Abstract
MYCN, a proto-oncogene normally expressed in the migrating neural crest, is in its amplified state a key factor in the genesis of human neuroblastoma (NB). However, the mechanisms underlying MYCN-mediated NB progression are poorly understood. Here, we present a MYCN-induced miRNA signature in human NB involving the activation and transrepression of several miRNA genes from paralogous clusters. Several family members derived from the miR-17∼92 cluster, including miR-18a and miR-19a, were among the up-regulated miRNAs. Expression analysis of these miRNAs in NB tumors confirmed increased levels in MYCN-amplified samples. Specifically, we show that miR-18a and miR-19a target and repress the expression of estrogen receptor-α (ESR1), a ligand-inducible transcription factor implicated in neuronal differentiation. Immunohistochemical staining demonstrated ESR1 expression in human fetal sympathetic ganglia, suggesting a role for ESR1 during sympathetic nervous system development. Concordantly, lentiviral restoration of ESR1 in NB cells resulted in growth arrest and neuronal differentiation. Moreover, lentiviral-mediated inhibition of miR-18a in NB cells led to severe growth retardation, outgrowth of varicosity-containing neurites, and induction of neuronal sympathetic differentiation markers. Bioinformatic analyses of microarray data from NB tumors revealed that high ESR1 expression correlates with increased event-free survival in NB patients and favorable disease outcome. Thus, MYCN amplification may disrupt estrogen signaling sensitivity in primitive sympathetic cells through deregulation of ESR1, thereby preventing the normal induction of neuroblast differentiation. Collectively, our findings demonstrate the molecular consequences of abnormal miRNA transcription in a MYCN-driven tumor and offer unique insights into the pathology underlying MYCN-amplified NB.
Keywords: oncogene, embryonic development, pediatric tumor, transcription factor, hormone receptor
Neuroblastoma (NB) represents a remarkably heterogeneous pediatric cancer derived from precursor cells of the sympathetic ganglionic lineage, with clinical behavior ranging from spontaneous regression to rapid progression and death (1, 2). Despite the frequent display of multiple genetic defects, including chromosomal gains and losses, aneuploidy, and amplification of chromosomal material, few established molecular genetic markers associate with disease outcome. Amplification of the MYCN locus, present in ≈20 to 30% of all cases, represents the most important genetic aberration, and is strongly related to poor clinical diagnosis (3). MYCN is expressed in the migrating neural crest and encodes a phosphoprotein that belongs to the Myc network of helix–loop–helix leucine zipper transcriptional regulators, which play key roles in governing cell growth, apoptosis, and differentiation (4). Transcriptional activation is mediated by binding of the Myc/Max dimer to the consensus E-box sequence CA(C/T)GTG in target gene promoters while the mechanism of Myc-mediated transcriptional repression is not fully understood (5). A direct link between MYCN expression and the transformed phenotype has been established in a range of studies, including a transgenic model in which targeted MYCN overexpression in migrating neural crest cells results in NB (2, 6). However, despite considerable progress, NB development linked to MYCN amplification remains to be fully elucidated.
MicroRNAs (miRNAs), small noncoding RNAs that negatively regulate gene expression through sequence-specific base pairing with the 3′-untranslated region (3′-UTR) of cognate mRNA targets (7), have recently been shown to play crucial roles in processes associated with development, differentiation, homeostasis, and cancer (8 –10). Aberrant expression and dysregulation of miRNAs occur in a wide range of neoplasias and their role in tumor initiation, development, and progression is becoming increasingly evident (11, 12). Several studies have reported deregulated miRNA expression in NB, including miRNAs derived from the miR-17∼92 cluster (13). Abnormal regulation of mRNAs identified downstream of NB-associated miRNAs include the Bcl-2 interacting mediator of cell death (BIM), the neurotrophin receptor tropomyosin-related kinase C (trkC), the p21Cip1/Waf1/Sdi1 cyclin-dependent kinase inhibitor (p21), and the potent transcriptional inducer of cell-cycle progression, E2F3 (13 –15). Thus, owing to miRNA misexpression, perturbations in critical pathways linked to proliferation, apoptosis, and cell cycle progression have been shown to play a central role in the development of NB.
In this study, we have investigated molecular mechanisms underlying MYCN-induced NB progression. Our findings show that hsa-miR-18a and hsa-miR-19a (hereafter referred to as miR-18a and miR-19a), from the oncogenic miR-17∼92 cluster, target and subsequently repress the expression of estrogen receptor-α (ESR1). We propose that ESR1 represents a previously undescribed MYCN target in NB and demonstrate a unique oncogenic circuitry in which the repression of ESR1 through MYCN-regulated miRNAs may play a fundamental role in NB tumorigenesis.
Results
Expression Analysis of miRNAs in Tet21N Cells Reveals Specific miRNA Seed-Sequence Signatures.
We used miRCURY Locked Nucleic Acid (LNA) arrays capable of assaying 558 human-specific miRNAs to identify MYCN-regulated miRNAs in Tet21N NB cells with inducible MYCN expression. In doxycycline (Dox)-free medium, Tet21N cells express exogenous MYCN in levels similar to common NB cell lines (16). We observed consistent up-regulation of several MYC-associated miRNAs previously identified (17, 18), including miRNAs from the oncogenic miR-17∼92 cluster (e.g., miR-17, miR-18a, and miR-19a) (19) and its paralogs on chromosome 7 and chromosome X (Fig. 1A, Fig. S1 A, and Table S1). Interestingly, we also noticed a robust down-regulation of miRNAs in Tet21N cells with high MYCN expression (Fig. 1A and Table S1). Sequence comparisons of the up-regulated miRNAs uncovered four sets of seed signatures based on the sequence identity of nucleotides 2 to 8 from the 5′-end of the mature miRNA (Fig. S1B). By Northern blotting, we verified that MYCN overexpression indeed resulted in a differential miRNA expression pattern (Fig. 1B). Analyses of several NB cell lines and tumor specimens revealed that expression levels of miRNAs from the oncogenic miR-17∼92 cluster were consistently higher in MYCN-amplified cell lines and tumors compared to the nonMYCN amplified samples (Fig. 1C and Fig. S1 C and D ). In addition, qPCR analysis for miR-18a and miR-19a showed that there is an overall correlation between all three methods (Fig. S1 E and F).
Fig. 1.
Identification of miRNA expression in Tet21N cells with high MYCN levels. (A) Relative transcription of array-identified miRNAs in Tet21N cells with high versus low MYCN expression (P-value cutoff = P < 0.05). The color code identifies miRNAs bearing the same seed sequence. Asterisks (*) indicate miRNAs (green = miR-18a; red = miR-17; blue = miR-19a; and black = miR-199a-5p) validated using Northern blot in (B). (B) Northern blot analysis of Tet21N cells with high and low MYCN levels showing differential expression levels of miR-17, miR-18a, miR-19a, and miR-199a-5p. Splicesomal U5 snRNA served as a loading control. (C) qPCR analysis of mir-17, miR-18a, and miR19a in MYCN-amplified (red triangles) and nonamplified (blue triangles) primary NB tumors. Results are shown as fold-change compared to the internal control small nucleolar U48 RNA.
MYCN Coordinates Transcriptional Regulation of miRNA Paralogs from Three Distinct Loci.
It was evident from our array analyses that the majority of up-regulated miRNAs originated from three distinct clusters/loci mapping to chromosome 13 (miR-17∼92), chromosome 7 (miR-106b∼25), and chromosome X (miR-106a∼363) (Fig. S1A). Inspection of the genomic regions surrounding the miRNA clusters revealed several putative MYCN E-box binding motifs. Using ChIP assays, we demonstrate that MYCN and Max associate with E-box sequences upstream of all polycistron-derived miRNAs identified (Fig. S2), including the host gene of the miR-106b∼25 cluster, MCM7. Furthermore, acetylation of histone H4 (α-AcH) was markedly enhanced in these regions (Fig. S2), suggesting that these positions represent regions of transcriptionally active chromatin (20).
Transient Knockdown of miR-18a and miR-19a Impedes Cell Proliferation in MYCN-Amplified NB Cells.
Next, we investigated putative target genes of the miRNAs encoded by the identified polycistrons. Because overexpression of the miR-17∼92 cluster strongly augments NB tumorigenesis (13), we focused on miRNAs derived from this locus, thereby narrowing our target predictions. Furthermore, although the oncgenic contribution of miR-17 and miR-20a has been described (13), the biological significance of miR-18a and miR-19a overexpression in NB has not been investigated in detail. We therefore examined the occurrence of Gene Ontology (GO) terms associated with miR-18a and miR-19a target predictions from TargetScanHuman 5.1. The top significant (P < 0.01) target categories were dominated by processes associated with cell cycle-related mechanisms, morphogenesis, and metabolism (Fig. S3A). Notably, the miR-18a target predictions were significanlty enriched with GO categories associated with the cell cycle, cell division, and cell growth. To test our GO analysis experimentally, we targeted miR-18a and miR-19a using LNA knockdown oligonucleotides in MYCN-amplified Kelly cells followed by EdU labeling and subsequent cell cycle analysis by FACS. Treatment with LNA-18a resulted in a robust decline in cell proliferation (7.5 ± 2.9%, P = 0.0087) compared to cells transfected with LNA scramble control (32.8 ± 5.9%) as measured by EdU incorporation (Fig. 2A). Inhibition of miR-19a also suppressed cell proliferation, although to a slightly lesser extent (9.8 ± 4.3%, P = 0.0199). Together, these data suggest that miR-18a and miR-19a overexpression provide MYCN-amplified cells with a proliferative advantage by deregulating messages linked to cell cycle progression.
Fig. 2.
Suppression of proliferation and neuronal differentiation following down-regulation of miR-18 in NB cells. (A) Cell proliferation as measured by EdU incorporation of Kelly cells transfected with LNA-inhibitors for miR-18a (LNA-18a), miR-19a (LNA-19a), or control (LNA-scramble). One representative FACS plot from four independent experiments is shown; the graph is the summary of all four experiments. (B) SK-N-BE(2) cells were transduced with lentiviral constructs expressing scramble or anti-miR-18a. (Upper) Phase contrast images. (Lower) GFP expression for identification of the transduced cells. (C) qPCR analysis of the neuronal differentiation markers SCG10, GAP43, and NPY in SK-N-BE(2) cells transduced with lenti-scramble or lenti-anti-miR-18a.
Stable Knockdown of miR-18a Results in Differentiation of MYCN-Amplified NB Cells.
To analyze the effect of long-term silencing of miR-18a and miR-19a, we transduced SK-N-BE(2) cells with lentiviral vectors encoding specific anti-sense microRNA sequences designed to inhibit their function. At first, we observed that SK-N-BE(2) cells infected with lenti-anti-miR-18a and lenti-anti-miR-19a grew noticeably slower than cells transduced with lenti-scramble control. Interestingly, after prolonged propagation, the cells that expressed anti-miR-18a underwent a noticeable morphological differentiation, manifested by outgrowth of varicosity-containing neurites that terminated in visible growth cones (Fig. 2B). In contrast, no apparent change in morphology was observed in scramble control or in anti-miR-19a transduced cells (Fig. 2B and Fig. S3B). We further observed a dramatic increase (4- to 6-fold) in expression of a set of neuronal sympathetic differentiation markers [superior cervical ganglia-10 (SCG10), growth-associated protein 43 (GAP43) and neuropeptide Y (NPY)] (21) in miR-18a-depleted cells compared with scramble control (Fig. 2C), indicating that miR-18a may represent a unique, key regulator of neuronal differentiation in cells derived from the sympathetic ganglionic lineage.
miR-18a and miR-19a Negatively Regulate ESR1 Expression via miRNA Elements in Its 3′-UTR.
To investigate the mechanisms through which miR-18a and miR-19a induces growth arrest and subsequent differentiation, we examined predicted overlapping target sets of these miRNAs using three algorithms: TargetScanHuman 5.1 (22), PicTar (23), and EIMMo (24). Several interesting genes were predicted to be targets of miR-18a and miR-19a, many of which are involved in neural or cancer-associated processes (Table S2). One gene in particular, ESR1, scored well in all algorithms used and was selected for further analysis. Inspection of the 3′-UTR of ESR1 revealed two potential miR-18a target sites that generate a palindrome-like miR-18 target region composed of two closely positioned 8mer seed-matched sites (Fig. S4A). Moreover, the ESR1 3′-UTR harbors two highly conserved binding sites for miR-19a in the distal 3′-end of its UTR (Fig. 3A). The presence of multiple cluster-related miRNA binding sites indicates that MYCN-dependent regulation of ESR1 can be achieved through the concomitant expression of miR-17∼92-derived miRNAs.
Fig. 3.
miR-18a and miR-19a negatively regulate ESR1 expression via its 3′-UTR. (A) Schematic representation of the human 3′-UTR of ESR1 indicating potential miR-18a and miR-19a binding sites. Triangles indicate possible binding sites for other miRNAs. Asterisks below the ESR1 3′-UTR display predicted poly(A) sites using a support vector machine as described in (43). The evolutionary conservation, shown below the ESR1 3′-UTR, was generated using the UCSC Genome Browser (human genome May 2004 assembly) (B) Histogram indicating the levels of luciferase activity in HEK-293 cells transfected with the miRNAs indicated together with the wild-type 3′-UTR (3′-ESR1-wt) reporter or with a mutant derivative (3′-ESR1-mut). Data shown are means of quintuplicate experiments and error bars represent standard deviations. Asterisks denote significant differences between indicated samples (*P < 0.05; **P < 0.01; *** P < 0.001 Student’s t test for unpaired data). (C) Western blot showing downregulation of ESR1 in MCF-7 cells following transfection with miRNA precursors or scramble control as indicated. Mock denotes untransfected cells and β-actin was used as loading control.
To demonstrate that miR-18a and miR-19a directly regulate ESR1 expression, we transfected a ESR1 3′-UTR (3′-ESR1-wt) luciferase reporter construct (Fig. S4B) together with miR-18a precursors into HEK-293 cells and noticed an ∼60% reduction in luciferase activity compared to cells transfected with the scramble control (Fig. 3B). Conversely, no reduction in luciferase activity was detected with the miR-18a seed-modified ESR1 3′-UTR (3′-ESR1-mut) reporter construct, demonstrating that the introduced target site mutations abolished the ability of miR-18a to regulate the ESR1 3′-UTR in these settings (Fig. 3B). MiR-19a also reduced 3′-ESR1--wt luciferase activity, albeit to a lesser degree (∼40%). Cotransfection of miR-18a and miR-19a precursors also resulted in a strong repression of 3′-ESR1--wt activity, even when the amount of each miRNA precursor was reduced by half (Fig. 3B). Taken together, these data suggest that ESR1 expression may be negatively regulated via both miR-18a and miR-19a 3′-UTR miRNA binding sites.
To assess miRNA-mediated repression of endogenous ESR1, we overexpressed miR-18a and miR-19a precursor molecules in MCF-7 cells. Ectopic expression of miR-18a resulted in a substantial down-regulation (∼90%) of ESR1 protein levels within 24 h of transfection, which was maintained throughout the time-course, indicating that miR-18a binding sites are highly efficient in conferring immediate and long-lasting repression of ESR1 (Fig. 3C). Forced miR-19a expression also resulted in reduced ESR1 levels 24 h posttransfection, although the degree of inhibition was less pronounced when compared to miR-18a (Fig. 3C). Over the next 48 h, ESR1 protein levels continued to drop until reaching a level comparable to miR-18a transfected cells. qPCR analysis demonstrated that the observed dampening of ESR1 protein coincided with a time-dependent ESR1 transcript reduction, indicative of mRNA destabilization, the major mode of repression for highly suppressed miRNA targets (25) (Fig. S4C ).
ESR1 Is Repressed by MYCN-Induced miRNAs and Contributes to Neuronal Differentiation of NB Cells.
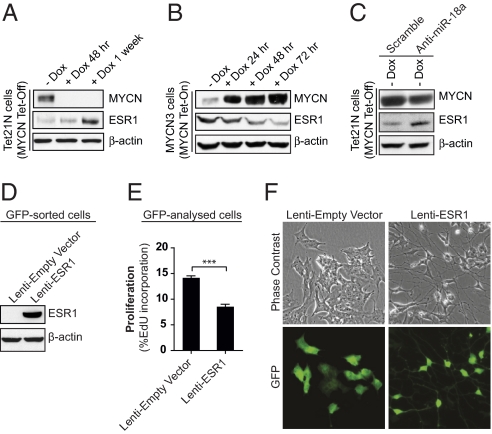
Long-term MYCN repression in Tet21N cells revealed a significant recovery of ESR1 protein over time, demonstrating that reduction of miR-18a and miR-19a expression through the prolonged dampening of MYCN protein levels alleviates translation and protein maturation of ESR1 (Fig. 4A and Fig. S5A). In MYCN3 cells, which contain a doxycycline-inducible (Tet-On) MYCN transgene (Fig. S5B), MYCN induction resulted in ESR1 protein loss over time (Fig. 4B), and was accompanied by significantly increased levels of miR-18a and miR-19a (Fig. S5C). Finally, we analyzed the effect of robust knockdown of miR-18a on ESR1 protein levels in MYCN expressing Tet21N cells. Infection with lentiviral particles encoding anti-miR-18a resulted in ESR1 protein recovery in miR-18a-depleted Tet21N cells, despite high MYCN levels (Fig. 4C). Collectively, our data provide a link between MYCN protein expression and subsequent modulation of an identified downstream target, ESR1, via the regulation miR-17∼92 cluster-derived miRNAs.
Fig. 4.
ESR1 is repressed by MYCN-induced miRNAs and contributes to neural differentiation of NB cells. (A) Expression of ESR1 protein in Tet21N cells following short-term (+Dox 48 h) and long-term (+Dox 1 week) MYCN repression. (B) ESR1 expression following MYCN induction of MYC3 cells during 24, 48, and 72 h. (C) MYCN expressing Tet21N cells (−Dox) were transduced with lenti-scramble or lenti-anti-miR-18a followed by analysis for ESR1. (D) ESR1 expression in SK-N-BE(2) cells transduced with lenti-ESR1 or lenti-empty vector control. (A–D) Western blot analysis, β-actin served as loading control. (E) Cell proliferation (as measured by percent-EdU incorporation) after FACS analysis of lenti-ESR1 and control (lenti-empty vector) transduced cells (asterisks denote significant differences between indicated samples; *** P < 0.001 Student’s t-test for unpaired data). (F) Morphology of SK-N-BE(2) cells stably transduced with lenti-ESR1 or lenti-empty vector.Upper panel shows phase contrast images, while the lower panel shows GFP expression for identification of the transduced cells.
Next, we analyzed the functional role of ESR1 expression in NB cells. Even though it is well-established that ESR1-mediated signaling contributes to proliferation of breast cancer cells (26), it has been reported that ligand-dependent activation of ESR1 in neuronal cells results in growth arrest and differentiation (27). To reaffirm this finding, we used lentiviral constructs expressing ESR1 cDNA and transduced SK-N-BE(2) cells. Western blot analysis of GFP-positive cells confirmed an enhanced expression of ESR1 at the protein level (Fig. 4D), and subsequent analysis of EdU incorporation indicated that re-establishment of ESR1 expression in SK-N-BE(2) cells resulted in a twofold decrease in proliferation (8.5 ± 0.3% EdU incorporation) compared to empty vector control (14.1 ± 0.2% EdU incorporation) (Fig. 4E). After prolonged propagation, we observed a robust ESR1-dependent morphological shift, marked by extensive neurite outgrowth that did not occur in control cells (Fig. 4F). Thus, reintroduction of ESR1 expression in NB cells resulted in a pronounced cell cycle arrest and morphological changes indicative of neuronal differentiation.
ESR1 Is Expressed During Human Fetal Neuronal Development and Is Associated with a Favorable Disease Outcome.
To establish the physiological relevance of ESR1 expression during normal development of the sympathetic nervous system, we stained for ESR1 in abdominal cross-sections from a 9-week-old human fetus. Expression of tyrosine hydroxylase was used as a marker for symphathetic ganglia (21) (Fig. 5A). We could indeed demonstrate nuclear, but also cytoplasmic, ESR1 expression in human fetal sympathetic ganglia (Fig. 5A), indicating that ESR1 has a role during human neuronal development. Furthermore, ESR1 expression analysis of the primary NB tumors used in Fig. 1 demonstrated an inverse relationship between MYCN and ESR1 levels (Fig. S6A). None of the MYCN amplified tumors showed strong ESR1 expression, while the majority of nonamplified tumors expressed moderate to high levels of ESR1. By querying clinical microarray data from several sources, including the GeneSapiens database, we could establish that ESR1 is expressed at low but detectable levels in NB specimens (28–30). Analysis of a NB microarray data set representing 251 children (28), demonstrated a weak but significant negative correlation between MYCN and ESR1 (r = −0.26, P < 0.001, n = 251) an observation that was strengthened when analyzing the MYCN amplified cases only (r = −0.37, P < 0.04, n = 33) (Fig. S6B). Furthermore, Kaplan-Meier survival analysis showed that high expression of ESR1 correlated to an increased event-free survival (P = 0.04, log-rank test, n = 251) (Fig. 5B). This relationship became more apparent when dividing the 251 patients into four quartile groups based on increasing ESR1 expression: patients with low expression of ESR1 (first quartile; blue line) had a significantly impaired event-free survival compared to patients with comparatively high ESR1 expression (fourth quartile; red line) (P = 0.002, log-rank test, n = 251) (Fig. 5B). In light of these data, ESR1 may represent a previously unidentified prognostic marker in MYCN-amplified NB patients.
Fig. 5.
ESR1 is expressed during human fetal neuronal development and correlates to increased event-free survival in NB. (A) Immunohistochemical demonstration of ESR1 protein in developing sympathetic ganglia in an abdominal human fetal (week 9) cross-section. Fetal sympathetic ganglia were identified by their location and by tyrosine hydroxylase (TH) positivity. Adjacent sections were stained with two different dilutions (1:5 and 1:10) of the anti-ESR1 antibody demonstrating cytoplasmic and nuclear staining. An ESR1 positive ductal breast carcinoma in situ (DCIS) was used as a positive staining control (anti-ESR1 antibody dilution 1:50). (B) ESR1 mRNA levels, as analyzed using gene expression microarrays (28) were correlated to event-free survival for a group of 251 NB patients. Patients were divided into two groups based on ESR1 expression levels above (red line) or below (green line) cohort median (Left). The two groups showed a significant difference in event-free survival (P = 0.04, log-rank test). Patients categorized into four equal-size quartile groups based on ESR1 expression showed a significant correlation between increasing ESR1 expression and improved event-free survival (P = 0.002, log-rank test) (Right).
Discussion
The biological impact of deregulated miRNA expression in NB has just begun to emerge (14, 15, 18, 31 –34), reinforcing the importance of miRNA biology in NB-associated tumorigenesis. Here, we describe a MYCN-mediated miRNA signature involving the activation and down-regulation of several miRNA genes from paralogous clusters. In line with previous reports, we show that MYCN transcriptionally activates oncogenic miRNAs from the miR-17∼92 cluster and its paralogs miR-106a∼363 and miR-106b∼25. This preferential selection of miRNAs bearing the same seed sequence suggests that MYCN regulates downstream targets through the coordinated control of specific miRNA families. Importantly, our array data show that activation of MYCN also leads to extensive suppression of miRNAs, several of which have tumor-suppressive like functions (14, 35). The expression of many of these miRNAs was recently reported to increase upon trans-retinoic acid-induced differentiation of the MYCN-amplified cell line SK-N-BE (14). All together, this implies that specific changes in miRNA expression levels may promote tumorigenic behavior of MYCN-amplified NB.
Our findings illustrate that the direct reprogramming of the miRNA transcriptome allows MYCN to modulate additional, previously unidentified downstream targets, such as ESR1. We demonstrate that MYCN activation of miR-18a and miR-19a results in the negative regulation of ESR1 expression. Both of the miR-18a and miR-19a target sites are located within highly conserved genomic regions, suggesting that these sites are under selective pressure to preserve their sequence, and presumably their functionality, across evolution. The presence of multiple cluster-related miRNA binding sites indicates that MYCN-dependent regulation of ESR1 can be achieved through the concomitant expression of miR-17∼92-derived miRNAs. Recently, several c-Myc-regulated miRNAs derived from the miR-17∼92 and the miR-106a∼363 clusters (including miR-18 and miR-19 family members) were shown to negatively regulate ESR1 expression in an estradiol-dependent manner in breast cancer cells (36), reinforcing the notion of a complex genetic circuitry involving Myc proteins, miR-17∼92-derived miRNAs, and ESR1.
Furthermore, we have demonstrated that inhibition of miR-18a and miR-19a expression results in a pronounced cell cycle arrest, suggesting that overexpression of these miRNAs in MYCN-amplified NB may interfere with normal induction of growth arrest and the subsequent onset of differentiation. Elevated levels of miR-18a, and in part miR-19a, may therefore provide MYCN-driven tumors with a proliferative advantage by deregulating messages linked to these processes. The GO categories associated with predicted miR-18a and miR-19a targets showed significant overlap with biological processes that are overrepresented in the Myc target gene network (37), implying that MYCN-responsive miRNAs act to synergise with the biological function of MYCN on a genome-wide level. Notably, we found that stable, long-term inhibition of miR-18a in MYCN amplified NB cells resulted in robust morphological and biochemical differentiation. It is tempting to speculate that miR-18a may be a promising therapeutic target for MYCN-amplified NB tumors. Likewise, the morphological changes observed following the restoration of ESR1 in NB cells together with our Kaplan-Meier survival analysis of 251 NB patients suggest that ESR1-positive NBs may represent a potential subgroup of tumors amenable to estrogen treatment.
To our knowledge, ESR1 represents a unique MYCN target in NB and is one of the key estrogen-receptor subtypes known to mediate the pleiotropic effects, including differentiation, of estrogen during the development and maturation of the nervous system. Our demonstration of ESR1 expression in human fetal sympathetic ganglia suggests that ESR1 plays an important role during the development of the sympathetic nervous system. Furthermore, ESR1 has been reported to induce growth arrest and differentiation of NB cells following estradiol treatment (27). Our data support this finding, as we observed ESR1-mediated neuronal differentiation of SK-N-BE(2) cells. The importance of estrogen and its receptors during neural development is recapitulated in embryonic neuronal stem cells, which undergo differentiation in response to estradiol exposure (38). Clearly, estrogen signaling plays a pivotal role in neuroendocrine biology, which if impaired, may have detrimental consequences for normal developmental processes, including proper maturation and differentiation of progenitor cells derived from the sympathetic nervous system. During human fetal life, the androgenic precursors dehydroepiandrosterone sulfate and 16α-hydroxyde-dehydroepiandrosterone sulfate of fetal and maternal adrenal origin are hydrolyzed in the placenta with the subsequent formation of estrogens (39, 40). Like other steroid hormones, estrogens easily pass through the placental barrier and enter the fetal adrenomedullary circulation (41). As a result, immature sympathetic cell groups that are in sufficient contact with the circulation may undergo differentiation in response to estrogen, given that they express estrogen receptors. Sympathetic neuroblasts are frequently seen in developing human fetal adrenal glands, which have a well-developed network of capillaries, venules, and large veins intended to provide surrounding cells with appropriate environmental cues, such as estrogens. Likewise, the small intensely fluorescent cells that reside in the sympathetic ganglia are closely associated with fenestrated capillaries and may also require molecular signals provided by the fetal circulation to further promote their development (42). Hence, the steroidogenic activity of the fetal adrenal gland can impart differentiation cues necessary for the elements of the sympathetic ganglionic lineage to acquire a fully differentiated phenotype. Aberrant regulation of ESR1 expression in primitive sympathetic cells by MYCN-driven miRNAs may interfere with the normal induction of neuroblast differentiation and thus represents a unique tumorigenic mechanism that may play a fundamental role in NB etiology.
Materials and Methods
Tet21N, a derivative of the SH-EP human NB cell line containing a doxycycline-repressible (Tet-Off) MYCN gene, was cultured as previously described (16). To repress MYCN expression, doxycycline was added at a final concentration of 1 μg mL−1. Chromatin immunoprecipitation assays and Western blots were performed as described (44, 45). Cell culture, miRNA microarray hybridization and data processing, qPCR, Northern blot, luciferase assays, flow cytometry analysis, transfections, immunohistochemistry, gene expression data analysis, lentiviral transductions, as well as antibodies, constructs, and primers are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Schwab and J. Shohet for cells, S. Beckman, E. Johansson, and E. Pivarcsi for excellent technical assistance, L.-G. Larsson for critical reading of the manuscript, M. Corcoran, J.-Å. Gustafsson, C. Williams, and members of our laboratory for stimulating discussions. J.L. and I.M. were supported by Karolinska Institutet and N. Z. by a stipend from the Cancer Research Institute (New York) and Concern Foundation (Los Angeles, CA). M.H. is a recipient of the Senior Investigator Award from the Swedish Cancer Society. This work was supported by funding from the Swedish Childhood Cancer Foundation and the Swedish Cancer Society (S.P and M.H), the Swedish Foundation for Strategic Research (S.P), the Hedlund Foundation and Karolinska Institutet (M.H).
Footnotes
The authors declare no conflict of interest.
Data deposition: Protocols, as well as raw and processed data for the miRNA microarrays, are publicly available at www.ebi.ac.uk/arrayexpress: E-MEXP-2006.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913517107/DCSupplemental.
References
- 1.Edsjö A, Holmquist L, Påhlman S. Neuroblastoma as an experimental model for neuronal differentiation and hypoxia-induced tumor cell dedifferentiation. Semin Cancer Biol. 2007;17:248–256. doi: 10.1016/j.semcancer.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Johnsen JI, Kogner P, Albihn A, Henriksson MA. Embryonal neural tumours and cell death. Apoptosis. 2009;14:424–438. doi: 10.1007/s10495-009-0325-y. [DOI] [PubMed] [Google Scholar]
- 3.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 4.Cole MD, Henriksson M. 25 years of the c-Myc oncogene. Semin Cancer Biol. 2006;16:241–330. doi: 10.1016/j.semcancer.2006.08.003. (Editors) [DOI] [PubMed] [Google Scholar]
- 5.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 6.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 9.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans . Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 10.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 11.Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–6196. doi: 10.1038/sj.onc.1209913. [DOI] [PubMed] [Google Scholar]
- 12.Deng S, Calin GA, Croce CM, Coukos G, Zhang L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle. 2008;7:2643–2646. doi: 10.4161/cc.7.17.6597. [DOI] [PubMed] [Google Scholar]
- 13.Fontana L, et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3:e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laneve P, et al. The interplay between microRNAs and the neurotrophin receptor tropomyosin-related kinase C controls proliferation of human neuroblastoma cells. Proc Natl Acad Sci USA. 2007;104:7957–7962. doi: 10.1073/pnas.0700071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 16.Lutz W, et al. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene. 1996;13:803–812. [PubMed] [Google Scholar]
- 17.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 18.Schulte JH, et al. MYCN regulates oncogenic MicroRNAs in neuroblastoma. Int J Cancer. 2008;122:699–704. doi: 10.1002/ijc.23153. [DOI] [PubMed] [Google Scholar]
- 19.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank SR, Schroeder M, Fernandez P, Taubert S, Amati B. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 2001;15:2069–2082. doi: 10.1101/gad.906601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoehner JC, et al. A developmental model of neuroblastoma: differentiating stroma-poor tumors’ progress along an extra-adrenal chromaffin lineage. Lab Invest. 1996;75:659–675. [PubMed] [Google Scholar]
- 22.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lall S, et al. A genome-wide map of conserved microRNA targets in C. elegans . Curr Biol. 2006;16:460–471. doi: 10.1016/j.cub.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Gaidatzis D, van Nimwegen E, Hausser J, Zavolan M. Inference of miRNA targets using evolutionary conservation and pathway analysis. BMC Bioinformatics. 2007;8:69. doi: 10.1186/1471-2105-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek D, et al. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 27.Ma ZQ, et al. Activated estrogen receptor mediates growth arrest and differentiation of a neuroblastoma cell line. Proc Natl Acad Sci USA. 1993;90:3740–3744. doi: 10.1073/pnas.90.8.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberthuer A, et al. Customized oligonucleotide microarray gene expression-based classification of neuroblastoma patients outperforms current clinical risk stratification. J Clin Oncol. 2006;24:5070–5078. doi: 10.1200/JCO.2006.06.1879. [DOI] [PubMed] [Google Scholar]
- 29.Kilpinen S, et al. Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological tissues. Genome Biol. 2008;9:R139. doi: 10.1186/gb-2008-9-9-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, et al. Integrative genomics identifies distinct molecular classes of neuroblastoma and shows that multiple genes are targeted by regional alterations in DNA copy number. Cancer Res. 2006;66:6050–6062. doi: 10.1158/0008-5472.CAN-05-4618. [DOI] [PubMed] [Google Scholar]
- 31.Afanasyeva EA, Hotz-Wagenblatt A, Glatting KH, Westermann F. New miRNAs cloned from neuroblastoma. BMC Genomics. 2008;9:52. doi: 10.1186/1471-2164-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res. 2007;67:976–983. doi: 10.1158/0008-5472.CAN-06-3667. [DOI] [PubMed] [Google Scholar]
- 33.Cole KA, et al. A functional screen identifies miR-34a as a candidate neuroblastoma tumor suppressor gene. Mol Cancer Res. 2008;6:735–742. doi: 10.1158/1541-7786.MCR-07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei JS, et al. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27:5204–5213. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang TC, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castellano L, et al. The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci USA. 2009;106:15732–15737. doi: 10.1073/pnas.0906947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang CV, et al. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Brännvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- 39.Albrecht ED, Haskins AL, Pepe GJ. The influence of fetectomy at midgestation upon the serum concentrations of progesterone, estrone, and estradiol in baboons. Endocrinology. 1980;107:766–770. doi: 10.1210/endo-107-3-766. [DOI] [PubMed] [Google Scholar]
- 40.Pepe GJ, Titus JA, Townsley JD. Increasing fetal adrenal formation of cortisol from pregnenolone during baboon (Papio papio) gestation. Biol Reprod. 1977;17:701–705. doi: 10.1095/biolreprod17.5.701. [DOI] [PubMed] [Google Scholar]
- 41.Pepe GJ, Albrecht ED. Regulation of the primate fetal adrenal cortex. Endocr Rev. 1990;11:151–176. doi: 10.1210/edrv-11-1-151. [DOI] [PubMed] [Google Scholar]
- 42.Hall AK, Landis SC. Principal neurons and small intensely fluorescent (SIF) cells in the rat superior cervical ganglion have distinct developmental histories. J Neurosci. 1991;11:472–484. doi: 10.1523/JNEUROSCI.11-02-00472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng Y, Miura RM, Tian B. Prediction of mRNA polyadenylation sites by support vector machine. Bioinformatics. 2006;22(19):2320–2325. doi: 10.1093/bioinformatics/btl394. [DOI] [PubMed] [Google Scholar]
- 44.Popov N, Wahlström T, Hurlin PJ, Henriksson M. Mnt transcriptional repressor is functionally regulated during cell cycle progression. Oncogene. 2005;24:8326–8337. doi: 10.1038/sj.onc.1208961. [DOI] [PubMed] [Google Scholar]
- 45.Xu D, Popov N, Hou M, Wang Q, Björkholm M, Gruber A, Menkel A, Henriksson M. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the hTERT promoter during differentiation of HL60. Proc Natl Acad Sci USA. 2001;98:3826–3831. doi: 10.1073/pnas.071043198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.