Abstract
Extracellular adenosine is a potent immunosuppressor that accumulates during tumor growth. We performed proof-of-concept studies investigating the therapeutic potential and mechanism of action of monoclonal antibody (mAb)-based therapy against CD73, an ecto-enzyme overexpressed on breast-cancer cells that catalyzes the dephosphorylation of adenosine monophosphates into adenosine. We showed that anti-CD73 mAb therapy significantly delayed primary 4T1.2 and E0771 tumor growth in immune-competent mice and significantly inhibited the development of spontaneous 4T1.2 lung metastases. Notably, anti-CD73 mAb therapy was essentially dependent on the induction of adaptive anti-tumor immune responses. Knockdown of CD73 in 4T1.2 tumor cells confirmed the tumor-promoting effects of CD73. In addition to its immunosuppressive effect, CD73 enhanced tumor-cell chemotaxis, suggesting a role for CD73-derived adenosine in tumor metastasis. Accordingly, administration of adenosine-5′-N-ethylcarboxamide to tumor-bearing mice significantly enhanced spontaneous 4T1.2 lung metastasis. Using selective adenosine-receptor antagonists, we showed that activation of A2B adenosine receptors promoted 4T1.2 tumor-cell chemotaxis in vitro and metastasis in vivo. In conclusion, our study identified tumor-derived CD73 as a mechanism of tumor immune escape and tumor metastasis, and it also established the proof of concept that targeted therapy against CD73 can trigger adaptive anti-tumor immunity and inhibit metastasis of breast cancer.
Keywords: adenosine, cancer, chemotaxis, immunosuppression, regulatory
The release of extracellular adenosine triphosphates (ATP) in response to cell death or cellular stress acts to activate immune responses (1, 2). In contrast, the hydrolysis of extracellular ATP into adenosine, a potent immunosuppressor, acts as a negative-feedback mechanism to prevent excessive inflammation and tissue damage (3 –5). Adenosine is generated in response to hypoxia, and it mediates its biological effects through four distinct receptors: pertussis toxin-sensitive A1 and A3 receptors and adenylyl cyclase-activating A2A and A2B receptors (6, 7). Extracellular adenosine accumulates in cancerous tissues and constitutes an important mechanism of tumor immune escape (8, 9). Among other effects, tumor-derived adenosine profoundly inhibits anti-tumor T cells through A2A adenosine receptors (8, 10). Accordingly, genetic deletion of A2A receptors can induce spontaneous T cell-dependent tumor rejection (8).
Despite the profound immunosuppressive and tumor-promoting effects of adenosine, the development of therapeutic strategies aimed at inhibiting adenosine-mediated immunosuppression has not been thoroughly explored in the context of cancer (6). Given the short half-life of adenosine, we here propose the development of targeted therapies against the ecto-enzyme CD73 (ecto-5′-nucleotidase) that catalyzes the dephosphorylation of extracellular nucleoside monophosphates into nucleosides such as adenosine. CD73 is a 70-kDa glycosylphosphatidylinositol (GPI)-anchored protein normally expressed on endothelial cells and subsets of hematopoietic cells (11, 12). CD73 is up-regulated by hypoxia-inducible factor (HIF)-1α (13, 14) and after exposure to type I interferons (15). In steady state, CD73 regulates vascular barrier function (14, 16), restricts lymphocyte migration to draining lymph nodes (17), and stimulates mucosal hydration (18).
CD73 expression on tumor cells has been reported in several types of cancer, including bladder cancer (19), leukemia (20), glioma (21), glioblastoma (22), melanoma (23), ovarian cancer (24), thyroid cancer (25), esophageal cancer (26), prostate cancer (27), and breast cancer (28, 29). Notably, CD73 expression has been associated with a prometastatic phenotype in melanoma and breast cancer (23, 30, 31). Although in vitro studies have suggested that CD73 expression can enhance breast-cancer cell migration and invasion (29), the mechanisms by which this occurs have remained elusive. In breast-cancer cells, CD73 expression has been shown to be regulated by estrogen receptors (ER), whereby loss of ER significantly enhances CD73 expression (28). We here investigated the immune-modulatory and prometastatic effects of CD73 on breast-cancer cells and evaluated, as a proof-of-concept study, the therapeutic potential and mechanism of action of anti-CD73 monoclonal antibody (mAb)-based therapy.
Results
Anti-CD73 mAb Therapy Inhibits Tumor Growth and Spontaneous Metastasis.
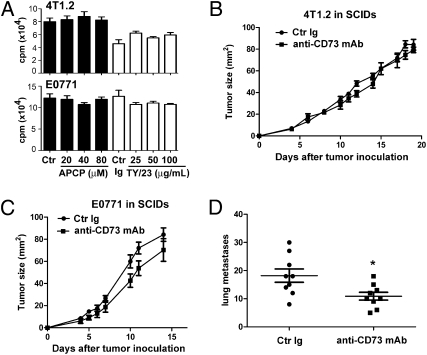
Cell-surface expression of CD73 was analyzed by flow cytometry on mouse breast-cancer cell lines. In vitro, 4T1.2 and E0771 cells, in contrast to a nonmetastatic variant of 4T1.2 (i.e., 67NR cells) (32), expressed high levels of CD73 (Fig. S1A). CD73-proficient 4T1.2 tumor cells, in contrast to 67NR cells, triggered the production of extracellular adenosine that was significantly inhibited by the CD73 inhibitor APCP (Table S1) or the anti-CD73 mAb clone TY/23 (Fig. S1 B–E). Notably, TY/23 also significantly decreased cell-surface expression of CD73, as determined by flow cytometry using a different anti-CD73 mAb (clone TY11.8) recognizing a distinct epitope (Fig. S1F). We investigated whether or not administration of anti-CD73 mAb TY/23 inhibited 4T1.2 and E0771 tumor growth in immune-competent mice. As shown in Fig. 1, anti-CD73 mAb therapy significantly delayed 4T1.2 (Fig. 1A) as well as E0771 (Fig. 1B) primary tumor growth. Because CD73 has been suggested to play a role in tumor metastasis (23, 30, 31), we investigated the effect of anti-CD73 mAb therapy on spontaneous 4T1.2 lung metastasis; 4T1.2 can readily and spontaneously metastasize after s.c. inoculation. Remarkably, anti-CD73 mAb therapy significantly decreased the number of spontaneous 4T1.2 lung metastases (Fig. 1C and Fig. S2), even when primary tumors were of equivalent sizes (Fig. 1D).
Fig. 1.
Anti-CD73 mAb therapy inhibits tumor growth and metastasis. (A) Female BALB/c mice were injected s.c. in the mammary fat pad with 105 4T1.2 cells and treated with 100 μg anti-CD73 mAb (TY/23) or isotype-matched control Ig (Mac4) two times weekly from day 3 (*, P < 0.05 by Mann–Whitney test; n = 4 per group; means ± SEs of one representative of four experiments are shown). (B) Female C57BL/6 mice were injected s.c. in the mammary fat pad with 5 × 105 E0771 cells and treated with anti-CD73 mAb or control Ig two times weekly from day 3 (*, P < 0.05 by Mann–Whitney test; n = 10 per group; means ± SEs of two experiments are shown). (C) After treatment with anti-CD73 mAb or control Ig, spontaneous 4T1.2 lung metastases were counted at day 20 after s.c. tumor injection (*, P < 0.05 by Mann–Whitney test; n = 8 per group; symbols represent individual mice; means ± SEs of two experiments are shown). (D) After treatment with anti-CD73 mAb or control Ig, spontaneous 4T1.2 lung metastases were counted when primary s.c. tumors reached 90 mm2 (*, P < 0.05 by Mann–Whitney test; n = 5–6 per group; symbols represent individual mice; means ± SEs are shown).
Anti-CD73 mAb Therapy Requires Adaptive Immunity.
Despite its anti-tumor activity in mice, anti-CD73 mAb treatment did not significantly affect 4T1.2 or E0771 tumor growth in vitro as measured by thymidine incorporation assays (Fig. 2A). Likewise, treatment with the CD73 inhibitor APCP also did not affect 4T1.2 or E0771 tumor growth in vitro (Fig. 2A). Given the immunosuppressive effect of adenosine generated by CD73, we hypothesized that the in vivo anti-tumor activity of anti-CD73 mAb was dependent on the generation of anti-tumor immune responses. To test our hypothesis, 4T1.2 and E0771 tumors were established in severe-combined immunodeficient (SCID) mice and treated with anti-CD73 mAb. As shown in Fig. 2, anti-CD73 mAb therapy was ineffective against primary 4T1.2 (Fig. 2B) or E0771 (Fig. 2C) tumors in SCID mice, whereas it had a modest, albeit significant, anti-metastatic effect (Fig. 2D). Our data thus support the development of an adaptive anti-tumor immune response posttherapy with anti-CD73 mAb administration. In addition, our data showed that anti-CD73 mAb therapy inhibited spontaneous lung metastasis independently of its effect on adaptive immunity.
Fig. 2.
Anti-CD73 mAb therapy requires adaptive immunity. (A) 4T1.2 (Upper) or E0771 (Lower) tumor cells were plated in complete media with increasing doses of APCP or anti-CD73 mAb (clone TY/23) for 4 days. Tritiated thymidine was added for the last 16 h, and cells were harvested and radioactivity measured with a β-counter (P > 0.05 by Mann–Whitney test; n = 5–6 per condition; means ± SEs of one representative of three experiments are shown). (B and C) Female SCID mice were injected s.c. in the mammary fat pad with 105 4T1.2 cells (B) or 5 × 105 E0771 cells (C) and treated with 100 μg anti-CD73 mAb (TY/23) or control Ig (Mac4) two times weekly from day 3 (P > 0.05 by Mann–Whitney test; n = 5 per group; means ± SEs of one representative of two experiments are shown). (D) After treatment, spontaneous 4T1.2 lung metastases were counted at day 20 after s.c. tumor injection (*, P < 0.05 by Mann–Whitney test; n = 9 per group; symbols represent individual mice; means ± SEs of two experiments are shown).
Tumor-Derived CD73 Inhibits Tumor Immunosurveillance.
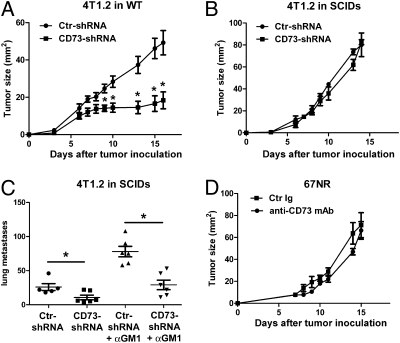
To further assess the immunosuppressive effect of tumor-derived CD73, we generated 4T1.2 tumor cells expressing a short-hairpin (sh) RNA against CD73. Flow cytometry analysis and TLC confirmed knockdown of CD73 expression and enzymatic activity, respectively (Fig. S3). As shown in Fig. 3A, knockdown of CD73 in 4T1.2 cells significantly decreased tumor growth in immune-competent mice. Consistent with our data with anti-CD73 mAb treatment, knockdown of CD73 in 4T1.2 cells had no impact on primary tumor growth in SCID mice (Fig. 3B), but it still significantly inhibited spontaneous lung metastasis (Fig. 3C). To investigate whether or not the prometastatic effect of CD73 was dependent on the suppression of natural killer (NK) cell function, SCID mice were depleted of NK cells using anti–asialo-GM1 (αGM1) antibody (Fig. S4A) and injected with 4T1.2 tumor cells expressing control- or CD73-shRNA. As expected, depletion of NK cells significantly enhanced 4T1.2 lung metastasis in SCID mice (Fig. 3C) without affecting primary tumor growth (Fig. S4B). However, knockdown of CD73 in 4T1.2 cells still significantly reduced the number of spontaneous lung metastases in SCID mice depleted of NK cells, showing that CD73 enhances lung metastasis independently of its effect on NK cells and adaptive immune cells.
Fig. 3.
Tumor-derived CD73 suppresses tumor immunosurveillance. Female wild-type BALB/c mice (A) or female SCID mice (B) were injected s.c. in the mammary fat pad with 105 4T1.2 cells expressing control-shRNA or CD73-shRNA (*, P < 0.05 by Mann–Whitney test; n = 7 per group; means ± SEs of one representative of two experiments). (C) Female SCID mice were depleted of NK cells by injecting rabbit anti–asialo-GM1 antibody 1 day before, 1 day after, and 7 days after s.c. inoculation of 105 4T1.2 cells expressing control-shRNA or CD73-shRNA. Spontaneous lung metastases were counted at day 20 after s.c. tumor injection (*, P < 0.05 by Mann–Whitney test; n = 5–6 per group; symbols represent individual mice; means ± SEs are shown). (D) Female BALB/c mice were injected s.c. in the mammary fat pad with 2 × 105 67NR cells and treated with anti-CD73 mAb (TY/23) or control Ig (Mac4) two times weekly from day 3 (P > 0.05 by Mann-Whitney; n = 4 per group; means ± SEs are shown).
Because host cells can express CD73, we next investigated whether or not anti-CD73 mAb therapy was effective against breast tumors that did not express CD73. For this purpose, mice were injected with 67NR tumor cells, a nonmetastatic variant of 4T1.2 (32), and were treated with anti-CD73 mAb. As shown in Fig. 3D, anti-CD73 mAb therapy was ineffective against 67NR tumors. Taken together, our data strongly suggest that tumor-derived CD73 is a potent suppressor of adaptive tumor immunosurveillance, CD73 promotes tumor metastasis independently of its effect on adaptive immunity or NK cells, and anti-CD73 mAb therapy targets tumor-derived CD73.
Anti-CD73 mAb Therapy Is Dependent on A2A on Hematopoietic Cells.
Because the immunosuppressive effect of adenosine largely depends on the expression of A2A adenosine receptors on hematopoietic cells (8), we investigated whether or not anti-CD73 mAb therapy was effective in mice lacking A2A receptors on hematopoietic cells. For this purpose, wild-type mice were irradiated and transplanted with bone-marrow cells isolated from congeneic A2A-deficient mice. After bone-marrow reconstitution (Fig. S5), mice were inoculated with E0771 tumor cells and treated with anti-CD73 mAb as described above. Compared with mice reconstituted with wild-type bone marrow (Fig. 4A), anti-CD73 mAb therapy was ineffective in mice reconstituted with A2A-deficient bone marrow (Fig. 4B), suggesting a role for host hematopoietic A2A adenosine receptors.
Fig. 4.
Anti-CD73 mAb therapy requires A2A adenosine receptors on hematopoietic cells. Wild-type (A) or A2A-deficient (B) bone-marrow reconstituted C57BL/6 mice were injected s.c. with 5 × 105 E0771 cells and treated with anti-CD73 mAb (TY/23) or control Ig (Mac4) two times weekly from day 3 (*, P < 0.05 by Mann–Whitney test; n = 4 per group; means ± SEs of one representative of two experiments).
CD73 Promotes Tumor Cell Migration and Metastasis.
Our observation that CD73 enhances lung metastasis independently of its effect on adaptive immune cells and NK cells led us to further investigate the role of CD73 in tumor-cell migration. Using in vitro transwell assays, we showed that CD73 inhibition with anti-CD73 mAb or APCP significantly suppressed 4T1.2 tumor-cell chemotaxis (Fig. 5A). Interestingly, addition of apyrase, which hydrolyzes extracellular ATP, also significantly suppressed 4T1.2 tumor cell chemotaxis (Fig. 5A). The addition of exogenous adenosine (ADO) significantly enhanced 4T1.2 chemotaxis in response to FCS (Fig. 5A), but it had no effect in the absence of FCS (Fig. 5B). Knockdown of CD73 in 4T1.2 cells also significantly inhibited their ability to migrate in response to FCS (Fig. 5B). Consistent with a role for adenosine in promoting tumor metastasis, administration of the adenosine analog NECA to 4T1.2 tumor-bearing mice significantly increased the number of spontaneous lung metastases (Fig. 5C) without any effect on primary tumor growth (Fig. S6A). Real-time PCR analysis revealed that 4T1.2 tumor cells expressed A1 and A2B adenosine receptors but not A2A or A3 adenosine receptors (Fig. 5D). Treatment of 4T1.2 cells with the A2B antagonist PSB1115 significantly decreased in vitro chemotaxis, whereas treatment with the A1 antagonist DPCPX had no effect (Fig. 5E). Consistent with a role for A2B receptors in promoting tumor metastasis, in vivo administration of the A2B antagonist PSB1115 to 4T1.2 tumor-bearing mice significantly reduced the number of spontaneous lung metastases, whereas administration of the A1 antagonist DPCPX had no effect (Fig. 5F). PSB1115 and DPCPX did not significantly affect primary 4T1.2 tumor growth (Fig. S6 B and C). Taken together, our data strongly suggest that the generation of extracellular adenosine by tumor-derived CD73 promotes breast-cancer metastasis to the lungs through the activation of A2B adenosine receptors.
Fig. 5.
CD73, adenosine, and A2B adenosine receptors promote 4T1.2 tumor-cell chemotaxis and metastasis. (A) 4T1.2 tumor cells were plated in serum-free media in the top chamber of a transwell plate and exposed to 5% FCS in the lower chamber to stimulate chemotaxis. After 4 h, migrating cells were fixed, stained, photographed using a digital camera mounted on a microscope, and counted. Where indicated, 40 μM APCP, 100 μg/mL anti-CD73 mAb (TY/23), 20 units/mL apyrase, or 0.1 μM adenosine was added to the top chamber (*, P < 0.05 by Mann–Whitney test; n = 8 per group; symbols represent individual mice; means ± SEs are shown). (B) Like in A, indicated 4T1.2 cells and adenosine-only, control-shRNA 4T1.2 or CD73-shRNA 4T1.2 cells were used. (C) Female BALB/c mice were injected s.c. in the mammary fat pad with 105 4T1.2 cells and treated two times weekly with 0.1% DMSO in PBS as control or 0.1% DMSO with the adenosine analog NECA (0.05 mg/kg intraperitoneally + 0.05 mg/kg s.c.). Spontaneous lung metastases at day 20 were counted (*, P < 0.05 by Mann–Whitney test; n = 5 per group; symbols represent individual mice; means ± SEs are shown). (D) Real-time PCR analysis of adenosine receptors mRNA expressed by 4T1.2 tumor cells in vitro (*, P < 0.05 by Mann–Whitney test). (E) Like in A, the A2B antagonist PSB1115 or the A1 antagonist DPCPX was added to the top chamber where indicated (*, P < 0.05 by Mann–Whitney test; n = 10 per group; symbols represent individual mice; means ± SEs are shown). (F) Like in C, female BALB/c mice were injected s.c., except that these mice were treated two times weekly with DPCPX (1 mg/kg i.p.; Left) or PSB1115 (10 mg/kg i.p.; Right). The primary s.c. tumors were surgically removed at day 15, and spontaneous 4T1.2 lung metastases were counted at day 25 (*, P < 0.05 by Mann–Whitney test; n = 4–6 per group; symbols represent individual mice; means ± SEs are shown).
Discussion
Herein, we have identified tumor-derived CD73 as a mechanism of tumor immune escape. We showed that CD73 expression on breast-cancer cells significantly inhibits endogenous adaptive anti-tumor immunosurveillance. In addition to its immunosuppressive effect, we have shown that CD73-derived adenosine enhances tumor-cell migration in vitro and metastasis in vivo through the activation of A2B adenosine receptors. Finally and most importantly, we have established the proof of concept that targeted therapy against tumor-derived CD73 can trigger adaptive anti-tumor immunity and significantly inhibit spontaneous lung metastasis of breast cancer.
The data presented here support previous studies that established extracellular adenosine as an important axis in tumor immune escape (8). Targeting tumor-derived CD73 may thus constitute an additional means to inhibit tumor immune escape, especially when tumor cells express high levels of CD73 such as ER-negative breast cancer. Blocking CD73, for instance, with a specific mAb, may rescue endogenous anti-tumor immune responses. Notably, the therapeutic efficacy that we have observed with anti-CD73 mAb as a single agent is comparable with the efficacy of other forms of immunotherapy using immune-stimulating antibodies such as anti-CD40 mAb or anti-41BB mAb against 4T1.2 breast tumors (33). In human cancer patients, however, endogenous immune responses to tumor antigens may be insufficient. Targeted CD73 therapies may, therefore, be most effective combined with other forms of immune-stimulating therapies, such as adoptive T cell transfer, immune-activating mAbs, cytokine therapy, or chemo-immunotherapy.
We propose that targeting CD73 will further synergize with standard and more recent cancer treatments. Accordingly, CD73 has been shown to increase the resistance of breast-cancer cells to doxorubicin (34). Although not fully understood, the increased resistance of CD73-expressing cancer cells to doxorubicin may reflect the high ATP requirement of multidrug-resistance cassettes and the resulting need for purine-salvage mechanisms in cancer cells. Under these conditions, purine salvage may require the combined action of ecto-enzymes such as CD73 and dipyridamole-sensitive carriers. Recently, CD73 has also been shown to enhance the resistance of Jurkat leukemia cells to tumor necrosis factor-related, apoptosis-inducing ligand (TRAIL)-mediated apoptosis (20). Intriguingly, this seems to be independent of the enzymatic activity of CD73, but dependent on the colocalization of CD73 with the TRAIL receptor DR5. Targeting CD73 with a mAb would potentially disrupt this interaction and enhance the therapeutic activity of proapoptotic receptor agonists such as TRAIL.
An important finding of our study is that adenosine and A2B adenosine receptors promoted spontaneous lung metastasis of 4T1.2 breast tumors. Others have previously shown that CD73 expression in breast tumor cells is associated with increased migratory potential (31). For instance, Zhi et al. (35) reported that small interfering RNA-mediated knockdown of CD73 in MB-MDA-231 human breast-cancer cells prevented their adhesion to extracellular matrix and inhibited their migration. However, previous studies did not address the mechanism of action by which CD73 may modulate tumor-cell migration. Our data confirmed a role for CD73 in regulating spontaneous breast-cancer cell metastasis to the lungs. Unlike other mouse models of breast cancer, 4T1.2 tumors can readily and spontaneously metastasize in immune-competent mice, closely mimicking human breast cancer. Our data suggest that the release of extracellular ATP from tumor cells and its subsequent hydrolysis into adenosine by tumor-derived CD73 enhances tumor-cell chemotaxis. The importance of extracellular ATP was evidenced by the decreased migratory potential of 4T1.2 cells treated with soluble apyrase, which hydrolyzes extracellular ATP and ADP. The effect of apyrase was somewhat unanticipated, because apyrase is expected to generate AMP, the substrate for CD73, and therefore increase adenosine levels. However, exogenous adenosine was clearly insufficient to trigger tumor-cell migration. Furthermore, in the presence of FCS, exogenous adenosine could only partly rescue chemotaxis of 4T1.2 cells when combined with apyrase. Our results are thus consistent with a model where both ATP-mediated and adenosine-mediated signaling act together to amplify chemotaxis. In this regard, our data are reminiscent of what has been previously described for neutrophils, whereby the release of extracellular ATP in response to a chemoattractant and its hydrolysis into adenosine act in concert to amplify the migratory cue (36). In 4T1.2 tumor cells, blocking A2B adenosine receptors significantly inhibited chemotaxis in vitro, suggesting that CD73-derived adenosine mediates its amplifying effect through A2B adenosine receptors. In vivo, activation of A2B receptors expressed on nontransformed host cells, such as endothelial cells, may also play an important role in tumor metastasis. It remains unclear at this point whether or not A2B adenosine receptors can promote metastasis of cancer cells from different tissues or metastasis to organs other than the lungs. We are also investigating the mechanism by which A2B activation and ATP signaling may promote tumor-cell migration. A2B adenosine receptors may act to enhance the levels or the nature of chemokine receptors (37), or modulate the surface expression of a peptidase, such as dipeptidyl peptidase IV (DPPIV), which can then alter the function of chemokines (38). Alternatively, A2B adenosine receptors may enhance tumor-cell motility through intracellular accumulation of cAMP and the activation of cAMP-dependent protein kinase A (PKA). Indeed, PKA-mediated phosphorylation of α4 integrins has been shown to be necessary for directional cell migration (39, 40).
Although our data strongly suggest that adenosine generated by CD73 enhances tumor-cell chemotaxis, the effect of CD73 on tumor metastasis in vivo may extend beyond its enzymatic activity. For instance, CD73 may directly enhance the adhesion of tumor cells to endothelial cells, thereby promoting their migration across the vascular endothelium. Indeed, earlier work showed that CD73 can promote the binding of human lymphocytes to endothelial cells in an lymphocyte function-associated antigen-1–dependent fashion (41). CD73 seems to play an important role in endothelial extravasation. This was recently suggested as the cause of resistance of CD73-deficient mice to experimental autoimmune encephalomyelitis (EAE) (42). Through the production of adenosine and the activation of adenosine receptors on endothelial cells, it was suggested that CD73 can trigger adhesion molecules such as ICAM-1 on endothelial cells (42).
In conclusion, the shift in the supply and demand of metabolites, such as glucose and oxygen, that is associated with tumor growth often results in the production of extracellular adenosine. One of the major sources of extracellular adenosine is the hydrolysis of extracellular AMP by CD73. We here showed that tumor-derived CD73 plays an important role in regulating both tumor immunosurveillance and tumor metastasis and anti-CD73 mAb therapy can stimulate endogenous anti-tumor immune responses and prevent spontaneous lung metastasis. We propose that targeting tumor-derived CD73 may constitute an effective means in combination with other therapeutic strategies to enhance endogenous anti-tumor immune responses and improve the treatment of metastatic breast cancer.
Materials and Methods
Cell Lines, Animals, and Antibodies.
4T1.2, E0771, and 67NR mouse breast-cancer cell lines were kindly provided by Robin Anderson (Peter MacCallum Cancer Centre, East Melbourne, Victoria, Australia) and have been previously described (32, 43). 67NR cells were confirmed to be nonmetastatic (32). BALB/c, C57BL/6, and SCID mice were bred and maintained at the Peter MacCallum Cancer Centre (Melbourne, Australia). A2A-deficient mice were kindly provided by J.F. Chen (Boston, MA) and bred and maintained at St. Vincent’s Hospital (Melbourne, Australia). CD73 expression was assessed using phycoerythrin (PE)-conjugated anti-mouse CD73 mAb (clone TY/23; BD Bioscience) and Pacific-blue–conjugated anti-mouse CD73 mAb (clone TY/11.8; BD Bioscience). PE-conjugated anti-mouse CD45.1 (A20), fluorescein (FITC)-conjugated anti-mouse Gr1 (RB6-8C5), FITC-conjugated anti-mouse CD4 (L3T4), and FITC-conjugated anti-mouse CD8 (53-6.7) were purchased from BD Bioscience. FITC-conjugated anti-mouse CD11b (M1/70) and allophycocyanin (APC)-conjugated anti-mouse CD45.2 (104) were purchased from eBioscience. Purified anti-CD73 mAb (clone TY/23) and control Ig (clone MAC4) were produced in house as previously described (44). TY/23 hybridoma was generously provided by Linda H. Thompson (Oklahoma City, OK). Relative CD73 expression was determined by flow cytometry on at least 5,000 cells using DIVA software and expressed using the formula of median intensity of treated cells over median intensity of untreated cells.
Chromatography.
For TLC, tumor cells (104 cells per well) were seeded overnight in a 24-well plate in complete medium. The next day, cells were replenished with fresh medium with or without anti-CD73 mAb (TY/23) or alpha,beta-methylene ADP (APCP; Sigma-Aldrich) as indicated; 1 μCi of tritiated AMP was added to the cells, and it was incubated for 1 h before a 10-μL aliquot of substrate. Products were separated on aluminum sheets (Silica gel 60 F254; Merck) using as solvent isobutanol:isoamylalcool:ethoxyethanol:ammonia:water (9:6:18:9:15), and autoradiography was measured using a BETA-Imager 2000 counter (Biospace). Data were analyzed using β-vision + software (Biospace), and CD73 activity was calculated using the formula [counts per minute (cpm) of adenosine in supernatant of treated cells/cpm of AMP in supernatant of treated cells]/(cpm of adenosine in supernatant of untreated cells/cpm of AMP in supernatant of untreated cells).
For HPLC, 5 × 103 4T1.2 tumor cells were plated in a 96-well plate in serum-free medium supplemented with 10 μM ethano-AMP (Sigma-Aldrich). One hour later, supernatants were collected, and substrate and products were separated as previously described (16).
In Vitro Proliferation Assays.
4T1.2 and E0771 tumor cells (104 cells per well) were cultured in 96-well plates in complete media for 4 days with or without anti-CD73 mAb (TY/23) or APCP as indicated; 1 μCi of tritiated thymidine per well was added for the last 16 h. Cells were harvested using a cell harvester, and radioactivity was measured using a Chameleon beta-counter (Hidex Ltd.).
In Vivo Tumor Treatment.
4T1.2 (105 cells), 67NR (2 × 105 cells), or E0771 (5 × 105 cells) tumor cells were injected s.c. in the mammary fat pad of female wild-type BALB/c mice (4T1.2 and 67NR), wild-type C57BL/6 mice (E0771), or SCID mice (4T1.2 and E0771). Mice were treated from day 3 twice weekly with 100 μg of anti-CD73 mAb (TY/23) or isotype-matched control Ig (Mac4) in PBS by i.p. injections. Tumor surface was measured by recording perpendicular diameters using a digital caliper. Lung metastases were counted under a dissecting microscope after perfusion of the lungs with Indian ink. Where indicated, mice were treated twice weekly with PBS 0.1% DMSO or adenosine-5′-N-ethylcarboxamide (NECA; Sigma-Aldrich) at 0.05 mg/kg intraperitoneally plus 0.05 mg/kg s.c., 4-(2,3,6,7-Tetrahydro-2,6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulfonic acid potassium salt (PBS1115; Tocris) at 10 mg/kg intraperitoneally, or 2-chloro-N(6)-cyclopentyladenosine (DPCPX; Sigma-Aldrich) at 1 mg/kg intraperitoneally diluted in PBS 0.1% DMSO. Doses were based on previous studies in mice (45, 46). Treated mice did not display any signs of distress or significant weight loss. For bone-marrow reconstitution, wild-type mice (CD45.1) were irradiated (17.6 Gy from a 137Cs source) and injected i.v. with 2 × 106 bone-marrow cells derived from congenic wild-type mice or previously described A2A-deficient gene-targeted mice (47). The resulting chimeric mice were housed in microisolators for 8 weeks before experimentation.
CD73 Knockdown.
Mouse CD73 shRNA (TI589875) and control shRNA (TR30003) constructs were purchased from Origene Technologies and cloned into a previously described murine stem cell virus retroviral vector (44). Phoenix-Eco 293 packaging cells were used to gene modify 4T1.2 tumor cells, and puromycin-resistant cells were expanded. For in vivo experiments, 105 gene-modified 4T1.2 cells were injected s.c. in wild-type BALB/c or SCID mice. For NK cell depletion, SCID mice were injected with 200 μL of control rabbit serum or rabbit anti–asialo-GM1 antibody according to the manufacturers’ instructions (Wako Chemicals) 1 day before, 1 day after, and 7 days after tumor inoculation; depletion was confirmed by flow cytometry using APC-conjugated anti-DX5 mAb (BD Biosciences).
In Vitro Chemotaxis Assays.
Tumor cells (2 × 105 cells) were plated in serum-free media in the top chamber of a 24-well transwell plate with 8-μm pores (Corning Costar) and exposed to 5% FCS in the lower chamber. After 4 h, cells on the top of the filter were scraped using cotton wool, and cells on the lower filter were fixed, stained with DAPI, and counted under fluorescence microscopy. When indicated, 40 μM APCP, 100 μg/mL anti-CD73 mAb (TY/23), 20 units/mL apyrase (Sigma-Aldrich), 0.1 μM adenosine (Sigma-Aldrich), PSB1115 (Tocris), or DPCPX (Sigma-Adrich) were added to the top chamber.
Real-Time PCR.
RNA was extracted using the RNeasy Mini Kit (Qiagen). Oligonucleotides and Taqman probe mix specific for mouse A1 (Mm01308023_m1), A2A (Mm00802075_m1), A2B (Mm01285229_s1), and A3 (Mm00802076_m1) adenosine receptors and mouse β-actin (4352933E) were purchased from Applied Biosystems. PCR reactions were performed using 7500 Fast Real-time PCR System (Applied Biosystems). Gene-expression relative quantification was determined as previously described (36) using the comparative Ct method and normalized to endogenous β-actin level using 7500 Fast System SDS Software (Applied Biosystems) and expressed in arbitrary units using the formula: (2−(average Ct adenosine receptor − average Ct β-actin)) × 10,000. Dilutions of cDNA from 4T1.2 cells were amplified in triplicates by real-time PCR using primers and probes for A1, A2b, and β-actin to compare PCR efficiencies and to validate the use of the comparative Ct method for relative quantification. Efficiencies were calculated as 100% for A1, 79% for A2b, and 85% for β-actin.
Supplementary Material
Acknowledgments
We thank Michelle Stirling for breeding and maintenance of the mice in this study and Dr. Andreas Möller for critical review of our manuscript. This work was supported by a US Department of Defense Breast Cancer Concept Award (W81XWH-08-1-0634) and the Susan G. Komen Breast Cancer Foundation. J.S. is supported by a Canadian Institutes of Health Research (CIHR) Fellowship. M.J.S is supported by a National Health and Medical Research Council of Australia Fellowship and Program Grant.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908801107/DCSupplemental.
References
- 1.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 2.Mizumoto N, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: Modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 3.Frick JS, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novitskiy SV, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: Tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 6.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden J. Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 8.Ohta A, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellegatti P, et al. Increased level of extracellular ATP at tumor sites: In vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review) Int J Oncol. 2008;32:527–535. [PubMed] [Google Scholar]
- 11.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 13.Synnestvedt K, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson LF, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemelä J, et al. IFN-beta regulates CD73 and adenosine expression at the blood-brain barrier. Eur J Immunol. 2008;38:2718–2726. doi: 10.1002/eji.200838437. [DOI] [PubMed] [Google Scholar]
- 16.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takedachi M, et al. CD73-generated adenosine restricts lymphocyte migration into draining lymph nodes. J Immunol. 2008;180:6288–6296. doi: 10.4049/jimmunol.180.9.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madara JL, et al. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stella J, et al. Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2009.01.035. 10.1016/j.urolonc.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Mikhailov A, et al. CD73 participates in cellular multiresistance program and protects against TRAIL-induced apoptosis. J Immunol. 2008;181:464–475. doi: 10.4049/jimmunol.181.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bavaresco L, et al. The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem. 2008;319:61–68. doi: 10.1007/s11010-008-9877-3. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig HC, Rausch S, Schallock K, Markakis E. Expression of CD 73 (ecto-5′-nucleotidase) in 165 glioblastomas by immunohistochemistry and electronmicroscopic histochemistry. Anticancer Res. 1999;19:1747–1752. [PubMed] [Google Scholar]
- 23.Sadej R, Spychala J, Skladanowski AC. Ecto-5′-nucleotidase (eN, CD73) is coexpressed with metastasis promoting antigens in human melanoma cells. Nucleosides Nucleotides Nucleic Acids. 2006;25:1119–1123. doi: 10.1080/15257770600894188. [DOI] [PubMed] [Google Scholar]
- 24.Cho SY, et al. In vitro evaluation of adenosine 5′-monophosphate as an imaging agent of tumor metabolism. J Nucl Med. 2006;47:837–845. [PubMed] [Google Scholar]
- 25.Kondo T, Nakazawa T, Murata SI, Katoh R. Expression of CD73 and its ecto-5′-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology. 2006;48:612–614. doi: 10.1111/j.1365-2559.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda K, et al. Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer. 2004;91:1543–1550. doi: 10.1038/sj.bjc.6602187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastie C, et al. Combined affinity labelling and mass spectrometry analysis of differential cell surface protein expression in normal and prostate cancer cells. Oncogene. 2005;24:5905–5913. doi: 10.1038/sj.onc.1208747. [DOI] [PubMed] [Google Scholar]
- 28.Spychala J, et al. Role of estrogen receptor in the regulation of ecto-5′-nucleotidase and adenosine in breast cancer. Clin Cancer Res. 2004;10:708–717. doi: 10.1158/1078-0432.ccr-0811-03. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, et al. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–372. doi: 10.1007/s00432-007-0292-z. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Lin EC, Liu L, Smith JW. Gene expression profiling of tumor xenografts: In vivo analysis of organ-specific metastasis. Int J Cancer. 2003;107:528–534. doi: 10.1002/ijc.11428. [DOI] [PubMed] [Google Scholar]
- 31.Leth-Larsen R, et al. Metastasis-related plasma membrane proteins of human breast cancer cells identified by comparative quantitative mass spectrometry. Mol Cell Proteomics. 2009;8:1436–1449. doi: 10.1074/mcp.M800061-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckhardt BL, et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res. 2005;3:1–13. [PubMed] [Google Scholar]
- 33.Takeda H, et al. Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med. 2006;12:395–397. doi: 10.1038/nm1386. [DOI] [PubMed] [Google Scholar]
- 34.Ujházy P, et al. Evidence for the involvement of ecto-5′-nucleotidase (CD73) in drug resistance. Int J Cancer. 1996;68:493–500. doi: 10.1002/(SICI)1097-0215(19961115)68:4<493::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Zhi X, et al. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24:439–448. doi: 10.1007/s10585-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 37.Richard CL, Tan EY, Blay J. Adenosine upregulates CXCR4 and enhances the proliferative and migratory responses of human carcinoma cells to CXCL12/SDF-1alpha. Int J Cancer. 2006;119:2044–2053. doi: 10.1002/ijc.22084. [DOI] [PubMed] [Google Scholar]
- 38.Tan EY, Mujoomdar M, Blay J. Adenosine down-regulates the surface expression of dipeptidyl peptidase IV on HT-29 human colorectal carcinoma cells: Implications for cancer cell behavior. Am J Pathol. 2004;165:319–330. doi: 10.1016/S0002-9440(10)63299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of alpha4 integrin phosphorylation regulates lamellipodial stability and alpha4beta1-dependent cell migration. J Cell Biol. 2003;162:731–741. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim CJ, et al. Integrin-mediated protein kinase A activation at the leading edge of migrating cells. Mol Biol Cell. 2008;19:4930–4941. doi: 10.1091/mbc.E08-06-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Airas L, Niemelä J, Jalkanen S. CD73 engagement promotes lymphocyte binding to endothelial cells via a lymphocyte function-associated antigen-1-dependent mechanism. J Immunol. 2000;165:5411–5417. doi: 10.4049/jimmunol.165.10.5411. [DOI] [PubMed] [Google Scholar]
- 42.Mills JH, et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Möller A, et al. Inhibition of Siah ubiquitin ligase function. Oncogene. 2009;28:289–296. doi: 10.1038/onc.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg J, et al. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci USA. 2008;105:16254–16259. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckle T, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 2009;4:e6784. doi: 10.1371/journal.pone.0006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.