Abstract
AB-type toxins, like other bacterial toxins, are notably opportunistic molecules. They rely on target cell receptors to reach the appropriate location within the target cell where translocation of their enzymatic subunits occurs. The anthrax toxin, however, times its own uptake, suggesting that toxin binding triggers specific signaling events. Here we show that the anthrax toxin triggers tyrosine phosphorylation of its own receptors, capillary morphogenesis gene 2 and tumor endothelial marker 8, which are not endowed with intrinsic kinase activity. This is required for efficient toxin uptake because endocytosis of the mutant receptor lacking the cytoplasmic tyrosine residues is strongly delayed. Phosphorylation of the receptors was dependent on src-like kinases, which where activated upon toxin binding. Importantly, src-dependent phosphorylation of the receptor was required for its subsequent ubiquitination, which in turn was required for clathrin-mediated endocytosis. Consistently, we found that uptake of the anthrax toxin and processing of the lethal factor substrate MEK1 are inhibited by silencing of src and fyn, as well as in src and fyn knockout cells.
Keywords: endocytosis, CMG2, TEM8, ubiquitination
To exert their virulence, extracellular bacteria often secrete protein toxins that subsequently alter the behavior of target mammalian cells to promote the survival and spreading of the pathogen. Most bacterial toxins contain a domain or subunit that harbors an enzymatic activity, the target of which is generally located in the host-cell cytoplasm (1). Therefore, the enzymatic subunit must be taken up by the cell and subsequently translocated across some intracellular membrane (endosomes or endoplasmic reticulum) to reach the cytoplasm (1). Extensive research over the last decade has shown that toxins evolved to hijack most, if not all, endocytic pathways to enter cells, including clathrin-dependent endocytosis, caveolae, and nonclathrin noncaveolar routes (2). Some of these pathways were actually uncovered through studies on bacterial toxins (3, 4).
The anthrax toxin, one of the two major virulence factors of Bacillus anthracis (5), is composed of three subunits, two of which are enzymes (6, 7). Edema factor (EF) is a calmodulin-dependent adenylate cyclase, whereas lethal factor (LF) is a zinc-dependent metalloprotease that cleaves MAP kinase kinases (8, 9). The third subunit, the protective antigen (PA), is required for cell surface binding of the toxin (10) and for escorting EF and LF to the cytoplasm (11).
More specifically, PA has the capacity to bind to surface receptors. It is produced by the bacterium as an 83-kDa form but requires processing to a 63-kDa form (PA63) either by plasma (12 –14) or cellular proteases (7). PA63 has the ability to oligomerize into heptameric (PA7mer) (15) or octameric (16) rings that act as the receptors for EF and LF (7). The hetero-oligomeric complex is then internalized preferentially via a clathrin-mediated pathway (17) although other routes may be taken (18), possibly depending on the cell type and/or the ligand doses (19). The toxin-receptor complex is then transported to endosomes where the acidic pH triggers membrane insertion of PA7mer—leading to the formation of the PA pore (pPA7mer) as well as to the partial unfolding of EF and LF. These are then translocated through the PA pore across the endosomal membrane.
Two receptors have been identified for the anthrax toxins: tumor endothelial marker 8 (TEM8) (20) and capillary morphogenesis gene 2 (CMG2) (7, 21). Both are type I membrane proteins, bearing an extracellular von Willebrand factor domain involved in PA binding. TEM8 and CMG2 share a high degree of similarity in both their extracellular and cytoplasmic domains (7).
We have previously shown that endocytosis requires toxin-induced ubiquitination of the receptor by the E3 ligase Cbl, a process that is required for uptake (22). Interestingly, although endocytosis of the toxin-bound receptor is rapid, that of the toxin-free receptor is slow (23), indicating that toxin endocytosis is ligand triggered (17). This in turn suggests that toxin binding and possibly heptamerization trigger intracellular signaling events to allow recruitment of the endocytic machinery.
Here we investigate whether tyrosine phosphorylation events control anthrax toxin endocytosis. Because anthrax toxin receptors lack an intrinsic kinase activity, signaling would require the assembly of a transduction machinery encompassing the receptors and other proteins, as observed for integrins (24). We found that PA triggers the activation of src-like kinases (SLKs), which in turn causes phosphorylation of the receptors and their subsequent ubiquitination. This series of events, initiated by active src, is necessary for rapid transport of PA to endosomes and delivery of LF to the cytoplasm.
Results and Discussion
Anthrax Toxin–Triggered Tyrosine Phosphorylation Promotes Uptake.
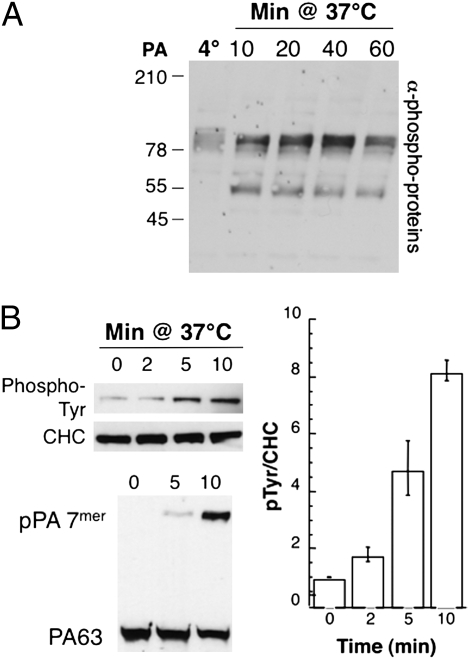
With the aim of understanding the mechanisms underlying PA-triggered endocytosis of the anthrax receptors, we investigated whether PA binding leads to tyrosine phosphorylation events. Extracts of cells, treated or not with PA, were analyzed by Western blotting using an anti-phospho-tyrosine antibody. Although binding of PA to cells at 4 °C did not lead to any signal above background, several tyrosine phosphorylated bands appeared in a PA-dependent manner in cells incubated at 37 °C (Fig. 1A). Interestingly, the kinetics of appearance and decrease differed for the two major bands, with the lower molecular band appearing at earlier times and being more transient.
Fig. 1.
Anthrax toxin triggers tyrosine phosphorylation events. HeLa cells were treated with 500 ng/mL of PA63 for 1 h at 4 °C and incubated for the indicated time at 37 °C. (A) Cell extracts (40 μg of proteins) were analyzed by SDS/PAGE and Western blotting to reveal phospho-tyrosine of total proteins. (B) Immunoprecipitates against clathrin heavy chain (CHC) were analyzed by SDS/PAGE and Western blotting against phospho-tyrosine proteins and CHC, and corresponding total cell extracts were blotted against PA. The plot represents the mean of three independent experiments. Error bars represent standard deviations.
The anthrax toxin enters cells via a clathrin-dependent pathway (17). Moreover, it has been reported that clathrin heavy chain can undergo phosphorylation during ligand-triggered endocytosis (25 –27), possibly to stabilize the clathrin coat. We found that PA can also trigger the phosphorylation of clathrin heavy chain (Fig. 1B) and that this event slightly preceded the appearance of the SDS-resistant PA heptamer (pPA7mer). It is important to note that the PA7mer that forms at the cell surface is initially SDS-sensitive but acquires SDS resistance upon exposure to acidic pH. Acquisition of SDS resistance in the cellular context thus constitutes a convenient readout for the formation, in endosomes, of the transmembrane PA7mer pore (labeled pPA7mer in Figs. 1–5).
Fig. 5.
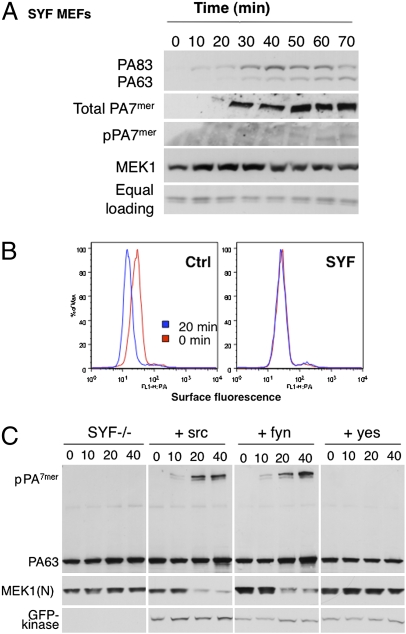
Endocytosis of the anthrax toxin is inhibited in SYF cells. (A) SYF–MEFs, deficient for src, yes, and fyn, were treated with 500 ng/mL of PA63 for 1 h at 4 °C and with 50 ng/mL LF for 1 h at 4 °C and for different times at 37 °C. Cell extracts (40 μg of proteins) were analyzed by SDS/PAGE and Western blotting to reveal PA SDS-resistant heptamer (intracellular), N-terminal part of MEK1, and tubulin as equal loading. Extracts (40 μg of proteins) previously described were treated for 10 min at room temperature with acid buffer and analyzed by SDS/PAGE and Western blotting to reveal all PA heptamers (at the surface and intracellular). (B) MEF cells or SYF–MEF cells were treated with 1 μg/mL of PA63 for 1 h at 4 °C (red) and incubated for 20 min at 37 °C (blue). After three washes at 4 °C with cold PBS, cells were trypsinized at 4 °C and stained for 30 min on ice with anti-PA antibodies, followed by staining for 30 min on ice with secondary fluorescent antibodies, washing in PBS + 1% FCS, and then evaluation on a FACSCalibur. (C) SYF–MEF cells were transfected or not for 48 h with src–GFP, fyn–GFP, or yes–GFP, and treated with 500 ng/mL of PA63 for 1 h at 4 °C and for different times at 37 °C. Cell extracts (40 μg of proteins) were analyzed by SDS/PAGE and Western blotting to reveal a PA SDS-resistant heptamer, PA63, N-terminal part of MEK1 [MEK1(N)], and kinase–GFP.
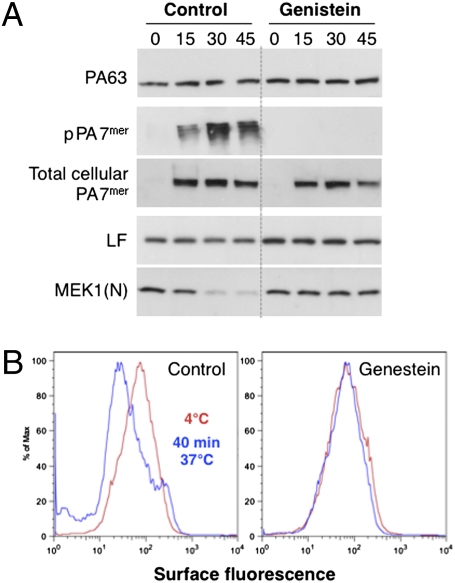
To test the importance of tyrosine phosphorylation events in toxin uptake, we analyzed the effects of the general tyrosine phosphorylation inhibitor genistein. Cleavage of the LF-target MEK1 was not observed in genistein-treated cells within the time frame of the experiment, in contrast to control cells (Fig. 2A), indicating that LF was not delivered to the cytoplasm. This was not due to a defect in binding of PA63 but to the fact that pPA7mer did not form (Fig. 2A). To test whether genistein affected the oligomerization process at the cell surface, we submitted cell extracts to an acid treatment to convert the heptameric PA prepores to an SDS-resistant form (total cellular PA7mer). Oligomerization was not inhibited (Fig. 2A), consistent with the observation that binding of LF—the receptor of which is PA7mer (15)—was not affected. Finally, to monitor endocytosis, we used a FACS assay that measures the disappearance of PA from the cell surface. Cells were treated at 4 °C with PA, subsequently incubated or not at 37 °C, and then incubated on ice with anti-PA antibodies. FACS analysis showed that under control conditions, the amount of surface PA was reduced upon incubation of cells at 37 °C. By contrast, uptake was not observed in genistein-treated cells (Fig. 2B).
Fig. 2.
Genistein treatment delays anthrax toxin endocytosis. HeLa cells were incubated for 2 h at 37 °C or not with genistein, then treated in the presence or absence of genistein with 500 ng/mL of PA63 and 50 ng/mL LF for 1 h at 4 °C, and incubated for different times at 37 °C. Cell extracts (40 μg of proteins) were analyzed by SDS/PAGE and Western blotting to reveal LF and the N-terminal part of MEK1 [MEK1(N)], PA63, and SDS-resistant intracellular heptamer. To measure total cellular heptamers, extracts (40 μg of proteins) were submitted for 10 min at room temperature to an acid treatment, to convert SDS-sensitive PA7mer to an SDS-resistant form detectable by SDS/PAGE and Western blotting. (B) HeLa cells were treated for 2 h at 37 °C or not with genistein. Cells were then treated in the presence or absence of genistein with 1 μg/mL of PA63 for 1 h at 4 °C (red) and incubated for 40 min at 37 °C (blue). After three washes at 4 °C with cold PBS, cells were trypsinized at 4 °C and stained for 30 min on ice with anti-PA antibodies, followed by staining for 30 min on ice with secondary fluorescent antibodies, washing in PBS + 1% FCS, and then evaluation on a FACSCalibur.
Anthrax Toxin Triggers Phosphorylation of Its Receptor.
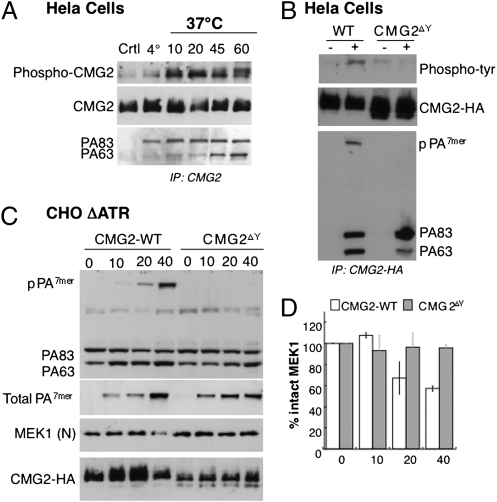
We next tested whether the toxin receptor itself underwent tyrosine phosphorylation. Cells transiently expressing HA-epitope-tagged CMG2 (isoform 4) were treated with toxin for different times and submitted to immunoprecipitation. A low level of phosphorylation of CMG2 was observed in untreated cells (incubated at 4 °C and 37 °C) and in cells treated with PA at 4 °C. Phosphorylation, however, strongly increased upon incubation at 37 °C (Fig. 3A). CMG2 contains four tyrosine residues in its cytoplasmic tail at positions 380, 381, 445, and 463 (Fig. S1A). We generated a quadruple mutant (CMG2ΔY) in which all four tyrosines were changed to alanine. As expected, no tyrosine phosphorylation signal was observed for CMG2ΔY upon toxin treatment (Fig. 3B). More importantly, pPA7mer could not be detected in the immunoprecipitates of the CMG2ΔY mutant (Fig. 3B), suggesting that endocytosis was impaired. To test the importance of the CMG2 cytoplasmic tyrosines in the mode of action of the toxin more directly, we transfected CHO cells defective in anthrax toxin receptors (CHOΔATR) with wild type and mutant CMG2ΔY. The kinetics of the appearance of pPA7mer and of the cleavage of MEK1 by LF were delayed in CMG2ΔY-expressing cells, whereas the oligomerization process at the cells surface was not (Fig. 3C and D), which is consistent with the notion that receptor phosphorylation promotes endocytosis of the toxin.
Fig. 3.
Tyrosine phosphorylation of CMG2 promotes endocytosis of the anthrax toxin. (A) HeLa cells were transfected for 48 h with CMG2-HA, treated or not (Ctrl) with 1 μg/mL of PA83 for 1 h at 4 °C, and incubated for the indicated time at 37 °C. Immunoprecipitates against HA were analyzed by SDS/PAGE and Western blotting against phospho-tyrosine proteins, CMG2-HA, and PA. (B) HeLa cells were transfected 48 h with CMG2-WT-HA or CMG2∆Y-HA mutated on four tyrosines: Y380A, Y381A, Y445A, and Y463A. Cells were treated (+) or not (−) with 1 μg/mL of PA83 for 1 h at 4 °C and for 20 min at 37 °C. Immunoprecipitates against HA were analyzed by SDS/PAGE and Western blotting against phospho-tyrosine proteins, CMG2-HA, and PA. (C) CHOΔATR cells were transfected for 48 h with CMG2-WT-HA or CMG2∆Y-HA mutated on four tyrosines: Y380A, Y381A, Y445A, and Y463A. Cells were then treated with 500 g/mL of PA83 and 50 ng/mL of LF for 1 h at 4 °C and an indicated time at 37 °C. Cell extracts (40 μg of proteins) were analyzed by SDS/PAGE and Western blotting to reveal different forms of PA: PA83, PA63, and the intracellular heptamer, SDS-resistant, N-terminal part of MEK1 and CMG2-HA. Total cellular PA7mer was visualized as in Fig. 2A through acid treatment of cell extracts. (D) MEK1 N-terminal part levels were quantified using the Typhoon scanner and normalized to 100% at time 0 (1 h at 4 °C). The plot represents the mean of three independent experiments described in C. Errors represent standard deviations.
We next investigated whether PA also triggered the phosphorylation of TEM8 isoform 1 (TEM8-1, Fig. S1A). It is worth mentioning that TEM8-1 is the most abundant receptor in HeLa cells, used in Figs. 1 and 2, and in the RNAi silencing experiments described below. Even in the absence of PA, a significant phosphorylation signal was observed for TEM8-1, which was absent as expected for TEM8 isoform 2, which has a much shorter cytoplasmic tail of only 25 residues and lacks tyrosine residues (Fig. S1B). Phosphorylation, however, was enhanced upon PA treatment (Fig. S1B). The four cytoplasmic tyrosine residues present in CMG2 are conserved in TEM8-1 (Fig. S1A). The later protein, however, has an additional tyrosine at position 425 (Fig. S1A). Mutation of Tyr-425 to alanine almost abolished the tyrosine phosphorylation signal observed in the absence of PA, rendering the toxin-induced increase in signal far more apparent (Fig. S1C). Altogether these observations show that PA binding and oligomerization trigger the phosphorylation of both anthrax toxin receptors.
Anthrax Toxin Triggers the Activation of src-like Kinases.
Potential candidates for the phosphorylation of the anthrax receptors are src-like kinases, known to be involved in the endocytosis of various receptors such as the EGF (25), the B-cell (26), and the T-cell (27) receptors. We therefore investigated whether PA triggers activation of SLKs. Phosphorylation of SLKs was not observed upon PA binding at 4 °C but increased, strongly but transiently, upon incubation at 37 °C (Fig. 4A). The kinetics and transient nature of the src phosphorylation, combined with its molecular weight, suggest that the lower phospho-tyrosine band observed in Fig. 1A might correspond to SLKs. Toxin-induced phosphorylation was not observed with a PA mutant that fails to oligomerize—due to mutations in the furin cleavage site (Fig. S2A)—indicating that PA heptamerization is important for src activation.
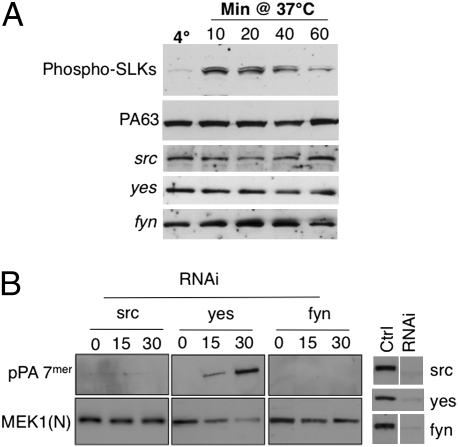
Fig. 4.
PA triggers activation of src-like kinases. (A) HeLa cells were treated with 500 ng/mL of PA63 for 1 h at 4 °C and incubated for the indicated time at 37 °C. Cell extracts (40 μg of proteins) were analyzed by SDS/PAGE and Western blotting to reveal phospho-tyrosine src-like kinases (Tyr-416), PA, src, yes, and fyn. (B) HeLa cells were transfected for 72 h with siRNAs against src, yes, or fyn. The efficiency of siRNas was analyzed on cell extracts (40 μg of proteins) by SDS/PAGE and Western blotting. Cells were incubated with 500 ng/mL PA63 and 50 ng/mL LF for 1 h at 4 °C and for different times at 37 °C, and cell extracts (40 μg of proteins) were analyzed by SDS/PAGE and Western blotting to reveal a PA SDS-resistant heptamer and N-terminal part of MEK1 [MEK1(N)].
To test the importance of src-like kinases, we silenced the expression of the three ubiquitous SLKs expressed in HeLa cells (Fig. 4B): src, yes, and fyn (SYF cells). Silencing was effective for all three kinases (Fig. 4B). Silencing of src, however, also led to a decrease in fyn and vice versa, so these two kinases could not be differentiated. Silencing of src/fyn, but not yes, inhibited the appearance of the pPA7mer and the LF-mediated cleavage of MEK1 (Fig. 4B), but did not affect heptamer formation at the cell surface (Fig. S2B). Silencing of src also inhibited PA-induced phosphorylation of the TEM8-Y425A mutant (Fig. S1C). Silencing of src, however, did not prevent PA-induced phosphorylation of the clathrin heavy chain (Fig. S2C), indicating that PA triggers multiple tyrosine kinase cascades.
These observations prompted us to test the effect of anthrax toxin on mouse embryonic fibroblasts (MEFs) derived from triple knockout mice for src, yes, and fyn (28). MEFs express both TEM8 and CMG2, but toxin sensitivity is mainly due to CMG2 (29). Whereas PA83 was able to bind to SYF cells and undergo processing to PA63 and subsequent oligomerization into PA7mer at the cell surface (Fig. 5A; PA7mer was converted to an SDS-resistant form using acid treatment of cell extracts as seen in Fig. 2), the SDS-resistant endosomal pPA7mer was undetectable, indicating that transport to early endosomes was impaired. This was confirmed by the absence of MEK1 cleavage by LF (Fig. 5A). FACS analysis showed that the first step of endocytosis—uptake from the cell surface—was impaired (Fig. 5B) in agreement with the fact that phosphorylation of CMG2 could not be detected in these cells (Fig. S2D). Recomplementation of the SYF cells with yes had no effect, as predicted by the RNAi gene-silencing experiments. In contrast, endocytosis of the toxin was restored in cells recomplemented with either src or fyn as indicated by the formation of the SDS-resistant pPA7mer and MEK1 cleavage by LF (Fig. 5C). Phosphorylation of CMG2 also was restored (Fig. S2D). Taken together, these observations show that anthrax toxin triggers the activation of SLKs and that the activation of src/fyn is required for rapid toxin uptake.
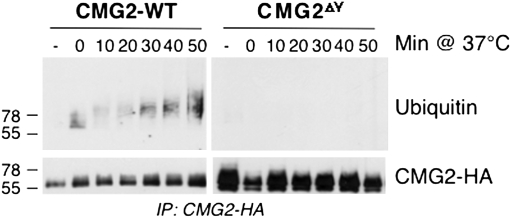
It is well known that phosphorylation creates docking sites for phospho-tyrosine–binding domains on appropriate partner molecules. We have previously shown that PA endocytosis requires ubiquitination of anthrax toxin receptors on cytoplasmic lysines by the E3 ligase Cbl (22). To investigate whether Cbl action is dependent upon phosphorylation of the receptor tail, we analyzed whether ubiquitination also occurred in the phosphorylation-deficient CMG2 mutant. Ubiquitination of CMG2 was strongly diminished in the case of CMG2ΔY when compared to wild type, suggesting that phosphorylation of the receptor tail promotes the recruitment of the E3 ligase (Fig. 6). Note that the kinetics of ubiquitination are slower than that of src activation and receptor phosphorylation, consistent with the sequence of events proposed here.
Fig. 6.
Phosphorylationof CMG2 is required for its ubiquitination. HeLa cells were transfected for 48 h with CMG2-WT-HA or CMG2∆Y-HA mutated on four tyrosines: Y380A, Y381A, Y445A, and Y463A. Cells were then treated with 500 g/mL of PA83 for indicated times at 37 °C. Immunoprecipitations were performed against HA, and the samples were analyzed by Western blotting both against the HA-tagged receptor and against ubiquitin.
Concluding Remarks.
The anthrax toxin receptors are typical representatives of transmembrane proteins present at the cell surface, which undergo relatively slow endocytosis in resting cells, possibly due to their engagement in cell–cell or cell–extracellular matrix interactions. We find that binding of the anthrax protective antigen to the extracellular domain of these proteins and its subsequent oligomerization trigger activation of src/fyn in the cytoplasm and phosphorylation by these kinases of the receptor cytoplasmic tail. This in turn allows receptor ubiquitination by the E3 ligase cbl and internalization of the receptor–toxin complex, leading to uptake and delivery of the enzymatic toxic subunits to the target cell cytoplasm.
Materials and Methods
Cells.
HeLa and anthrax-toxin-receptor–deficient CHO cells were grown as described (22). Wild-type and SYF MEFs, kindly provided by M. Way, were grown in complete DMEM (Gibco) supplemented with 10% FCS and 2 mM L-glutamine, penicillin, and streptomycin.
Toxins, Antibodies, and Reagents.
All toxin subunits and antibodies against them were a gift from S. Leppla. Antibodies against the N-terminal peptide of MEK1 were produced in our laboratory; anti-HA, anti-GFP monoclonals, and anti-HA-agarose–conjugated beads were from Roche; protein G-agarose–conjugated beads from GE Healthcare; the monoclonals anti-src, anti-fyn, and anti-yes from Invitrogen; anti-CHC from Affinity Bioreagents; anti-phospho-src (TYR416) from Cell Signaling; anti-phospho-tyrosine (clone 4G10) from Upstate; anti-ubiquitin (P4D1) from Santa Cruz Biotechnology; HRP secondary antibodies from Pierce; and Alexa-conjugated secondary antibodies from Molecular Probes. Genistein was purchased from Calbiochem and used at a final concentration of 200 μM in medium without serum at 37 °C for 2 h before toxin addition.
Plasmids and Transfection.
Human TEM8-1-HA was cloned in pIREShyg2 as described (22). TEM8-1 GFP was cloned into PHS001 and TEM8-2-GFP in pKB319. The human CMG2 (isoform 4) gene tagged with an HA epitope in the C terminus was cloned in pIREShyg2 expression vector. The four tyrosines Y380, Y381, Y445, and Y463 of CMG2 and tyrosine 425 of TEM8-1 were mutated to alanine with Quickchange (Stratagene) following the manufacturer's instructions. Plasmids were transfected into HeLa cells for 48 h (2 μg cDNA/9.6 cm2 plate) using Fugene (Roche Diagnostics).
RNAi Experiments.
Validated siRNA of human src (SI02664151), human yes (SI00302218) and human fyn (SI02659545) were purchased from Qiagen. For the control siRNA, we used the following target sequence of the viral glycoprotein VSV-G: attgaacaaacgaaacaagga. For gene silencing, HeLa cells were transfected for 72 h with 100 pmol/9.2 cm2 dish of siRNA using oligofectamine (Invitrogen) transfection reagent.
Total Cell Extracts and Western Blot Analysis.
HeLa cells were harvested, washed with PBS, and homogenized by passage through a 22G injection needle in HB (HB: 2.9 mM imidazole and 250 mM sucrose, pH 7.4) containing a phosphatase inhibitor mixture (Sigma) and a mini tablet protease inhibitor mixture (Roche). Protein quantification, Western blotting, and immunoprecipitations were performed as described (22). To convert surface PA7mer to an SDS-resistant form, cell extracts were incubated at room temperature for 10 min with 145 mM NaCl and 20 mM Mes–Tris, pH 4.5.
Immunoprecipitation.
For immunoprecipitations, cells were lysed 30 min at 4 °C in IP buffer (0.5%Nonidet P-40, 500 mM Tris-HCl pH 7.4, 20 mM EDTA, 10 mM NaF, 2 mM benzamidine, and a mixture of protease inhibitors, Roche), centrifuged 3 min at 2000 g and supernatants were precleared with protein G-agarose conjugated beads and supernatants were incubated 16 h at 4 °C with antibodies and beads.
Flow Cytometric Analysis.
HeLa cells were incubated for 1 h at 4 °C with 1 μg/mL PA63, washed, and incubated at different times at 37 °C and then washed at 4 °C and incubated for 5 min on ice with cold trypsin. Loosely attached cells were harvested by pipetting and stained for 30 min on ice with anti-PA antibodies, followed by staining for 30 min on ice with secondary fluorescent antibodies, washing in PBS + 1% FCS, and evaluation on a FACSCalibur (Becton Dickinson). FACS data were analyzed using FlowJo software (FlowJo).
Supplementary Material
Acknowledgments
We thank S. Leppla for the anthrax toxin, TEM8 cDNA plasmids, and anti-PA antibodies and Michael Way for the SYF cells as well as for the GFP-tagged constructs of src, yes, and fyn. This work was supported by the Swiss National Science Foundation and the Fondation Leenaards. F.G.v.d.G. is an international Fellow of the Howard Hughes Medical Institute and a member of the Endocyte Marie Curie training network.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910782107/DCSupplemental.
References
- 1.Schiavo G, van der Goot FG. The bacterial toxin toolkit. Nat Rev Mol Cell Biol. 2001;2:530–537. doi: 10.1038/35080089. [DOI] [PubMed] [Google Scholar]
- 2.Sandvig K, van deurs B. Delivery into cells: Lessons learned from plant and bacterial toxins. Gene Ther. 2005;12:865–872. doi: 10.1038/sj.gt.3302525. [DOI] [PubMed] [Google Scholar]
- 3.Sandvig K, van Deurs B. Transport of protein toxins into cells: Pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002;529:49–53. doi: 10.1016/s0014-5793(02)03182-4. [DOI] [PubMed] [Google Scholar]
- 4.Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7:495–504. doi: 10.1038/nrm1959. [DOI] [PubMed] [Google Scholar]
- 5.Frankel AE, et al. Pathophysiology of anthrax. Front Biosci. 2009;14:4516–4524. doi: 10.2741/3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young JA, Collier RJ. Anthrax toxin: Receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 7.van der Goot FG, Young JA. Receptors of anthrax toxin and cell entry. Mol Aspects Med. 2009;6:406–412. doi: 10.1016/j.mam.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duesbery NS, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 9.Vitale G, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 10.Scobie HM, Young JA. Interactions between anthrax toxin receptors and protective antigen. Curr Opin Microbiol. 2005;8:106–112. doi: 10.1016/j.mib.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Puhar A, Montecucco C. Where and how do anthrax toxins exit endosomes to intoxicate host cells? Trends Microbiol. 2007;15:477–482. doi: 10.1016/j.tim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Ezzell JW, Jr, Abshire TG. Serum protease cleavage of Bacillus anthracis protective antigen. J Gen Microbiol. 1992;138:543–549. doi: 10.1099/00221287-138-3-543. [DOI] [PubMed] [Google Scholar]
- 13.Moayeri M, Wiggins JF, Leppla SH. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect Immun. 2007;75:5175–5184. doi: 10.1128/IAI.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman DL, Zeng WY, Rivera J, Nakouzzi A, Casadevall A. Human serum contains a protease that protects against cytotoxic activity of Bacillus anthracis lethal toxin in vitro. Clin Vaccine Immunol. 2008;15:970–973. doi: 10.1128/CVI.00064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogridge J, Cunningham K, Collier RJ. Stoichiometry of anthrax toxin complexes. Biochemistry. 2002;41:1079–1082. doi: 10.1021/bi015860m. [DOI] [PubMed] [Google Scholar]
- 16.Kintzer AF, et al. The protective antigen component of anthrax toxin forms functional octameric complexes. J Mol Biol. 2009;392:614–629. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boll W, Ehrlich M, Collier RJ, Kirchhausen T. Effects of dynamin inactivation on pathways of anthrax toxin uptake. Eur J Cell Biol. 2004;83:281–288. doi: 10.1078/0171-9335-00373. [DOI] [PubMed] [Google Scholar]
- 19.Sigismund S, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 21.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beauregard KE, Collier RJ, Swanson JA. Proteolytic activation of receptor-bound anthrax protective antigen on macrophages promotes its internalization. Cell Microbiol. 2000;2:251–258. doi: 10.1046/j.1462-5822.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 24.Legate KR, Wickström SA, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 25.Wilde A, et al. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 26.Stoddart A, et al. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–462. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- 27.Crotzer VL, Mabardy AS, Weiss A, Brodsky FM. T cell receptor engagement leads to phosphorylation of clathrin heavy chain during receptor internalization. J Exp Med. 2004;199:981–991. doi: 10.1084/jem.20031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newsome TP, Weisswange I, Frischknecht F, Way M. Abl collaborates with Src family kinases to stimulate actin-based motility of vaccinia virus. Cell Microbiol. 2006;8:233–241. doi: 10.1111/j.1462-5822.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, et al. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci USA. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.