Abstract
The calcium-activated K+ channel KCa3.1 plays an important role in T lymphocyte Ca2+ signaling by helping to maintain a negative membrane potential, which provides an electrochemical gradient to drive Ca2+ influx. To assess the role of KCa3.1 channels in lymphocyte activation in vivo, we studied T cell function in KCa3.1−/− mice. CD4 T helper (i.e., Th0) cells isolated from KCa3.1−/− mice lacked KCa3.1 channel activity, which resulted in decreased T cell receptor–stimulated Ca2+ influx and IL-2 production. Although loss of KCa3.1 did not interfere with CD4 T cell differentiation, both Ca2+ influx and cytokine production were impaired in KCa3.1−/− Th1 and Th2 CD4 T cells, whereas T-regulatory and Th17 function were normal. We found that inhibition of KCa3.1−/− protected mice from developing severe colitis in two mouse models of inflammatory bowel disease, which were induced by (i) the adoptive transfer of mouse naïve CD4 T cells into rag2−/− recipients and (ii) trinitrobenzene sulfonic acid. Pharmacologic inhibitors of KCa3.1 have already been shown to be safe in humans. Thus, if these preclinical studies continue to show efficacy, it may be possible to rapidly test whether KCa3.1 inhibitors are efficacious in patients with inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis.
Keywords: T helper cells, potassium channels, T-cell signaling, inflammatory bowel disease, calcium signaling
Ca2+ influx from the extracellular space is critical for the activation of immune cells, including T lymphocytes. The generation of inositol 1,4,5-triphosphate in response to T cell receptor (TCR) activation results in ER Ca2+ depletion, leading to the oligomerizaton of the ER Ca2+ sensor STIM1 and the subsequent activation of the Ca2+ release–activated channel, resulting in the rapid influx of extracellular Ca2+ (1, 2). This increase in cytosolic Ca2+ results in the assembly of NFAT transcription complexes, which then mediates transcriptional activation of genes critical for T cell activation (3, 4).
One of the consequences of the rapid influx of Ca2+ is the depolarization of the membrane potential which, if left unchecked, limits further Ca2+ influx by removing the favorable electrochemical gradient. Thus, lymphocytes require activation of a K+ channel that, by mediating the efflux of K+, maintains a hyperpolarized membrane potential that is critical for sustained calcium entry into these cells via Ca2+ release–activated channels (5, 6). Several K+ channels exist in the lymphocyte. Whereas resting naïve human T cells and activated CCR7− effector memory T cells predominantly express Kv1.3 (2, 5, 7 –10), resting naïve mouse T cells have been described to express several voltage-gated K+ channels such as Kv1.3, Kv1.1, and Kv1.6 in CD4+ T cells and Kv3.1 in CD8+ T cells (6, 11). Both human and mouse T cells have an additional K+ channel, the calcium-activated channel KCa3.1, which is rapidly up-regulated following T cell activation and is subsequently required for maximal Ca2+ influx and proliferation during the reactivation of naïve and central memory T cells (5, 9, 10). Mouse T effector memory cells differ from similar T cells in humans and rats in up-regulating KCa3.1 in place of Kv1.3.
After antigen stimulation, naïve CD4 T lymphocytes proliferate and differentiate into a number of different subsets of effector T lymphocytes that have distinct functions and produce distinct cytokines (12, 13). T helper (Th)–1 cells are mainly characterized by the production of IFN-γ and play a critical role in delayed type hypersensitivity and in mounting responses to intracellular pathogens. In contrast, Th2 cells secrete the cytokines IL-4, IL-5, and IL-13, which function to stimulate antibody production by B cells and to mediate clearance of extracellular pathogens (14). With regard to autoimmunity, increased Th1 response has been invariably linked to autoimmunity whereas a robust Th2 response has been linked to allergy and atopy (13). More recently, a great deal of attention has been focused on Th17 cells, which have emerged as a third effector CD4 T cell subset that has been shown to function alone or together with Th1 cells to mediate a number of different autoimmune disease (15 –17). There have been only a limited number of studies evaluating the role for K+ channel in these various Th subsets. A previous study demonstrated that Th2 cells express lower KCa3.1 currents than Th1 cells and this difference contributes to differences in calcium signaling (18).
Several previous studies have demonstrated that inhibition of Kv1.3 in T-effector memory CD4 T cells may be a good therapeutic target for autoimmune diseases (8, 19, 20). To gain further insight into KCa3.1’s role in activation of various CD4 T cell subsets, and to identify autoimmune diseases in which interfering with KCa3.1 function may be beneficial, CD4 T cell function was assessed in KCa3.1−/− mice. We show that Th1 and Th2 KCa3.1−/− T cells are markedly defective in TCR-stimulated Ca2+ flux and cytokine production, whereas KCa3.1−/− Th17 and T-regulatory (Treg) function was similar to WT cells. Moreover, studying two different models of murine colitis, we found that genetic deletion and pharmacological blockade of KCa3.1 reduced disease severity suggesting that KCa3.1 may be a target for the treatment of inflammatory bowel disease (IBD).
Results
T and B Cell Development Is Normal in KCa3.1−/− Mice.
KCa3.1−/− mice were born at the expected Mendelian frequency and were phenotypically normal, with the exception of mild splenomegaly in some of the mice as previously reported (21, 22). FACS analysis of spleen, lymph nodes, peripheral blood, and thymus demonstrated that CD4 and CD8 T cells as well as CD19-positive B cells were present in similar amounts in WT and KCa3.1−/− mice (Fig. S1 A and B). The number of CD4+CD25+FOXP3+ T cells was also similar between WT and KCa3.1−/− mice, indicating that Treg development is normal in KCa3.1−/− mice (Fig. S1C).
Defective KCa3.1 Channel Activity, Ca2+ Flux, and Cytokine Production in Th0 Cells from KCa3.1−/− Mice.
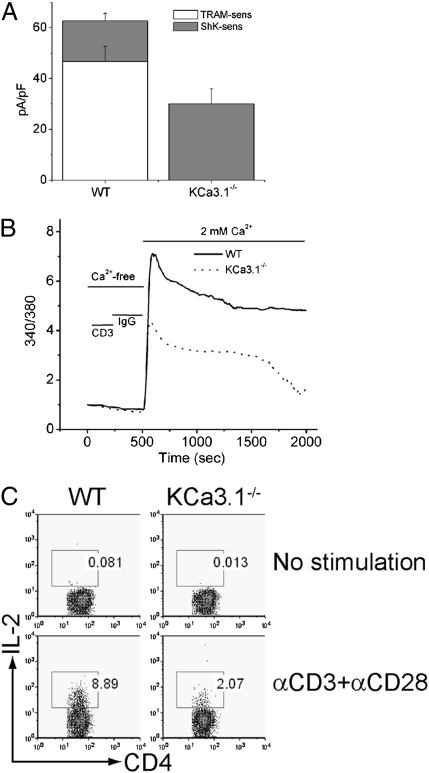
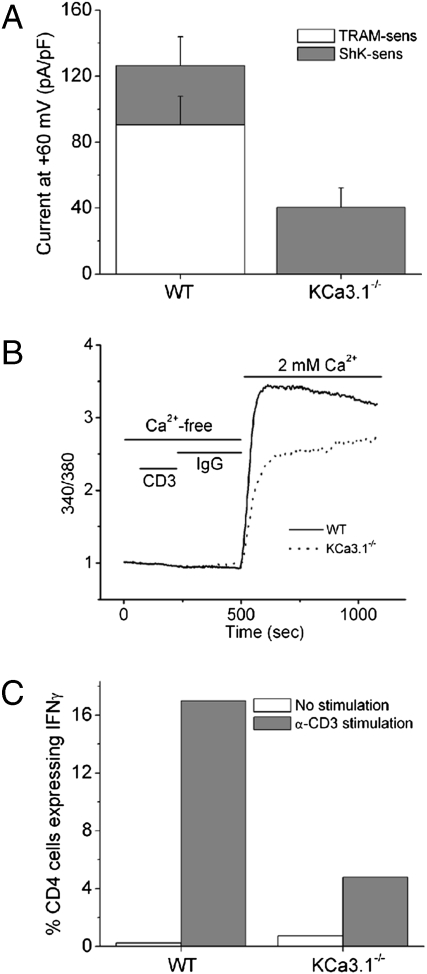
CD4 T cells were isolated from WT and KCa3.1−/− mice, and KCa3.1 channel activity was assessed 48 h after stimulation with anti-CD3 and anti-28 antibodies. In WT activated T cells, approximately two thirds of the K+ channel activity was attributed to KCa3.1 based on the sensitivity of the current to Ca2+ and the specific KCa3.1 blocker TRAM-34 (23). The remaining third of the K+ current was sensitive to ShK, suggesting that it was primarily carried by Kv1.3 and/or Kv1.1 or Kv1.6 (Fig. 1A) (11). KCa3.1 channel activity was not detected in KCa3.1−/− T cells, although there was a statistically significant (P < 0.05) twofold increase in Kv current density in these cells (Fig. 1A). The ShK-insensitive current (possibly Kv1.2) is 9 pAmps/pF ± 1.2 (n = 10 cells) and accounts for approximately 12% of the total K+ current. Nevertheless, total K+ channel activity was still <50% of WT CD4 T cells.
Fig. 1.
Decreased TCR-stimulated Ca2+ flux and cytokine production in KCa3.1−/− Th0 cells. (A) Whole-cell patch-clamp experiments of CD4 T cells isolated from spleens of WT or KCa3.1−/− mice following stimulation for 48 h with anti-CD3, anti-CD28 antibodies. Bar graph summary of n = 10–15 cells. KCa3.1 current was measured as the TRAM-34 (1 μM)–inhibited current and Kv current as the ShK (1 nM)–inhibited current. (B) Cells in A were rested overnight and after loading with Fura-2 AM (5 μM), Ca2+ flux was determined at excitation wavelengths of 340 and 380 nm after cross-linking with anti-CD3 antibodies. (C) Cells in A were rested overnight and after stimulating with anti-CD3 and anti-CD28, IL-2 production was determined by flow cytometry following intracytoplasmic staining with anti–IL-2 antibodies. All these experiments are representative experiments that were repeated at least five times from CD4 T cells isolated from independent mice.
We next tested whether Ca2+ influx was defective in KCa3.1−/− Th0 cells. Stimulation of WT CD4 T cells with anti-CD3 led to a marked increase in Ca2+ influx that was sustained for more than 30 min. In contrast, both the initial increase in Ca2+ influx and the plateau phase of Ca2+ influx were markedly decreased in KCa3.1−/− Th0 cells (Fig. 1B). KCa3.1−/− Th0 CD4 T cells were also defective in anti-CD3/anti-CD28–stimulated IL-2 production (Fig. 1C).
KCa3.1−/− Th1 and Th2 Cells Are Defective in TCR-Stimulated Ca2+ Flux and Cytokine.
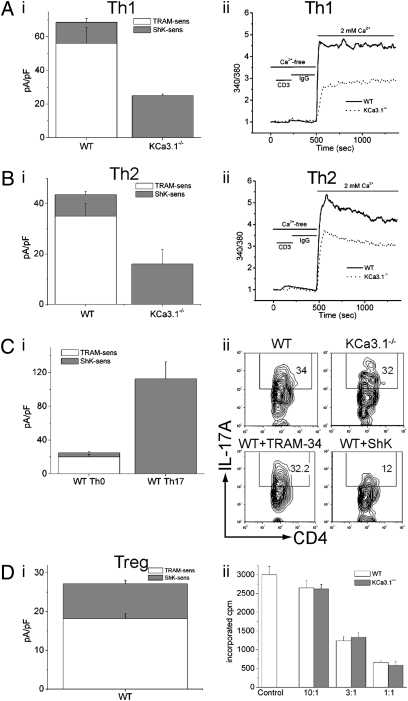
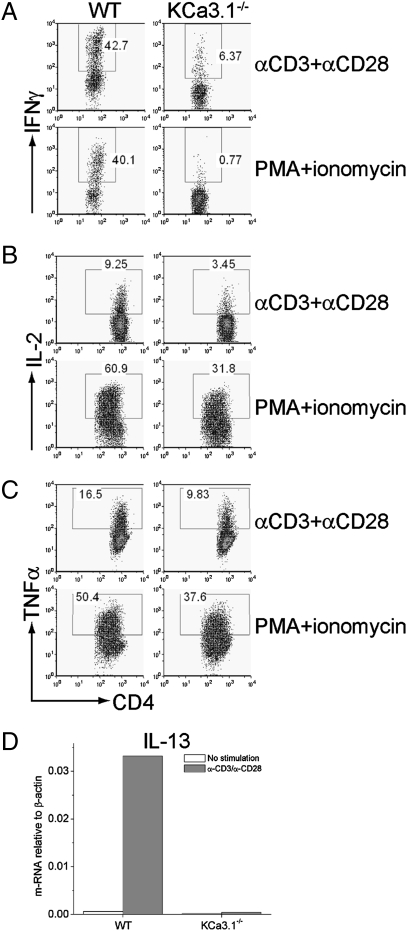
We found that naïve KCa3.1−/− CD4 T cells differentiated normally into Th1 and Th2 cells when exposed to polarizing conditions in vitro as assessed by T-bet and GATA-3 mRNA expression (Fig. S2A). Whole-cell patch-clamp experiments of WT Th1 and Th2 cells demonstrated functional KCa3.1 channels and Th2 cells expressing less KCa3.1 channels than Th1 cells, and these TRAM-34 sensitive channels were absent from KCa3.1−/− Th1 (Fig.2A(i)) and Th2 cells (Fig. 2B(i)). Although Kv current was also upregulated in KCa3.1−/− Th1 and Th2 cells, total K+ channel activity was reduced by more than 50% in KCa3.1−/− cells, which led to impaired peak Ca2+ flux in both cell types (Fig. 2A(ii) and B(ii)). Moreover, the generation of IFN-γ and IL-2 was severely impaired in KCa3.1−/− Th1 cells (Fig. 3 A and B). TNF-α production was also reduced in KCa3.1−/− Th1 cells, although less than the other cytokines (Fig. 3C). In addition, production of the Th2 cytokine IL-13 was markedly impaired in KCa3.1−/− Th2 cells (Fig. 3D).
Fig. 2.
Impaired activation of KCa3.1−/− Th1 and Th2, but not Th17 or Treg CD4 T cells. Whole-cell patch-clamp experiments were performed on WT and KCa3.1−/− (A(i)) Th1 and (B(i)) Th2 cells as described in figure 1A (n = 10–12 cells). Th1 and Th2 cells were rested overnight, and anti-CD3-stimulated Ca2+ flux was assessed in Th1 (A(ii)) and Th2 (B(ii)) as described in figure 1B. (C) (i) Whole-cell patch-clamp data (n = 15 cells) from WT Th17 cells (for details refer to SI Materials and Methods). (ii) Cells were differentiated into Th17, and IL-17 expression was assessed by intracellular staining after stimulation with PMA and ionomycin. Expression of IL-17 in WT cells was also assessed in the presence of the KCa3.1 inhibitor TRAM-34, or the Kv inhibitor ShK. (D) (i) CD4+CD25hi Treg cells isolated from WT spleens were subject to whole-cell patch-clamp experiments (n = 10 cells). (ii) Suppression Assay: CD4+CD25- WT effector cells were co-cultured with either WT or KCa3.1−/− CD4+CD25+ suppressor Tregs at an effector:suppressor ratio of 10:1, 3:1, or 1:1 and proliferation was determined by assessing uptake of [3H]thymidine.
Fig. 3.
Decreased cytokine production by KCa3.1−/− Th1 and Th2 cells. WT and KCa3.1−/− Th1 cells were stimulated with anti-CD3 and anti-CD28 or PMA and ionomycin, and IFN-γ (A), IL-2 (B), and TNF(C) were assessed by flow cytometry following intracytoplasmic staining with antibodies as indicated. (D) WT and KCa3.1−/− Th2 cells were stimulated with anti-CD3 and anti-CD28, and IL-13 expression was determined 4 h after stimulation by real-time PCR. Shown is the relative amount of IL-13 standardized to β-actin control.
In contrast, Th17 and Treg differentiation (Fig. S1C and Fig. S2B) and function (Fig. 2 C(ii) and D(ii))were normal in cells from KCa3.1−/− mice. The production of IL-17 by Th17 cells was similar between WT and KCa3.1−/− cells (Fig. 2C(ii)). In addition, ShK, but not TRAM-34, inhibits IL-17 production by Th17 cells (Fig. 2C(ii)). Th17 cells express ShK sensitive K+ channels but not KCa3.1 (Fig. 2C(i)), suggesting why Th17 differentiation (Fig. S1C) and function are normal in KCa3.1−/− mice. Although KCa3.1 is expressed in freshly isolated CD4+CD25+ Tregs (Fig. 2D(i)), KCa3.1−/− CD4+CD25+ Tregs suppressed the in vitro proliferation of WT CD4+CD25– effector cells to a similar extent as WT CD4+CD25+ cells (Fig. 2D(ii)).
Decreased Function of KCa3.1 in CD4 T Cells Protects Mice from Developing Severe Colitis in Two Murine Models.
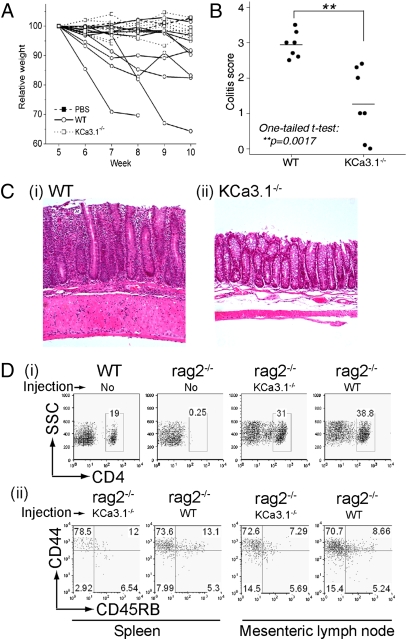
Th1 cells have been shown to contribute to the development of colitis in a number of different mouse models (14, 24, 25). We used the adoptive transfer model of colitis in which either WT or KCa3.1−/− naïve CD4+CD25−CD45RBhi T cells were transferred into a rag2−/− host (26). This model of colitis is characterized by mucosal infiltration of immune cells associated with chronic diarrhea and weight loss. rag2−/− mice injected with WT T cells lost more weight than mice that received KCa3.1−/− T cells (Fig. 4A), suggesting KCa3.1−/− T cells induce less severe disease. This was confirmed on histological evaluation of colons that demonstrated significantly decreased inflammatory cell infiltration and tissue destruction after transfer of KCa3.1−/− T cells compared with WT T cells (Fig. 4 B and C; P = 0.0017 by one-tailed t test).
Fig. 4.
Decreased severity of colitis induced by KCa3.1−/− CD4+CD25-CD45RBhi donor T cells. WT or KCa3.1−/− CD4+CD25-CD45RBhi were transferred into rag2−/− mice, and weight (A) and histologic score (B) on 7 pairs of animals were determined. (C) Example of histology of colon from Rag2−/− mice adoptively transferred WT (i) and KCa3.1−/− (ii) cells. (D) Adoptively transferred WT or KCa3.1−/− T cells were recovered from rag2−/− spleens or mesenteric lymph nodes and stained with antibodies to CD4 (i) or CD44) and CD45RB (ii) followed by FACS analysis. In i and ii, WT/no-injection is from a control C57BL/6 mouse, and rag2−/−/no-injection is from a control rag2−/− mouse.
Spleens and mesenteric lymph nodes from rag2−/− mice reconstituted with KCa3.1−/− or WT T cells contained a similar number of CD4 T cells, indicating that decreased reconstitution of rag2−/− mice by KCa3.1−/− T cells does not explain the difference in disease severity (Fig. 4D(i)). The CD4 T cells isolated from spleens of both KCa3.1−/− and WT reconstituted rag2−/− mice are most consistent with T effector memory as they are CD44hiCD45RBloCD62lo (Fig. 4D(ii) and Fig. S3). These results suggest that decreased severity of disease is a result of impaired activation of KCa3.1−/− Th1 cells.
To directly test whether adoptively transferred KCa3.1−/− cells have impaired TCR-stimulated activation, CD4 T cells were isolated from spleens of reconstituted rag2−/− mice, and KCa3.1 channel activity, TCR-stimulated Ca2+ flux, and cytokine production were assessed. KCa3.1 channels were absent from KCa3.1−/− T cells and total K+ channel activity was approximately one third of WT T cells (Fig. 5A). In addition, TCR-stimulated Ca2+ uptake and production of the Th1 cytokine IFN-γ were impaired in KCa3.1−/− T cells compared with WT T cells (Fig. 5 B and C).
Fig. 5.
KCa3.1−/− cells recovered from rag2−/− mice are defective in TCR-stimulated Ca2+ flux and cytokine production. CD4 T cells recovered from rag2−/− mice underwent whole-cell patch-clamp experiment (A) or stimulation with anti-CD3 to assess Ca2+ flux (B) and IFN-γ production (C) as described in Fig. 1. Shown is a representative experiment from CD4 T cells isolated from three independent reconstituted rag2−/− mice.
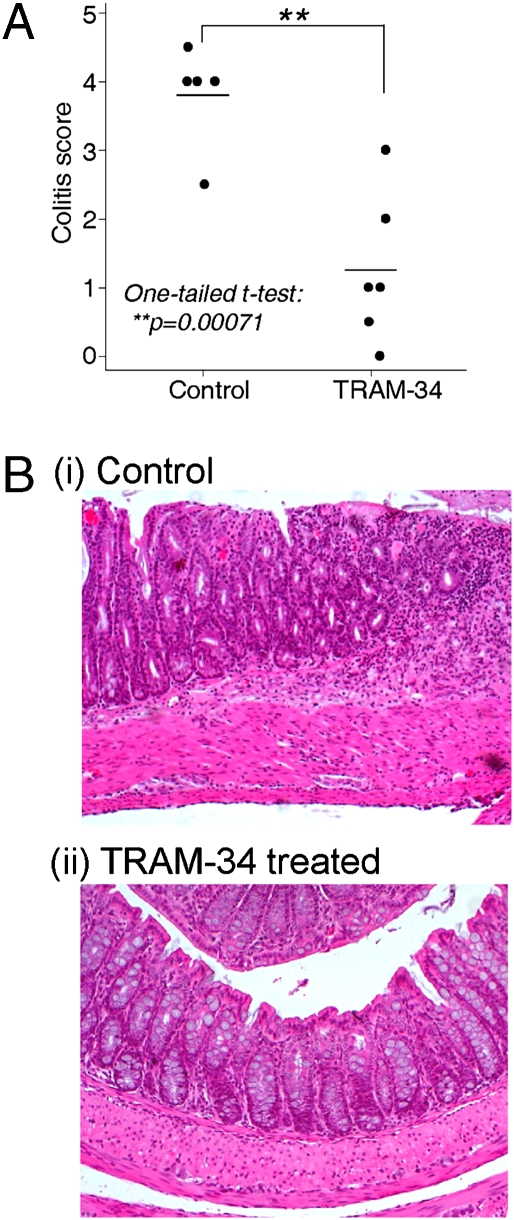
Another well studied colitis model is induced by administration of the hapten trinitrobenzene sulfonic acid (TNBS) in 40% ethanol into the rectum of SJL mice (27). TNBS-induced colitis is at least partially dependent on Th1 cells and has been extensively used to assess the efficacy of therapeutic agents in IBD (27). In contrast to TNBS-treated mice that received the vehicle control, mice treated for 6 d with the selective KCa3.1 inhibitorTRAM-34 (23, 28) had significantly less severe inflammation assessed by macroscopic appearance and histological analysis (Fig. 6; P = 0.00071 by one-tailed t test).
Fig. 6.
TRAM-34, a KCa3.1 inhibitor, inhibits TNBS-induced colitis. TNBS in 40% ethanol was administered rectally to SJL mice that were untreated or treated with TRAM-34, and histological analysis was performed at day 6 after TNBS treatment. (A) Summary of histological analysis of 6 pairs of mice. (B) Example of histological analysis of control (i) or TRAM-34–treated colon(ii).
Discussion
Although previous studies have demonstrated critical roles for KCa3.1 in TCR-stimulated Ca2+ flux and proliferation of preactivated CD4 T cells (5, 10, 19, 29), there have been only limited studies of the role of KCa3.1 in vivo in various mouse CD4 T cell subsets. We now demonstrate that KCa3.1 plays a critical role in TCR-stimulated activation of mouse Th0, Th1, and Th2 cells, whereas Th17 and Treg functions are normal in KCa3.1−/− mice. In addition, inhibition of KCa3.1, either through genetic deletion or by treatment with the KCa3.1 inhibitor TRAM-34, markedly decreased the development of colitis in two mouse models.
KCa3.1 is expressed at low levels in resting naïve CD4 T cells and is transcriptionally activated following TCR stimulation, leading to increased numbers of KCa3.1 channels in activated cells (10, 30, 31). We found that KCa3.1 channel activity is also markedly up-regulated following differentiation into Th1 and Th2 cells, and KCa3.1 accounts for the majority of the K+ current in these cells. KCa3.1 channel activity is then required for full activation of these cells. In contrast, mouse Th17 cells lack KCa3.1 channel activity and are dependent on ShK-sensitive Kv channels for activation.
By determining the CD4 T cell subsets that are dependent on KCa3.1 activity for activation, we hoped to identify possible diseases in which interfering with KCa3.1 function could have therapeutic potential. For example, our finding that Th17 function is normal in KCa3.1−/− mice would suggest that KCa3.1−/− mice should not be protected from Th17-mediated diseases such as experimental allergic encephalitis (17). In fact, we found that KCa3.1−/− mice were not protected from the induction of experimental allergic encephalitis following immunization with MOG35-55 peptide. Conversely, Th1 cells have been shown to be an important mediator of colitis in several mouse models based upon their dependence on IFN-γ, as well as T-bet and IRF-1, both of which are critical for Th1 development (25, 32, 33). More recently, O’Connor et al. provided further evidence that classic Th1 associated cytokines play a critical role in initiation of colitis using an adoptive transfer model of colitis (24). However, IL-23 and IL-17 have also been linked to IBD. Genome-wide association studies have identified several IL-23R variants that are linked to IBD (34), and antibodies to the unique p19 subunit of IL-23 or adoptive transfer of IL-23 mutant cells protects against the disease (35). The finding that IL-23 is also necessary for the maintenance of Th17 cells has suggested that Th17 cells are also important in colitis (36, 37). However, although some studies have demonstrated that interfering with IL-17 is beneficial (14, 35, 38), other studies have found that IL-17 is not important and may even function to inhibit colitis by interfering with the differentiation of effector Th1 cells (14, 24, 26, 39). IL-23 may also indirectly activate Th1 cells by inhibiting differentiation of Treg cells, which are required to constrain Th1 cells (26). Thus, a consensus is emerging that Th1 cells play an important role in the development of colitis in these models, and that IL-23 and possibly IL-17 cooperate in a still incompletely defined way (14, 39).
Based on these observations, together with our finding that activation of Th1 cells is impaired in KCa3.1−/− cells, we tested whether knockout on pharmacologic inhibition of KCa3.1, protected mice from colitis. Using both an adoptive transfer model of colitis as well as the immunogenic hapten TNBS, we found that inhibition of KCa3.1 protected mice from colitis in both these models. Although both the WT and KCa3.1−/− cells recovered from rag2−/− mice displayed a similar memory cell phenotype, KCa3.1−/− cells were defective in both TCR-stimulated Ca2+ flux and cytokine production. These findings indicate that, although KCa3.1−/− cells differentiate normally into memory cells, they require KCa3.1 channel activity for activation in vivo. These findings also reinforce a critical role for Th1 effector cells in these two murine models of colitis, and are consistent with a recent report demonstrating that IL-17 may have more of a protective function by interfering with the generation of Th1 cells (24). Thus, by not inhibiting Treg and Th17 cell function, KCa3.1 blockers may have a unique therapeutic profile to inhibit procolitis-inducing Th1 and Th2 cells, without interfering with the beneficial function of Treg and possibly Th17 cells.
IBD in humans is characterized by a chronic relapsing intestinal inflammatory disorder that is classified into two major forms, Crohn’s disease (CD) and ulcerative colitis (UC). In the past, CD has been considered a Th1-driven disease (40 –42) and has been associated with aberrant activation of Th1 cells leading to high levels of IFN-γ, whereas UC has been classified as a Th2-driven disease that arises from high levels of IL-4 resulting in aberrant expression of IL-13 (40, 43). With the discovery of IL-23 and IL-17, it has become clear that designating these diseases simply as Th1- or Th2-driven was an oversimplification, and in fact interfering with IL-23 has been shown to be beneficial in patients with CD (44). However, it is hard to ignore all the data demonstrating the importance of Th1 and Th2 cells to disease severity in patients with IBD. Our findings reported here suggests that pharmacologic inhibitors of KCa3.1 may be useful to treat patients with IBD. However, it will be important to demonstrate that KCa3.1 channels play a similar role in human CD4 T cells, as a number of differences in choice of K+ channels between mouse and human CD4 T cell subsets have been previously described (6).
Materials and Methods
Cell Purification and Differentiation.
CD4+ T cells were purified on MACS beads (Miltenyi Biotech) from WT or KCa3.1−/− spleens as previously described (26). Various CD4 T cell subsets were generated by culturing CD4 cells purified from spleen cells under Th1 polarizing conditions (100 U/mL IL-12 and anti–IL-4), Th2 polarizing conditions (100 U/mL IL-4, anti–IFN-γ), Th17 polarizing conditions (5 ng/mL TGF-β, 20 ng/mL IL-6, anti-IL-4, anti–IFN-γ), or Treg polarizing conditions (5 ng/mL TGF-β, 50 U/mL IL-2) for 4 to 6 d.
Whole-Cell Patch-Clamp Experiments.
Whole-cell patch-clamping was performed on activated CD4 T cells 48 h after stimulation with anti-CD3 and anti-CD28 antibodies as described (19) with some modification (30).
Intracellular Ca2+ Activity.
Intracellular Ca2+ concentrations were determined as previously reported (29) and detailed in SI Materials and Methods.
Mice.
KCa3.1−/− mice were obtained from J. Melvin (Rochester, NY) and were backcrossed into C57BL/6 as previously described (21). Recombinase-activating gene-deficient 2(rag2−/−) C57BL/6 mice were obtained from Jackson Labs and used at 6 weeks of age.
T Cell Transfer Experiments.
For transfer experiments into rag2−/− mice, a population of CD4+CD25-CD45RBhi T cells were obtained by cell sorting by MoFlo (Dako) after staining WT or KCa3.1−/− spleen cells with anti-CD4, anti-CD25, and anti-CD45RB antibodies (26, 45). CD4+CD25-CD45RBhi WT or KCa3.1−/− T cells (0.4 × 106) were then transferred intraperitoneally into syngeneic rag2−/− mice as previously described (26). Mice were then killed 8 to 12 weeks following transfer when symptoms became apparent in control animals.
TNBS-Induced Colitis.
TNBS (2–3 mg) in 40% ethanol was instilled into the rectum through a 3.5 F catheter in lightly anesthetized SJL mice as previously described (27, 46). To assess whether inhibition of KCa3.1 protects against TNBS-induced colitis, mice were treated s.c. with TRAM-34 (100 mg/kg/d) in peanut oil or vehicle control starting 1 d before TNBS treatment until day 5 as previously described (47).
Histology.
Colons were removed from mice 8 to 12 weeks after T cell reconstitution or 5 to 7 d after TNBS induction and fixed in buffered 10% formalin. Six-micrometer paraffin-embedded sections were cut and stained, with hematoxylin and eosin and graded semiquantitatively from 0 to 5 in a blinded fashion as described in the SI Materials and Methods (48).
FACS Analysis and Intracellular Cytokine Staining.
Cell suspensions were stimulated with anti-CD3 (5 μg/mL) and anti-CD28 (5 μg/mL) or PMA (50 ng/mL) and ionomycin (500 ng/mL) for 4 h in the presence of brefeldin A (20 μg/mL). After stimulation, cells were stained with anti-CD4 antibodies, followed by fixation with a Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences). This was followed by staining with antibodies to IFN-γ, IL-2, or TNF-α.
Quantitative Real-Time PCR.
Total RNA was isolated and reverse-transcribed, and quantitative PCR was then assessed using SYBR Green 1 by iCycler iQ (BioRad) using gene-specific primers purchased from Qiagen.
In Vitro Proliferation and Suppression Assay.
CD4+CD25+ and CD4+CD25− lymphocytes were purified from spleens of WT and KCa3.1−/− mice yielding a >90% CD4+CD25+ Treg population. To assess the effect of the CD4+CD25+ Treg population on cell proliferation, 2 × 104 CD4+CD25− WT effector cells, 2 × 104 mitomycin C–treated spleen cells from TCRα−/−β−/− mice, and anti-CD3 (0.5 μg/mL) were plated in 96-well plates either alone, or together with CD4+CD25+ WT or KCa3.1−/− lymphocytes at an effector:suppressor ratio of 1:1, 3:1, or 10:1 as previously described (45). 3[H]thymidine (1 μCi/well) was added on day 3 and was assessed 6 h later by scintillation counting.
Supplementary Material
Acknowledgments
We thank James Melvin (University of Rochester) for the KCa3.1−/− mice, Vijay Kuchroo and Mohamed Oukka (Harvard Medical School) for the IL-23R GFP reporter mice, and Jun Huh for help in purifying IL-17 cells. E.Y.S. is supported by National Institutes of Health Grants R01 GM084195 and R01 AI052459.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910133107/DCSupplemental.
References
- 1.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 2.Luik RM, Lewis RS. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends Mol Med. 2007;13:103–107. doi: 10.1016/j.molmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 4.Winslow MM, Crabtree GR. Immunology. Decoding calcium signaling. Science. 2005;307:56–57. doi: 10.1126/science.1108163. [DOI] [PubMed] [Google Scholar]
- 5.Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr Opin Drug Discov Devel. 2003;6:640–647. [PubMed] [Google Scholar]
- 6.Beeton C, Chandy KG. Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist. 2005;11:550–562. doi: 10.1177/1073858405278016. [DOI] [PubMed] [Google Scholar]
- 7.Beeton C, et al. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 2005;67:1369–1381. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beeton C, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- 10.Ghanshani S, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 11.Liu QH, et al. Modulation of Kv channel expression and function by TCR and costimulatory signals during peripheral CD4(+) lymphocyte differentiation. J Exp Med. 2002;196:897–909. doi: 10.1084/jem.20020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 13.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 14.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 16.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanger CM, Neben AL, Cahalan MD. Differential Ca2+ influx, KCa channel activity, and Ca2+ clearance distinguish Th1 and Th2 lymphocytes. J Immunol. 2000;164:1153–1160. doi: 10.4049/jimmunol.164.3.1153. [DOI] [PubMed] [Google Scholar]
- 19.Wulff H, et al. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matheu MP, et al. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begenisich T, et al. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel, Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 22.Grgic I, et al. Disruption of the Gardos channel (KCa3.1) in mice causes subtle erythrocyte macrocytosis and progressive splenomegaly. Pflugers Arch. 2009;458:291–302. doi: 10.1007/s00424-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 23.Wulff H, et al. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor W., Jr A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 26.Izcue A, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- 28.Wulff H, Gutman GA, Cahalan MD, Chandy KG. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem. 2001;276:32040–32045. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava S, et al. Histidine phosphorylation of the potassium channel KCa3.1 by nucleoside diphosphate kinase B is required for activation of KCa3.1 and CD4 T cells. Mol Cell. 2006;24:665–675. doi: 10.1016/j.molcel.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava S, et al. Phosphatidylinositol-3 phosphatase myotubularin-related protein 6 negatively regulates CD4 T cells. Mol Cell Biol. 2006;26:5595–5602. doi: 10.1128/MCB.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava S, et al. Protein histidine phosphatase 1 negatively regulates CD4 T cells by inhibiting the K+ channel KCa3.1. Proc Natl Acad Sci USA. 2008;105:14442–14446. doi: 10.1073/pnas.0803678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito H, Fathman CG. CD45RBhigh CD4+ T cells from IFN-gamma knockout mice do not induce wasting disease. J Autoimmun. 1997;10:455–459. doi: 10.1016/s0896-8411(97)90152-9. [DOI] [PubMed] [Google Scholar]
- 33.Neurath MF, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 37.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 38.Leppkes M, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Awasthi A, Kuchroo VK. IL-17A directly inhibits TH1 cells and thereby suppresses development of intestinal inflammation. Nat Immunol. 2009;10:568–570. doi: 10.1038/ni0609-568. [DOI] [PubMed] [Google Scholar]
- 40.Fuss IJ, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 41.Okazawa A, et al. Th1-mediated intestinal inflammation in Crohn’s disease may be induced by activation of lamina propria lymphocytes through synergistic stimulation of interleukin-12 and interleukin-18 without T cell receptor engagement. Am J Gastroenterol. 2002;97:3108–3117. doi: 10.1111/j.1572-0241.2002.07107.x. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka K, et al. T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn’s disease. Gut. 2004;53:1303–1308. doi: 10.1136/gut.2003.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heller F, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Sandborn WJ, et al. Ustekinumab Crohn’s Disease Study Group. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 46.Neurath MF, et al. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996;183:2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toyama K, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.