Abstract
Cocaine use during pregnancy is deleterious to the newborn child, in part via its disruption of placental blood flow. However, the extent to which cocaine can affect the function of the fetal primate brain is still an unresolved question. Here we used PET and MRI and show that in third-trimester pregnant nonhuman primates, cocaine at doses typically used by drug abusers significantly increased brain glucose metabolism to the same extent in the mother as in the fetus (∼100%). Inasmuch as brain glucose metabolism is a sensitive marker of brain function, the current findings provide evidence that cocaine use by a pregnant mother will also affect the function of the fetal brain. We are also unique in showing that cocaine’s effects in brain glucose metabolism differed in pregnant (increased) and nonpregnant (decreased) animals, which suggests that the psychoactive effects of cocaine are influenced by the state of pregnancy. Our findings have clinical implications because they imply that the adverse effects of prenatal cocaine exposure to the newborn child include not only cocaine’s deleterious effects to the placental circulation, but also cocaine’s direct pharmacological effect to the developing fetal brain.
Keywords: brain function, in vivo, pregnancy, imaging, in utero
Brain function and potentially cognitive experiences of the fetus in utero are not well-understood and are likely (if at all) to occur only in the most mature third-trimester fetal brain. Recently, discussions around the increase in fetal surgical procedures has raised the question of whether the fetus feels pain and the need for adequate analgesia at least in third-trimester fetuses when thalamocortical pain fibers are maturing (1). There is also a serious lack of information in regards to whether drugs taken by women during pregnancy, such as antidepressants or drugs of abuse (e.g., cocaine and nicotine), are pharmacologically active in the fetal brain and linked with behavioral or cognitive changes occurring in the child later in life. For example, in the adult brain cocaine induces a feeling of euphoria, an effect that is mediated in part by fast and supraphysiological increases in dopamine in the brain’s reward circuits (including the nucleus accumbens) secondary to cocaine’s blockade of the dopamine transporter. However, the functional changes of the fetus’ brain in response to maternal use of cocaine are not known.
PET and 2-deoxy-2-[18F]fluoro-D-glucose (18FDG) can measure brain glucose metabolism noninvasively, which serves as a marker of brain function and has been used extensively to evaluate the effects of psychoactive drugs (including drugs of abuse) in the live brain (2 –6). In the case of cocaine, PET studies in cocaine abusers, showed that its i.v. administration produced a 14% global reduction in the cerebral metabolic rate of glucose (CMRglu) that was associated with the subjective experience of euphoria (4). Similar reductions in CMRglu have been demonstrated in nonanesthetized nonhuman primates after short-term cocaine self-administration (7) and in pregnant sheep, where cocaine was infused directly into the fetus using invasive approaches (8). In the rodent, cocaine’s pharmacological action in the fetus has also been demonstrated using quantitative autoradiography (9, 10). However, the question of whether cocaine can modify brain function in the primate fetal brain when the mother is exposed to cocaine at a dose equivalent to that abused by cocaine abusers remains unanswered.
Because the reinforcing effects of cocaine are associated with its ability to block dopamine transporters, thereby increasing dopamine (11), a prerequisite for cocaine to act as a drug reinforcer in the fetal brain would at least require the presence of dopamine synthesis, functioning dopaminergic receptors, dopaminergic nerve fibers, and dopamine transporters. In vitro studies on monkey and human tissues have demonstrated the presence of dopamine receptors in second- and third-trimester nonhuman primate fetuses (12), and dopamine transporters by day 70 of gestation (13). There is also clear evidence of dopamine synthesis and spontaneous dopamine release in the fetal rat brain, starting in the second half of uterine development (14). In the nonhuman primate fetal brain, the information is more limited; however, tyrosine hydroxylase-labeled axons and varicosities have been demonstrated in the frontal cortex of rhesus monkeys at the time of birth (15). Extrapolating from these data, it appears that all of the necessary neurochemical components are present in the fetal brain for cocaine to be pharmacologically active and to have reinforcing effects (provided that sufficient amounts of cocaine reaches the fetal brain).
We have developed a noninvasive method for visualizing pharmacokinetic profiles of drugs concurrently in the mother and fetus of nonhuman primates with PET and radio-labeled ligands, including [11C]cocaine (16). With this imaging paradigm, we have shown that there is significant uptake of cocaine in the nonhuman primate fetal brain (≈80% of the cocaine uptake by the mother’s brain). We now investigate the effect of maternal intake of cocaine on the fetal brain function by noninvasively measuring the fetal CMRglu, which serves as a marker of brain function (17), using PET and 18FDG in third-trimester pregnant M. radiata. We hypothesized similar effects of cocaine on brain glucose metabolism in the fetal and the maternal brain.
Results
The main purpose of this study was to evaluate the effect of 1-mg/kg cocaine, which is a dose in good correspondence to doses used by cocaine abusers (18), administered i.v. to pregnant nonhuman primates concurrently on the maternal and fetal CMRglu. For this purpose, we applied a noninvasive dynamic 18FDG PET scan approach in which the 18FDG time-activity curve measured in the cardiac cavity of the mother is used as an estimation of the arterial input function for calculation of CMRglu in the maternal, as well as the fetal, brain. Twelve healthy pregnant nonhuman primates (Macaca radiata) and five healthy nonpregnant baboons were used in these studies and divided into three groups (Table 1, and Methods). Group 1 (n = 8) comprised pregnant M. radiata primates who did not receive cocaine (pregnant control group); group 2 (n = 4) comprised pregnant M. radiata primates exposed to 1 mg/kg i.v. cocaine; and group 3 comprised nonpregnant baboons tested twice with and without exposure to 1 mg/kg i.v. cocaine and in whom the CMRglu was calculated using a conventional invasive 18FDG arterial input function acquired during the dynamic PET scan (Methods and Table 1).
Table 1.
Experimental groups
| Experimental group | Species | Pregnant | PET 18FDG | Cocaine 1 mg/kg i.v. | MRI scan |
| 1 (n = 8) | M. radiata | Yes | Yes | No | Yes |
| 2 (n = 4) | M. radiata | Yes | Yes | Yes | Yes |
| 3 (n = 5) | Papio papio | No | Yes | Yes | No |
Physiological Data.
All subjects survived the scanning procedures without morbidity or mortality and all of the pregnant M. radiata delivered their babies without complications within 1 to 2 weeks after the experiment.
There were no significant differences in the mean arterial blood pressure during the dynamic PET scan between group 1 (control) subjects and group 2 subjects exposed to 1 mg/kg i.v. cocaine. Importantly, the average i.v. anesthesia infusion rates between the two groups were also within similar ranges (138 ± 32 μg/kg per min versus 150 ± 22 μg/kg per min) during the PET scanning procedures, suggesting that all subjects were exposed to the same anesthetic depth during the studies. Similarly, in group 3 (baboons) the mean arterial blood pressure and anesthesia infusion rates were identical between the baseline control study and during exposure to 1 mg/kg i.v. cocaine, and within the infusion rate range of that used in the M. radiata. In the baboons, the average plasma glucose during the first PET was 3.25 ± 0.23 mmol/L and was significantly higher (P = 0.028) during the second PET scan acquired during exposure to 1 mg/kg cocaine (3.49 ± 0.28 mmol/L).
Cerebral Metabolic Rate of Glucose Utilization.
To verify that the noninvasive approach for calculation of the CMRglu generated reliable results, we first compared the CMRglu of the striatum obtained from the pregnant M. radiata during control conditions with the corresponding CMRglu calculated conventionally and invasively via an 18FDG arterial input function in the nonpregnant baboons. This analysis demonstrated that the CMRglu values in the striatum measured in the pregnant M. radiata were not statistically different from those in the baboons (10.1 ± 7.6 μmol/100 g per min vs. 11.6 ± 2.8 μmol/100 g per min, P > 0.05), signifying that the noninvasive approach was assessing the CMRglu correctly (Tables 2 and 3). Our data in the pregnant nonhuman primates also showed that under control conditions and with anesthesia, the CMRglu of the fetal brain was significantly lower (40–50%) than that of the maternal brain (P < 0.04; Table 2).
Table 2.
CMRglu of the fetal brain compared with that of the maternal brain
| Group | Mother CMRglu, μmol/min/100 g | Fetus CMRglu, μmol/min/100 g |
| Group 1 (n = 8) (control) | 10.1 ± 7.6 | 5.1 ± 3.1* |
| Group 2 (n = 4) (1 mg/kg cocaine) | 20.5 ± 3.9† | 11.5 ± 2.5* |
Fetal values that differ statistically from maternal values in group 1 (P < 0.05).
Values of group 1 that differ statistically from group 2 (P < 0.05).
Table 3.
CMRglu and K i for group 3 (n = 5) for nonpregnant baboons
| Baseline | Cocaine (1 mg/kg) | |
| CMRglu (μmol/min/100 g) | 11.6 ± 2.8 | 8.0 ± 1.9* |
| Ki | 0.0189 ± 0.0052 | 0.0132 ± 0.0032* |
Values are mean ± SD.
Baseline values of group 3 that differ statistically from those obtained during cocaine exposure (P < 0.05).
It has been reported in the literature that an acute i.v. cocaine challenge decreases the CMRglu in human subjects (4) and in nonhuman primates (7). In accordance with prior studies, we also showed that the same dose of cocaine decreased CMRglu in the nonpregnant baboons (Fig. 1). Thus, in the nonpregnant baboons the CMRglu was 11.6 ± 2.8 μmol/100 g per min under control conditions, and decreased to 8.0 ± 1.9 μg/100 g per min (P = 0.028) during the acute challenge with 1-mg/kg cocaine (Table 3). In contrast to the CMRglu decreases observed in the nonpregnant baboons, the acute i.v. challenge of cocaine significantly increased the CMRglu in the pregnant maternal brain (∼100%%) as well as in the fetal brain (∼100%) when compared to the CMRglu in the pregnant controls (group 1) that did not received cocaine (Fig. 2 and Table 2). Specifically, the maternal CMRglu increased from 10.1 ± 7.6 μg/100 g per min to 20.5 ± 3.9 μg/100 g min (P = 0.037) during exposure to 1-mg/kg cocaine. Similar to the maternal CMRglu, the CMRglu of the fetal brain increased 2-fold (P < 0.01) from 5.1 ± 3.1 μg/100 g per min to 11.5 ± 2.5 μg/100 g min during exposure to 1 mg/kg cocaine (Table 2).
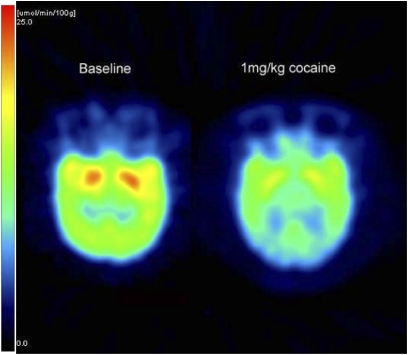
Fig. 1.
Parametric PET images of CMRglu acquired at baseline (Left) and during 1-mg/kg i.v. cocaine (Right) in an anesthetized nonpregnant female baboon brain (transaxial views) from group 3. The scale bar at the bottom of the PET images provides the color-coded hue correspondence of the glucose utilization rate measured as micromole per 100 g per minute. It is clear from the quantitative PET CMRglu images that the acute i.v. challenge of cocaine reduces the CMRglu globally in the baboon brain. On average, in group 3, the CMRglu at baseline was 11.6 ± 2 μg/100 g per min and decreased significantly to 8.0 ± 1.9 μmol/100 g per min (P = 0.028) during 1 mg/kg cocaine exposure.
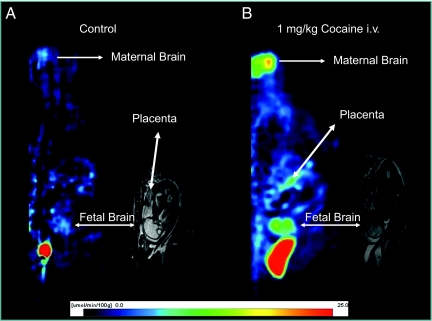
Fig. 2.
(A) A parametric PET image of an anesthetized pregnant M. radiata (sagittal view) of the local metabolic rate of glucose calculated based on the fetal brain time activity curve (as well as a fetal plasma glucose of 2.8 μmol/L) in a normal, pregnant M. radiata (group 1) and the corresponding T2-weighted MRI image, demonstrating the anatomical position of the fetal brain. The scale bar at the bottom of the PET images provides the color-coded hue correspondence of the glucose utilization rate measured as micromole per 100 g per min in the parametric image (except for the area corresponding to the maternal bladder, which appears as a bright red homogenous area). The color-coded scale helps illustrate that the CMRglu of the fetal brain approximated 7 to 8 μmol/100 g per min in this particular M. radiata fetus. (B) Parametric PET image of the local metabolic rate of glucose, also calculated based on the fetal brain time activity curve (fetal plasma glucose of 2.8 mmol/L) in a pregnant M. radiata exposed to 1-mg/kg cocaine i.v. (group 2); and the corresponding T2-weighted MRI image demonstrating the position of the fetal brain. Again, the scale bar at the bottom of the PET images provides the color-coded hue correspondence of the glucose utilization rate measured as micromole per 100 g per min in the parametric PET image (except for the maternal bladder) and is scaled identical to the control image in A for direct comparison. The blue colors represent the low values (0–10 μmol/100 g per min), and yellow and red colors represent higher values (approximately >10–15 μmol/100 g per min). The average CMRglu of the fetal brain in this particular M. radiata fetus approximated 10 μmol/100 g per min. Note the two symmetrical hot spots in the fetal brain, which appear in a location likely to correspond to the striatum. On average, the fetal CMRglu increased 2-fold (P < 0.01) from 5.1 ± 3.1 μg/100 g per min to 11.5 ± 2.5 μg/100 g per min during exposure to 1-mg/kg cocaine, while the maternal CMRglu increased from 10.1 ± 7.6 μg/100 g per min to 20.5 ± 3.9 μg/100 g per min (P = 0.037).
Comparisons of the CMRglu in the pregnant M. radiata and the nonpregnant baboons, both with and without cocaine exposure, showed that even though they did not differ during control conditions (no cocaine exposure), the CMRglu with cocaine exposure was significantly higher in the M. radiata that in the nonpregnant baboon (20.5 ± 3.9 vs. 8.0 ± 1.9 μg/100 g per min, P < 0.01).
Discussion
Our PET imaging data provide direct evidence that cocaine, when administered i.v. to a pregnant nonhuman primate, is pharmacodynamically active in the fetal brain. More specifically, we are unique in showing that the nonhuman primate fetus’ brain function measured as changes in CMRglu increases significantly in response to cocaine. Moreover, cocaine’s effects are similar in the pregnant mother’s brain and in the fetal brain in that the drug increases CMRglu to the same extent (≈100%), but opposite to nonpregnant females in whom cocaine decreased metabolism. We speculate that the opposite effects may reflect the interaction between cocaine and high levels of circulating hormones, including progesterone, estriol, and estetrol in the pregnant female and fetus (19). Indeed, there is extensive data that both estrogen and progesterone modify the pharmacological effects of cocaine (20, 21), and epidemiological data demonstrating that cocaine use in humans addicted to cocaine is lowest in the third trimester (22).
It is also possible that the increase in CMRglu we observed in the maternal brain with cocaine is caused by a slower metabolism of cocaine in pregnancy, resulting in higher than normal levels of cocaine and its active metabolites when compared to the nonpregnant female baboon brains. However, Jatlow showed no differences in the pharmacokinetic profiles for cocaine and its major metabolites (e.g., benzoylecgonine) in pregnant versus nonpregnant subjects (23), eliminating this possibility. Further work is needed to identify the mechanisms responsible for the opposite effects of cocaine on CMRglu between pregnant and nonpregnant animals.
In this study we chose to be as noninvasive as technically possible for safety reasons, as this M. radiata colony is known to periodically shed the Herpes B virus, which is structurally related to herpes simplex viruses I and II of humans (24), and to avoid manipulations that might interfere with normal delivery. Therefore, instead of collecting maternal and umbilical arterial blood for calculation of 18FDG time activity curves (TAC), we positioned the pregnant M. radiata transverse to the aperture of the PET camera so that we could noninvasively obtain the dynamic TACs from the maternal brain, maternal heart, and the fetus simultaneously during the PET-scanning procedures. The reliability of using the noninvasive approach for calculation of the CMRglu was strongly supported by the finding that the average CMRglu during control conditions of the pregnant M. radiata was similar to the average CMRglu of the nonpregnant female baboon brains calculated using an arterial input function. We recognize that the use of nonpregnant female baboons as a comparison control group to the pregnant M. radiata in our study design comprises a potentially confounding factor, given the possibility of interspecies differences. However, we were not able to include or supplement with additional nonpregnant M. radiata control subjects for technical as well as safety reasons.
The method of using a noninvasive-derived arterial input function from the left ventricular blood pool to estimate the metabolic rate of glucose utilization in the heart with 18FDG and a Gjedde-Patlak graphical analysis was originally described by Gambhir et al. (25). Ideally, the derived cardiac input function should be corrected for spillover of activity between the left ventricular blood pool and the myocardium using plasma samples obtained during the latter part of the dynamic scan (26). As we did not attempt to correct for partial volume effects (i.e., spillover from the left ventricular chamber blood pool to the myocardium—and vice versa—in the Macaca radiate), the cerebral input function, and consequently calculations of CMRglu, might therefore be slightly underestimated by 10 to 20% (26). However, the potential underestimation of the CMRglu would be the same for group 1 (control) and group 2 (cocaine) and, therefore, the outcome and conclusion of the analysis would still be valid.
The CMRglu of the fetal brain calculated based on the maternal left ventricle TAC was observed to be about 40% lower than that of the maternal brain. These findings are consistent with those reported in the literature, which also showed lower CMRglu in the fetal brain of guinea pigs (27) and rats (28) compared with the maternal CMRglu. Even though one may have predicted higher metabolism in the fetal than in the mother’s brain because the brain is rapidly growing, this may be counteracted by lower functional activity, alternative sources of energy (ketone bodies and lactate), or greater sensitivity to the effects of anesthesia. Additionally, partial volume effects would tend to have a larger impact in the smaller fetal brain but, if anything, this would lead to underestimation of the fetal CMRglu.
All of the experiments in this study were performed in anesthetized animals and it is therefore possible that the pharmacodynamic effect of cocaine was altered under these circumstances, explaining the observed increase in CMRglu of the maternal as well as the fetal brain. We used a combination of remifentanil and propofol for anesthesia and both drugs are known to cross the placenta: metabolites of remifentanil have been isolated from the fetal circulation (29–31). Data reported in the literature does confirm that the effect of cocaine on cerebral metabolism and cerebral blood flow is critically dependent on the anesthetic used (32 –34) and, in some cases, different from that seen in the awake state. On the other hand, our parallel data obtained in female adult-nonpregnant baboons did demonstrate a cocaine-induced decrease in CMRglu, as previously reported in awake human subjects and nonhuman primates, suggesting that (i) the propofol/remifentanil anesthesia did not alter the pharmacodynamic profile of cocaine as measured by CMRglu and (ii) the pharmacodynamic effect of cocaine in third-trimester pregnancy is indeed different when compared to the nonpregnant state.
Conclusions
The unique finding of this noninvasive imaging study is that an acute challenge of cocaine at a dose (1 mg/kg i.v.) abused for recreational purposes by cocaine abusers increased brain glucose metabolism to the same extent in the maternal and the fetal nonhuman primate brain. Inasmuch as brain glucose metabolism is a sensitive marker of brain function, the current findings provide evidence that cocaine use by a pregnant mother will also affect the function of the fetal brain. We also show that cocaine’s effects on the brain glucose metabolism differed in pregnant and nonpregnant animals, which suggests that the psychoactive effects of cocaine are influenced by the state of pregnancy. Our findings have clinical implications, as they suggest that the adverse effects of prenatal cocaine exposure to the newborn child include not only cocaine’s deleterious effects to placental circulation, but also cocaine’s direct pharmacological effect to the developing fetal brain.
Methods
Subjects.
Twelve pregnant, female Bonnet macaques (M. radiata) from the Primate Laboratory, Department of Psychiatry, State University of New York (SUNY) Downstate and five nonpregnant adult female baboons (Papio papio) from the vivarium at Brookhaven National Laboratory (BNL) were used in this study (Table 1). The pregnant monkeys were judged to be in the third-trimester gestational stage by the veterinarian after a brief physical examination and ultrasound. The monkeys were transported to BNL 2 weeks before the study and were housed according to National Institute of Health guidelines at the BNL vivarium. The five adult female baboons (Papio papio) were used as nonpregnant controls to validate our noninvasive approach to quantify the cerebral metabolic rate of glucose utilization (CMRglu) using 18FDG PET (Table 1) and to compare the effects of acute cocaine on the CMRglu between pregnant and nonpregnant animals. The experimental protocol was approved by the Institutional Animal Care and Use Committees at BNL and SUNY Downstate.
Anesthesia.
All animals were anesthetized with a continuous infusion of propofol (100–200 μg/kg per min; Diprivan, AstraZeneca) and Remifentanil hydrochloride (0.1 μg/kg per min; ULTIVA). Mechanical ventilation was implemented during the imaging procedures, as previously described (16, 35). Physiological parameters (blood pressure, heart rate, oxygen saturation, body temperature, and end-tidal CO2) were monitored continuously and kept within normal ranges. No muscle relaxant was used. At the conclusion of imaging experiments, the anesthesia was discontinued and the animals recovered spontaneous breathing within 2 to 5 min, were extubated, and returned to their cages. All animals delivered their babies approximately within 2 to 3 weeks of the experiment.
MRI of Pregnant M. radiata.
All MRI data were acquired on a Varian/Siemens 4T instrument equipped with a high-performance gradient system (Sonata, Siemens, Inc). A 27-cm inner diameter radiofrequency transceiver coil was used for all studies. MRI data were collected under scan-synchronous ventilation using a two-dimensional (2D) multislice dual spin-echo sequence; proton density (TE15/TR3000) and T2-weighted (TE50/TR3000) MRIs were typically acquired using 65 2-mm slices with a nominal in-plane resolution of 0.63 mm × 0.78 mm using a matrix of 512 × 256, and a total acquisition time of 40 min. Only the maternal thorax, abdomen, and pelvis were included in the field of view.
Dynamic FDG PET Studies.
All PET scans were performed on an ECAT EXACT HR+ tomograph (Siemens/CTI) with a 56-cm transaxial and 15.5-cm axial field of view surrounded by detector blocks of 4 × 4-mm bismuth germanate crystals 30 mm long.
The 12 pregnant M. radiata were divided into two groups: group 1 (n = 8) served as controls; in group 2 (n = 4), the mother received an acute i.v. challenge with 1mg/kg cocaine at the time of the 18FDG injection. To obtain the 18FDG time course of activity in both maternal and fetal brain, as well as the maternal heart, the animal was positioned transverse to the scanner axis as previously described (16). Emission data were collected using acquisition windows of 350 to 650 keV for energy and 12 ns for coincidence timing. To maximize sensitivity, we used 3D mode (septa retracted), which gives a spatial resolution at the center of the field of view of 4.6 mm transaxially and 4.2 mm axially (using National Electrical Manufacturers Association NU-2 protocols). We obtained a separate 15-min 68Ge transmission scan before 18FDG injection (1.5–5 mCi) to correct for attenuation by eliminating emission contamination, and in 2D mode (septa extended) to minimize acceptance of scattered events. PET data were processed in a fully quantitative manner using the vendor ECAT software. Random coincidence events were subtracted “on-line” using a delayed coincidence method. Attenuation correction sinograms were segmented before application to the emission data to reduce statistical noise propagation, and additional corrections for system dead time, detector normalization, and scattered coincidences were performed. The 3D emission sinograms were subsequently reduced to a set of 2D sinograms using Fourier rebinning and then reconstructed into a 256 × 256 (transverse) × 63 (axial) image matrix using the attenuation-weighted ordered-subsets expectation maximization algorithm. Six iterations with 16 subsets were used, and the images were postfiltered with a Gaussian kernel of 4 mm in full width at half maximum to control noise. All studies were performed using a dynamic acquisition sequence with the following 18FDG time framing: 10 × 30 s, 5 × 60 s, 6 × 120 s, 1 × 180 s, 2 × 300 s, 1 × 480 s, and 1 × 720 s.
The five nonpregnant female baboons (group 3) were exposed to a baseline 18FDG dynamic PET scan without cocaine and 2 to 4 weeks later a repeat 18FDG dynamic PET scan was performed with 1 mg/kg cocaine coadministered i.v. at the same time as the 18FDG. An arterial input function via a femoral arterial line was obtained during both scans.
Data Analysis.
Pregnant M. radiata.
All PET data analysis was performed using PMOD (version 2.65; PMOD Technologies) and required several steps. First, the whole-blood arterial input function was determined, which involved accurately defining the lumen of the maternal left cardiac ventricle. This was accomplished by visualizing the heart in a short-axis view on the earliest PET time frames and using markers to carefully outline the left ventricle chamber in the long-axis view. Second, the striatum was identified as distinct “hot” spots in the maternal forebrain after carefully viewing all three orthogonal views. Third, for the fetus, the exact position and rotation of the fetal brain on the anatomical MRI images were explored and used to guide identification of the fetal forebrain and the hot spot areas within the striatum on the corresponding PET images. For example, on the MRI images, all three orthogonal planes were carefully investigated to identify the fetal brain in the sagittal plane that best visualized the fetal forebrain, striatum, and cerebellum. Using PMOD software, the forebrain/striatum and adjacent landmarks, such as the placenta, was marked on the MRI image and identified on the corresponding PET image. Next, a region of interest was positioned in the areas of hot spots on the PET images assessed to correspond to the fetal striatum, with careful motion adjustment if necessary. Finally, the maternal left heart-chamber TAC was used as the input function for the subsequent kinetic analysis (Fig. 3). We used the Gjedde-Patlak fitting routine in PMOD to determine the maternal and fetal CMRglu and the corresponding slope of the regression curve fit. The Gjedde-Patlak Plot estimations of CMRglu are critically dependent on correct values of the plasma glucose concentration, because K i varies inversely with the plasma glucose concentration. We did not measure the plasma glucose concentration in either the mother or the fetus during the PET study. However, from previous data it is known that the fasting plasma-glucose concentration of the adult M. radiata is in the range of 2 to 4 mmol/L. We used a value of 3.5 mmol/L for the mother for both group 1 and group 2. Furthermore, assuming that the fetal plasma glucose concentration is known to be in a constant ratio to the mother’s but ∼20% lower (36), we therefore used a plasma glucose concentration value of 2.8 mmol/L for calculation of the fetal CMRglu. In the PMOD Gjedde-Patlak model (PKIN), the original tissue activity measurements are transformed according to the algorithm and a regression line is fitted to the curve within a range that can be set manually, so that only a certain portion of the curve is fitted. The ‘Lin’ start point was adjusted to only fit the last three to four points of the curve (corresponding to measurements taken >30 min following the 18FDG injection).
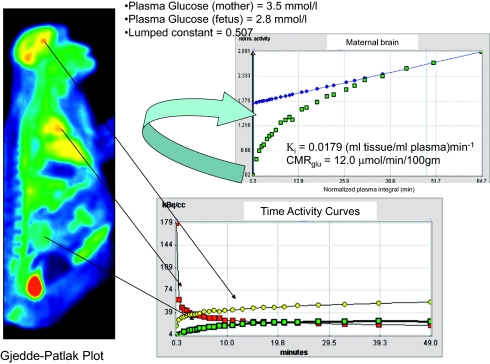
Fig. 3.
Calculation of the CMRglu in the maternal brain using commercially available software (PMOD) is illustrated. The maternal left-ventricle time-activity curve is obtained from the maternal heart and used as the arterial input function for calculation of CMRglu using the Gjedde-Patlak plot approach. The graphical analysis of the Gjedde-Patlak plot is shown; the regression line is fitted to the last three to four points of the curve and the slope of the linear phase of the plot is the uptake rate constant Ki (ml tissue/mL plasma)min−1 from which the CMRglu is calculated (41).
Lumped constant.
The lumped constant for 2-deoxyglucose (DG) in monkeys is known [0.344 (37)]. However, as far as we are aware, the lumped constant for 18FDG in monkeys has not been measured. However, in rats the DG lumped constant and FDG lumped constant are 0.48 (38) and 0.71 (39), respectively. Therefore, for all our calculations, we used a LC of (0.71/0.48) × 0.344 = 0.507. Furthermore, the LC is known to be relatively stable, also under conditions with changes in blood ketone and lactate concentrations (40).
Baboons.
The PET data analysis of the baboon brains was straightforward, as both the arterial input function and plasma glucose was known. The analysis was also performed using the PMOD Gjedde-Patlak model and by fitting to the last three to four points of the transformed curves from the regions of interest placed in the striatal area. We also used a lumped constant of 0.507 for these calculations.
Statistics.
The calculated parameters included the CMRglu of the maternal and fetal brains derived from the slope of the corresponding regression curve fits (K i) is shown in Fig. 3. All parameters are presented as mean ± SD. Differences between the CMRglu of the maternal and fetal brains were assessed using a paired Student t test (two-tailed). Differences between pregnant group 1 (control) and group 2 (cocaine) CMRglu values were assessed using an unpaired Student t test (two-tailed). Differences between CMRglu values in the nonpregnant baboon brains at baseline and after 1 mg/kg cocaine was tested with a paired Student t test (one-tailed). The differences were considered statistical significant at P < 0.05.
Acknowledgments
We thank Donald Warner and David Alexoff for assistance with the PET scan acquisitions; Pauline Carter, Payton King, and Barbara Hubbard for help with the preparation, anesthesia, and imaging of the nonhuman primates; and Dr. L. Rosenblum for allowing us access to pregnant M. radiata from the Primate Laboratory at State University of New York Downstate. This research was supported by Department of Energy Office of Biological and Environmental Research (Brookhaven National Laboratory Contract DE-AC02-98CH10886), National Institutes of Health, and New York State Office of Science, Technology and Academic Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.A. is a guest editor invited by the Editorial Board.
Data deposition: All PET and MRI imaging data sets are available via our database upon request at: http://www3.bnl.gov/ifeinstein/viewPET.php.
References
- 1.Lee SJ, Ralston HJ, Drey EA, Partridge JC, Rosen MA. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–954. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]
- 2.Bell RD, Alexander GM, Schwartzman RJ. Methylphenidate decreases local glucose metabolism in the motor cortex. Pharmacol Biochem Behav. 1983;18:1–5. doi: 10.1016/0091-3057(83)90241-1. [DOI] [PubMed] [Google Scholar]
- 3.Breit S, Reimold M, Reischl G, Klockgether T, Wüllner U. [(11)C]d-threo-methylphenidate PET in patients with Parkinson’s disease and essential tremor. J Neural Transm. 2006;113:187–193. doi: 10.1007/s00702-005-0311-7. [DOI] [PubMed] [Google Scholar]
- 4.London ED, et al. Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47:567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- 5.Schreckenberger M, et al. “Ecstasy”-induced changes of cerebral glucose metabolism and their correlation to acute psychopathology. An 18-FDG PET study. Eur J Nucl Med. 1999;26:1572–1579. doi: 10.1007/s002590050497. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154:50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- 7.Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burchfield DJ, Abrams RM. Cocaine depresses cerebral glucose utilization in fetal sheep. Brain Res Dev Brain Res. 1993;73:283–288. doi: 10.1016/0165-3806(93)90148-4. [DOI] [PubMed] [Google Scholar]
- 9.Dow-Edwards DL, Freed-Malen LA, Gerkin LM. Sexual dimorphism in the brain metabolic response to prenatal cocaine exposure. Brain Res Dev Brain Res. 2001;129:73–79. doi: 10.1016/s0165-3806(01)00184-5. [DOI] [PubMed] [Google Scholar]
- 10.Dow-Edwards DL, Freed LA, Milhorat TH. Stimulation of brain metabolism by perinatal cocaine exposure. Brain Res. 1988;470:137–141. doi: 10.1016/0165-3806(88)90209-x. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Bergson C, Howard RL, Lidow MS. Differential expression of D1 and D5 dopamine receptors in the fetal primate cerebral wall. Cereb Cortex. 1997;7:711–721. doi: 10.1093/cercor/7.8.711. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y, Ronnekleiv OK. Cocaine upregulates the dopamine transporter in fetal rhesus monkey brain. J Neurosci. 1999;19:8966–8978. doi: 10.1523/JNEUROSCI.19-20-08966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borisova NA, Sapronova AY, Proshlyakova EV, Ugrumov MV. Ontogenesis of the hypothalamic catecholaminergic system in rats: synthesis, uptake and release of catecholamines. Neuroscience. 1991;43:223–229. doi: 10.1016/0306-4522(91)90429-r. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 16.Benveniste H, et al. Maternal and fetal 11C-cocaine uptake and kinetics measured in vivo by combined PET and MRI in pregnant nonhuman primates. J Nucl Med. 2005;46:312–320. [PubMed] [Google Scholar]
- 17.Sokoloff L. Relation between physiological function and energy metabolism in the central nervous system. J Neurochem. 1977;29:13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, et al. Cocaine doses equivalent to those abused by humans occupy most of the dopamine transporters. Synapse. 1996;24:399–402. doi: 10.1002/(SICI)1098-2396(199612)24:4<399::AID-SYN7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Braunstein GD. In: Williams Textbook of Endocrinology, 11th Edition. Kronenberg H, Melmed S, Polonsky K, Larsen PR, editors. Saunders, Elsevier, Philadelphia, PA; 2008. pp. 741–745. [Google Scholar]
- 20.Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.10.002. Oct 9, 2009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jatlow P. Cocaine: analysis, pharmacokinetics, and metabolic disposition. Yale J Biol Med. 1988;61:105–113. [PMC free article] [PubMed] [Google Scholar]
- 24.Scharf BA, et al. Lethargy, ulcers, bronchopneumonia and death in two aged female bonnet macaques presumed to be caused by Cercopithicine herpes virus I. J Med Primatol. 2008;37(Suppl 1):60–64. doi: 10.1111/j.1600-0684.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 25.Gambhir SS, et al. Simple noninvasive quantification method for measuring myocardial glucose utilization in humans employing positron emission tomography and fluorine-18 deoxyglucose. J Nucl Med. 1989;30:359–366. [PubMed] [Google Scholar]
- 26.Lin KP, et al. Correction of spillover radioactivities for estimation of the blood time-activity curve from the imaged LV chamber in cardiac dynamic FDG PET studies. Phys Med Biol. 1995;40:629–642. doi: 10.1088/0031-9155/40/4/009. [DOI] [PubMed] [Google Scholar]
- 27.Berger R, et al. Extension of the 2-deoxyglucose method to the fetus in utero: theory and normal values for the cerebral glucose consumption in fetal guinea pigs. J Neurochem. 1994;63:271–279. doi: 10.1046/j.1471-4159.1994.63010271.x. [DOI] [PubMed] [Google Scholar]
- 28.Dyve S, Gjedde A. Glucose metabolism of fetal rat brain in utero, measured with labeled deoxyglucose. Acta Neurol Scand. 1991;83:14–19. [PubMed] [Google Scholar]
- 29.He YL, et al. Effects of protein binding on the placental transfer of propofol in the human dually perfused cotyledon in vitro. Br J Anaesth. 2000;85:281–286. doi: 10.1093/bja/85.2.281. [DOI] [PubMed] [Google Scholar]
- 30.Gin T, Yau G, Chan K, Gregory MA, Oh TE. Disposition of propofol infusions for caesarean section. Can J Anaesth. 1991;38:31–36. doi: 10.1007/BF03009160. [DOI] [PubMed] [Google Scholar]
- 31.Volikas I, Butwick A, Wilkinson C, Pleming A, Nicholson G. Maternal and neonatal side-effects of remifentanil patient-controlled analgesia in labour. Br J Anaesth. 2005;95:504–509. doi: 10.1093/bja/aei219. [DOI] [PubMed] [Google Scholar]
- 32.Tsukada H, et al. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res. 1999;849:85–96. doi: 10.1016/s0006-8993(99)02018-1. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt KF, et al. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology (Berl) 2006;185:479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du C, et al. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26:11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benveniste H, et al. PET study; MRI study. Maternal-fetal in vivo imaging: a combined PET and MRI study. J Nucl Med. 2003;44:1522–1530. [PubMed] [Google Scholar]
- 36.Brusati V, et al. Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res. 2005;58:700–704. doi: 10.1203/01.PDR.0000180549.86614.73. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L. Local cerebral glucose utilization in the normal conscious macaque monkey. Ann Neurol. 1978;4:293–301. doi: 10.1002/ana.410040402. [DOI] [PubMed] [Google Scholar]
- 38.Sokoloff L, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 39.Tokugawa J, Ravasi L, Nakayama T, Schmidt KC, Sokoloff L. Operational lumped constant for FDG in normal adult male rats. J Nucl Med. 2007;48:94–99. [PubMed] [Google Scholar]
- 40.Hasselbalch SG, et al. Brain metabolism during short-term starvation in humans. J Cereb Blood Flow Metab. 1994;14:125–131. doi: 10.1038/jcbfm.1994.17. [DOI] [PubMed] [Google Scholar]
- 41.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]