Abstract
The population component of a species’ niche corresponds to the distribution of individuals across environments within a region. As evolutionary clades of species diversify, they presumably fill niche space, and, consequently, the rate of increase in species numbers slows. Total niche space and species numbers appear to be relatively stable over long periods, and so an increase in the species richness of one clade must be balanced by decrease in others. However, in several analyses, the total population niche space occupied per clade is independent of the number of species, suggesting that species in more diverse clades overlap more in niche space. This overlap appears to be accommodated by variation in the populations of each species, including their absence, within suitable niche space. I suggest that the uneven filling of niche space results from localized outcomes of the dynamic coevolutionary interactions of populations with their pathogens or other antagonists. Furthermore, I speculate that relationships with pathogens might constrain diversification if pathogen diversity increased with host diversity and resulted in more frequent host switching and emergent disease. Many indirect observations are consistent with these scenarios. However, the postulated influence of pathogens on the filling of niche space and diversification of clades primarily highlights our lack of knowledge concerning the space and time dimensions of coevolutionary interactions and their influence on population distribution and species diversification.
Keywords: mosaic evolution, niche breadth, pathogen
Each species occupies a part of the ecological space available in the environment, usually referred to as its “ecological niche.” Although the niche has been difficult to define precisely, most treatments consider conditions of the physical environment, characteristics of resources—whether soil nutrients, plants, or animal prey—and the traits of other interacting species (i.e., competitors, mutualists, predators, and pathogens) as important axes of ecological space that are partitioned among species (1, 2). In this essay, I distinguish individual and population niche space, the former reflecting primarily evolutionary adaptations of individuals to exploit resources and the latter, primarily reflecting demographic processes maintaining populations at a particular place. Of course, the population component of the niche also is influenced by adaptations of individuals to the range of environmental conditions over space within a region.
Both components of niche space can be defined for any particular species and also for a set of species, of which those comprising an evolutionary clade descended from a common ancestor are most relevant here. Adaptive radiations of species fill niche space by evolutionary diversification, yet both phylogenetic analyses (3, 4) and paleontological studies (5, 6) suggest that diversification is constrained and that individual clades typically enjoy a limited period of rapid radiation early in their existence. The number of species varies widely among clades, reflecting ecological constraints including competitors, the positions of clades with respect to phases of diversification and decline, and inherent variation among clades in the rate of diversification. Regardless of the cause, ecologists poorly understand the way in which clades of different size fill available niche space and whether niche filling constrains further diversification. Although diversification also can create niche space, evolution probably has little influence on the sum over species of the population component of niche space within a region, which is determined primarily by variation in climate and other physical variables.
In this essay, I consider several aspects of niche filling by clades from which one may infer that populations of species in larger clades do not exhibit reduced average breadth of the population niche over gradients of ecological conditions but rather fill suitable habitat less completely. I speculate that uneven filling of population niche space might represent varying local outcomes of pathogen–host coevolutionary interactions. Moreover, the diversity, frequency, and/or intensity of these interactions might increase with clade size and provide a mechanism for diversity-dependent feedbacks on the rate of diversification. Conceivably, periods of rapid diversification by small clades follow the acquisition of genetic resistance factors by host lineages; evolutionary responses by pathogens might subsequently constrain further species formation and filling of niche space. Although these ideas receive indirect support from different quarters, there is a need for continuing local field studies on niche filling and host–pathogen coevolutionary relationships and for phylogenetic analyses of the history of host–pathogen associations on appropriate scales as well as the development of suitable theory and simulation analyses to model these processes.
The Niche Concept
Although the niche has been a key concept in the development of ecological thinking, it defies precise definition. The niche is variously thought of as a place in the natural world, particularly a habitat, microhabitat, or range of suitable environmental conditions (7), or as the role a species plays in the ecosystem defined, in part, by its resources and consumers (8). Interest in niche approaches to understanding ecological communities (1) and geographic distributions (9) has increased recently, in part as a result of two analytical/conceptual developments. One of these developments is ecological niche modeling, based on ecological conditions at locations recorded for a species; this modeling can be used to predict the distribution of a species when ranges are poorly sampled (10 –12). The modeled niche and predicted distribution correspond to the population component of niche space.
A second conceptual development arose out of the idea of evolutionary niche conservatism and the notion, dating at least to Charles Darwin, that closely related species are similar ecologically and therefore likely to compete intensely for ecological resources (13, 14). Accordingly, closely related species might exclude each other locally, although their similar tolerances of environmental conditions might lead to closer association when viewed at broader environmental scales (15, 16).
Although these developments are closely allied with earlier ideas about the niche, they also reflect the dual nature of the niche emphasized here, pertaining partly to how individuals fit in their local habitats and partly to the distribution of populations over space within regions. Ecological niche modeling draws attention to the population niche as it is distributed across spatial environmental gradients, whereas phylogenetic community analysis emphasizes the evolutionary diversification of individual traits and the influence of ecological interactions on the niche space occupied by individuals (13, 17).
Individual and Population Components of the Niche.
Ecologists traditionally distinguish between fundamental and realized niches (2, 12, 18). The first represents the combinations of conditions and resources over which an individual or a population could persist in the absence of competitors, predators, and pathogens. Those other species can exclude a population from parts of the local environment or from regions which have marginally supportive conditions; the remaining distribution is the realized niche. Species in nature rarely, if ever, fill out their fundamental niches. Moreover, because each species is sensitive to the presence of others, the realized niche is context-dependent.
Viewed from a different perspective, the niche is seen to have two complementary parts. The individual component reflects the adaptations of the organism to use resources and avoid sources of mortality. This component expresses the activities of the individual and is defined locally, that is, within the individual’s spatial range of activity. The population component reflects the persistence of a species over its geographic distribution. Over space, physical conditions of the environment and the availability of resources change, influencing the birth and death rates of individuals and the ability of a population to maintain itself locally. In addition, where individuals produce offspring in excess of the number needed to maintain a population locally, dispersal to less favorable areas can extend the boundaries of the population niche—a phenomenon variously called a “mass effect” (19), “core–satellite relationship” (20), or “source–sink relationship” (21, 22).
The distinction between the individual and population components of the niche strikes me as important because these components reflect different, albeit interacting, properties of species. The individual component expresses evolutionary adaptations of the phenotype to attributes of the habitat within which it lives: food types, foraging substrates, escape space, and so on. Different factors additionally influence the distributions of populations (23). For example, regional gradients in conditions such as precipitation and temperature (24, 25) cannot be partitioned by individuals of different species within a habitat. The partitioning of environmental space among populations within a region is an epiphenomenon, or emergent property, expressing the adaptations of individuals through population demography. This population component of the niche also reflects interactions with other species. Thus, the ability of a population to maintain itself over a particular range of environments cannot be predicted exclusively from the adaptations of its individuals but rather depends as much on the other species that are competing for the same resources or using the focal species as a resource.
Lability of the Population Niche.
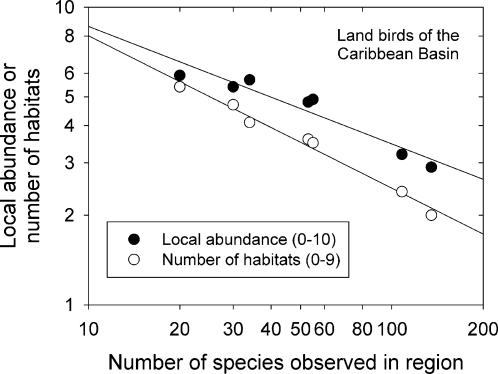
The breadth and relative abundance of population niches are sensitive to competitors and consumers. For example, islands support fewer species than continental assemblages and therefore have reduced interspecific competition and predation. As a result, many island species exhibit “ecological release” in the form of higher local densities and broader distributions across environments (26, 27). On islands in the West Indies and surrounding continental areas in the Caribbean Basin, the local relative abundance and habitat distribution of individual species of small land birds across matched environments varied from 2-fold to 3-fold in inverse relationship to the 6-fold variation in number of species per area, presumably reflecting the increasing pressure of interspecific competition (Fig. 1) (28). The density of all populations considered together was similar across areas, regardless of species richness (29), as one might expect from the similar biological productivity of each of these areas.
Fig. 1.
Decrease in local abundance and habitat breadth of species observed over nine matched habitats ranging from grassland to cloud forest on five islands in the West Indies and two continental areas (Trinidad and Panama) of the Caribbean Basin. [From Ricklefs (28) based on data in Cox and Ricklefs (29) and Wunderle (109).]
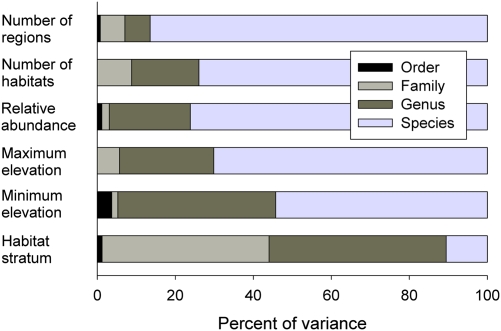
The evolutionary lability of the population component of the niche is apparent in nested analyses of variance that partition the total variance in a trait into components representing differences among species within genera, genera within families, families within orders, and so on. Measures of population density and geographic range size typically are most variable at low taxonomic levels, primarily among species within genera, reflecting a high degree of niche lability (30 –32). For example, among nonraptorial land birds of South America, most of the variance in the number of zoological regions (a measure of geographic distribution over continental ranges of environmental conditions), number of habitats occupied (referring to vegetation types), and relative abundance resides at the level of differences between closely related species (Fig. 2). Only the habitat stratum within which a species is most active—ground, forest understory, midlevel, or canopy—is conserved taxonomically; stratum partly reflects morphological adaptations for movement over substrates that are shared among species in higher taxa (genera and families) (33).
Fig. 2.
Taxonomically nested analysis of variance (order, family, genus, species) in measures of geographic range, habitat breadth, and relative abundance. Data refer to 3,033 species of nonraptorial land birds of South America and are from Stotz et al. (72), who reported the number of zoological regions (of 22) and number of forested (of 14) and open (of 14) habitats occupied by each species. Abundance was assigned to one of four ordinal categories (rare, fairly common, common, or abundant). Habitat stratum was assigned to one of five ordinal categories (ground, understory, midcanopy, canopy, or aerial).
Similarly, for the 250 species of trees on the 50-ha forest dynamics plot on Barro Colorado Island, Panama (https://ctfs.arnarb.harvard.edu/webatlas/datasets/bci/abundance/bciN100.html), 68% of the variance in abundance (average number of individuals per species in six 5-year censuses) resides at the level of species within genera and 32% at the level of genera within families, mostly representing variation among monospecific genera in this analysis.
General and Specific Variation in Niche Breadth.
Variations in average habitat niche breadth and local population density of birds among regions in the Caribbean Basin represent general community-wide differences in competition and exploitation pressure. Among species within a region, as among birds within the continent of South America or among trees on Barro Colorado Island, such variations reflect specific differences in the productivity of populations influencing the population component of niche breadth. Because most of the variance occurs among congeneric species, this variation in the population component of the niche cannot reflect general environmental conditions, to which all species are exposed, or conserved adaptations within clades. Instead, this variation must signal particular relationships of individual species to their environments—for example, between each species and its specialized pathogens or other antagonists. Coevolutionary relationships between pathogens and their hosts likely shift the balance of productivity back and forth between host and parasite populations, a phenomenon shown experimentally years ago by David Pimentel (34) and incorporated into the taxon cycle concept of expanding and contracting ranges by Ricklefs and Cox (35). I suggest that localized coevolutionary outcomes can cause a population to be absent from otherwise suitable environments and furthermore that the suppressing effects of pathogens might increase with species richness within a clade.
Evidence for highly specific and evolutionarily dynamic interactions can be seen in the different susceptibilities of closely related species to pathogens. For example, the North American gray squirrel introduced to the British Isles harbors an endemic viral pathogen that is fatal to the closely related native red squirrel (36). Similarly, the native Scandinavian crayfish Astacus astacus was decimated by a freshwater fungal pathogen introduced to European lakes with the related North American crayfish Pacifastacus leniusculus, which resists the disease but effectively carries the fungus (37). The invasive chytrid fungus Batrachochytrium dendrobatidis, which is responsible for declines in many anuran populations worldwide, selectively causes extinction of primarily locally distributed species, whereas other widespread anurans serve as reservoirs for the pathogen (38).
Local (island-specific) and independent (species-specific) coevolutionary outcomes in host–pathogen interactions are evident in endemic biotas, as well. For example, in surveys of hemosporidian parasites in populations of avian hosts in the Lesser Antilles, the prevalence of a particular blood parasite (Haemoproteus coatneyi) in a single host varies strikingly across islands, often in inverse relationship to its prevalence in an alternative host (39). Why could such coevolutionary interactions not influence the abundance, and even the presence, of a species in an otherwise suitable location? Variation in relative abundance and in the breadth of ecological distribution among closely related species suggests, at least, that the population component of the niche might reflect the outcomes of coevolutionary interactions that occur on time scales within the life span of a species.
Evolutionary Diversification and the Filling of Niche Space
Thinking about diversification is colored by adaptive radiations of species in isolated archipelagoes (40 –44), where colonists experience few constraints from established biotas. The fossil record reveals similar bursts of adaptive radiation following mass extinctions or within taxa that have entered, according to Simpson’s (5) terminology, new “adaptive zones” following the evolution of “key innovations.” Species richness and morphological variation typically increase quickly (e.g., ref. 45), filling previously unoccupied niche space, before a clade begins to dwindle toward extinction (46).
These often spectacular occurrences obscure the continuing diversification and replacement of evolutionary lineages (clades) within apparently saturated niche space. Paleontological evidence often reveals long periods of relatively constant numbers of species and morphological variety. For example, the numbers of species of mammals (47) and tropical forest trees (48, 49) have varied little (probably within a factor of 2) over the 60 million years since the early Tertiary recovery following the end-Cretaceous extinctions.
Constrained overall diversity need not imply stasis in the sizes of clades but rather that increases in some clades are balanced, on average, by declines in others. Molecular phylogenetic reconstructions of ancestral relationships within contemporary clades frequently reveal initial phases of rapid species diversification (4, 50 –52), often in association with generation of morphological disparity (45, 53, but see ref. 54). These transient phases of evolutionary activity are followed by relative stasis in the number of ancestral lineages (3), consistent with the general absence of a correlation between clade size and clade age (55 –59). Apparently, ecological space on much of the earth is now relatively filled and has been filled for a long time. By implication, niche space resists finer subdivision, and species richness has become largely self-limiting through diversity-dependent feedbacks on speciation and extinction (3, 6, 60 –62).
Rapid diversification early in the history of modern clades, followed by relative stasis, has several implications for understanding the evolutionary filling of niche space. Assuming that overall species richness has been relatively constant, diversification within some clades must be balanced by the shrinking of others. Growing clades either fill niche space vacated by extinction or force other clades out of occupied niche space, causing species extinction. Potential drivers of diversification and extinction can be extrinsic, including persistent environmental changes that shift the spectrum of niche space and cause extinction of species but also open new ecological opportunities. These drivers also can be intrinsic, including stochastic variation in speciation and extinction as well as the origin of evolutionary novelty in lineages that subsequently diversify at the expense of others. Adaptations that increase the occupation of niche space also might promote diversification and drive the replacement of species over time within the same niche space through competitive exclusion. How such diversifying clades become self-limited is not understood but possibly is related to the way in which clades fill niche space.
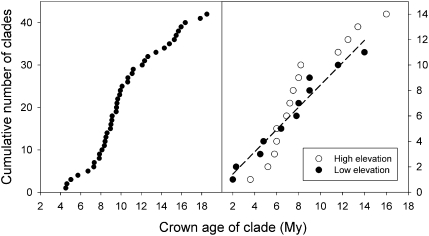
Extrinsic and intrinsic drivers of species turnover might result in different temporal patterns in the initiation of clade diversification. Clumping of clade ages would suggest a role for external drivers acting during periods of rapid environmental change. Conversely, clades whose diversification was independently initiated by internal drivers might exhibit random distribution of origins over time. The origin of a particular clade can be taken as its crown age, that is, the age of the initial lineage split in the reconstructed phylogeny. In a global dataset of avian clades assembled by Phillimore and Price (4) from available time-calibrated molecular phylogenies (not necessarily a random sample of clades), clade ages were clumped between about 8 and 10 Mya (Fig. 3), possibly associated with the late Miocene cooling and drying of the global climate (63). The same climate trends evidently opened new areas of arid habitat for plant diversification in southern Africa, as is apparent in phylogenetic reconstructions (64).
Fig. 3.
(Left) Cumulative number of avian clades (•) compiled by Phillimore and Price (4) as a function of clade age. (Right) Cumulative number of primarily genus-level clades assembled by Weir (52) for high-elevation (○) and low elevation (• and regression line) South American land birds. The slope of the relationship between cumulative clades and time is the rate of clade origination in the respective samples.
Weir (52) assembled phylogenies for mostly genus-level clades of lowland and Andean tropical South American birds. The crown ages of the lowland clades were distributed evenly between 2 and 12 Mya (Fig. 3); in contrast, the majority of high-elevation clades originated between 5 and 8 Mya, corresponding to a period of rapid uplift of the northern Andes that also was associated with diversification of plant clades (65 –67). At least in the case of Neotropical lowland birds, clade initiation appears unrelated to persistent environmental change (see also ref. 3).
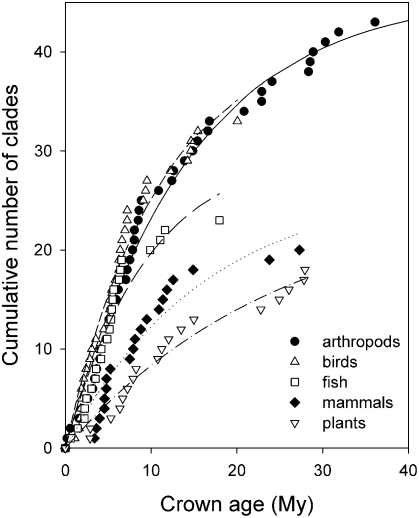
Whereas clades begin to diversify at intervals over time, they also dwindle to extinction at some rate. Thus, as one goes back in time, the cumulative number of clades with progressively older origins should approach an equilibrium value in the same way that the number of species on an island approaches a colonization–extinction equilibrium (68, 69). If clades originated at rate C per million years, and extant clades went extinct at rate E, then, at equilibrium, the cumulative number of clades (N) would increase with clade age (t) according to N = (C/E)[1 − exp(−Et)], toward a steady-state number equal to C/E. Applied to a sample of crown ages for clades of various higher organisms, estimates of clade extinction rate varied between 0.040 and 0.105 per million years, equivalent to average persistence times between about 10 and 25 million years (Fig. 4).
Fig. 4.
Increase in the cumulative number of clades with increasing age in a sample assembled according to other criteria by McPeek and Brown (110) and McPeek (3). Estimated rates of extinction were arthropods, 0.070 ± 0.003; birds, 0.093 ± 0.010; fish, 0.105 ± 0.021; mammals, 0.062 ± 0.014; flowering plants (Magnoliaphyta), 0.040 ± 0.009.
Although clade definition is arbitrary, most clades that are the focus of phylogenetic studies include modest numbers (average 23 in this sample) of closely related but distinctive species, often belonging to a single genus. The fitted cumulative curves in Fig. 4 suggest an underlying dynamic in the appearance and dwindling of clades over time. It is unlikely that extinctions leading to a decrease in clade size occur at random, because the average persistence time of a clade of size S under random extinction is on the order of S species durations (70), a period that, typically being more than a million years per species, is too long. Accordingly, clade extinction would appear to be more deterministic than random, resulting either from environmental change or pressure from other competing or exploiting species. In the latter case, clade turnover would result from evolutionary processes intrinsic to a biota.
Species Richness and Niche Space
Niche Packing.
Larger clades must occupy more niche space overall, pack niche space more densely (either through less niche space per individual species or greater niche overlap between species), or utilize some combination of the two strategies. Increased competition for resources limits population productivity and causes a decrease in the abundance or distribution of each population, or both, on average. Moreover, partitioning of the individual and population components of niche space among species, as a mechanism to reduce interspecific competition, is widely thought to be a prerequisite for species coexistence (71). This partitioning should be apparent as reduced local density and geographic distribution of individual populations.
Using the number of zoogeographic zones and number of habitats occupied per species compiled by Stotz et al. (72) for the land birds of South America, Ricklefs (59) showed that both the average number of habitats and the average number of zoogeographic zones occupied per species were independent of the size of family-level clades. Thus, regardless of the size of a clade, individual species occupy population niches of the same average breadth. With population niches of more or less constant breadth, variation in diversity among clades could be accommodated by the total niche space occupied by all the species in a clade. However, of 14 types of forested habitat and 14 types of open habitats, only 0.4 and 0.8 habitats, respectively, were added to the population niche space occupied by each clade for each 10-fold increase in number of species (59).
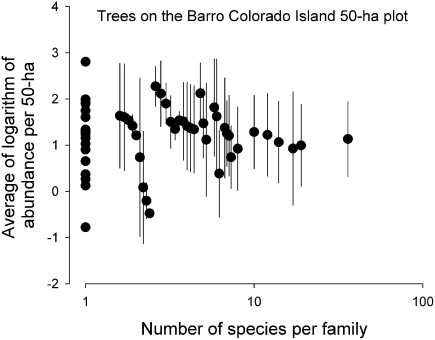
These findings suggest that higher species richness within clades results in more dense packing of population niches. Accordingly, and assuming that clades do not overlap strongly in ecological niche space, the populations in larger clades would bear more competition from closely related species and exhibit lower local density on average. I tested this prediction with censuses of passerine birds in three local census plots (ca. 10 ha) in tropical rainforests in Peru (73), French Guiana (74), and Panama (75) and found no relationship between the average density of populations and the number of co-occurring species per family (59). The sums of the population densities within each family increased in direct proportion to the local number of species, and these “family” densities were highly correlated among plots, indicating consistent patterns of niche filling by clades across tropical South and Central America. The generality of this pattern is shown by a similar analysis for tree species on Barro Colorado Island, which revealed the same absence of a relationship between the number of species in each family-level taxon and the average abundance of each species in that taxon (Fig. 5).
Fig. 5.
Average abundances of species of trees within family-level taxa on the 50-ha forest dynamics plot on Barro Colorado Island, Panama, based on the average number of individuals for each of 250 species in six 5-year censuses. Families with the same number of species are separated slightly to show the standard deviations of abundance among species. (Data from https://ctfs.arnarb.harvard.edu/webatlas/datasets/bci/abundance/bciN100.html.) Neither the family mean abundance (F 1,54 = 0, P = 0.95) nor the SD of abundance within families (F 1,34 = 1.35, P = 0.25) was related to the number of species per family.
This unexpected lack of diversity-dependence in the breadth of population niches and local population density across clades of different size might reflect increasing total within-habitat (individual) niche space occupied as a function of clade size. Individual trees, at least for species that reach the canopy of the forest, occupy nonoverlapping physical space and do not obviously partition local ecological space, particularly where the environment is relatively uniform (76, 77). Thus, species-rich clades of trees probably do not command more within-habitat niche space than species-poor clades.
In contrast, many species of bird have overlapping activity spaces locally, and the summed within-habitat niche space used by members of a clade could increase with the number of species (78). Globally, the log-transformed standard deviation of morphological variation within family-level clades of passerine birds increases about 12% as rapidly as the logarithm of the number of species (e.g., ref. 79). Thus, the volume of morphological space, which might be regarded as a surrogate for ecological space (80, 81), increases slowly compared with species richness. Overlap between clades can be estimated by misassignment of species to clades using morphology-based discriminant analysis. Among 25 family-level clades of passerine birds for which I have measured 10 or more species, 629 of 1,221 species, or 52%, were correctly placed (R. E. Ricklefs, unpublished data). The correct placement per family averaged 60% and decreased by 25% for each 10-fold increase in number of species. Thus, on a global basis, larger clades occupy more morphological space and overlap with other clades to a greater degree, but not enough to explain the diversity-independence of local population density.
Saturation of the Population Niche Space.
One explanation for diversity-independence of population components of the niche, at least as observed in passerine birds, is that as the number of species increases, the filling of suitable niche space by each population (i.e., its presence or absence in an appropriate environment) declines. Using Breeding Bird Surveys in North America, Hurlbert and White (31, 82) found that many species of breeding land birds were not detected in suitable habitats within their geographic ranges. Range occupancy was positively correlated with mean abundance and niche breadth within the occupied geographic area; however, all three measures might be considered as components of niche filling.
Not all space within a geographic range provides appropriate habitat for a species, but aspects of habitat suitability, such as climate, can be included in assessing distributions by ecological niche modeling, which develops predictive relationships between the presence/absence or the abundance of species and particular environmental variables (10, 11, 83). As in the case of range occupancy, however, species often are absent from locations that otherwise are judged to be appropriate (84 –86). Of course, niche models might lack critical environmental variables for a particular species and thus fail to characterize its niche space adequately.
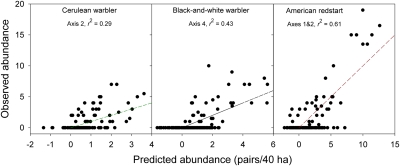
Another approach is to let the distributions of species across locations indicate suitable niche space. Ordination methods place localities in a multivariate space based on the distribution of species across the localities (87) and presumably allow a fauna to indicate the general suitability of sites on multiple derived axes. One might assume that the statistically most important longer axes represent a fauna-eye view of variation in niche space, with the shorter axes representing preferences of a small number of specialist species or stochastic variation in abundance. Even with this approach, which may delineate consistently occupied parts of the niche space, most species are sparsely distributed within or even absent from large portions of apparently suitable niche space (Fig. 6).
Fig. 6.
Abundances of three species of warbler (Parulinae) compared with predictions based on multiple regressions of their abundances on five Bray-Curtis ordination axes for 56 species of nonraptorial land birds recorded in 128 Breeding Bird Censuses from terrestrial habitats in North America.
Coevolution and the Filling of Niche Space.
What causes geographic variation in the presence or abundance of the population of a species in suitable niche space? Presumably, adaptations of individuals to the physical conditions and resources of the environment should not be a factor, because individual species often are absent from suitable locations, as shown by the distribution of their populations elsewhere (88). Dispersal limitation can result in the absence of species where they might persist otherwise, particularly in regions with dynamic climates (89), but probably cannot explain holes in geographic distributions over reasonably uniform regions. Allee effects (18, 90, 91) and priority effects (92, 93) also might be important. Alternatively, variation in the occupation or saturation of suitable niche space might reflect the balance of locally coevolved interactions of a population with predators and pathogens. Sufficient demographic pressure, particularly from pathogens, might drive a host population to extinction locally, creating a hole in the suitable niche space.
The elements necessary for depopulating suitable niche space are broadly understood: profound population impacts of emerging diseases with rapid dynamics of host–pathogen coevolution, sometimes revealed by the release of invasive exotic species from pathogen, predator, or herbivore pressure (94); occurrence of localized sink populations in unsuitable habitat, with insufficient dispersal from productive areas to balance death and emigration (21); and localized coevolutionary outcomes (95 –99), which are the foundation of the mosaic of coevolution (100). The temporal and spatial dimensions of coevolutionary outcomes are poorly understood and presumably depend on population characteristics of the interacting species, particularly the rate of evolution compared with the rate of dispersal.
Poor dispersal within a population relative to the shifting balance of host and pathogen adaptations is prerequisite to establishing a mosaic of coevolutionary outcomes. For example, the prevalence of individual avian hemosporidian lineages can vary strikingly within the same host species in neighboring, ecologically similar islands in the Lesser Antilles, between which there is little host dispersal (101) but exhibits more uniform distribution over continental areas with less restricted host and parasite movement (102). Virulence can shift through host and pathogen evolution within a few generations, as in the case of the myxomatosis virus in Australian rabbits (103), but phases of expansion and contraction in the distributions of West Indian birds, which might reflect coevolutionary outcomes based on the appearance of rare mutations (35, 104), appear to occur at intervals on the order of hundreds of thousands of years (69). Clearly, the temporal and spatial dimensions of coevolutionary interactions causing changes in host populations deserve further investigation.
Clade Size and Constraints on Diversification.
With respect to clade diversification, several observations require explanation: (i) the initial phase of rapid diversification apparent in many clades; (ii) subsequent constraint on further increase in species richness; and (iii) variation in niche space and population distribution among species within a clade. Each of these aspects can be tied speculatively to varying outcomes of host–pathogen coevolution. Achieving resistance to particularly important pathogens (i.e., pathogens that constrain population size) might initiate a phase of rapid diversification. Suppression of pathogens leading to high population productivity, saturation of niche space, and broad geographic distribution might increase opportunities for population subdivision and species formation. I have argued elsewhere that specialized pathogens might prevent secondary sympatry by sister taxa and reduce diversification within clades (105, 106). Suppression of pathogens would reduce this impediment to diversification. In this case, pathogen resistance could be regarded as a key innovation.
In this scenario, rapid diversification within a clade could be brought to an end by subsequent coevolution of pathogens, tending to reduce host population sizes and restrict their geographic distribution. Specialized pathogens also might constrain diversification if rates of host switching were to increase in proportion to the diversity of hosts and their pathogens. In a more diverse clade, each host species might be liable to invasion from a greater source pool of pathogens endemic to a larger set of closely related host species. Not only would diverse pathogens reduce host population size and distribution, they also might reduce the potential for achieving secondary sympatry and therefore slow the accumulation of local diversity and reduce the potential for further allopatric speciation. Localized outcomes of host–pathogen coevolutionary interactions also could account for variation in niche space and population distribution among closely related species—lability in the population component of the niche—leading to the sporadic occupation of otherwise suitable niche space.
Although the elements of these ideas are plausible and fit what is known about the distributions of species and the outcomes of host–pathogen interactions, our knowledge in these areas is insufficient to characterize the underlying mechanisms. Without knowing which pathogens exert constraining influences on host populations, the temporal and spatial extent of their coevolutionary outcomes, the degree of host specialization, and rates of switching between hosts, it will be difficult to evaluate these ideas. Consideration of these hypotheses exposes how little we know about the interactions of species in nature and the processes that control diversification, species coexistence, and the filling of ecological niche space. Moreover, to the extent that specialized pathogens limit host population size, niche space will have almost as many dimensions as the number of important pathogens in the environment, and the important niche dimensions for each species will change through time with the varying outcomes of coevolutionary relationships.
Predictions arising from coevolutionary hypotheses for the filling of niche space might be testable to varying degrees. More extensive assessments of population niche filling are needed to construct an empirical foundation. Spatial variation in coevolutionary outcomes is accessible through reciprocal transplant and reciprocal exposure experiments (e.g., refs. 107, 108). Moreover, host population size should vary inversely with the presence of virulent pathogens. If pathogens constrained diversification within clades through reducing the probability of successful secondary sympatry, one also would expect species in larger clades to exhibit a higher diversity of specialized pathogens. Possibly, nonconstraining pathogens would exhibit similar patterns and might serve as model systems relating parasite and host diversity.
Clearly, the ecological space for every species includes the distribution and abundance of coevolving pathogenic and mutualistic species. Testing the ideas outlined in this essay requires that such influences on host populations be assessed with reference to space within regions and to their distribution within clades of hosts. Although niche theory in the past has focused primarily on competitive relationships between species and the partitioning of ecological resources, it is possible that pathogens and other antagonist populations reduce host/prey populations in a shifting pattern of local coevolutionary outcomes that allow the species within a clade to coexist within otherwise similar niche space. A more speculative extension of this idea is that pathogen diversification and host-switching also might constrain the diversification of their hosts in a diversity-dependent fashion and thus limit clade size. These possibilities present a major challenge for ecologists and evolutionary biologists and emphasize the importance of integrated training across fields that span microbiology and ecology as well as the need for basic data on the distribution and abundance of populations in the context of clade diversification and the filling of the population component of the niche space.
Acknowledgments
Jonathan Chase, Jason Knouft, Brian McGill, A. Townsend Peterson, Susanne Renner, and Mark Vellend provided insightful discussion and helpful comments on the manuscript. I acknowledge the generous support of the Board of Curators of the University of Missouri and the Alexander von Humboldt Foundation.
Footnotes
The author declares no conflict of interest.
References
- 1.Chase JM, Leibold MA. Ecological Niches. Linking Classical and Contemporary Approaches. Chicago: University of Chicago Press; 2003. [Google Scholar]
- 2.Hutchinson GE. Concluding remarks. Cold Spring Harb Symp Quant Biol. 1957;22:415–427. [Google Scholar]
- 3.McPeek MA. The ecological dynamics of clade diversification and community assembly. Am Nat. 2008;172:E270–E284. doi: 10.1086/593137. [DOI] [PubMed] [Google Scholar]
- 4.Phillimore AB, Price TD. Density-dependent cladogenesis in birds. PLoS Biol. 2008;6:e71. doi: 10.1371/journal.pbio.0060071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson GG. The Major Features of Evolution. New York: Columbia University Press; 1953. [Google Scholar]
- 6.Stanley SM. Macroevolution: Pattern and Process. San Francisco: W. H. Freeman; 1979. [Google Scholar]
- 7.Grinnell J. The niche-relationships of the California Thrasher. Auk. 1917;34:427–433. [Google Scholar]
- 8.Elton CS. Animal Ecology. New York: Macmillan; 1927. [Google Scholar]
- 9.Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10:1115–1123. doi: 10.1111/j.1461-0248.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 10.Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Modell. 2000;135:147–186. [Google Scholar]
- 11.Austin MP. Spatial prediction of species distribution: An interface between ecological theory and statistical modeling. Ecol Modell. 2002;157:101–118. [Google Scholar]
- 12.Soberón J, Peterson AT. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics. 2005;2:1–10. [Google Scholar]
- 13.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annu Rev Ecol Syst. 2002;33:475–505. [Google Scholar]
- 14.Wiens JJ, Graham CH. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst. 2005;36:519–539. [Google Scholar]
- 15.Cavender-Bares J, Keen A, Miles B. Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology. 2006;87:S109–S122. doi: 10.1890/0012-9658(2006)87[109:psofpc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Cavender-Bares J, Kozak K, Fine P, Kembel S. The merging of community ecology and phylogenetic biology. Ecol Lett. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 17.Ackerly DD. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci. 2003;164:S165–S184. [Google Scholar]
- 18.Holt RD. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc Natl Acad Sci USA. 2009;106:19659–19665. doi: 10.1073/pnas.0905137106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shmida A, Wilson MV. Biological determinants of species diversity. J Biogeogr. 1985;12:1–20. [Google Scholar]
- 20.Hanski I. Dynamics of regional distribution: The core and satellite hypothesis. Oikos. 1982;38:210–221. [Google Scholar]
- 21.Pulliam HR. Sources, sinks, and population regulation. Am Nat. 1988;132:652–661. [Google Scholar]
- 22.Pulliam HR. On the relationship between niche and distribution. Ecol Lett. 2000;3:349–361. [Google Scholar]
- 23.Brown JH. On the relationship between the abundance and distribution of species. Am Nat. 1984;124:255–279. [Google Scholar]
- 24.Currie DJ. Energy and large scale patterns of animal and plant species richness. Am Nat. 1991;137:27–49. [Google Scholar]
- 25.Hawkins BA, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- 26.Terborgh JW, Faaborg J. Saturation of bird assemblages in the West Indies. Am Nat. 1980;116:178–195. [Google Scholar]
- 27.MacArthur RH, Recher H, Cody ML. On the relation between habitat selection and species diversity. Am Nat. 1966;100:603–609. [Google Scholar]
- 28.Ricklefs RE. The relationship between local and regional species richness in birds of the Caribbean Basin. J Anim Ecol. 2000;69:1111–1116. [Google Scholar]
- 29.Cox GW, Ricklefs RE. Species diversity, ecological release, and community structuring in Caribbean land bird faunas. Oikos. 1977;29:60–66. [Google Scholar]
- 30.Gaston KJ. Species-range size distributions: Products of speciation, extinction and transformation. Philos Trans R Soc Lond B Biol Sci. 1998;353:219–230. [Google Scholar]
- 31.Hurlbert AH, White EP. Ecological correlates of geographical range occupancy in North American birds. Glob Ecol Biogeogr. 2007;16:764–773. [Google Scholar]
- 32.Scheuerlein A, Ricklefs RE. Prevalence of blood parasites in European passeriform birds. Proc R Soc Lond B Biol Sci. 2004;271:1363–1370. doi: 10.1098/rspb.2004.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles DB, Ricklefs RE. The correlation between ecology and morphology in deciduous forest passerine birds. Ecology. 1984;65:1629–1640. [Google Scholar]
- 34.Pimentel D. Population regulation and genetic feedback. Science. 1968;159:1432–1437. doi: 10.1126/science.159.3822.1432. [DOI] [PubMed] [Google Scholar]
- 35.Ricklefs RE, Cox GW. Taxon cycles in the West Indian avifauna. Am Nat. 1972;106:195–219. [Google Scholar]
- 36.Tompkins DM, White AR, Boots M. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol Lett. 2003;6:189–196. [Google Scholar]
- 37.Josefsson M, Andersson B. The environmental consequences of alien species in the Swedish lakes Malaren, Hjalmaren, Vanern and Vattern. Ambio. 2001;30:514–521. doi: 10.1579/0044-7447-30.8.514. [DOI] [PubMed] [Google Scholar]
- 38.Smith KG, Lips KR, Chase JM. Selecting for extinction: Nonrandom disease-associated extinction homogenizes amphibian biotas. Ecol Lett. 2009;12:1069–1078. doi: 10.1111/j.1461-0248.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 39.Fallon SM, Bermingham E, Ricklefs RE. Host specialization and geographic localization of avian malaria parasites: A regional analysis in the Lesser Antilles. Am Nat. 2005;165:466–480. doi: 10.1086/428430. [DOI] [PubMed] [Google Scholar]
- 40.Givnish TJ, Sytsma KJ, editors. Molecular Evolution and Adaptive Radiation. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 41.Schluter D. The Ecology of Adaptive Radiation. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- 42.Givnish TJ. In: The Biology of Biodiversity. Kato M, editor. Tokyo: Springer-Verlag; 1999. pp. 67–90. [Google Scholar]
- 43.Losos JB, Ricklefs RE. Adaptation and diversification on islands. Nature. 2009;457:830–836. doi: 10.1038/nature07893. [DOI] [PubMed] [Google Scholar]
- 44.Grant PR, editor. Evolution on Islands. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- 45.Harmon LJ, Schulte JA, Larson A, Losos JB. Tempo and mode of evolutionary radiation in iguanian lizards. Science. 2003;301:961–964. doi: 10.1126/science.1084786. [DOI] [PubMed] [Google Scholar]
- 46.Foote M. The evolution of morphological diversity. Annu Rev Ecol Syst. 1997;28:129–152. [Google Scholar]
- 47.Alroy J. Successive approximations of diversity curves: Ten more years in the library. Geology. 2000;28:1023–1026. [Google Scholar]
- 48.Jaramillo C, Rueda MJ, Mora G. Cenozoic plant diversity in the Neotropics. Science. 2006;311:1893–1896. doi: 10.1126/science.1121380. [DOI] [PubMed] [Google Scholar]
- 49.Wing SL, et al. Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest. Proc Nat Acad Sci USA. 2009;106:18627–18632. doi: 10.1073/pnas.0905130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lovette IJ, Bermingham E. Explosive speciation in the New World Dendroica warblers. Proc R Soc Lond B Biol Sci. 1999;266:1629–1636. [Google Scholar]
- 51.Rabosky DL, Lovette IJ. Density-dependent diversification in North American wood warblers. Proc R Soc London B Biol Sci. 2008;275:2363–2371. doi: 10.1098/rspb.2008.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weir JT. Divergent timing and patterns of species accumulation in lowland and highland Neotropical birds. Evolution. 2006;60:842–855. [PubMed] [Google Scholar]
- 53.Kozak KH, Larson AA, Bonett RM, Harmon LJ. Phylogenetic analysis of ecomorphological divergence, community structure, and diversification rates in dusky salamanders (Plethodontidae: Desmognathus) Evolution. 2005;59:2000–2016. [PubMed] [Google Scholar]
- 54.Adams DC, Berns CM, Kozak KH, Wiens JJ. Are rates of species diversification correlated with rates of morphological evolution? Proc R Soc Lond B Biol Sci. 2009;276:2729–2738. doi: 10.1098/rspb.2009.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricklefs RE, Renner SS. Species richness within families of flowering plants. Evolution. 1994;48:1619–1636. doi: 10.1111/j.1558-5646.1994.tb02200.x. [DOI] [PubMed] [Google Scholar]
- 56.Magallón S, Sanderson MJ. Absolute diversification rates in angiosperm clades. Evolution. 2001;55:1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 57.Ricklefs RE. Estimating diversification rates from phylogenetic information. Trends Ecol Evol. 2007;22:601–610. doi: 10.1016/j.tree.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Ricklefs RE, Losos JB, Townsend TM. Evolutionary diversification of clades of squamate reptiles. J Evol Biol. 2007;20:1751–1762. doi: 10.1111/j.1420-9101.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 59.Ricklefs RE. In: Speciation and Patterns of Diversity. Butlin R, Bridle J, Schluter D, editors. Cambridge, UK: Cambridge University Press; 2009. pp. 257–277. [Google Scholar]
- 60.Sepkoski JJ., Jr A kinematic model of Phanerozoic taxonomic diversity II. Early Phanerozoic families and multiple equilibria. Paleobiology. 1979;5:222–251. [Google Scholar]
- 61.Rosenzweig ML. In: Ecology and Evolution of Communities. Cody ML, Diamond JM, editors. Cambridge, MA: Harvard University Press; 1975. pp. 121–140. [Google Scholar]
- 62.Rosenzweig ML. Species Diversity in Space and Time. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- 63.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 64.Verboom GA, et al. Origin and diversification of the Greater Cape flora: Ancient species repository, hot-bed of recent radiation, or both? Mol Phylogenet Evol. 2009;51:44–53. doi: 10.1016/j.ympev.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 65.Antonelli A, Nylander JAA, Persson C, Sanmartín I. Tracing the impact of the Andean uplift on Neotropical plant evolution. Proc Natl Acad Sci USA. 2009;106:9749–9754. doi: 10.1073/pnas.0811421106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregory-Wodzicki KM. Uplift history of the Central and Northern Andes: A review. Geol Soc Am Bull. 2000;112:1091–1105. [Google Scholar]
- 67.Hughes C, Eastwood R. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proc Natl Acad Sci USA. 2006;103:10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton, NJ: Princeton University Press; 1967. [Google Scholar]
- 69.Ricklefs RE, Bermingham E. Nonequilibrium diversity dynamics of the Lesser Antillean avifauna. Science. 2001;294:1522–1524. doi: 10.1126/science.1065005. [DOI] [PubMed] [Google Scholar]
- 70.Leigh EG. The average lifetime of a population in a varying environment. J Theor Biol. 1981;90:213–239. doi: 10.1016/0022-5193(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 71.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 72.Stotz DF, Fitzpatrick JW, Parker TA, III, Moskovits DK. Neotropical Birds. Ecology and Conservation. With Ecological and Distributional Databases by Theodore A. Parker III, Douglas F. Stotz, and John W. Fitzpatrick. Chicago: University of Chicago Press; 1996. [Google Scholar]
- 73.Terborgh J, Robinson SK, Parker TA, III, Munn CA, Pierpont N. Structure and organization of an Amazonian forest bird community. Ecol Monogr. 1990;60:213–238. [Google Scholar]
- 74.Thiollay J-M. Structure, density and rarity in an Amazonian rainforest bird community. J Trop Ecol. 1994;10:449–481. [Google Scholar]
- 75.Robinson WD, Brawn JD, Robinson SK. Forest bird community structure in central Panama: Influence of spatial scale and biogeography. Ecol Monogr. 2000;70:209–235. [Google Scholar]
- 76.Harms KE, Condit R, Hubbell SP, Foster RB. Habitat associations of trees and shrubs in a 50-ha Neotropical forest plot. J Ecol. 2001;89:947–959. [Google Scholar]
- 77.Hubbell SP, et al. Light-cap disturbances, recruitment limitation, and tree diversity in a Neotropical forest. Science. 1999;283:554–557. doi: 10.1126/science.283.5401.554. [DOI] [PubMed] [Google Scholar]
- 78.Ricklefs RE, Travis J. A morphological approach to the study of avian community organization. Auk. 1980;97:321–338. [Google Scholar]
- 79.Ricklefs RE. Cladogenesis and morphological diversification in passerine birds. Nature. 2004;430:338–341. doi: 10.1038/nature02700. [DOI] [PubMed] [Google Scholar]
- 80.Karr JR, James FC. In: Ecology and Evolution of Communities. Cody ML, Diamond JM, editors. Cambridge, MA: Belknap Press of Harvard University Press; 1975. pp. 258–291. [Google Scholar]
- 81.Ricklefs RE, Miles DB. In: Ecological Morphology: Integrative Organismal Biology. Wainwright PC, Reilly SM, editors. Chicago: University of Chicago Press; 1994. pp. 13–41. [Google Scholar]
- 82.Hurlbert AH, White EP. Disparity between range map- and survey-based analyses of species richness: Patterns, processes and implications. Ecol Lett. 2005;8:319–327. [Google Scholar]
- 83.Peterson AT. Predicting species’ geographic distributions based on ecological niche modeling. Condor. 2001;103:599–605. [Google Scholar]
- 84.Murphy HT, VanDerWal J, Lovett-Doust J. Distribution of abundance across the range in eastern North American trees. Glob Ecol Biogeogr. 2006;15:63–71. [Google Scholar]
- 85.Sagarin RD, Gaines SD, Gaylord B. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol Evol. 2006;21:524–530. doi: 10.1016/j.tree.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 86.VanDerWal J, Shoo LP, Johnson CN, Williams SE. Abundance and the environmental niche: Environmental suitability estimated from niche models predicts the upper limit of local abundance. Am Nat. 2009;174:282–291. doi: 10.1086/600087. [DOI] [PubMed] [Google Scholar]
- 87.Legendre P, Legendre L. Numerical Ecology. Amsterdam: Elsevier; 1998. [Google Scholar]
- 88.McGill B, Collins C. A unified theory for macroecology based on spatial patterns of abundance. Evol Ecol Res. 2003;5:469–492. [Google Scholar]
- 89.Svenning JC, Skov F. Limited filling of the potential range in European tree species. Ecol Lett. 2004;7:565–573. [Google Scholar]
- 90.Case TJ, Holt RD, McPeek MA, Keitt TH. The community context of species’ borders: Ecological and evolutionary perspectives. Oikos. 2005;108:28–46. [Google Scholar]
- 91.Etienne R, Wertheim B, Hemerik L, Schneider P, Powell J. The interaction between dispersal, the Allee effect and scramble competition affects population dynamics. Ecol Modell. 2002;148:153–168. [Google Scholar]
- 92.Belyea LR, Lancaster J. Assembly rules within a contingent ecology. Oikos. 1999;86:402–416. [Google Scholar]
- 93.Jenkins DG, Buikema AL. Do similar communities develop in similar sites—a test with zooplankton structure and function. Ecol Monogr. 1998;68:421–443. [Google Scholar]
- 94.Torchin ME, Mitchell CE. Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ. 2004;2:183–190. [Google Scholar]
- 95.Gandon S. Local adaptation and the geometry of host–parasite coevolution. Ecol Lett. 2002;5:246–256. [Google Scholar]
- 96.Morgan AD, Gandon S, Buckling A. The effect of migration on local adaptation in a coevolving host–parasite system. Nature. 2005;437:253–256. doi: 10.1038/nature03913. [DOI] [PubMed] [Google Scholar]
- 97.Greischar M, Koskella B. A synthesis of experimental work on parasite local adaptation. Ecol Lett. 2007;10:418–434. doi: 10.1111/j.1461-0248.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- 98.Nuismer SL, Gandon S. Moving beyond common-garden and transplant designs: Insight into the causes of local adaptation in species interactions. Am Nat. 2008;171:658–668. doi: 10.1086/587077. [DOI] [PubMed] [Google Scholar]
- 99.Thrall PH, Hochberg ME, Burdon JJ, Bever JD. Coevolution of symbiotic mutualists and parasites in a community context. Trends Ecol Evol. 2007;22:120–126. doi: 10.1016/j.tree.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- 101.Fallon SM, Bermingham E, Ricklefs RE. Island and taxon effects in parasitism revisited: Avian malaria in the Lesser Antilles. Evolution. 2003;57:606–615. doi: 10.1111/j.0014-3820.2003.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 102.Fallon SM, Fleischer RC, Graves GR. Malarial parasites as geographical markers in migratory birds? Biol Lett. 2006;2:213–216. doi: 10.1098/rsbl.2005.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fenner F, Ratcliffe FN. Myxomatosis. Cambridge: Cambridge University Press; 1965. [Google Scholar]
- 104.Ricklefs RE, Bermingham E. The concept of the taxon cycle in biogeography. Glob Ecol Biogeogr. 2002;11:353–361. [Google Scholar]
- 105.Ricklefs RE, Bermingham E. The causes of evolutionary radiations in archipelagoes: Passerine birds in the Lesser Antilles. Am Nat. 2007;169:285–297. doi: 10.1086/510730. [DOI] [PubMed] [Google Scholar]
- 106.Ricklefs RE. Host-pathogen coevolution, secondary sympatry, and species diversification. Philos Trans R Soc Lond B Biol Sci. 2009 doi: 10.1098/rstb.2009.0279. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klironomos JN. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology. 2003;84:2292–2301. [Google Scholar]
- 108.Gandon S, Van Zandt PA. Local adaptation and host–parasite interactions. Trends Ecol Evol. 1998;13:214–216. doi: 10.1016/s0169-5347(98)01358-5. [DOI] [PubMed] [Google Scholar]
- 109.Wunderle JM. An ecological comparison of the avifaunas of Grenada and Tobago, West Indies. Wilson Bull. 1985;97:356–365. [Google Scholar]
- 110.McPeek MA, Brown JM. Clade age and not diversification rate explains species richness among animal taxa. Am Nat. 2007;169:E97–E106. doi: 10.1086/512135. [DOI] [PubMed] [Google Scholar]