Abstract
Oncolytic viruses constitute a promising therapy against malignant gliomas (MGs). However, virus-induced type I IFN greatly limits its clinical application. The kinase mammalian target of rapamycin (mTOR) stimulates type I IFN production via phosphorylation of its effector proteins, 4E-BPs and S6Ks. Here we show that mouse embryonic fibroblasts and mice lacking S6K1 and S6K2 are more susceptible to vesicular stomatitis virus (VSV) infection than their WT counterparts as a result of an impaired type I IFN response. We used this knowledge to employ a pharmacoviral approach to treat MGs. The highly specific inhibitor of mTOR rapamycin, in combination with an IFN-sensitive VSV-mutant strain (VSVΔM51), dramatically increased the survival of immunocompetent rats bearing MGs. More importantly, VSVΔM51 selectively killed tumor, but not normal cells, in MG-bearing rats treated with rapamycin. These results demonstrate that reducing type I IFNs through inhibition of mTORC1 is an effective strategy to augment the therapeutic activity of VSVΔM51.
Keywords: innate antiviral immunity, malignant gliomas, mTORC1, oncolytic viruses
Malignant gliomas (MGs) are by far the most frequent, aggressive, and lethal primary brain tumor variants (1, 2). Patients with MGs have a median survival time of approximately 1 y and respond poorly to most available therapeutic modalities (3 –5). Thus, more effective treatments are needed.
Recent evidence implicates the PI3K/mammalian target of rapamycin (mTOR) signaling pathway as one of the main oncogenic signaling pathways whose deregulation may underlie gliomagenesis (6, 7). mTOR exists in two complexes: mTOR complex 1 (mTORC1), which is sensitive to the drug rapamycin and regulates mRNA translation, and mTORC2, which is rapamycin-insensitive and regulates the organization of the actin cytoskeleton (reviewed in refs. 8 –10). mTORC1 stimulates type I IFN production via phosphorylation of its target proteins 4E-BPs and S6K1/2 (11). Evidence for the critical role of the mTORC1 signaling pathway in innate immunity emerged from the findings that the mTORC1 inhibitor rapamycin suppresses type I IFN in plasmacytoid dendritic cells (pDCs), which are the major producers of systemic type I IFN (12). In addition, genetic deletion of the mTOR downstream target S6K1/2 leads to impaired type I IFN response (see Results). In contrast, we recently found that the lack of the translational repressors 4E-BP1/2 leads to enhanced type I IFN production (13).
Oncogenic transformation is associated with a deficient type I IFN response, which constitutes the first line of defense against virus infection (14, 15). Oncolytic viruses are studied as effective anticancer agents because they exploit this selective defect (16 –19). One of the best characterized oncolytic viruses, whose replication is extremely sensitive to the inhibition by IFN, is vesicular stomatitis virus (VSV) (20). However, there are several reasons that limit the use of oncolytic viruses for the treatment of MGs. First, some MGs exhibit a robust type I IFN response, which may preclude virus oncolysis in vivo and in vitro. Second, oncolytic viruses are extracellular pathogens, thus the immune system impedes their replication and spread, even within the tumor. Finally, MGs are very heterogeneous, which contributes to their therapeutic resistance. As a consequence, MGs tend to evade single-targeted therapeutic approaches designed to inhibit the proliferation and survival of MGs (4). Therefore, a combinatorial approach that suppresses tumor cell survival and at the same time selectively promotes virus replication in malignant cells should lead to more effective tumor treatments and ultimately boost their translation from the laboratory to the clinic. Thus, we developed a “pharmacoviral” approach to treat MGs. Here, we demonstrate that the combination of rapamycin, which specifically silences mTORC1 activity, with VSVΔM51 significantly prolongs the survival of immunocompetent rats bearing malignant gliomas. Furthermore, we determine the precise molecular mechanism underlying this process.
Results
Malignant Glioma Cells Respond to and Produce Type I IFN.
Although VSV typically replicates in human glioma cell lines (21), MGs are thought to elicit a type I IFN response (16, 22), which could be sufficient to impede the intratumoral spread of the virus. Indeed, when pretreated with human IFN-β, both human glioma cell lines and freshly excised glioma cell are protected against VSVΔM51 (an exquisitely IFN sensitive VSV-mutant strain and the prototype for VSV-based oncolytic therapies; Fig. 1 A and B and Fig. S1). In addition, freshly excised glioma cells and human and rat glioma cell lines generate type I IFN, as determined by VSV protection assays and HEK-Blue type I IFN assay (InvivoGen; Fig. 1 C–E). Importantly, the generation of type I IFN was prevented when glioma cell lines were pretreated with rapamycin (Fig. 1F). These data indicate that inhibition of mTORC1 blocks type I IFN production in vitro.
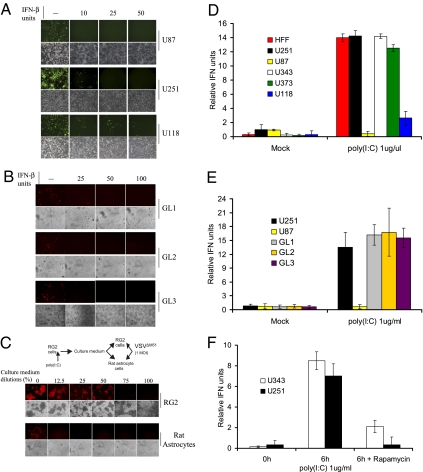
Fig. 1.
Glioma cell lines are responsive to and produce type I IFN. (A) Addition of IFN-β protects glioma cells against VSV infection. The human glioma cell lines U87, U251, and U118 were pretreated with increasing amounts of human IFN-β for 6 h before infection with VSVΔM51-GFP (MOI of 1). At 24 h after infection, GFP fluorescence and CPE were analyzed by phase-contrast and fluorescent microscopy. (B) Freshly excised glioma cells were treated as described and infected with VSVΔM51-RFP (MOI of 10). (C) Poly(I:C) stimulates type I IFN in RG2 cells and conditioned media protect cells against VSV infection. Scheme of the assay: RG2 cells were transfected with poly(I:C) dsRNA (1 μg/mL) for 6 h. Cultured medium was used to treat either RG2 or Rat astrocytes for 12 h before infection with VSVΔM51-RFP (MOI of 1). RFP fluorescence and CPE were analyzed as described. (D) Detection of type I IFN production. Human foreskin fibroblast and the indicated human glioma cell lines were treated with poly(I:C) (1 μg/mL) for 24 h. Supernatant was collected and 30 μL was used to condition HEK-Blue type I IFN cells (InvivoGen). OD was measured by colorimetric assay at 650 nm using the Quanti-Blue reagent (InvivoGen). IFN production was plotted as relative IFN units. (E) Freshly excised glioma cells were treated with poly(I:C) (1 μg/mL) for 24 h and type I IFN production was assessed as in D. (F) Rapamycin reduces the type I IFN response in glioma cells. Human glioma cell lines U251 and U343 were pretreated with DMSO or rapamycin (20 nM) for 1 h before poly(I:C) stimulation at 1 μg/mL, and HEK-Blue type I IFN colorimetric assay was performed 6 h after poly(I:C) stimulation.
Rapamycin Potentiates VSV Oncolysis of Gliomas in Vivo.
In light of these findings, we reasoned that blocking mTORC1 activity in vivo with rapamycin would inhibit systemic type I IFN production and allow the replication of VSVΔM51 in IFN-producing MG. To test this hypothesis, we used a pharmacoviral approach whereby we combined rapamycin with VSVΔM51 in an RG2 immunocompetent rat glioma model (23, 24). The design of the in vivo experiments was based on our previous work using this immunocompetent rat model of MG (23, 25). Briefly, rats were injected intracranially with RG2 cells and subsequently i.p.-treated with either vehicle or rapamycin for 10 d. One day following rapamycin injection, VSVΔM51 was i.v.-administered at a multiplicity of infection (MOI) of 5 × 108 pfu. To determine whether rapamycin blocks systemic type I IFN production, which is induced by VSVΔM51 in vivo, serum was collected 2 d after VSVΔM51 infection, and serially diluted aliquots were added to RG2 cells before their infection with VSVΔM51 at an MOI of 0.1. Strikingly, serum from rapamycin-treated animals was less potent (25 fold) in protecting RG2 cells against VSVΔM51 infection than serum from nontreated animals, as determined by VSVΔM51-RFP fluorescence and cytopathic effect (CPE) (Fig. 2A). In sharp contrast, nontransformed rat astrocytes and fibroblast (Rat1) cells required much lower (5–10 fold) serum concentrations from rapamycin-treated animals to become protected against VSVΔM51 (Fig. S2). These data demonstrate that mTORC1-mediated reduction in type I IFN production protects normal, but not glioma, cells against VSVΔM51-mediated oncolysis. In agreement with the in vitro data, VSVΔM51-GFP was detected in the gliomas (but not in normal brain tissue) from rapamycin-treated animals 3 d after injection, as determined by GFP fluorescence and immunohistochemistry (Fig. 2B; for quantification see Fig. S3). The combination rapamycin-VSVΔM51 greatly increased survival of immunocompetent rats bearing MGs and caused a reduction in tumor size (Fig. 2 D and E and Fig. S3). In addition, in MG-bearing rats treated with rapamycin, the virus exclusively replicated in the tumor, but not in normal cells, as determined by quantitative real-time PCR (Fig. S3D). It is noteworthy that the virus alone failed to improve survival, indicating that the tumor is indeed IFN-competent (Fig. 2E). These data demonstrate that mTORC1-mediated reduction in systemic type I IFN in vivo sensitizes glioma cells, but not normal cells, to virus-mediated oncolysis.
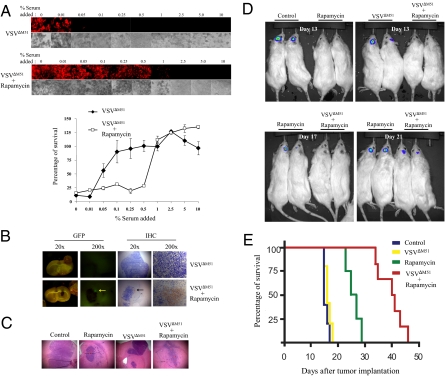
Fig 2.
Rapamycin increases VSVΔM51 oncolysis by reducing type I IFN production. Rapamycin reduces the level of antiviral cytokines that is generated upon VSVΔM51 infection. (A) Rats were treated or untreated with rapamycin (5 mg/kg) 1 d before and every day after i.v. infection with VSVΔM51-GFP (MOI of 5 × 108 pfu per rat). At 48 h after infection, sera were collected and different dilutions were added to RG2 cells for 1 h before infection with VSVΔM51-RFP (MOI of 0.1). At 20 h after infection, RFP fluorescence and CPE were assessed as in Fig. 1A. Remaining cell viability was further confirmed by MTT assay. (B) Presence of VSV in glioma tumors. GFP fluorescence and immunohistochemistry of VSV proteins on brain slices from rats infected with VSVΔM51-GFP (72 h after infection) pretreated or untreated with rapamycin. The combination of VSVΔM51 and rapamycin prolongs the survival and reduces tumor growth of a rat model of malignant glioma. (C) H&E staining of RG2 tumors from the different conditions on d 13 post RG2 intracranial injection. (D) On d 0, rats were intracranially implanted with RG2 rat glioma cells expressing luciferase (RG2-Luc; 1 × 104 cells). On d 6, rapamycin (5 mg/kg) was administered by i.p. injection for 10 d. Control rats received vehicle only. On d 7, VSVΔM51-GFP or PBS solution was administered by i.v. injection (MOI of 5 × 108 pfu). Photograph showing the luciferase expression of RG2-Luc tumors at d 13, d 17, and d 21 from representative rats of each group. (E) Kaplan-Meier survival curve. Control median survival, 15.6 d; VSVΔM51-GFP, 16.4 d; rapamycin, 22 d; VSVΔM51-GFP + rapamycin, 39.8 d. Control and VSVΔM51-GFP log-rank test, P = 0.2402; control and rapamycin log-rank test, P = 0.0038; control and VSVΔM51-GFP plus rapamycin log-rank test, P = 0.0007; rapamycin and VSVΔM51-GFP plus rapamycin log-rank test, P = 0.0011.
Innate Antiviral Response Is Impaired in Mice and Cells Lacking S6K1 and 2.
How does mTORC1 control type I IFN production and VSV-mediated oncolysis? 4E-BPs negatively regulate the production of type I IFN (13). In addition, pDCs lacking the mTORC1 downstream targets, S6K1 and S6K2, failed to generate type I IFN in response to Toll-like receptor stimulation (12). Therefore, it was pertinent to study the role of S6Ks in virus replication. To this end, we used both mouse embryonic fibroblasts (MEFs) and mice deficient in S6K1/2 (S6K1/2 DKO) (26). MEFs were infected at an MOI of 10 pfu per cell and VSV protein synthesis was determined at various times after infection by [35S]methionine pulse labeling (13, 27). VSV proteins were first detected at 6 h after infection in S6K1/2 DKO MEFs (Fig. 3 A and B), whereas in WT MEFs, VSV proteins were only weakly detected by Western blotting at 8 h after infection (Fig. 3B), indicating that S6Ks positively control VSV propagation. Consistent with published reports (28, 29), at early times after infection, WT MEFs were remarkably resistant to low amounts of VSV (MOI of 1), which might be a result of the genetic background of the MEFs (30). However, at higher MOIs (Fig. S4A) or later times after infection (Fig. S4B), VSV proteins were clearly observed in WT MEFs; nonetheless, the kinetics of VSV replication were consistently faster in S6K1/2 DKO MEFs (Fig. S4B). Importantly, introduction of S6K1 and S6K2 into S6K1/2 DKO MEFs rescued the susceptibility of the cells to VSV infection (Fig. S5), demonstrating that the VSV-sensitive phenotype of S6K1/2 DKO MEFs is caused by the lack of S6K1/2. The VSV-induced CPE at 12 h after infection was more pronounced in S6K1/2 DKO MEFs (Fig. 3C), as they generated >100 times more infectious virus particles than WT control MEFs (Fig. 3D). Consistent with these data, virus-induced type I IFN production was impaired in the serum of S6K1/2 DKO mice in vivo (Fig. 3E), as determined by ELISA. Consequently, 40% of the S6K1/2 DKO mice succumbed to VSV infection whereas all WT mice survived (Fig. 3F). In addition, VSV yield was significantly increased (approximately 10-fold) in the lungs of S6K1/2 DKO compared with WT mice (Fig. 3F).
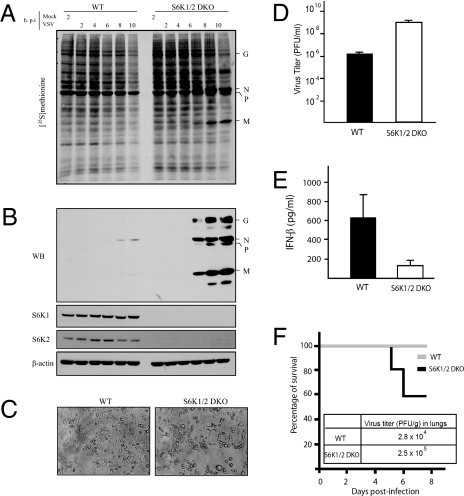
Fig. 3.
MEFs and mice lacking S6K1/2 are sensitive to VSV infection. WT and S6K1/2 DKO MEFs were infected with VSV (MOI of 10) and viral infection was followed up to 10 h after infection. (A) MEFs were incubated with [35S]methionine for 1 h at the indicated times after infection. Proteins were subjected to SDS-PAGE and transferred to a membrane. An autoradiogram is shown. Viral proteins are indicated on the right. G, glycoprotein; M, matrix protein; N/P, nucleocapsid protein/phosphoprotein. The infection was also confirmed by Western blotting analysis using antibodies against VSV proteins, S6K1, S6K2 and β-actin (B), VSV-induced CPE at 12 h after infection (C), and viral titers determined by plaque assay at 30 h after infection at an MOI of 1 (D) (mean ± SD of three independent experiments). (E) WT and S6K1/2 DKO mice (n = 3 per group) were infected with VSV (1 × 108 pfu) and serum was collected at 48 h after infection. IFN-β levels were measured by ELISA. (F) Survival of WT and S6K1/2 DKO infected with VSV. Mice (n = 10) were intranasally infected with VSV (1 × 108 pfu) and their survival was plotted as a Kaplan-Meier curve. Viral titers found in lung tissues 3 d after infection are shown.
To determine whether S6K1/2 DKO MEFs exhibit an impaired type I IFN response, WT and S6K1/2 DKO MEFs were transfected with poly(I:C), a potent inducer of type I IFN (Fig. 4). Type I IFN production was determined by measuring the inhibition of virus replication, VSV-induced CPE, and ELISA (13). Consistent with the enhanced susceptibility of S6K1/2 DKO mice to VSV infection, S6K1/2 DKO MEFs failed to protect cells from VSV infection and produced less type I IFN compared with WT MEFs (Fig. 4 A–E). We next asked whether the VSV-susceptible phenotype in S6K1/2 DKO MEFs could be rescued by the addition of exogenous type I IFN. WT and S6K1/2 DKO MEFs were primed with increasing amounts of exogenous type I IFN (IFN-α/β) and subsequently infected with VSV-GFP. Ten times more exogenous type I IFN was necessary to protect S6K1/2 DKO MEFs against VSV-GFP infection compared with WT MEFs (Fig. 4 F and G). Furthermore, type I IFN gene expression was deficient in MEFs lacking S6K1 and S6K2, as determined by the IFN-promoter reporter assay and the preferential replication of the IFN-hypersensitive VSV strain (VSVΔM51) in these cells (Fig. S6). Collectively, these data demonstrate that type I IFN production is impaired in MEFs and mice lacking S6K1/2.
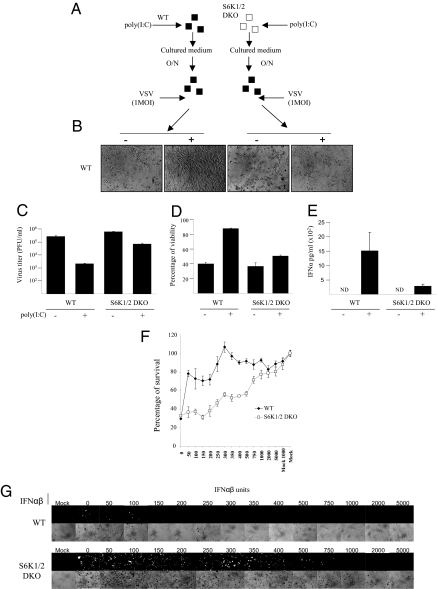
Fig. 4.
MEFs lacking S6K1/2 have an impaired type I IFN response. (A) Scheme of the protection assay experiment: WT and S6K1/2 DKO MEFs were mock treated or treated with 1 μg/mL of synthetic dsRNA poly(I:C) for a period of 6 h. Supernatants from cells were subsequently collected and put onto WT cells overnight. The next day, WT cells were infected with VSV at an MOI of 1 for 36 h. Viral infections were assessed by cytopathic effects (B), virus titers as determined by plaque assays (C), remaining viability as measured by MTT assay (D), and IFN-α production measured by ELISA (E). (Error bars correspond to the mean ± SD of experiments done in triplicate.) Impaired response to exogenous type I IFN in S6K1/2 DKO MEFs. WT and S6K1/2 DKO MEFs were treated with mouse IFN-α/β for 6 h, followed by infection with VSV-GFP (MOI of 1). Infection was monitored at 36 h after infection by MTT assay (F) and GFP fluorescence and CPE (G).
To investigate the molecular mechanisms by which S6K1/2 regulates type I IFN production, we studied the role of the IFN regulatory factor-7 (IRF-7), which is essential for virus-induced type I IFN production (31). Expression of a constitutively active IRF-7 protein protected both WT and S6K1/2 DKO MEFs against VSV infection (Fig. S7). However, WT IRF-7 failed to protect S6K1/DKO MEFs against VSV compared with WT MEFs. These data suggest that activation (i.e., phosphorylation) of IRF-7 by S6K1/2 is a crucial step in the antiviral response. These results are consistent with those recently published by Cao et al. (12), which show that rapamycin prevents IRF-7 phosphorylation in pDCs.
Discussion
Oncolytic viruses are thought to replicate and kill many types of malignant cells in vitro and in vivo as a result of an impaired type I IFN response in cancer cells (16, 18). However, in addition to the fact that many cancer cells produce type I IFN (16, 32), the virus-induced “systemic” type I IFN response also impedes the utility of oncolytic viruses such as VSVΔM51 as efficient therapeutic agents. Thus, the effectiveness of VSVΔM51 oncolytic therapeutic is determined by the combination of two main factors: (i) type I IFN deficiency and (ii) tumor-specific virus replication. Here we report a method that overcomes these obstacles, which combines a drug (rapamycin) with the oncolytic virus VSVΔM51 to treat MGs. At the molecular level, we demonstrated that mTORC1, through its downstream targets 4E-BPs and S6Ks, regulates type I IFN production and virus propagation (13) (Figs. 3 and 4 and Figs. S4–S7). Rapamycin blocks mTORC1-mediated 4E-BPs and S6K1/2 phosphorylation, therefore preventing IRF-7 translation and activation (12, 13). Consistent with these data, rapamycin blocks systemic type I IFN and antiviral cytokines (Fig. 2), most likely through inhibition of mTORC1 activity in pDCs (12). The reduction in the amounts of circulating systemic type I IFN and antiviral cytokines protects normal, but not MG cells, against virus infection. Consequently, the combination of rapamycin and VSVΔM51 not only prolongs survival, but significantly reduces the tumor size of MG-bearing rats (Fig. 2). Although rapamycin in conjunction with other viruses (e.g., myxoma virus, vaccinia virus, or adenovirus) augment oncolysis (23, 33 –36), the molecular mechanism by which this process occurs remained elusive. Autophagy and the antiangiogenic effect induced by rapamycin were postulated to promote viral oncolysis (35, 37). Here we demonstrate that the inhibition of systemic type I IFN production by rapamycin is the primary mechanism by which VSVΔM51 (and most likely the other oncolytic viruses) exhibits superior therapeutic efficacy against MGs compared with VSVΔM51 or rapamycin alone. More important, VSVΔM51 selectively replicates (and kills) in cancer cells but not normal cells from MG-bearing rats treated with rapamycin. In addition, normal cells required much lower amounts of IFN to be completely protected from VSV infection compared with cancer cells (Fig. 1C and Fig. S1–S3). These data indicate that normal cells, but not glioma cells, are able to respond to low levels of IFN and mount an effective antiviral response. Thus, there may be a threshold at which low amounts of IFN causes the protection of only normal cells, and an additional threshold at which too much IFN production protects both normal and cancer cells against virus infection. Taken together, our data raise the interesting possibility that mTORC1 inhibitors in combination with IFN-sensitive oncolytic virus could be used to enhance clinical outcomes in patients with MG in future clinical trials.
Materials and Methods
Cell Lines and Viruses.
The rat glioblastoma cell line RG2, the human glioma cell lines U87, U251, U373, U343, U118, and the human foreskin fibroblast cell line HFF were obtained from the American Type Culture Collection. The rat astrocyte cell line DI TNCI and the fibroblast RAT1 cell line were obtained from T. Hebert (Montreal, QC, Canada) and S. Meloche (Montreal, QC, Canada), respectively. All cell lines were cultured in DMEM plus 10% FCS. Freshly excised glioma cells from patients were obtained with approval from the Montreal Neurological Institute and cultured as described (21). To generate the luciferase-RG2 cell line, the modified firefly luciferase gene plasmid (pGL3 enhancer vector; Promega) was cotransfected into RG2 cells as previously reported (38). Firefly luciferase plasmid was provided by B. Wilson and E. Moriyama (Toronto, ON, Canada). MEFs derived from WT and S6K1/2 DKO mice (26) were immortalized by sequential passaging. The Indiana serotype of VSV, VSVWT-GFP, VSVΔM51-RFP (red fluorescent protein), and VSVΔM51-GFP (green fluorescent protein) have been described (16, 21). Virus titers were determined by a standard plaque assay method (17).
In Vivo Efficacy and Virus Replication Studies in Rodent Orthotropic Glioma Model in Immunocompetent Hosts.
Female Fischer 344 rats under anesthesia (80 mg/kg ketamine and 8 mg/kg xylazine, i.p.) were fixed to a stereotactic apparatus, and 1 × 104 of RG2-Luc cells suspended in 2 to 3 μL of PBS solution were implanted intracranially as described before (23). First, to determine the effect of VSVΔM51 and rapamycin on survival, animals were divided into the following treatment groups 1 d after implantation of RG2 cells: (i) PBS solution control, (ii) rapamycin alone via i.p. administration 1 d before virus injection (rapamycin 5 mg/kg five times a week for a total of 2 weeks), (iii) VSVΔM51 via i.v. administration (5 × 108 pfu in 100 μL PBS solution on d 2 and d 5 for a total of two injections, and (i.v.) VSVΔM51 plus rapamycin (same dose as described previously). Animals in each group were monitored daily and killed when symptoms developed (loss of ≥20% of body weight or difficulty ambulating, feeding, or grooming). Second, to examine viral distribution within the brain tumor, RG2 cells (1 × 104) were implanted into the frontal lobe of the rats as described earlier. Ten days later, animals were grouped and treated as described for the survival study. Rapamycin was administered i.p. with 5 mg/kg/d 1 d before virus administration. Animals were killed at 24 and 72 h after treatment. Animals were anesthetized and perfused with 30 mL of saline solution, followed by 30 mL of phosphate-buffered 10% formalin via a cardiac catheter. The brains were imaged in situ using a stereotactic microscope with a GFP filter, then embedded with ornithine carbamyl transferase and sectioned for immunohistochemistry as described previously (15) or kept frozen for RNA extraction and quantitative real-time PCR analysis.
Mice were infected intranasally with VSV at 1 × 108 pfu and monitored until d 8 for survival or killed 2 to 3 d after infection for serum or tissue collection. Sera were collected by cardiac puncture and lungs were aseptically removed and snap-frozen in liquid nitrogen. Specimens were homogenized in 0.5 mL of PBS solution on ice, and virus titers were determined in BHK21 cells.
VSV RNA Quantification.
Brains were cut in half and the tumors were excised from the half that contained them. The tumors, tumor-free brain, and lungs from rapamycin-treated animals were placed in 1 mL of PBS solution and homogenized with a tissue homogenizer. Homogenized tissue (100–300 μL) was used for the RNA extraction. RNA extraction was performed using the Qiagen RNEasy kit according to the manufacturer’s protocol. Samples were stored at −80 °C. One microgram of RNA was subject to reverse transcription using random primers. Reverse transcriptase was from Invitrogen (SSRT II). Samples were stored at 4 °C. Quantitative PCR was performed on the RotorGene instrument using 1 μL of the RT reaction using SYBR Green as the fluorophore and Platinum Taq Polymerase (Invitrogen) as the polymerase. Primers were specific to VSV-N: forward primer, 5′-GCTGCATTGGCAACATTTGG-3′; reverse primer, 5′-GCTGCATTGGCAACATT TGG-3′. Standard curves were generated using the pT7Blue-3 plasmid with full length VSV-N inserted into it.
In Vivo Bioluminescence Imaging.
Real-time monitoring of tumor growth and treatment response were performed with an IVIS 200 system (Xenogen) to record bioluminescent signal emitted from tumors. On the indicated days after RG2-Luc tumor implantation, rats treated with PBS solution, rapamycin (5 mg/kg i.p. given on d 6 and every following day for 2 weeks), VSVΔM51 (d 7 i.v., 5 × 108 pfu) and VSVΔM51 plus rapamycin (4 rats per group) were imaged. Anesthesia was induced in an induction chamber with 2.5% isoflurane in 100% oxygen at a flow rate of 1 L/min and maintained in the IVIS system with a 1.5% mixture at 0.5 L/min. Rats were i.p. injected with D-luciferin (126 mg/kg; Xenogen) dissolved in PBS solution (15 mg/mL). Subsequently, bioluminescence was measured until the maximum signal was reached or until the signal returned to background level in the pharmacokinetic studies. Data were analyzed based on total photon flux emission (photons/sec) in the region of interest over the intracranial space (39). Imaging results were confirmed using H&E at d 13 after tumor implantation.
Virus Infections and Protection Assays.
Virus infections and metabolic labeling were performed as described (13). The VSV protection assay was performed as followed: WT and S6K1/2 DKO MEFs, or RG2 cells, were either mock treated or transfected with 1 μg/mL of poly(I:C) (Sigma) using FuGENE 6 transfection reagent (Roche) according to the manufacturer’s protocol. Supernatants were collected at 6 h from mock or stimulated cells and were subsequently added to the cells overnight. The next day, cells were infected with VSVΔM51-RFP or VSV at an MOI of 0.1 or 1 for a 24-h period. For the protection assay with the serum experiment, rat sera from control, rapamycin, VSVΔM51, and VSVΔM51/rapamycin were collected 48 h after virus (VSVΔM51-GFP) injection i.v. at an MOI of 5 × 108 pfu. Serial dilutions of the sera were added to RG2, rat astrocyte cells, or Rat1 cells 1 h before their infection with VSVΔM51-RFP at an MOI of 0.1 or 1. Shown are the representative results from two sera obtained from each group. The exogenous IFN protection assay was performed by treating WT and S6K1/2 DKO MEFs, or freshly excised human glioma cells and glioma cell lines with increasing amount of mouse, rat, or human type I IFNα/β (Sigma/Biosource) for 6 h before VSVWT-GFP, VSVΔM51-GFP or VSVΔM51-RFP infection at an MOI of 0.1 or 1. Cell viability was measured at 24 or 36 h after infection by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazoium bromide (MTT) assay as described (25).
ELISA and HEK-Blue Type I IFN Assays.
WT and S6K1/2 DKO MEFs were transfected with poly(I:C) using FuGENE 6 as described earlier. Cultured medium was recovered at 6 h after transfection. Murine IFN-α production was detected in the cultured medium by ELISA according to the manufacturer’s procedure (PBL Biomedical Laboratories). The HEK-Blue type I IFN assay was performed per the manufacturer protocol (InvivoGen). Briefly, human glioma cells plated in 24-well plates were treated with 1 μg/mL of poly(I:C) as described for a period of up to 24 h. For experiments including rapamycin, cells were treated with rapamycin (20 nM) 1 h before poly(I:C) stimulation. Supernatant from mock and stimulated cells was collected and 30 μL were added onto HEK-Blue type I IFN cells (InvivoGen) plated in 96-well plates at 37 °C overnight. The next day, 30 μL of supernatant from HEK-Blue cells was added to 170 μL of Quanti-Blue reagent (InvivoGen) for a period of 30 min at 37 °C. The colorimetric reaction was measured at 650 nm on a plate reader.
Western Blot Analysis.
MEFs were homogenized in RIPA buffer supplemented with a protease inhibitors mixture (Roche), 20 mM B-glycerophosphate, 0.25 mM Na3VO4, 10 mM NaF, and 1 mM PMSF, and then incubated for 30 min at 4 °C. Cell debris was removed by centrifugation at 10,000 × g for 5 min at 4 °C and total protein content was determined using a BioRad assay. S6K1 and phospho-240–244 S6 antibodies were from Cell Signaling and β-actin antibody from Sigma. VSV antibody was as described (13). S6K2 and IRF-7 antibody were purchased from Santa Cruz Biotechnology.
Plasmid Transfection and Luciferase Assay.
The plasmids encoding the ISRE, IFN-β, and NF-κB promoters linked to firefly luciferase (FL) were transfected with a TK-promoter linked to Renilla luciferase (RL; gift from D. Muruve, Calgary, AB, Canada), using Lipofectamine and Plus reagent (Invitrogen) according to the manufacturer’s instructions. Cell extracts were prepared in passive lysis buffer (Promega) 48 h after transfection and assayed for RL and FL activity in a Lumat LB95507 bioluminometer (EG & G Bertold) using a dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions. FL activity was normalized against RL activity, which was used as a transfection control. The plasmids pCAGGS-COOH (empty) and those encoding wt-IRF-7 (pCAGGS IRF7A) and a constitutively active form of IRF-7 (pMSV IRF7-2D) were provided by M. Gale (Seattle, WA). Plasmids were transfected as described for 48 h before infection. For the rescue experiments, pBABE-S6K1, pBABE-S6K2, and empty vector constructs were transfected into phoenix-293-T packaging cells. After 48 h, virus-containing medium was collected, filtered, and used to infect S6K1/2 DKO MEFs in the presence of 5 mg/mL of Polybrene (Sigma). Cells were reinfected the next day and selected with puromycin (5 μg/mL; Sigma) for 5 d before the viral infection experiments.
Supplementary Material
Acknowledgments
We thank M. Gale for the IRF-7 constructs and D. Muruve for the IFN-related promoter reporters, S. Meloche for the Rat1 cell line, T. Hebert for the DI TNC1 cell line, K. Petrecca for the human glioma samples, and C. Lister, A. Sylvestre, and P. Kirk for assistance. This work was supported by funds from the National Cancer Institute of Canada and the Canadian Cancer Society (N.S., J.B. and P.A.F). M.C-M. is a Searle scholar. T.A. holds fellowships from the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research. X.L. is supported by the Kids Cancer Care Foundation of Alberta.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912344107/DCSupplemental.
References
- 1.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 2.Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–1378. doi: 10.4161/cc.7.10.5954. [DOI] [PubMed] [Google Scholar]
- 3.Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. modeling adult gliomas using RCAS/t-va technology. Transl Oncol. 2009;2:89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shai R, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22:4918–4923. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, van den Bent MJ, Hegi ME. Optimal role of temozolomide in the treatment of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5:198–206. doi: 10.1007/s11910-005-0047-7. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous; Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 10.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Costa-Mattioli M, Sonenberg N. RAPping production of type I interferon in pDCs through mTOR. Nat Immunol. 2008;9:1097–1099. doi: 10.1038/ni1008-1097. [DOI] [PubMed] [Google Scholar]
- 12.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colina R, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 14.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 15.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 16.Stojdl DF, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 17.Stojdl DF, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 18.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 19.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belkowski LS, Sen GC. Inhibition of vesicular stomatitis viral mRNA synthesis by interferons. J Virol. 1987;61:653–660. doi: 10.1128/jvi.61.3.653-660.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lun X, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 22.Wollmann G, Robek MD, van den Pol AN. Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells. J Virol. 2007;81:1479–1491. doi: 10.1128/JVI.01861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lun XQ, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 24.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 25.Yang WQ, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 26.Pende M, et al. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa-Mattioli M, Svitkin Y, Sonenberg N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol Cell Biol. 2004;24:6861–6870. doi: 10.1128/MCB.24.15.6861-6870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berlanga JJ, et al. Antiviral effect of the mammalian translation initiation factor 2alpha kinase GCN2 against RNA viruses. EMBO J. 2006;25:1730–1740. doi: 10.1038/sj.emboj.7601073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baltzis D, et al. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2alpha kinases PERK and PKR. J Virol. 2004;78:12747–12761. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durbin RK, Mertz SE, Koromilas AE, Durbin JE. PKR protection against intranasal vesicular stomatitis virus infection is mouse strain dependent. Viral Immunol. 2002;15:41–51. doi: 10.1089/088282402317340224. [DOI] [PubMed] [Google Scholar]
- 31.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 32.Nguyên TL, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci USA. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lun XQ, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonso MM, et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007;14:756–761. doi: 10.1038/sj.cgt.7701067. [DOI] [PubMed] [Google Scholar]
- 35.Alonso MM, et al. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther. 2008;16:487–493. doi: 10.1038/sj.mt.6300400. [DOI] [PubMed] [Google Scholar]
- 36.Stanford MM, et al. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol Ther. 2008;16:52–59. doi: 10.1038/sj.mt.6300348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homicsko K, Lukashev A, Iggo RD. RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res. 2005;65:6882–6890. doi: 10.1158/0008-5472.CAN-05-0309. [DOI] [PubMed] [Google Scholar]
- 38.Moriyama EH, Bisland SK, Lilge L, Wilson BC. Bioluminescence imaging of the response of rat gliosarcoma to ALA-PpIX-mediated photodynamic therapy. Photochem Photobiol. 2004;80:242–249. doi: 10.1562/2004-02-20-RA-088. [DOI] [PubMed] [Google Scholar]
- 39.Szentirmai O, et al. Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts: correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect. Neurosurgery. 2006;58:365–372. doi: 10.1227/01.NEU.0000195114.24819.4F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.