc CKO/LSL-Kras mice presented with primary tumors exclusively in the distal colon. Of the 55 mice that were infused with adeno-cre, we were able to detect tumors, by colonoscopy (see below), in 53 (96%) of the mice in as little as 3 weeks after viral administration, with an average tumor burden of 3.6 lesions per mouse. Tumor histology was assessed using the same criteria as was used for tumors from the Apc CKO mice. Of the 42 tumors examined, 27 (64%) were adenomas and the remaining 15 (36%) were carcinomas. However, carcinomas first presented 20 weeks after adeno-cre injection. Of the 30 tumors that were examined after this time, 15 (50%) were carcinomas (Fig. 2 A–C). Furthermore, spontaneous gross liver metastases were noted starting 24 weeks after adeno-cre injection (Fig. 2D). Of the 25 mice that were examined after this time, 5 (20%) mice showed these lesions. Upon histological examination, these lesions were classified as adenocarcinomas (Fig. 2E). To confirm that the metastatic tumors were of intestinal origin, both primary and metastatic tumors were stained with the intestinal-specific transcription factor cdx-2 (Fig. 2 F and G). Wnt activation was noted in metastatic tumors by nuclear β-catenin staining. (Fig. 2H). These results suggest that the addition of an activated Kras allele can accelerate tumor progression and lead to eventual metastasis.
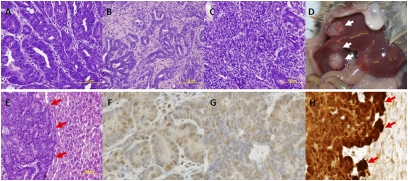
Fig. 2.
Adeno-cre treatment of Apc CKO/LSL-Kras mice accelerates tumor progression. Adeno-cre administered to the distal colon of Apc CKO/LSL-Kras mice results in primary tumors that present as (A) adenomas, (B) carcinomas, and (C) invasive carcinomas. Furthermore, in a subset of animals (D) gross (white arrows) and (E) histological liver metastases (red arrows) are seen. Nuclear staining for cdx-2 demonstrates (F) primary and (G) metastatic tumors are of intestinal origin. (H) Nuclear β-catenin staining (red arrows) demonstrates Wnt pathway is activated in metastatic tumors.
Endoscopic Assessment of Mouse Sporadic Colon Cancers.
The ability to longitudinally follow the development of tumors provides a number of opportunities to study progression. Although murine colonoscopy systems have been developed, they have proven to be not useful because the tumors in germline mutant models are predominantly located in the small intestine (18, 19). Because the model described above develops primary tumors only in the distal colon, we were able to use a custom-made colonoscopy system to monitor the natural history of tumor growth. To visualize tumors, the distal colon was cleansed with PBS, and air was carefully insufflated to distend the colon, avoiding perforation. This procedure was well tolerated by all animals. The colonoscope was inserted into the intestinal tract through the anus and white light images were obtained. Representative results from a few such colonoscopies are shown in Fig. 3A. These results suggest that colonoscopy can be used to follow the natural history of tumor formation in this model.
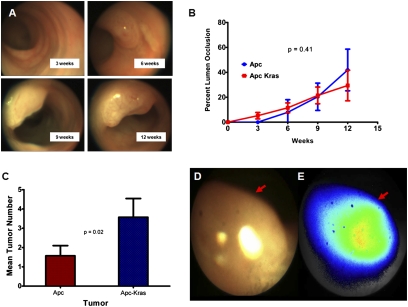
Fig. 3.
Endoscopic assessment of mouse sporadic colon cancers. (A) Representative serial endoscopic images after adeno-cre administration to the distal colon of Apc CKO mice. (B) Tumor growth curves of adeno-cre-induced Apc and Apc/Kras mutant mice do not show a significant difference (P = 0.406). (C) Early endoscopic determination in Apc CKO and Apc CKO/LSL-Kras animals after adeno-cre induction shows an increase in tumor multiplicity (P = 0.02). Endoscopic imaging of mouse colon tumors (red arrows) after injection with Prosense 680: (D) white light imaging and (E) near-infrared imaging.
Activated Kras Accelerates Growth of Early Lesions.
We next assessed the feasibility of quantitating the rate of tumor growth by colonoscopy. To accomplish this, endoscopic images obtained in real time were saved for later offline analysis. Assessing the absolute tumor size from these images is difficult because tumor size in these images is a function of the distance between the endoscopic probe and the tumor itself. Therefore, we hypothesized that if the colon were fully insufflated, the relative lumen sizes could be used as a normalization factor. We used ImageJ software to determine the ratio of the tumor and lumen cross-sectional areas. To validate this ratio as a surrogate metric for tumor size, mice underwent colonoscopy followed by immediate necropsy. The dissected tumor size was then assessed by standard caliper measurements. Comparison of the ratio of the tumor/lumen cross-sectional area and the absolute ex vivo tumor size showed good correlation (r = 0.87, n = 12). On the basis of this approach, we generated growth curves for tumors in the induced Apc CKO and Apc CKO/LSL-Kras mice (Fig. 3B). For the Apc CKO mice, tumors were seen in as little as 6 weeks. However, for the Apc CKO/LSL-Kras mice initial lesions were seen in as little as 3 weeks. Nonetheless, the difference between the overall composite growth curves was not significant (P = 0.41).
A significant advantage of the colonoscopy approach is the ability to assess tumor multiplicity at the earliest stages of tumor formation. If tumor multiplicity were only assessed at necropsy, multiple initial tumors may have coalesced and appear as a single tumor. However, initial tumor multiplicities can be assessed at the time of earliest tumor formation by colonoscopy. We compared the tumor multiplicity of Apc CKO and Apc CKO/LSL-Kras at each endoscopic time of assessment. When the Kras mutation is activated, the overall tumor multiplicity increased from 1.3 to 3.6 in a statistically significant manner (P = 0.002, Fig. 3C). Taken together, these results suggest that the presence of K-Ras mutations may provide an added advantage for the tumor clone to survive and develop into a tumor rather than providing a specific advantage in the rate of established tumor growth.
Assessment of Colonoscopy Adjuvants.
In the clinical arena, optical colonoscopy is the gold standard for colon cancer screening, but a significant number of polyps are still missed (20). There is growing interest in the use of molecular beacon protease-activated synthetic probes for improved detection of early stage cancers including flat adenomas. Prosense 680 is a long circulating graft copolymer that markedly increases its near-infrared fluorescence intensity after selective enzymatic cleavage of lysine-lysine bonds within it, primarily by cathepsin B in vivo. Our previous mass spectrometry-based study of the plasma proteome of intestinal tumor-bearing Apc Δ580 mice revealed high levels of plasma cathepsin B (21). Immunohistochemical analysis indicated that cathepsin B was preferentially expressed in tumor compared with normal mucosa. Ex vivo imaging after injection with Prosense 680 demonstrated highly specific staining for intestinal tumors. To assess whether Prosense 680 could be used as an endoscopic adjuvant, the same agent was i.v. injected into adeno-cre-induced Apc CKO mice with established tumors and imaged with near-infrared colonoscopy. Representative results are shown in Fig. 3 D and E. Near-infrared endoscopic images revealed that Prosense 680 signal correlates well with tumor lesions and that it can be a useful adjunct to white light colonoscopy. These results demonstrate the utility of this model in assessment of colonscopic adjuvant strategies.
mTOR Pathway Is Activated in Tumors from Mouse Sporadic Colon Cancer Models.
Recent studies have revealed the importance of the mTOR growth pathway in a mouse model for FAP (22). To assess whether this pathway was activated in our mouse sporadic colon cancer model, we examined the levels of phosphophorylated S6 ribosomal protein (S6-P) as a metric for mTOR activation. Comparison of tumor and normal epithelium by Western blot analysis showed similarly increased levels of S6-P in both Apc CKO and Apc CKO/LSL-Kras mice (Fig. 4A). These results were confirmed by tumor immunohistochemistry in these mice (Fig. 4B). Taken together, it appears that the mTOR pathway is activated in tumors derived from Apc CKO and Apc CKO/LSL-Kras mice.
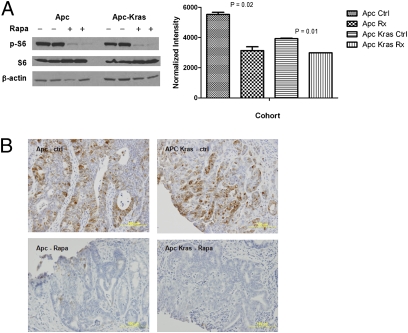
Fig. 4.
Rapamycin treatment blocks the mTOR pathway in Apc and Apc-Kras mutant tumors. Phospho-S6 levels are decreased after rapamycin treatment in both Apc and Apc-Kras mutant tumors by (A) Western blot analysis and (B) immunohistochemistry.
Rapamycin Treatment Causes Tumor Regression Only in Apc CKO Mice.
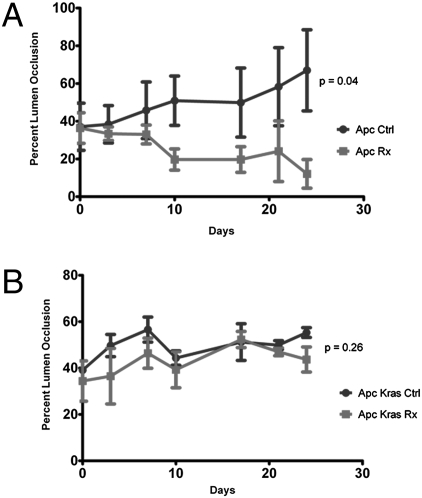
Because the mTOR pathway is activated in both Apc CKO and Apc CKO/LSL-Kras mice, we hypothesized that these tumors might be “oncogenically addicted” to mTOR signaling. If so, mTOR blockade might be expected to result in growth arrest or even regression of established tumors. To assess this feature, we treated adeno-cre-induced Apc CKO and Apc CKO/LSL-Kras mice with established tumors as determined by colonoscopy (n = 8) with the mTOR inhibitor rapamycin. Mice were treated with daily i.p. injection of rapamycin (4 mg/kg) and subsequent tumor growth was followed by twice weekly colonoscopy. After 24 days of rapamycin treatment, tumors from Apc CKO mice showed a 82% reduction in tumor size as compared to control animals (Fig. 5A, P = 0.04). Interestingly, the growth curves between treated case and control Apc CKO/LSL-Kras mice (n = 8) was not significantly different (Fig. 5B, P = 0.26). To assess whether the treatment failure was simply due to increased drug requirements secondary to mutated Kras, Western blot and immunohistochemistry were used to demonstrate that S6-P was equivalently decreased in both the treated Apc CKO and Apc CKO/LSL-Kras mice (Fig. 4 A and B). These results suggest that rapamycin has powerful anticolonic tumor effects that are abrogated in the presence of activated Kras alleles.
Fig. 5.
mTOR blockade results in tumor regression that is dependent upon Kras mutational status. Endoscopic growth curves demonstrate decrease in tumor size with rapamycin treatment in (A) Apc mutant tumors (P = 0.04) but not (B) Apc-Kras (P = 0.26).
Mutant Kras Activates the MAPK Pathway.
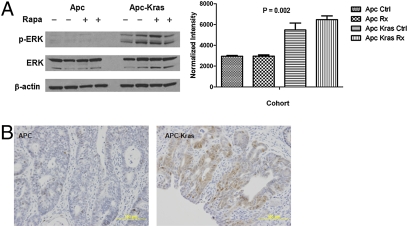
We reasoned that the additional mutant Kras allele might provide an alternate signaling pathway that allows these tumors to escape inhibition of “oncogenic addiction” to mTOR signaling. As Kras is known to activate the MAPK pathway, we assessed its activation using levels of phosphorylated ERK (p-ERK) as a metric. Western blot analysis revealed that p-ERK was increased in tumors from Apc CKO/LSL-Kras mice as compared to those from Apc CKO (Fig. 6A). These results were also confirmed by immunohistochemistry analysis (Fig. 6B). These results suggest that the activation of the MAPK pathway might serve as a parallel signaling pathway that permits tumor growth in the face of mTOR blockade.
Fig. 6.
Apc-Kras tumors show increased levels of MAPK activation. Phospho-ERK levels are increased in Apc-Kras tumors by (A) Western blot analysis and (B) immunohistochemistry.
Discussion
Several GEM models for human colon cancer have been described that show an intestinal tumor predisposition phenotype. Many of these incorporate genetic modification in the Apc gene, which is mutated in a majority of human colorectal cancers, whereas others have mutations in DNA mismatch repair genes (4). Although these are excellent models for hereditary gastrointestinal cancer, none of them are appropriate models for sporadic colorectal cancer. Because the majority of human colon cancers are sporadic in nature, having a robust mouse model for such cancers would prove to be an important adjunct for our understanding of tumor initiation, progression, and treatment.
A faithful sporadic GEM model of colon cancer must have the following features: (i) the mice should develop one or a very few colonic adenomas that have the capacity to progress to adenocarcinoma and metastasis, (ii) the tumor(s) must be restricted to colon, (iii) the tumors should involve loss of function of the Apc gene leading to activation of Wnt signaling, (iv) the tumor must develop in the context of normal colonic epithelium and stroma, and (v) the model should have a short and predictable timeline for tumor progression. The mouse we have described in this report meets all of these expectations. We began with a mouse that is homozygous for a floxed allele of the Apc locus (13). We developed a reliable and reproducible strategy to inactivate the Apc locus in a small subset of colonic epithelial cells, leading to the development of one to two tumors only in the distal colon. These tumors develop very rapidly in the setting of normal colonic epithelium and stroma with the earliest macroscopically detectable tumors visualized in as little as 6 weeks after the genetic modification.
Because loss of germline Apc gene function results in embryonic lethality, only mice that are heterozygous for an Apc mutation can be used in most mouse models. In models carrying different Apc mutations, the latency period required before detection of tumors can be quite variable. In the sporadic model described here, we have initiated tumor formation through simultaneous modification of both floxed Apc alleles, thereby reducing the time of latency required for tumor development. The short latency period and high tumor penetrance are ideal for preclinical drug development studies.
Our studies provide an explanation as to why the development of a robust sporadic colon cancer mouse model using a simple adenoviral enema strategy has been problematic. X-gal staining after adeno-lacZ administration revealed that the majority of infection occurs in the superficial intestinal epithelium, which would result in gene modification of already well-differentiated cells that would not have the capacity to develop into a tumor. Our surgically based infection strategy physically confines the adenovirus to the distal colon for an adequate period to increase the effective local concentration and to allow for infection of the cells. Without this surgical approach, we have determined that simple adenoviral enemas result in leakage of the solution containing the virus to either proximally into the right colon, cecum, or small intestine, or distally out through the anus, resulting in suboptimal adenoviral infection. In addition, our surgical strategy allows direct visualization of the desired region to confirm the absence of stool that might decrease the overall infection efficiency. Most importantly, this approach physically constrains tumor formation to the distal colonic region that is flanked by the surgical clips, thereby preventing the formation of extracolonic tumors and allowing for subsequent endoscopic tumor assessment.
Traditional mouse models, such as ApcMin, can present with greater than 100 intestinal tumors, show a high level of morbidity, and die by 4–5 months of age secondary to anemia (4). In contrast, our adenoviral induction strategy mimics human sporadic colon cancer in its very low colonic tumor burden. As a result, these mice remain healthy for extended periods past initial tumor detection, allowing development of the full spectrum of tumors that are found in human colon cancer, i.e., adenomas and carcinomas. The ability to follow the development and progression of one or a few tumors in each mouse provides excellent opportunities to study the natural history of tumors. None of the models for hereditary tumor predisposition syndromes (e.g., ApcMin or Apc 1638) have provided the opportunity to study this process, because each of the models develops many tumors that are predominantly located in the small intestine and therefore not accessible for serial observation. The combination of having a robust colon cancer model combined with unique colonoscopic methods now provides an excellent opportunity to follow the progression of individual tumors throughout their life history (18). This endoscopic capability is important in that it now allows us to study the dynamic longitudinal history of colonic tumor formation, as opposed to a single snapshot at necropsy. In these studies, we used the ratio of the tumor area to the visible colonic lumen as a metric for tumor size. Our initial characterization studies show good correlation between this metric and absolute tumor size. A method for more precise quantitation using a reference rod to determine tumor size through geometric construction has been described (19). Additional refinements to the precise measurement of tumor size and the ability to biopsy the tumors at different stages would facilitate the usefulness of the model.
Activating mutations in KRAS are frequent in human colonic cancers with as many as 50% of all tumors having such mutations (5). To assess the significance of Kras mutations in colon cancer, we have simultaneously inactivated the Apc gene and activated the Kras gene in our model. Histologic analysis did reveal that the addition of the Kras allele promotes the progression from adenomas to carcinomas and metastasis. Similar findings have been reported in Apc/Kras-based mouse models by other groups, as well (23 –25). Interestingly, whereas the multiplicity of the tumors in the Apc CKO/LSL-Kras mice increased threefold, the overall growth rate of the established tumors was not increased significantly. These results suggest that activation of the Wnt signaling pathway through Apc inactivation is necessary for the initiation of tumors, but that additional changes are required for tumor progression. Similar findings in a recent study using zebrafish and human cells have described a requirement for Kras activation to induce nuclear localization of β-catenin and subsequent progression from adenomas to carcinomas (26).
Colonoscopy is considered the best method to detect colonic polyps and tumors in humans, but recent clinical studies suggest that a significant proportion of colonic tumors are not detected through routine colonoscopy (20). Adenomas that are obscured by intestinal folds, flat adenomas, and dysplasia in the setting of inflammatory bowel disease are also significant challenges to the clinical endoscopist. For these reasons, there is increasing interest in the development of molecular beacon synthetic probes that can be activated by tumor-specific proteases to improve detection. Such compounds can be injected into the mouse where they are cleaved at the tumor site by specific proteases, thus permitting enhanced detection at the site of interest. We have previously shown that the Prosense 680 agent can be used to visualize intestinal tumors in an ex vivo setting (21). As proof of principle, we used a custom-built endoscope designed for both white light and near-infrared imaging to demonstrate the feasibility of detecting tumors using Prosense 680 as an endoscopic adjuvant. Our results indicate that it is indeed possible to detect the tumors accurately using this method. These results also suggest that the model described here can be used to quickly assess the suitability of various molecular beacons for subsequent clinical testing.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that integrates multiple signals to regulate cell growth and proliferation through control of mRNA translation, ribosome synthesis, autophagy, and metabolism (27). As this pathway has been shown to be activated in a wide variety of cancers, there is strong interest in mTOR blockade as an anticancer strategy (28). These tumors presumably rely on mTOR activation, i.e., oncogenic addiction, such that blockade would result in therapeutic efficacy. Indeed, a recent study in the ApcΔ716 mouse model for FAP demonstrated that mTOR blockade decreased both the number and size of intestinal polyps (22). We used the mTOR inhibitor rapamycin to treat two types of mice (Apc CKO and Apc CKO/LSL-Kras) that represent the majority of cases of human colon cancer. We observed that mice with Apc mutation alone respond to treatment with rapamycin, but that those with both mutations do not. Similar results have been demonstrated in a recent study assessing rapamycin sensitivity of eight different tumor-derived colon cancer cell lines. The most sensitive cell lines possess wild-type KRAS alleles, whereas the remaining cell lines are known to possess mutations in KRAS (29).
We have examined several biochemical pathways that are critical during the development of tumors. We observed that mouse tumors that were initiated by inactivation of the Apc gene have phosphorylated S6. There is evidence to suggest that the PI3K and mTOR pathways are activated by default in Apc mutant mice, perhaps resulting in oncogenic addiction to mTOR activation (22, 30). However, with the additional incorporation of an activated Kras allele, we find a significant increase in phosphorylated ERK. This implies that the addition of a mutant Kras gene may allow a parallel signaling pathway that abrogates oncogenic addiction to mTOR, thus enabling these tumors to survive pharmacologic mTOR blockade. Taken together, examination of the Kras mutational status and activation of the MAPK pathway may be critical before the administration of mTOR inhibitors. Furthermore, colon cancer with activating KRAS mutations might be amenable to combination therapy with rapamycin and a MEK inhibitor, as has recently been demonstrated in a mouse lung cancer model using combination treatment with a PI3K and a MEK inhibitor (31).
In summary, we present the results of our efforts to develop a robust GEM model for sporadic colon cancer. This model mimics human disease in that it presents along the entire spectrum from adenomas to invasive cancer to metastatic cancer. The restricted location in the colon will allow more specific biomarker studies and preclinical therapeutic trials in both primary and metastatic tumors. Our preclinical studies suggest that the use of mTOR inhibitors warrants further investigation in human colorectal cancer. However, these also suggest that such agents might fail in the setting of patients that possess activating KRAS mutations. This phenomenon might explain why the efficacy of mTOR inhibitors against colon cancer has been underappreciated. As such, future clinical trials should perhaps focus on wild-type KRAS patient populations. Taken together, this treatment paradigm may add to the personalized medicine arsenal against colon cancer.
Methods
Animals.
Apc conditional knockout (Apc CKO) mice were used as has been previously described (16). To generate compound mutants in Apc and Kras, the above mice were crossed to Kras mice bearing a latent mutant Kras allele (7).
Adenoviral Infection of Colonic Epithelium.
Mice were fasted overnight and anesthetized using 2% isoflurane. A midline incision was performed and the distal colon was clamped 3 cm from the anus. After washing with PBS, 100 μL trypsin was injected into the colon for 10 min. The lining of the distal colon was then mechanically abraded using a small caliber brush. After washing with PBS, 109 pfu of adenovirus in 100 μL PBS was injected into the colon for 30 min. For all incubations, a second clamp was placed 1 cm from the anus to ensure localization during the entire incubation period. After the infection period, both clamps were removed and the abdominal wall was closed in two layers. These procedures were well tolerated by all animals. All of the above procedures were approved by the Harvard Medical Area Standing Committee on Animals. Ad5CMVcre, Ad5CMVempty, and Ad5CMVntLacZ adenoviruses were obtained from the Gene Transfer Vector Core, University of Iowa.
Mouse Colonoscopy.
Mice were fasted overnight and anesthetized using 2% isoflurane. The colon was washed with PBS to cleanse the bowel. A custom-made colonoscopy system was used as previously described (32). Air was carefully insufflated into the colon to allow full visualization, but avoid perforation. Endoscopic images and movies were saved for later offline analysis by ImageJ software to calculate the ratio of the tumor area relative to that of the lumen (33). For cathepsin imaging, Prosense 680 (VisEn Medical) was injected IV at a dose of 2 nmol/mouse 24 h before colonoscopy. White light colonoscopy and simultaneously acquired near-infrared imaging using a filter set optimized for Cy5.5 was performed on a custom-made colonoscopy system as previously described (21, 32).
Rapamycin Therapy.
Rapamycin (LC Laboratories) working stock was prepared at 50 mg/mL in 100% ETOH. For injection, a fresh working solution of rapamycin was prepared with a final concentration of 4% ethanol, 5% PEG400, and 5% Tween 80. Mice were treated with daily i.p. 250-μL injections of 4 mg/kg rapamycin for 3 weeks. Control animals were treated with diluents vehicle alone.
For further details on β-galactosidase staining, Kras sequencing, Western blotting, and immunohistochemistry, please see SI Text.
Supplementary Material
Acknowledgments
We thank Eric Martin and Alain Charest for critical review of the manuscript. The work reported here is supported by grants from the American Gastroenterological Association (Research Scholar Award) and from the National Institute of Diabetes and Digestive and Kidney Diseases, Grant 5K08DK078033 (to K.H.); from the National Institute of Biomedical Imaging and Bioengineering, Grant 5R01EB001872 (to U.M.); and from the National Cancer Institute, Grant 5U01CA084301 (to R.K.) and Grant 5P50CA127003 (to U.M. and R.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908682107/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 3.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 4.Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136:780–798. doi: 10.1053/j.gastro.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 6.Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nat Rev Cancer. 2002;2:251–265. doi: 10.1038/nrc777. [DOI] [PubMed] [Google Scholar]
- 7.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meuwissen R, Linn SC, van der Valk M, Mooi WJ, Berns A. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K-Ras oncogene. Oncogene. 2001;20:6551–6558. doi: 10.1038/sj.onc.1204837. [DOI] [PubMed] [Google Scholar]
- 9.Harada N, et al. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 10.Colnot S, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci USA. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Dinulescu DM, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 13.Shibata H, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 14.Alencar H, et al. Colonic adenocarcinomas: near-infrared microcatheter imaging of smart probes for early detection—study in mice. Radiology. 2007;244:232–238. doi: 10.1148/radiol.2441052114. [DOI] [PubMed] [Google Scholar]
- 15.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuraguchi M, et al. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2006;2:e146. doi: 10.1371/journal.pgen.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoemaker AR, Luongo C, Moser AR, Marton LJ, Dove WF. Somatic mutational mechanisms involved in intestinal tumor formation in Min mice. Cancer Res. 1997;57:1999–2006. [PubMed] [Google Scholar]
- 18.Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protoc. 2006;1:2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
- 19.Hensley HH, et al. Endoscopic imaging and size estimation of colorectal adenomas in the multiple intestinal neoplasia mouse. Gastrointest Endosc. 2009;69(3 Pt 2, Suppl):742–749. doi: 10.1016/j.gie.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bressler B, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Hung KE, et al. Comprehensive proteome analysis of an Apc mouse model uncovers proteins associated with intestinal tumorigenesis. Cancer Prev Res (Phila Pa) 2009;2:224–233. doi: 10.1158/1940-6207.CAPR-08-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA. 2008;105:13544–13549. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haigis KM, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen K-P, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology. 2006;131:1096–1109. doi: 10.1053/j.gastro.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Sansom OJ, et al. Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo. Proc Natl Acad Sci USA. 2006;103:14122–14127. doi: 10.1073/pnas.0604130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelps RA, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 28.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Buck E, et al. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 30.Moran AE, et al. Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6J-Min/+ mice. J Biol Chem. 2004;279:43261–43272. doi: 10.1074/jbc.M404276200. [DOI] [PubMed] [Google Scholar]
- 31.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funovics MA, Alencar H, Montet X, Weissleder R, Mahmood U. Simultaneous fluorescence imaging of protease expression and vascularity during murine colonoscopy for colonic lesion characterization. Gastrointest Endosc. 2006;64:589–597. doi: 10.1016/j.gie.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 33.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.