Abstract
The role of lung epithelial stem cells in maintenance and repair of the adult lung is ill-defined, and their identity remains contentious because of the lack of definitive markers for their prospective isolation and the absence of clonogenic assays able to measure their stem/progenitor cell potential. In this study, we show that replication of epithelial–mesenchymal interactions in a previously undescribed matrigel-based clonogenic assay enables the identification of lung epithelial stem/progenitor cells by their colony-forming potential in vitro. We describe a population of EpCAMhi CD49fpos CD104pos CD24low epithelial cfus that generate colonies comprising airway, alveolar, or mixed lung epithelial cell lineages when cocultured with EpCAMneg Sca-1pos lung mesenchymal cells. We show that soluble fibroblast growth factor-10 and hepatocyte growth factor partially replace the requirement for mesenchymal support of epithelial colony formation, allowing clonal passaging and demonstration of their capacity for self-renewal. These data support a model in which the adult mouse lung contains a minor population of multipotent epithelial stem/progenitor cells with the capacity for self-renewal and whose descendants give rise to airway and alveolar epithelial cell lineages in vitro.
Keywords: colony-forming assay, lung epithelium, lineage specificity, differentiation, EpCAM
During lung development in the mouse, progenitors of the anterior foregut endoderm undergo directed differentiation to establish distinct respiratory epithelial cell compartments. The tracheobronchial airways are composed predominantly of ciliated, Clara, and basal cells as well as a lesser number of goblet and neuroendocrine cells. Submucosal glands are restricted to the highest reaches of the cartilaginous tracheal airway. In the distal lung, the bronchiolar epithelium is composed largely of Clara and ciliated cells with intermittent clusters of neuroendocrine cells, whereas type I and type II alveolar epithelial cells (AEC I/II) make up the gas exchange surface of the alveoli. However, in comparison to our understanding of the mechanisms regulating epithelial cell proliferation and differentiation during lung development (1), our knowledge of the organization and regulation of endogenous lung stem and progenitor cells involved in maintenance, remodeling, regeneration, and repair of the postnatal lung is relatively poor.
For the most part, cell lineage tracing studies and the analysis of mouse lung injury models suggest that the adult lung epithelium is maintained by divergent progenitor cells residing in discrete microenvironmental niches along the proximal-distal axis of the respiratory tree (2), consistent with the existence of a “nonclassical” stem cell hierarchy in which relatively quiescent differentiated progenitor cells function as facultative stem cells (3). However, Rawlins et al. (4) recently provided compelling evidence of the existence of multipotent stem/progenitor cells in the fetal lung distal tip that are able to self-renew and contribute to all lung epithelial cell lineages during development. Others have also posited that the processes of adult lung regeneration and repair are regulated by highly conserved mechanisms that recapitulate ontogeny (5). These processes are key to understanding normal and pathophysiological lung stem/progenitor cell behavior, which, in turn, leads to the identification of cellular and molecular therapeutical targets that could be exploited for the attenuation or reversal of intractable lung diseases. However, their elucidation has been confounded by a lack of specific markers and functional assays for the prospective isolation and characterization of adult lung epithelial stem and progenitor cells and the measurement of their proliferative and differentiative potential.
In this study, we describe a previously undescribed and robust organotypic epithelial colony-forming assay which has enabled us to identify and characterize a minor population of renewing EpCAMhi CD49fpos CD104pos CD24low epithelial cfus in the adult mouse lung which give rise to more committed clonogenic airway and alveolar epithelial progenitor cells in vitro.
Results
Mesenchymal Progenitor Cells Regulate the Growth of Lung Epithelial Stem/Progenitor Cells in Vitro.
To separate lung epithelial cells from mesenchymal cells (6), we sorted nonhematopoietic (CD45neg) and nonendothelial (CD31neg) cells from disaggregated adult lung cell suspensions on the basis of the differential expression of a panepithelial cell marker (EpCAM; CD326) and the Sca-1 antigen. We show that EpCAMpos epithelial cells (40.6 ± 2.0%, mean ± SEM, n = 7) are easily resolved from EpCAMneg Sca-1pos mesenchymal cells (Fig. 1A), which generate mesenchymal cell colonies in matrigel (Fig. 1B) defined by Oil-Red O (lipofibroblasts) and α-smooth muscle actin (SMA; myofibroblasts) expression (Fig. S1A).
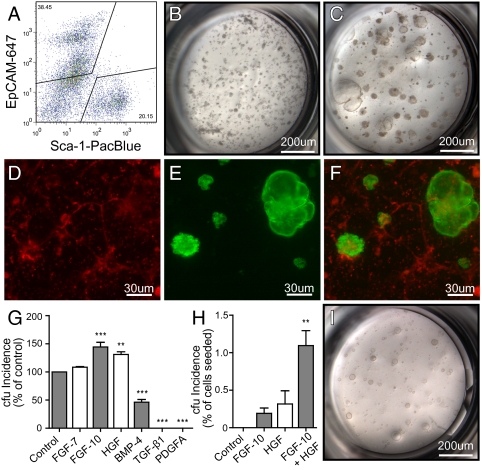
Fig. 1.
Colony-forming potential of EpCAMpos epithelial stem/progenitors is mediated by EpCAMneg Sca-1pos mesenchymal cells. (A) Gating strategy for subsetting EpCAMpos epithelial cells and EpCAMneg Sca-1pos mesenchymal cells. (B) EpCAMneg Sca-1pos cell culture. (C) Coculture of EpCAMpos cells with EpCAMneg Sca-1pos cells. (D–F) Red and green overlay of fluorescence from coculture of eGFPpos EpCAMpos and RFPpos EpCAMneg Sca-1pos cells. (G) Effect of growth factor addition on colony growth of EpCAMpos cells cocultured with EpCAMneg Sca-1pos mesenchymal cells. Data are presented relative to no factor control (% of control + SEM, n = 3 per group). (H) Incidence of cfus (% of cells seeded + SEM, n ≥ 3 per group) of EpCAMpos cells supplemented with FGF-10 and/or HGF in stromal-free cultures. A statistically significant difference (**P < 0.01; ***P < 0.001) between growth factor-treated groups and controls was determined using one-way ANOVA with a Tukey posttest. (I) Image of colonies generated in stromal-free cultures with FGF-10 and HGF.
In contrast, EpCAMpos cells did not exhibit clonogenic growth when cultured alone in matrigel but did generate complex epithelial cell colonies (Fig. 1C) when cocultured with EpCAMneg Sca-1pos mesenchymal cells. Significantly, when EpCAMneg Sca-1pos mesenchymal cells were grown in the lower chamber of the matrigel transwell culture separated from EpCAMpos epithelial cells, epithelial colony formation was not supported, demonstrating that the recapitulation of physiological epithelial mesenchymal cell and/or paracrine interactions is critical for lung epithelial colony formation in vitro. Counterstaining of cocultures for α-SMA showed that αSMApos mesenchymal cells were tightly wrapped around epithelial colonies (Fig. S1B), also suggesting that, as in lung morphogenesis during development, adult lung epithelial cell fate specification is orchestrated by smooth muscle progenitors in the lung mesenchyme (7 –9).
Epithelial cfus Are Enriched in the EpCAMpos Cell Fraction.
Cell mixing experiments using eGFPpos EpCAMpos cells and RFPpos EpCAMneg Sca-1pos cells (Fig. 1F) showed that RFPpos EpCAMneg Sca-1pos cells are uniformly dispersed throughout the matrigel culture (Fig. 1D) and that all epithelial colonies were derived exclusively from eGFPpos EpCAMpos cells (Fig. 1E). This observation further confirms that lung epithelial cfus are enriched in the EpCAMpos epithelial cell fraction but require coculture with EpCAMneg Sca-1pos mesenchymal cells to reveal their proliferative potential.
Fibroblast Growth Factor-10 and Hepatocyte Growth Factor Regulate Epithelial cfus Colony-Forming Potential.
To determine the growth factor requirements of epithelial cfus, organotypic cocultures were supplemented with combinations of cytokines known to be involved in lung development (Fig. 1G). The incidence of epithelial colonies in organotypic coculture was significantly increased in the presence of epithelial growth factors fibroblast growth factor (FGF)-10 (P < 0.001) or hepatocyte growth factor (HGF) (P < 0.01) and was unaltered by addition of FGF-7. Conversely, the addition of mesenchymal growth factors [bone morphogenic protein (BMP-4), TGF-β1, or platelet-derived growth factor (PDGF)-AA] resulted in a significant reduction or complete inhibition of epithelial colony growth.
Next, we assessed whether FGF-10 and HGF could replace the requirement for mesenchymal support of epithelial cfus (Fig. 1H). These experiments showed that addition of FGF-10 or HGF to stromal-free matrigel cultures of EpCAMpos cells supports the generation of epithelial colonies. A supraadditive increase in both size and incidence of epithelial cfus was observed when FGF-10 and HGF were used in combination, indicating that these growth factors acted synergistically to support the proliferation of epithelial cfus. Under these conditions, epithelial cfus proliferated robustly to generate simple spheroid colonies after 1 week in culture (Fig. 1I). Interestingly, parallel studies by Rock et al. (10) have demonstrated that mouse basal cells isolated from tracheal epithelium can self-renew and form “tracheospheres” in matrigel culture in the absence of stroma when supplemented with cholera toxin, EGF, and bovine pituitary extract.
Lung Epithelial cfus Comprise a Minor Fraction of Lung Epithelial Cells.
Epithelial cfus comprise less than 0.05% of EpCAMpos lung epithelial cells (determined by linear regression analysis of the incidence of epithelial colonies relative to the number of EpCAMpos cells seeded; Fig. S1C). To enrich further for epithelial cfus, we fractionated the EpCAMpos epithelial cells based on the relative expression of EpCAM and CD49f (α-6 integrin; Fig. 2A and Fig. S2A) and showed that epithelial cfus were predominantly restricted to the EpCAMhi CD49fpos cell fraction (Fig. S2B), which give rise to large lobular cystic colonies and small dense saccular colonies. Small saccular colonies were also observed in cultures of the EpCAMmed CD49flow cell fraction (Fig. S2C), whereas very few colonies grew from the EpCAMlow CD49fneg/low cell fraction (Fig. S2D).
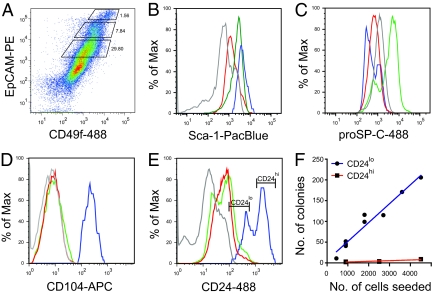
Fig. 2.
Lung epithelial cfus are enriched in the EpCAMhi CD49fpos CD104pos CD24low cell fraction. (A) Fractionation of CD45neg CD31neg lung cells based on EpCAM and CD49f expression. Sca-1 (B), proSP-C (C), CD104 (D), and CD24 (E) expression on EpCAMhi (blue), EpCAMmed (green), and EpCAMlow (red) cell fractions and isotype controls (gray). (F) Cloning efficiency of EpCAMhi CD24low (blue) and CD24hi (red) cells with linear regression analysis (r 2 = 0.9481, 1/slope = 22.45, n = 7).
Fig. 1B shows that lung epithelial cfus can be resolved from mesenchymal cells on the basis of EpCAM vs. Sca-1 expression. Here, we show that the EpCAMhi and EpCAMmed cell fractions are also Sca-1low (Fig. 2B). This is noteworthy in that it shows that previous fluorescence-activated cell sorting gating strategies used to sort Sca-1pos lung cell fractions based on the differential expression of CD45/CD31 vs. Sca-1 antigens (6) would result in potential overlap of Sca-1pos cells at the boundary of the EpCAMpos Sca-1low gate (Fig. S2E). This would explain why we and others have previously detected rare epithelial colonies within the CD45neg CD31neg Sca-1pos cell fraction (6, 11, 12), thus helping to resolve the ongoing debate regarding the ambiguous status of Sca-1 as a selectable marker for lung stem cell populations.
Additional phenotypic analysis of the respective cell fractions demonstrated that proSP-Cpos cells were found only within the EpCAMmed cell fraction (Fig. 2C) and that the EpCAMhi cell fraction was exclusively CD104pos (β-4 integrin; Fig. 2D). In the adult mouse lung, immunofluorescent staining demonstrated that CD104 has a distinct localization to endothelial cells and the basal plasma membrane of virtually all epithelial cells in the bronchi and most cells in the terminal bronchioles (Fig. S3 A–C) but did not colocalize with proSP-Cpos cells in the alveoli (Fig. S3 D and F) and the bronchioalveolar duct junction (BADJ; Fig. S3 E and F). Together, this suggests that the majority of epithelial cfus reside in the CD104pos bronchiolar epithelium. However, the EpCAMmed proSP-Cpos cell fraction also generated small saccular colonies (Fig. S2C), suggesting that proSP-Cpos cells in the alveoli and/or BADJ may have limited colony-forming potential.
Further enrichment of epithelial cfus in the EpCAMhi cell fraction was achieved by subsetting on the basis of high or low expression of CD24 (heat-stable antigen; Fig. 2E). Organotypic culture of the CD24hi and CD24low cell subsets showed that epithelial cfus were resolved in the CD24low cell fraction. Linear regression analysis (Fig. 2F) shows that the incidence of colony formation is directly proportional to the number of CD24low cells plated and that the cloning efficiency of this population is ≈4.5%. In the adult mouse lung, heterogeneity in CD24 staining could not be observed at the level of immunofluorescent staining on tissue sections, thus precluding localization of the CD104pos CD24low cells.
Immunofluorescent staining of primary sorted EpCAMhi CD49fpos CD104pos CD24low (abbreviated to EpCAMhi CD24low) and CD24hi cells showed that CCSPpos Clara cells were abundant in both the CD24low (54%) and CD24hi (32%) cell fractions (Fig. S2F). This was confirmed by real-time RT-PCR, which showed a 1.9-fold increase in scgb1a1 expression in CD24low cells relative to CD24hi cells (CD24low: ΔCt = 4.201 ± 0.051, CD24hi: ΔCt = 5.117 ± 0.067, where ΔCt is the difference in the cycle threshold of the sample relative to an 18s RNA control). In addition, expression of Foxj1 (ciliated cells) was detected in both fractions, albeit enriched 22.4-fold in CD24hi cells (CD24low: ΔCt = 17.332 ± 0.088, CD24hi: ΔCt = 12.848 ± 0.088). Therefore, although both the CD24hi and CD24low cell fractions comprise Clara and ciliated bronchiolar epithelial cells, only CD24low cells demonstrate cfu potential, suggesting that only a restricted subpopulation of EpCAMhi CD49fpos CD104pos CD24low bronchiolar epithelial cells have the capacity to serve as stem/progenitor cells. This is at variance with previous reports suggesting that the majority of Clara cells in the bronchioles can self-renew and generate differentiated progeny (13). The relation of our EpCAMhi CD24low population to the Clara or variant Clara cell is unclear (3) and will require identification of previously undescribed Clara cell markers for further subsetting.
Epithelial Colonies Are Clonally Derived.
Previous studies have shown that heterogeneous embryonic lung cells comprising both epithelial and mesenchymal elements are able to undergo organotypic rearrangement to generate complex epithelial structures in Matrigel without the requirement for proliferation (14). To demonstrate the clonal proliferation of epithelial cfus, we performed mixing experiments using equal numbers of RFPpos and GFPpos EpCAMhi CD24low cells in coculture with WT EpCAMneg Sca-1pos mesenchymal cells (Fig. 3A). All epithelial colonies (n = 259) were exclusively monochromatic (RFPpos or eGFPpos), whereas surrounding mesenchymal cells were nonfluorescent (Fig. 3 B–D), confirming that epithelial colonies are clonally derived from the proliferation of single EpCAMhi CD24low cfus rather than by aggregation, which would give rise to colonies comprising both RFPpos and eGFPpos cells.
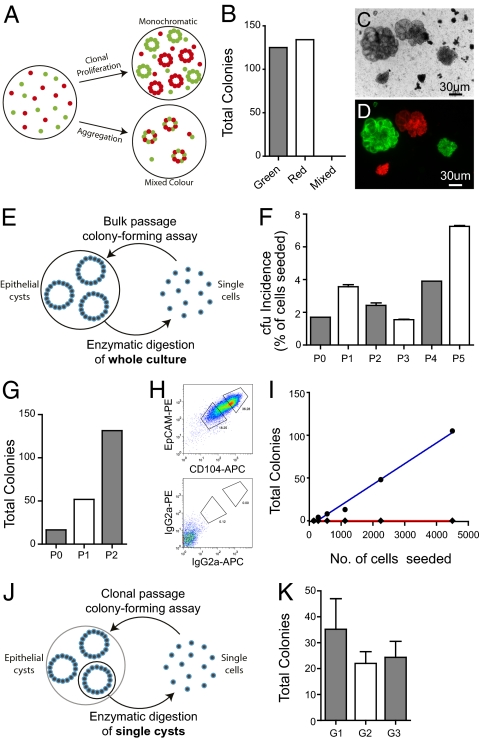
Fig. 3.
Lung epithelial cfus are capable of clonal proliferation and self-renewal in vitro. (A) Schematic representation of potential outcomes of mixing experiment. (B) Total colonies that were red, green, or mixed color. (C) Phase contrast image of colonies. (D) Overlay of fluorescence from cocultures using RFPpos and eGFPpos EpCAMhi CD24low cells with WT EpCAMneg Sca-1pos cells. (E) Schematic representation of stromal-free bulk serial passaging. (F) Incidence of cfus (% of cells seeded + SEM, n ≥ 2) of EpCAMhi CD24low cells after bulk serial passage in stromal-free cultures with FGF-10 and HGF. (G) Increase in total number of epithelial cfus generated after serial passaging. (H) EpCAM vs. CD104 subsetting of passaged cells (P3). (I) Cloning efficiency of EpCAMhi CD104hi (blue) and EpCAMlow CD104low (red) passaged cells (P3) replated in stromal-free culture with FGF-10 and HGF. (J) Schematic representation of stromal-free clonal serial passaging. (K) Incidence of cfus (mean number of colonies + SEM, n ≥ 3 colonies for each generation) from serial recloning of single colonies.
Lung Epithelial cfus Possess the Capacity for in Vitro Self-Renewal.
To determine whether lung epithelial cfus exhibit the capacity for self-renewal, primary EpCAMhi CD24low cell-derived colonies grown in stromal-free cultures supplemented with FGF-10 and HGF were dispersed by enzymatic digestion and serially recloned to measure their secondary and subsequent epithelial colony-forming potential. Initially, bulk passaging of whole cultures (Fig. 3E) demonstrated that a subset of EpCAMhi CD104pos epithelial cfus retain their colony-forming potential after serial passaging (Fig. 3F). Importantly, when epithelial colonies are dissociated and replated, there is a progressive increase in cfu number (Fig. 3G), suggesting that self-renewal rather than cell survival is occurring. The reanalysis of these serially propagated cells after three passages also demonstrated that the majority of propagated cells maintained their EpCAMhi CD104pos phenotype, although the remainder exhibited a reduction in EpCAM and CD104 expression (Fig. 3H), correlated with preservation and loss of colony-forming potential, respectively (Fig. 3I). Significantly, if transformation had occurred within the colonies or during serial passaging, it would be expected that colony-forming potential would be uncoupled from the level of EpCAM expression. More importantly, serial passaging of single colonies (Fig. 3J) demonstrated the robust self-renewal of single cfus over three generations (Fig. 3K).
Lung Epithelial cfus Comprise Lineage-Committed and Multilineage Subtypes.
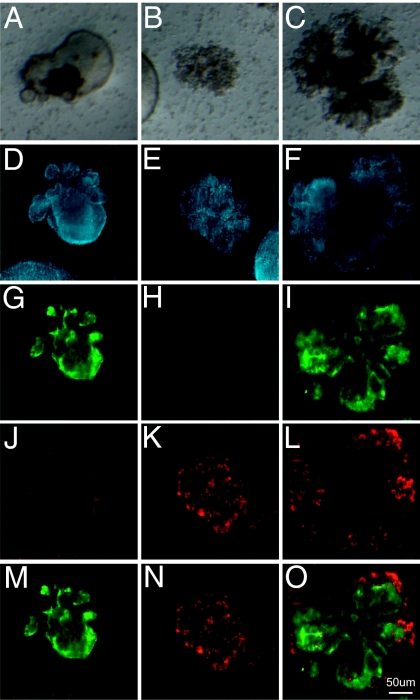
Morphological characterization of epithelial colonies grown in organotypic cultures demonstrates the generation of three distinct subtypes of epithelial colonies from the EpCAMhi CD24low cell fraction, including large airway-like lobular cystic colonies with a clearly defined lumen (46% of colonies, n = 150 of 326; Fig. 4A), small dense saccular colonies (35% of colonies, n = 114 of 326; Fig. 4B), and colonies of mixed phenotype with distinct budding (19% of colonies, n = 62 of 326; Fig. 4C). Immunofluorescent labeling of epithelial colonies in fixed whole-mount organotypic cultures confirmed the presence of three distinct colony subtypes. In cystic colonies, cells expressed the polymeric mucin MUC5AC, which was secreted into the lumen but did not express the AEC II marker, proSP-C (Figs. 4 D, G, J, and M). Fluid could be seen circulating within the lumen of cystic colonies along with beating cilia in cell patches on the inner surface of the lumen. Given that MUC5AC is produced specifically by airway mucous-secreting epithelial cells, these data suggest that cystic colonies comprise cells of the airway lineage. In contrast, the majority of cells in the smaller saccular colonies expressed proSP-C but did not stain for MUC5AC (Fig. 4 E, H, K, and N), suggesting that these colonies comprised alveolar AEC II epithelial cells and their progeny. Mixed colonies showed immunoreactivity for MUC5AC and proSP-C (Fig. 4 F, I, L, and O), suggesting that both airway and alveolar epithelial lung cell lineages can be derived from a multipotent lung epithelial cfu. Interestingly, proSP-C staining in mixed colonies was only observed on cells at the peripheral tips of the colonies.
Fig. 4.
Generation of distinct epithelial colony subtypes. Bright-field images of lobular cystic airway-like colonies (A), dense saccular alveolar-like colonies (B), and colonies with mixed morphologies (C). Fluorescent confocal images of DAPI (blue) (D–F), MUC5AC (green) (G–I), proSP-C (red) (J–L), and overlay of MUC5AC and proSP-C staining of representative colonies (M–O).
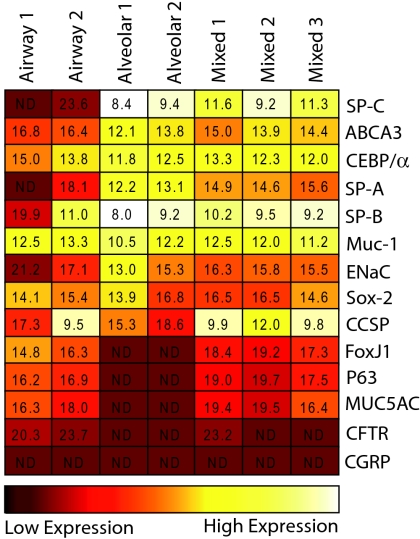
Real-time RT-PCR gene expression analysis of individual colonies (Fig. 5) established that genes encoding airway lineage markers, including FoxJ1 (Foxj1; ciliated cells), P63 (Trp63; basal cells), MUC5AC (Muc5ac; goblet cells) and CFTR (Cftr; anion secretory cells) were exclusively detected in cystic and mixed cfus. The alveolar markers, ABCA3 (Abca3), CEBP/alpha (Cebpa) and SP-C (Sftpc), as well as SP-A (Sftpa1) were highly expressed in saccular and mixed colonies, but were not detected (SP-C and SP-A) or expressed at lower levels (ABCA3 and CEBP/alpha) in cystic colonies. The Clara cell marker, CCSP (Scgb1a1) was enriched in cystic and mixed colonies, but also detected in saccular colonies, and SP-B (Sftpb), MUC-1 (Muc1), ENaC (Scnn1g) and Sox-2 (Sox2) were expressed by all epithelial colony subtypes. CGRP (Calca) was not detected in any of the epithelial colonies examined suggesting that neuroendocrine cells are part of a separate lineage. Taken together, the differential expression of airway versus alveolar lineage markers supports the concept that different epithelial colony subtypes are derived from either lineage-committed epithelial cfus or multipotent lung epithelial cfus.
Fig. 5.
Multilineage differentiation of mixed lung epithelial cfus. Heat map shows relative real-time RT-PCR gene expression levels in cells harvested from single airway, alveolar, and mixed epithelial colonies. Values represent average ΔCt values of genes relative to 18s RNA control. ND, not detected (Ct <40).
Evidence of an Adult Lung Epithelial Stem/Progenitor Cell Hierarchy.
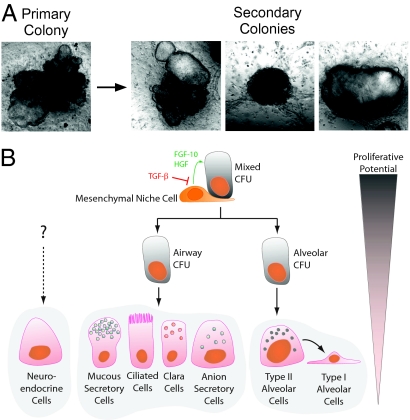
In support of the hypothesis that an epithelial stem/progenitor cell hierarchy exists in the adult mouse lung, we have shown that dissociation and reseeding of primary mixed colonies in coculture with EpCAMneg Sca-1pos stromal cells resulted in the generation of secondary mixed, airway and alveolar colonies (Fig. 6A). In contrast, cystic airway and saccular alveolar colonies failed to generate secondary colonies, suggesting that these progenitor cells have only limited proliferative potential. However, it is not possible at this stage to categorically exclude the possibility that mixed colonies may be derived from a multipotent regenerative cfu, consisting of juxtaposed cells rather than a single multipotent stem cell. Ultimately, single cell deposition would be required to resolve this issue. The complex growth requirements of these cells and their low incidence have made this goal elusive to date.
Fig. 6.
Evidence of an epithelial hierarchy. (A) Images of a primary colony that was enzymatically dissociated and the secondary colonies generated after subsequent reseeding of disaggregated cells. (B) Proposed lineage hierarchy of different lung epithelial cfu subsets.
Taken together, these findings suggest that the adult mouse lung contains a population of multipotent epithelial stem/progenitor cells with the capacity for self-renewal, whose fate and specificity is regulated by cell-cell and/or paracrine interactions with the surrounding mesenchyme. We propose a model in which descendants of mixed cfus give rise to lineage-committed progenitors with limited proliferative potential (Fig. 6B) which in turn give rise to airway or alveolar epithelial lineages in the adult lung. Although we have not directly investigated the differentiation of AEC I in this system, it is generally believed that these cells are descendants of AEC II cells, as indicated in the hierarchy schematic (Fig. 6B).
Discussion
In this study, we have identified epithelial stem/progenitor cell subsets in the adult mouse lung that give rise to clonally derived airway alveolar or mixed lung epithelial colonies in vitro, providing evidence that the adult lung epithelium may be organized in a hierarchical manner such that lung epithelial stem cells give rise to differentiated lung epithelium via a series of lineage-committed progenitors. We have shown that both mixed and lineage-committed cfus can be harvested from the adult mouse lung, with the latter being more abundant, suggesting that lineage-committed cfus may be the primary reserve for maintenance of distinct lineages in vivo.
To date, there is no convincing evidence to suggest that a multipotent stem cell in the bronchiolar airways is capable of migrating to alveoli to replenish alveolar epithelium in vivo. Indeed, there is a strong belief that alveolar and bronchiolar epithelial cells are maintained by distinct lineages, questioning the biological relevance of multipotent lung epithelial stem cells. However, the existence of a quiescent multipotent lung epithelial stem cell pool should not be discounted. Rawlins et al. (4) recently provided compelling evidence using a lineage-tracing strategy, based on the restricted expression of Id2 (inhibitor of differentiation 2), that multipotent progenitors in the fetal lung distal tip are able to self-renew and contribute to both bronchiolar and alveolar lineages during development of the lung (4). Importantly, it should also be noted that although we demonstrate that multipotent lung epithelial cfus can generate mixed-lineage colonies in vitro, this does not necessarily mean that they exhibit this potential in vivo. The capacity for multilineage differentiation of EpCAMhi CD104pos CD24low cells in the adult lung may be indicative of an enduring developmental potential characterizing cells with the capacity to replenish regional facultative stem cell pools whose fate specification is regulated by the niche in which they reside.
We propose that EpCAMneg Sca-1pos mesenchymal elements of lung epithelial stem/progenitor cell niches provide instructional cues for proliferation and differentiation of EpCAMhi CD24low lung epithelial cfus by the local release of growth factors, particularly FGF-10 and HGF. In the developing lung, FGF-10 is expressed in mesenchymal progenitors adjacent to epithelial buds, where it regulates branching morphogenesis (15), and several studies have shown that it is important for maintaining epithelial proliferation during development (16, 17). HGF is thought to be involved in signaling between the mesenchyme and developing epithelium, acting in synergy with FGF-10 (18). In addition, HGF has been shown to stimulate epithelial cell proliferation during postpneumonectomy compensatory lung growth (19) and also to ameliorate the effects of elastase-induced emphysemas in mice (20). Conversely, the effect of FGF-10 in inducing epithelial growth has been shown to be antagonized by BMP-4 (21), which would explain the reduction of epithelial colony generation in cultures supplemented with exogenous BMP-4. In the same way, TGF-β1 and PDGF-AA are known to regulate branching morphogenesis via modulation of mesenchymal cells, promoting smooth muscle cell differentiation and subsequently inhibiting the action of FGF-10 on the underlying epithelium, preventing further proliferation and promoting proximal differentiation (9, 22). These data suggest that there may be a common regulatory mechanism for embryonic lung epithelium and adult epithelial stem cells.
In conclusion, our data support a model in which the adult mouse lung contains a population of multipotent epithelial stem/progenitor cells with the capacity for self-renewal and whose descendants give rise to airway and alveolar epithelial lineages. Thus far, we have not detected proliferating or differentiating neuroendocrine cells in our culture system. Consequently, the relation of the neuroendocrine cell lineage to the cfus we describe remains unresolved. It is possible that the dissociation technique employed in this study is not optimal for harvesting all cells with stem/progenitor cell activity. Likewise, defining the proliferative and differentiative potential of other putative stem/progenitor cell cohorts in the lung will depend on defining the optimal requirements and culture conditions to support their growth. Importantly, this in vitro clonogenic assay provides a powerful tool for identification of stem/progenitor cell populations in the lung and the factors that regulate their fate and specificity.
Materials and Methods
Mice.
All mice were maintained on the C57BL/6 background. Mice expressing nuclear-localized enhanced GFP derived by ES cell insertion of the pCagg promoter (chicken β-actin) driving an enhanced GFP gene were obtained from Klaus Mathaei (Australian National University, Canberra, Australian Capital Territory, Australia). C57BL/6 RFP mice derived from ES cells expressing a pbActin-CMV-DsRed T3 transgene were obtained from Patrick Tam (Children’s Medical Research Institute, Sydney, New South Wales, Australia).
Lung Cell Preparations and Flow Cytometry.
A detailed methodology for the preparation of lung cells is provided in SI Materials and Methods. Briefly, mouse lung cell suspensions were prepared by collagenase digestion of mouse lungs following removal of upper airways as previously described (6, 9). Low-density cells were then isolated by density gradient centrifugation (Nycoprep 1.077A; Nycomed Pharma) and then resuspended and incubated in 2% vol/vol FCS or newborn calf serum (PBS-2% vol/vol Se) (5 × 107 cells/mL, 20 min on ice) in an optimally pretitered mixture of antibodies, including anti-CD45, anti-CD31, anti-Sca-1, anti-EpCAM, anti-CD49f, anti-CD24, anti-CD104, and relevant isotype controls (Biolegend). Labeled cells were washed in PBS-2% Se, resuspended at 5–10 × 106 cells/mL, and held on ice for flow cytometric analysis and sorting. Viability was determined by propidium iodide (1 μg/mL) staining, and doublets excluded by forward scatter (height) vs. forward scatter (area) gating. For proSP-C analysis, cells were fixed using a Fix ‘n’ Perm kit (Invitrogen). Sorting was performed using a BD Influx cell sorter (Becton Dickinson). Analysis was done using a BD LSRII bench top analyzer (Becton Dickinson) and data were analyzed using FlowJo (Tree Star).
Real-Time RT-PCR.
RNA from individual colonies or primary sorted cells was prepared using the RNAqueous MicroElute RNA isolation kit (Applied Biosystems), and cDNA was prepared using the high-capacity RNA-to-cDNA kit (Applied Biosystems). For real-time PCR, isolated cDNA was subjected to 40 cycles of amplification using Applied Biosystems TaqMan gene expression assays (Table S1) and 18s RNA endogenous control as per the manufacturer’s instructions. Reactions resulting in a Ct of less than 40 indicated the presence of target cDNA in the sample, and data were expressed as the number of cycles that the reaction sample differed (ΔCt) from an 18s RNA control. Relative expression of the target gene was expressed as raw ΔCt values relative to the endogenous control.
Cell Culture.
Sorted cells resuspended in 90 μL of Matrigel (BD Biosciences) prediluted 1:1 (vol/vol) with media were added to a 24-well transwell filter insert (Millicell-CM; Millipore) in a 24-well tissue culture plate containing 400 μL of media. For cocultures, epithelial cells were mixed with EpCAMneg Sca-1pos cells (2 × 106 cells/mL) in Matrigel. DMEM/F12 plus L-glutamine plus 2.438 g/L sodium bicarbonate (Invitrogen) was supplemented with 10% newborn calf serum, penicillin/streptomycin, insulin, transferrin, and selenium for all cultures. Where specified, FGF-7 (100 ng/mL; Millipore), FGF-10 (50 ng/mL; R&D Systems), HGF (30 ng/mL; R&D Systems), BMP-4 (100 ng/mL; R&D Systems), TGF-β1 (10 ng/mL; Peprotech), and PDGF-AA (50 ng/mL; Millipore) were added to the media. Cultures were incubated at 37°C in a humidified incubator (5% vol/vol O2, 10% vol/vol CO2, 85% vol/vol N2) and refed three times weekly. All images are representative of cultures grown for 14–16 days unless otherwise specified. For bulk passaging, whole cultures were dissociated in 1 mg/mL Collagenase Type I plus 3 mg/mL Dispase (Roche) in PBS to generate a single-cell suspension. For clonal passaging, single colonies were picked and dissociated in the Collagenase/Dispase solution. Single cells were reseeded in stromal-free cultures supplemented with FGF-10 and HGF.
Immunohistochemistry.
Whole-mount cultures were fixed with 4% vol/vol paraformaldehyde and removed from inserts, washed in PBS, subjected to antigen retrieval by boiling in 10 mM citrate buffer for 20 min, and incubated in blocking buffer (1 h; 5% wt/vol BSA, 1% skim milk, 0.05% Triton X-100 in PBS). Cultures were then incubated overnight with antibodies against proSP-C (goat anti-proSP-C, clone C-19; Santa Cruz Biotechnology, or rabbit anti-proSP-C; Millipore), CCSP (goat anti-CCSP; Santa Cruz Biotechnology), MUC-1 (rabbit anti-MUC-1; Abcam), and MUC5AC (biotin-labeled mouse anti-MUC5AC) and were then washed in PBS (0.05% Tween 20) and incubated with Alexafluor-488, -568, or -647–conjugated anti-goat, anti-rabbit, or streptavidin secondary reagents (Invitrogen) for 3 h and then washed. Nuclei were stained with DAPI, and sections were mounted in Vectashield (Vecta Laboratories). Images were acquired using a Leica SP confocal microscope and colored and overlaid using Adobe Photoshop (Adobe Systems). Bright-field images and whole-mount fluorescent images of cultures were taken using an Olympus SZX16 stereo dissecting microscope.
Supplementary Material
Acknowledgments
We thank Kate Rutherford and Daniela Cardozo for assistance with experimental animals and Andrew Fryga, Darren Ellemor, and Kathryn Flanagan for assistance with flow cytometry. This study was supported by the Australian Stem Cell Centre, National Health and Medical Research Council of Australia (Grant 400323), and National Health and Medical Research Council Peter Doherty Fellowship 384367 (to J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909207107/DCSupplemental.
References
- 1.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 3.Stripp BR. Hierarchical organization of lung progenitor cells: Is there an adult lung tissue stem cell? Proc Am Thorac Soc. 2008;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi W, Xu J, Warburton D. Development, repair and fibrosis: What is common and why it matters. Respirology. 2009;14:656–665. doi: 10.1111/j.1440-1843.2009.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQualter JL, et al. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 7.Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Taderera JV. Control of lung differentiation in vitro. Dev Biol. 1967;16:489–512. doi: 10.1016/0012-1606(67)90061-9. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl P, et al. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 10.Rock JR, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol. 2007;37:152–159. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondrinos MJ, et al. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12:717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 15.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 16.Ramasamy SK, et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol. 2007;307:237–247. doi: 10.1016/j.ydbio.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol. 2008;8:2. doi: 10.1186/1471-213X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmichi H, Koshimizu U, Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development. 1998;125:1315–1324. doi: 10.1242/dev.125.7.1315. [DOI] [PubMed] [Google Scholar]
- 19.Sakamaki Y, et al. Hepatocyte growth factor stimulates proliferation of respiratory epithelial cells during postpneumonectomy compensatory lung growth in mice. Am J Respir Cell Mol Biol. 2002;26:525–533. doi: 10.1165/ajrcmb.26.5.4714. [DOI] [PubMed] [Google Scholar]
- 20.Hegab AE, et al. Intranasal HGF administration ameliorates the physiologic and morphologic changes in lung emphysema. Mol Ther. 2008;16:1417–1426. doi: 10.1038/mt.2008.137. [DOI] [PubMed] [Google Scholar]
- 21.Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- 22.Boström H, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.