Abstract
Cholesterol is essential for the growth and function of all mammalian cells, but abnormally elevated levels of circulating low-density lipoprotein cholesterol (LDL-C) are a major risk factor for the development of atherosclerotic cardiovascular disease (ASCVD). For many years, statin drugs have been used to effectively lower LDL-C, but ASCVD still persists in most of the world. Hence, additional LDL-C lowering is now recommended, and the search for therapeutic strategies that work in synergy with statins has now begun. Intestinal absorption and biliary excretion of cholesterol represent two major pathways and continue to show promise as druggable processes. Importantly, both of these complex physiological pathways are tightly regulated by key proteins located at the apical surface of the small intestine and the liver. One of these proteins, the target of ezetimibe Niemann-Pick C1-Like 1 (NPC1L1), was recently identified to be essential for intestinal cholesterol absorption and protect against excessive biliary sterol loss. In direct opposition of NPC1L1, the heterodimer of ATP-binding cassette transporters G5 and G8 (ABCG5/ABCG8) has been shown to be critical for promoting biliary cholesterol secretion in the liver, and has also been proposed to play a direct role in intestinal disposal of sterols. The purpose of this review is to summarize the current state of knowledge regarding the function of these opposing apical cholesterol transporters, and provide a framework for future studies examining these proteins.
Keywords: ezetimibe, statin, cholesterol, phytosterol, sterolin, fecal sterol loss, sitosterolemia, biliary sterol secretion
INTRODUCTION
Atherosclerosis is the disease process underlying the clinical complications of coronary heart disease (CHD), an epidemic that has cost more lives in this country over the past century than the next four causes combined [1]. It was estimated that in 2007, cardiovascular diseases would account for $431.8 billion in direct and indirect costs in the United States alone, a significant increase from $393.5 billion in 2005. The most accurate predictor of CHD incidence is the plasma concentrations of low-density lipoprotein cholesterol (LDL-C). Hence, lowering LDL-C has been the primary therapeutic goal for decades, and statins have been the main drugs used to accomplish this since the late 1980s. However, even with the substantial LDL-C lowering achieved with statin treatments, these drugs have reduced CHD-associated mortality and morbidity by only ~30% [2]. Therefore, emphasis has now shifted towards developing novel therapies that would work in synergy with statins to further lower LDL-C and associated CHD risk. Currently, the two most attractive synergistic therapeutic strategies are drugs that work to 1) prevent intestinal cholesterol absorption, or 2) augment biliary secretion of cholesterol in order to promote fecal sterol loss. Interestingly, both of these complex physiological processes are tightly controlled by identical transport proteins that reside on the apical membrane of both enterocytes in the small intestine and hepatocytes in the liver. These proteins, Niemann-Pick C1-Like 1 (NPC1L1) and ATP-binding cassette transporters G5 and G8 (ABCG5/ABCG8), serve as opposing gatekeepers in the liver and intestine to tightly regulate whole body sterol homeostasis (Fig. 1). The purpose of this review is to summarize the current state of knowledge regarding the biology of NPC1L1 and ABCG5/ABCG8, and summarize why these proteins are attractive targets for future non-statin based therapeutics.
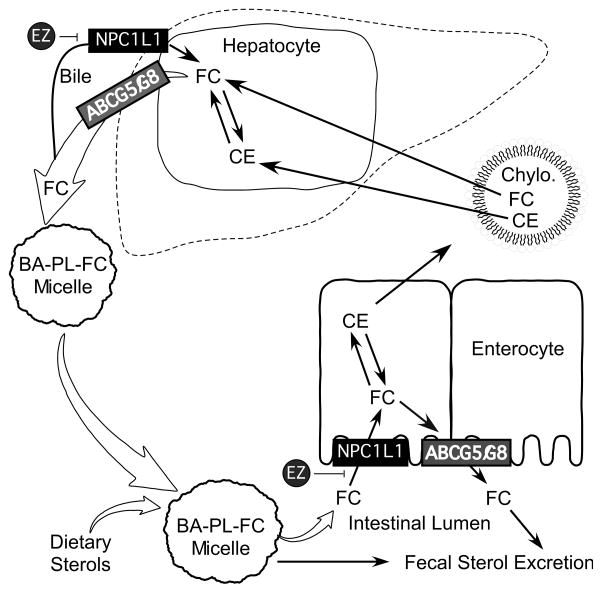
Fig. 1.
Role of NPC1L1 and ABCG5/ABCG8 in enterohepatic circulation of sterols. In the gut lumen, free cholesterol (FC) derived from diet and bile is mixed with bile acids (BA) and phospholipids (PL) to form micelles. FC in micelles is transported by NPC1L1 into the absorptive enterocyte where the majority of FC is converted to cholesteryl ester (CE) for the assembly into chylomicrons (Chylo.) for delivery into the body. A proportion of FC in enterocyte is presumably secreted back into the gut lumen by ABCG5/ABCG8. Chylomicrons deliver their cargo (FC and CE) to the liver. In hepatocytes, a proportion of FC is secreted into the canalicular bile by ABCG5/ABCG8. FC in the canalicular bile may be recaptured by NPC1L1 back into hepatocyte or delivered to the gut lumen for another round of enterohepatic circulation. Importantly, ezetimibe (EZ) blocks NPC1L1-dependent sterol uptake at both the apical surface of enterocytes and the canalicular membrane of hepatocytes.
CHOLESTEROL’S WAY IN: NIEMANN PICK-C1 LIKE-1 (NPC1L1)
Discovery of a Dedicated Protein That Facilitates Intestinal Cholesterol Absorption: A New Hypolipidemic Target
Cholesterol is essential for the growth and function of all mammalian cells. Therefore, the body has devised many elegant pathways to maintain cellular cholesterol levels at a set point. It is well appreciated that cholesterol can either be obtained through dietary sources or synthesized from endogenous precursors. Both of these pathways have been intensively studied, and several effective drugs have surfaced targeting both pathways. Statins, the champion of hypocholesterolemic drugs, work very effectively to limit de novo synthesis of cholesterol. A breakthrough in the late 1990s [3] gave rise to a new hypolipidemic drug that specifically blocks intestinal absorption of dietary and biliary cholesterol known as ezetimibe (Zetia; SCH 58235; 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone). For many years intestinal cholesterol absorption was thought to be a passive process, but the second order kinetics [4] and sterol specificity of this process [5,6], and the fact that ezetimibe could specifically and dose-dependently inhibit the process [3,7] lead to a search for candidate ezetimibe-sensitive sterol transport protein(s) in the intestine.
At nearly the same time ezetimibe was discovered, the first clues into the identity of this elusive intestinal sterol transport protein surfaced [8]. Through a genomic and bioinformatics approach, Yiannis Ioannou and colleagues first identified a protein that shared a high sequence identity (42%) and similiarity (51%) with the late endosomal protein Niemann-Pick C1 (NPC1), and therefore named this protein Niemann-Pick C1-Like 1. Like NPC1, NPC1L1 contains a putative sterol-sensing domain [8], and shares similarity with the resistance-nodulation-division family of prokaryotic permeases that are involved in the transport of lipophilic drugs, detergents, fatty acids, and bile acids across the plasma membrane, suggesting a potential role in sterol transport. Given this information, and the coincident availability of a powerful tool to inhibit intestinal cholesterol absorption (ezetimibe), the stage was set for further investigation into molecular mechanisms regulating intestinal cholesterol absorption.
NPC1L1 Is Critical For Intestinal Cholesterol Absorption and Is the Target of Ezetimibe
In 2004, in a landmark paper, scientists at Schering-Plough provided the first insight into the function of NPC1L1 [9]. This work clearly demonstrated that mice lacking NPC1L1 have severely diminished intestinal cholesterol absorption, a finding that has been consistently substantiated by multiple investigators [10–13]. Furthermore, ezetimibe administration inhibited cholesterol absorption to the same level seen in NPC1L1 knockout mice, and ezetimibe treatment in NPC1L1 knockout mice caused no further reduction in cholesterol absorption, providing indirect evidence that NPC1L1 may be the target of ezetimibe [9]. More direct evidence for this connection was first published by Garcia-Calvo and colleagues [14]. These investigators showed that the glucuronidated form of ezetimibe binds with high affinity to cells ectopically overexpressing NPC1L1 and to intestinal brush border membranes from wild type mice, but did not bind to intestinal membranes from NPC1L1 knockout mice [14]. The interaction site of ezetimibe binding has been traced to a 61 amino acid region of the extracellular domain in dog NPC1L1, and Phe-532 and Met-543 seem to be especially critical for binding [15]. In further support of direct binding, Hawes and colleagues demonstrated that the well-documented species-specific responsiveness to ezetimibe correlates directly with NPC1L1 binding affinity [16]. The most impressive preclinical demonstration of the importance of the ezetimibe-NPC1L1 pathway was a study where ApoE knockout mice either received ezetimibe treatment or had NPC1L1 genetically deleted [13]. This study clearly demonstrated that inhibition of NPC1L1 results in dramatic LDL-C lowering, and complete protection against murine atherosclerosis [13].
NPC1L1 Is Critical For Hepatobiliary Cholesterol Secretion: Another Target of Ezetimibe Action
Interestingly, rodents express NPC1L1 almost exclusively in the small intestine [9,17]. In contrast, humans express high levels of NPC1L1 mRNA in the liver and the small intestine [9,17]. We recently discovered that NPC1L1 protein is abundantly expressed in human liver, and localizes to the canalicular membrane of both primate and human hepatocytes [18]. Based upon these findings, we hypothesized that hepatic NPC1L1 may facilitate the recapture of newly secreted biliary cholesterol by hepatocytes, and that ezetimibe may be able to disrupt this pathway. To test this hypothesis, we generated transgenic mice expressing human NPC1L1 specifically in hepatocytes. Hepatic overexpression of NPC1L1 resulted in a greater than 90% decrease in biliary cholesterol concentration, but had no effect on biliary phospholipid and bile acid concentrations. Quite strikingly, biliary cholesterol concentrations in these animals were returned virtually to normal with ezetimibe treatment. These findings suggest that in humans, ezetimibe may reduce plasma cholesterol by inhibiting NPC1L1 function in both the intestine and liver, and hepatic NPC1L1 may have evolved to protect the body from excessive biliary loss of cholesterol. Collectively, we believe that in humans NPC1L1 may have evolved at two sites (apical membrane of enterocytes and canalicular membrane of hepatocytes) to facilitate cholesterol uptake, protecting the body against fecal and biliary loss of cholesterol.
NPC1L1 Is a Clinically Relevant Target in Humans: LDL-C Lowering by Ezetimibe
Given the attractive pharmacological profile seen in rodent models, the clinical relevance of the target of ezetimibe (NPC1L1) was examined in primates and humans. In cynomolgus monkeys, treatment with an ezetimibe analogue significantly reduced the cholesterol content of postprandial chylomicrons, without affecting triglycerides or apoB-48 levels [19]. Ezetimibe treatment in mildly hypercholesterolemic [20] or vegetarian [21] humans resulted in a dramatic 54% or 58% reduction in cholesterol absorption, respectively. Importantly, in these studies, ezetimibe treatment significantly reduced LDL-C by 17–20% [20,21]. These studies clearly demonstrated that ezetimibe was effective in blocking the intestinal absorption of both dietary and biliary cholesterol, with parallel reductions of LDL-C in humans. The safety and efficacy of ezetimibe monotherapy have been subsequently tested in two randomized double-blind studies [22,23]. In a pooled analysis from both of these studies it was shown that ezetimibe effectively lowered LDL-C by 18.2%, lowered plasma triglycerides, and also significantly increased high density lipoprotein cholesterol (HDL-C) [24]. Importantly, ezetimibe treatment was shown to be extremely safe and was well tolerated across all groups [22–24].
Since ezetimibe works by inhibiting cholesterol absorption [9–13, 19–21] and promoting biliary cholesterol secretion [18], it positions itself as an ideal complimentary LDL-C-lowering drug for use as a dual therapy with a statin. Therefore, several large clinical trials have tested whether coadministration of ezetimibe and statin could provide additional LDL-C lowering above statin treatment alone. In the ezetimibe add-on to statin for effectiveness (EASE) trial, greater than three thousand patients were randomized into groups receiving either statin plus ezetimibe or statin plus placebo [25]. This study showed that statin plus ezetimibe dual therapy was well tolerated and resulted in a 26% decrease in LDL-C, whereas statin plus placebo only reduced LDL-C by 2.7%. In a separate study, ezetimibe plus statin dual therapy was given to patients with homozygous familial hypercholesterolemia (FH), and demonstrated that dual therapy resulted in an additional LDL-C lowering of 14–21%, when compared to statin monotherapy [26]. This study provided important new information that LDL-C lowering by ezetimibe does not rely on functional LDL receptors, and offers FH patients another viable treatment option [26]. In addition to these trials, four different statins (simvastatin, atorvastatin, lovastatin, and pravastatin) have been given in combination with ezetimibe in independent phase III studies [27–30], with strikingly similar results. Regardless of the type of statin used, ezetimibe plus low dose statin (10 mg) reduced LDL-C to a similar extent as the highest statin dose (40–80 mg, according to statin) [27–30]. Collectively, these studies provided strong evidence that this dual therapy was extremely effective in lowering LDL-C in humans, and may provide benefit above statin monotherapy [27–30].
Given this information, a fixed dose combination of simvastatin and ezetimibe (Vytorin®) was developed and approved by the FDA in 2004, and it dramatically lowered LDL-C by 45–60% over a 10–80 mg simvastatin dose range [31]. Subsequent studies with this dual therapy demonstrated that ezetimibe/simvastatin was superior to atorvastatin (80 mg) in the percent LDL-C reduction [32], and that at each dose (10, 20, 40, or 80 mg) of statin the ezetimibe/simvastatin dual therapy reduced LDL-C to a greater extent than statin alone [33]. Furthermore, in the high-risk patients with type II diabetes, ezetimibe/simvastatin dual therapy resulted in a greater percentage of patients who attained a LDL-C level of <70 mg/dL, when compared to statin alone [34]. Collectively, studies looking at the benefit of adding ezetimibe to statin therapy have provided striking evidence that this dual therapy is the most effective LDL-C lowering therapy known to date. Therefore, four highly publicized randomized, double-blind, clinical outcomes trials (ENHANCE = Ezetimibe and Simvastatin in Hypercholerolemia Enhances Atherosclerosis Regression study; SEAS = Simvastatin and Ezetimibe in Aortic Stenosis study; SHARP = Study of Heart and Renal Protection; and IMPROVE IT = the Improved Reduction of Outcomes: Vytorin Efficacy International Trial) have been initiated to answer a long-standing question in the field: Does the dramatic LDL-C lowering seen with inhibition of cholesterol absorption and de novo sterol synthesis translate into reduced ASCVD and microvascular complications? Results of these studies are just now coming to light [35,36], and have sparked an unfortunate controversy in the field [37,38] that will only be solved with additional studies.
The Cell Biology of NPC1L1: Cholesterol-Sensitive Endocytic Recycling
With strong in vivo evidence in hand regarding the role of NPC1L1 in intestinal cholesterol absorption and biliary sterol transport, several investigators have attempted to define the molecular mechanism by which NPC1L1 facilitates cellular cholesterol transport. However, initial attempts at developing a functional cell-based assay for NPC1L1-dependent cholesterol transport was unexpectedly difficult. In 2005, our laboratory was able to first demonstrate a functional cellular assay for NPC1L1-dependent sterol transport [39]. The rationale for development of this assay stemmed from previous work that had demonstrated that NPC1L1 could localize to either the plasma membrane [39,40], or to an ill-defined intracellular compartment [17,40]. Based on this, we reasoned that NPC1L1 could be dynamically trafficking to and from the plasma membrane through an endocytic recycling event. After testing a number of conditions known to affect endocytic recycling, we serendipitously treated hepatoma cells overexpressing NPC1L1 with methyl-β-cyclodextrin (Mβ CD) to deplete the cells of cholesterol. We found that under these conditions NPC1L1 translocates from the endocytic recycling compartment to an apical subdomain in the plasma membrane, where it can facilitate cellular cholesterol transport [39,41]. From these studies we now appreciate that initial attempts to establish a functional assay for NPC1L1 likely failed simply due to the fact that the majority of NPC1L1 proteins reside intracellularly in sterol-replete cells, where they do not actively facilitate sterol transport. In agreement with our studies, independent investigators have demonstrated endocytic recycling-dependent, NPC1L1-driven cholesterol transport in a variety of cell models [41–45]. Furthermore, we have demonstrated that NPC1L1 is a unidirectional transporter of unesterified cholesterol, and not cholesterol ester [41], clearly making this pathway distinct from other cholesterol receptors such as the LDL receptor [46] or the scavenger receptor class B type I (SR-BI) [47]. Importantly, ezetimibe dose-dependently inhibits NPC1L1-dependent cellular cholesterol transport [39, 42, 43, 45], further establishing NPC1L1 as a direct target of ezetimibe.
Until very recently, the molecular mechanism by which ezetimibe inhibits NPC1L1-dependent sterol transport was not well understood. In an elegant study, Ge and colleagues provided the first clues into the mechanism of ezetimibe action [45]. Interestingly, following cholesterol depletion-driven movement of NPC1L1 to the plasma membrane (i.e. Mβ CD treatment), adding back cholesterol to the cells resulted in rapid internalization of NPC1L1 via clathrin/AP2-dependent vesicular endocytosis [45]. Importantly, ezetimibe functionally blocks cholesterol-induced NPC1L1 internalization and cholesterol uptake [45]. Currently, published data support a model where NPC1L1 acts not as a permease, but rather as a free cholesterol receptor that is internalized via cholesterol-sensitive and ezetimibe-inhibitable clathrin-mediated endocytosis [39–45]. However, more work is needed to comprehensively define this clinically important pathway.
The Sterol Specificity of NPC1L1: Does It Discriminate?
It has long been known that vegetarian humans and animals consume only plant-derived sterols in their diets, yet all the cells in their body contain large amounts of cholesterol and no plant sterols [48,49]. Observations like these have intrigued many investigators, and have lead to the discovery that plant sterols are completely excluded from the body, yet may compete with cholesterol for incorporation into micelles in the intestinal lumen, thereby indirectly blocking the intestinal absorption of cholesterol [50–53]. Importantly, although mutations in ABCG5 and ABCG8 (discussed in detail below) cause the accumulation of plant sterols in the body [54,55], the rank order of intestinal absorption among animal and plant sterols is maintained in ABCG5/ABCG8 knockout mice [56], indicating that ABCG5/ABCG8 is a gatekeeper rather than the intestinal discriminator. The search for protein mediators of sterol selectivity at the level of the intestine has continued for decades, without a clear understanding. It remains possible that NPC1L1 may be one such protein contributing to intestinal sterol selectivity, but more work is needed for clarification since current data are conflicting.
It is quite clear that NPC1L1 knockout mice have significantly reduced plant sterol absorption [12]. In agreement, ezetimibe treatment effectively blunts the accumulation of plant sterols seen in genetically sitosterolemic patients [57] and mice [58]. Furthermore, we have recently shown that targeted deletion of NPC1L1 prevents plant sterol accumulation in the body in genetically sitosterolemic mice (ABCG5/ABCG8 knockouts) [11]. All of the in vivo observations strongly support that NPC1L1 does not discriminate, and facilitates the intestinal absorption of both cholesterol and plant sterols. However, data generated in minimal cell models of NPC1L1-dependent transport have not substantiated this [41,43,45]. In hepatoma cells overexpressing NPC1L1, we were able to demonstrate robust NPC1L1-dependent transport of cholesterol, but not β-sitosterol [41]. In fact, the kinetics of cholesterol and β-sitosterol transport were strikingly different in hepatoma cells, indicating different transport mechanisms for the two sterols [41]. In agreement, it has been shown that the NPC1L1-dependent transport of β-sitosterol is 60% lower than that of cholesterol in CaCo2 cells [43]. Furthermore, when examining the sterol-dependent endocytic recycling of NPC1L1, Ge and colleagues [45 found that clathrin-dependent internalization of NPC1L1 could only be induced by cholesterol, but not plant sterols, further indicating sterol specificity. Collectively, in several cellular models of NPC1L1-dependent sterol transport, it seems that NPC1L1 can indeed discriminate between sterols of plant and animal origin.
Generally in vivo data trumps in vitro data, but in the case of NPC1L1 one must consider both arguments. One possible explanation for the reported decreases in sitosterol absorption seen in mice lacking NPC1L1 is that NPC1L1 protein is the transporter for phytosterols, but its affinity to these sterols is much lower than to cholesterol. Alternatively, the lack of phytosterol accumulation in NPC1L1-deficient mice may be indirect, and may instead be mediated by the drastic reduction in enterocytic cholesterol content seen when intestinal absorption is blocked, resulting in activation or repression of sterol-regulated transcription factors. For instance, Davis and colleagues [12] reported that mice lacking NPC1L1 showed dramatic decreases in intestinal ABCA1 mRNA expression, most likely resulting from diminished cholesterol-driven liver X receptor (LXR)-mediated transcription. Interestingly, intestinal ABCA1 expression has been recently implicated in promoting intestinal cholesterol absorption [59,60], and has been shown to mediate the basolateral transport of β-sitosterol out of CaCo-2 cells [61], Therefore the decreases in intestinal ABCA1 seen in NPC1L1-knockout mice may partially explain the diminished intestinal β-sitosterol absorption. It is not hard to imagine that, in addition to ABCA1, the expression of many sterol-sensitive genes are altered in the intestine of NPC1L1 knockout mice, and one of the genes may be critical for plant sterol transport. Furthermore, the fact that ezetimibe normalizes elevated plasma sitosterol levels in sitosterolemic humans [57] and mice [58] could also be a secondary effect of the potent cholesterol absorption inhibiting effects of ezetimibe (i.e. altering sterol-driven gene expression in the intestine), and not a direct effect on NPC1L1 per se. Although it is quite clear that NPC1L1 readily transports cholesterol through a clathrin-mediated endocytic pathway [39,41–43,45], the same may not be true for plant sterols [41,43,45]. Additional work is needed to clarify the route by which plant sterols are transported into intestinal enterocytes.
Transcriptional Regulation of NPC1L1
In addition to posttranslational control (endocytic recycling), there is emerging data that NPC1L1 may be transcriptionally controlled by sterol-sensing transcription factors. The first evidence of this was presented by Duval and colleagues, who showed that mice treated with a synthetic LXR agonist had reduced NPC1L1 expression in the intestine [62]. The mechanism behind the reduction has yet to be elucidated. Further work has demonstrated that intestinal NPC1L1 mRNA levels are downregulated in response to a cholesterol/cholate-containing diet [12]. It has also been shown that dietary fish oil can cause reductions in intestinal NPC1L1 expression [63]. In addition, in two genetic mouse models of altered intestinal cholesterol metabolism (acyl-CoA:cholesterol acyltransferase 2 knockouts and phospholipid transfer protein knockouts), NPC1L1 mRNA is diminished, indicative of transcriptional regulation [60,64]. More direct evidence of sterol-sensitive transcriptional control was demonstrated recently by Alrefai and colleagues [65]. This study demonstrated that the human promoter for NPC1L1 contains two functional sterol response elements, and demonstrated that NPC1L1 was indeed a target for the sterol regulatory element-binding protein 2 (SREBP2). In a subsequent study it was shown that hepatocyte nuclear factor 4α (HNF4α) works synergistically with SREBP2 in the NPC1L1 promoter for sterol-sensitive repression [66]. Although our understanding of the transcriptional control of NPC1L1 is in its infancy, it seems that NPC1L1 expression is regulated by sterol-sensitive transcription factors.
Inhibiting NPC1L1 May Have Multiple Therapeutic Benefits: NPC1L1’s Future
Over the last eight years NPC1L1 has solidified itself as a critical gatekeeper of cholesterol absorption and biliary sterol secretion, thus positioning itself as an attractive target for prevention of cholesterol-driven diseases such as atherosclerosis. However, inhibition of NPC1L1 may provide benefits for other important metabolic diseases as well. It has recently been shown that ezetimibe-mediated inhibition of intestinal cholesterol absorption prevents diet-induced increases in biliary cholesterol secretion in hamsters [67]. In parallel, ezetimibe decreases biliary cholesterol levels and gallstone formation in mice fed a lithogenic diet [68], and in patients presenting with cholesterol gallstones [68,69]. In fact ezetimibe treatment was able to facilitate the dissolution of gallstones that were already present prior to treatment in mice [68]. Collectively, these data have shown that ezetimibe-mediated inhibition of intestinal cholesterol absorption may prevent the formation of cholesterol gallstones and potentially act as an effective cholelitholytic agent [67–69]. In addition, ezetimibe has been shown to ameliorate hepatic steatosis, and now has potential as an antisteatotic therapy [70,71]. Ezetimibe has also been shown to blunt high fat diet-induced obesity and insulin resistance in mice [72]. Most recently, the genetic inactivation of NPC1L1 in mice was shown to attenuate hepatic steatosis caused by an LXR agonist [10] and protect against high fat diet-induced obesity [72] (Yu L, unpublished observation). More work is needed to understand the molecular mechanisms underlying these intriguing results. Simply by regulating how much cholesterol enters the body, NPC1L1 has the potential to be a master regulator of metabolic diseases. The future is bright for NPC1L1 as a viable therapeutic target in humans, yet additional work is needed.
CHOLESTEROL’S WAY OUT: ATP-BINDING CASSETTE PROTEINS G5 AND G8 (ABCG5/ABCG8)
Discovery of ABCG5/ABCG8: Sitosterolemia and the Power of Human Genetics
As described above, most humans consume large amounts of dietary plant sterols, yet these sterols are excluded from the body, primarily at the level of the intestine. [48–53]. A major breakthrough regarding this issue came when Bhattacharayya and Connor described a novel disease in which two sisters presented to the clinic with tendon xanthomas, and were surprisingly shown to have extremely elevated levels of plasma plant sterols (primarily β-sitosterol) [73]. Hence, the disease was named β-sitosterolemia, and the authors proposed that this disease was caused by a single genetic defect that permitted the intestinal absorption of plant sterols. Sitosterolemic patients have elevated fractional absorption of dietary sterols and diminished ability to secrete multiple sterols into bile [73–76]. As a result, sitosterolemia manifests as the accumulation of both plant and animal sterols in the plasma, skin, tendons, and coronary arteries, and most affected individuals suffer from premature CHD [77–80]. Sitosterolemia was subsequently mapped to a single locus (STSL, sitosterolemia locus) on human chromosome 2p21 [54,55,81]. The STSL locus was found to contain two genes ABCG5 and ABCG8 that were positioned in a head-to-head orientation, and mutations in either of these genes were shown to be causative of sitosterolemia [54,55]. These two genes were shown to encode two distinct proteins known as sterolin-1 (ABCG5) and sterolin-2 (ABCG8).
Since the discovery of the genetic basis for sitosterolemia, there has been intensive effort put forth to understand the function of both ABCG5 and ABCG8. Like NPC1L1, ABCG5 and ABCG8 are expressed almost exclusively on the apical membrane of enterocytes in the intestine and hepatocytes in the liver [54,55,82,83,84]. As will be discussed in detail later, ABCG5 and ABCG8 are obligate heterodimers [84], explaining why single mutations in either of these genes causes sitosterolemia. It was originally hypothesized that the ABCG5/ABCG8 heterodimeric complex served as an efflux pump to remove sterols (cholesterol and plant sterols) from cells, trafficking them either back into the lumen of small intestine in the enterocyte or into bile in the hepatocyte. If this were true, ABCG5/ABCG8 would likely play a rate-limiting role in both intestinal sterol absorption and hepatobiliary sterol secretion by opposing the actions of NPC1L1, which experimental evidence has now substantiated.
The Role of ABCG5/ABCG8 in Intestinal Sterol Absorption
In contrast to NPC1L1, which directly transports cholesterol from the gut lumen into enterocytes, ABCG5/ABCG8 likely plays an opposing role, thereby indirectly regulating net sterol absorption. A potential role for ABCG5/ABCG8 in intestinal cholesterol absorption was first discovered in sitosterolemic patients, who have elevated retention of both plant sterols and cholesterol in the body [74–76, 85–87]. In fact, sitosterolemic patients retain roughly 20–30% of dietary sitosterol, compared to <5% absorption in unaffected individuals [73,74,85,87]. Importantly, mice lacking either ABCG5 alone [88], ABCG8 [89,90] alone, or mice lacking both transporters [56] exhibit sitosterolemia, which can likely in part be explained by increased intestinal absorption of phytosterols. In support of this concept, in mice lacking both ABCG5 and ABCG8 fed a chow diet, intestinal absorption of cholesterol was not significantly altered, yet the absorption of sitosterol, cholestanol, campesterol was significantly increased [56]. Furthermore, in a recent study by Wang and colleagues, it was elegantly demonstrated the lymphatic transport rate of cholesterol and sitostanol was increased by ~40% and 500%, respectively in mice lacking ABCG8 [90]. In agreement, mice transgenically overexpressing ABCG5 and ABCG8 in the intestine and liver exhibit a 50% reduction in fraction cholesterol absorption, and fecal neutral sterol levels were shown to increase 3–6 fold [91]. Collectively, work done in both sitosterolemic humans and animal models has supported an important role for ABCG5/ABCG8 in intestinal sterol absorption. The ABCG5 and ABCG8 heterodimer in the intestine is thought to be an efflux pump to deliver sterols from enterocytes back into the lumen of the small intestine for fecal disposal, but direct experiment evidence of the concept is still lacking.
ABCG5/ABCG8 Is Critical For Promoting Hepatobiliary Cholesterol Secretion
Opposing the previously described function for NPC1L1 in the liver [18], ABCG5 and ABCG8 are critical for promoting the removal of neutral sterols from the body through biliary secretion. In fact, sitosterolemic patients have severely impaired ability to secrete multiple sterols into bile [74,76,85]. In parallel, in mice lacking both ABCG5 and ABCG8, cholesterol concentrations in gall bladder bile were reduced by >90%, yet biliary phospholipids and bile acids concentrations were normal [56]. Similar effects have been seen in mice lacking ABCG5 [88] or ABCG8 [89,90] alone. Also, mice transgenically overexpressing ABCG5 and ABCG8 in the intestine and liver had a > 5-fold increase in biliary cholesterol output [91]. In addition to these data, it has been shown that ABCG5 and ABCG8 mRNA expression is highly correlated with biliary cholesterol secretion rates in a variety of different mouse strains and conditions [92]. Collectively, there is a large body of evidence that the ABCG5/ABCG8 heterodimer represents the major route for delivery of sterols into bile. However, there is emerging support for an alternative ABCG5/ABCG8-independent route for biliary sterol secretion under some conditions [92–94], which requires further exploration.
The LDL-C Lowering and Atheroprotective Role of ABCG5/ABCG8
ABCG5/ABCG8 plays a crucial role in cholesterol excretion from the body. Based on this, it seemed logical to assume that mice lacking the heterodimer should have increased atherosclerotic burden, while mice transgenically overexpressing ABCG5/ABCG8 should be protected against atherosclerosis. These hypotheses have been tested, and provide useful insight into the potential for ABCG5/ABCG8 to become a viable therapeutic target for ASCVD. In LDL receptor knockout mice, as expected high-level overexpression of ABCG5/ABCG8 in both the intestine and liver results in reduced LDL-C and significantly less atherosclerosis [95]. In contrast, hepatocyte-specific ABCG5/ABCG8 overexpression, which elevated biliary sterol secretion ~2-fold, did not protect against atherosclerosis in the LDL receptor or apolipoprotein E (ApoE) knockout backgrounds [96]. This result was very confusing, since it was anticipated that a 2-fold increase in biliary cholesterol output would result in atheroprotection. In a subsequent study, these same authors clarified this confusing outcome, and showed that ezetimibe treatment in mice overexpressing ABCG5/ABCG8 specifically in the liver produced profound protection against atherosclerosis [97]. These data strongly support the opposing roles of ABCG5/ABCG8 and NPC1L1, and demonstrate that therapies that simply increase biliary sterol output may not be effective at altering sterol balance and atherosclerosis, since these sterols can re-enter the body via the actions of intestinal NPC1L1 [98]. However, dual therapies that increase biliary sterol output and block re-uptake of biliary sterols by the intestine continue to hold promise.
It is well known that many sitosterolemic patients suffer from premature ASCVD [77–80]. However, whether this ASCVD is caused by the massive accumulation of plant sterols in the blood or the associated mild to moderate hypercholesterolemia has been a matter of debate. Without a doubt hypercholesterolemia is a major driving force in ASCVD progression, but little is known about the consequences of elevated blood plant sterols because in organisms with intact ABCG5/ABCG8 plant sterols are efficiently excluded from the body. Therefore, sitosterolemic patients and ABCG5/ABCG8 knockout mice provide a unique research opportunity to ask the age-old question: How are plant sterols metabolized, and do plant sterols like their animal counterparts contribute to atherogenesis? Importantly, the plant sterol stigmasterol has been shown regulate cholesterol metabolism by directly inhibiting SREBP2 processing and activating LXR-driven gene expression [98]. In addition the plant sterol sitosterol has recently been shown to drive macrophage cell death [99], an important feature of the late stages of ASCVD. These and many other findings have promoted a potential role for plant sterols in the progression of ASCVD in sitosterolemic patients. Recently, a study examined this relationship in both ABCG5/ABCG8 knockout mice and in sitosterolemic patients [100]. It was found that plasma levels of plant sterols did not correlate with atherosclerosis extent in either of these sitosterolemic models, leading the authors to conclude that perhaps elevated plasma cholesterol levels are responsible for the development of premature ASCVD in sitosterolemic patients [100]. At this time the atherogenicity of plant sterols remains in question, but without a doubt more studies are needed to understand why sitosterolemic patients suffer from premature CHD.
The Cell Biology of ABCG5/ABCG8: Obligate Heterodimers Regulating Apical Sterol Transport
Although the in vivo relevance of ABCG5 and ABCG8 in both plant sterol and cholesterol homeostasis has been clearly established in humans and genetically modified mice, our understanding of the cell biology of these proteins is still incomplete. What has been clearly demonstrated is that ABCG5 and ABCG8 must heterodimerize in order to transport sterols across membranes [84,101,102]. This is supported by data demonstrating that, when expressed together, the proteins colocalize in the endoplasmic reticulum (ER) and the plasma membrane [84,101], they can be co-immunoprecipitated [84], and the exit of these proteins from the ER to the plasma membrane requires coexpression of both proteins [84,101,102]. From these studies it was postulated that ABCG5 and ABCG8 heterodimerize in the ER, traffic together through the Golgi apparatus, and subsequently target to apical subdomains in the plasma membrane [101]. It has been further demonstrated that both ABCG5 and ABCG8 undergo N-linked glycosylation, and that glycosylation at Asn-619 in ABCG8 is critical for efficient trafficking of the heterodimer [102]. It has also been shown that the ABCG5/ACBG8 heterodimer requires the molecular chaperones, calnexin [102] and calreticulin [102,103] for proper folding and trafficking out of the ER. Subsequent site-directed mutagenesis experiments demonstrated that the majority of mutants causative of sitosterolemia exhibit impaired transport of the heterodimer from the ER to the plasma membrane [102].
Although there is a strong body of work examining the subcellular trafficking and dimerization of ABCG5 and ABCG8, characterization of the sterol transport properties of the heterodimer is still incomplete. Recently, it has been shown that overexpression of ABCG5 and ABCG8 in human kidney and gallbladder epithelial cells promotes the efflux of cholesterol and plant sterols [104,105]. In these studies it was shown that ABCG5/ABCG8-dependent efflux was dependent on the presence of mixed bile salt micelles as an acceptor, whereas other cholesterol acceptors such as apolipoprotein AI, HDL, or Mβ CD failed to efficiently promote ABCG5/ABCG8-dependent efflux [104,105]. More recently, ABCG5/ABCG8-dependent sterol transport has been successfully reconstituted in vitro [106,107]. From these studies it was demonstrated that, with either a recombinant [106] or purified native ABCG5/ABCG8 [107], the heterodimer could directly transport sterols, sterol esters, and phospholipids from donor vesicles to proteoliposomes in an ATP-dependent fashion. With this established in vitro assay [106,107] and complimentary cell-based models [104,105] for ABCG5/ABCG8-dependent sterol transport we now have the tools available to address unanswered questions. For instance, does the ABCG5/ABCG8-transported pool of sterols originate from a cytosolic pool, or does the heterodimer act primarily on a membrane-associated pool as a flippase? Are other proteins required for ABCG5/ABCG8-dependent sterol transport? Additionally, what is the substrate specificity for ABCG5/ABCG8?
The Sterol Specificity of the ABCG5/ABCG8 Heterodimer: Does It Discriminate?
There is now overwhelming evidence that mutations in either ABCG5 or ABCG8 cause abnormal accumulation of plant sterols in the body [54,55,85–90]. Given this fact, most have assumed that the ABCG5/ABCG8 heterodimer is the primary, if not the sole protein, responsible for sterol discrimination in the intestine. However, the current knowledge base does not firmly support this conclusion. In support of this concept, genetically sitosterolemic patients [75], rats [108], and mice [56] still possess the ability to discriminate between cholesterol, campesterol, and sitosterol at the level of intestinal absorption, indicating that ABCG5/ABCG8 is a gatekeeper rather than the intestinal sterol discriminator. Given this information, caution should be taken when assuming the ABCG5/ABCG8 heterodimer is the sole mediator of sterol selectivity in the intestine. Much more work is needed in this area, and with recent progress using cell-based and in vitro systems for ABCG5/ABCG8-dependent sterol transport, we now have the tools to gain further mechanistic insight into the critical question of substrate specificity.
Transcriptional Regulation of ABCG5/ABCG8
Both ABCG5 and ACBG8 seem to be primarily controlled at the transcriptional level. The sterol-sensing transcription factors LXRα and LXRβ seem to be the major regulator of ABCG5 and ABCG8 mRNA expression. In support of this, both dietary cholesterol and synthetic LXR agonists upregulate ABCG5 and ABCG8 mRNA expression in the small intestine and liver of wild type mice, but not LXR knockout mice [54,109–111]. This is indicative of a direct effect, but the presence of a bona fide LXR response element in the ABCG5/ABCG8 intergenic promoter or surrounding areas has not been demonstrated. The physiological relevance of LXR-driven upregulation of ABCG5 and ABCG8 mRNA has recently been highlighted in two separate studies. It was first shown that LXR-driven increases in hepatobiliary and fecal cholesterol excretion rely on functional ABCG5 and ABCG8, since ABCG5/ABCG8 knockout mice could not elevate biliary and fecal sterol secretion in response to a synthetic LXR agonist [112]. In agreement, using ABCG5/ABCG8 knockout mice, Calpe-Berdiel and colleagues demonstrated that LXR-mediated induction of macrophage to feces reverse cholesterol transport requires functional ABCG5/ABCG8 [113].
Another transcriptional activator of the STSL locus is the liver receptor homolog-1 (LRH-1) [114]. However, it is important to point out that a functional LRH-1 binding site is only present in the human gene, and rodent homologs do not possess LRH-1-sensitive transcriptional activation [114]. More recently, it has been shown that three additional transcription factors known as HNF-4α, GATA4, and GATA6 act in a cooperative fashion to transactivate the human intergenic promoter [115]. There is also evidence that bacterial endotoxin can down-regulate ABCG5 and ABCG8 mRNA levels [116], yet the transcription factors involved in this response have yet to be clearly elucidated. Collectively, it is quite clear that ABCG5 and ABCG8 are coordinately regulated at the transcriptional level. More work in this area may prove to be critical for future ABCG5/ABCG8-centered therapies.
Are The Sterolins Viable Therapeutic Targets? ABCG5/ABCG8’s Future
Inter-individual variations exist in responses to statins [117], which were thought to be attributable to differences in baseline cholesterol absorption and/or endogenous cholesterol synthesis among individuals [118,119]. Stimulation of ABCG5/ABCG8-mediated cholesterol excretion not only promotes fecal disposal of cholesterol, but also increases endogenous cholesterol synthesis [120]. Coadministration of a statin and a yet-to-be developed ABCG5/ABCG8 activator is expected to have a synergistic cholesterol-lowering effect. Consistent with this notion, transgenic overexpression of ABCG5/ABCG8 in the liver and intestine confers mice hypersensitive to the cholesterol-lowering capacity of lovastatin [121].
Unlike the success story for NPC1L1 and its inhibitor ezetimibe, a specific pharmacological modulator for ABCG5/ABCG8-dependent sterol transport has yet to be developed. Most current attempts at modulating ABCG5/ABCG8-dependent sterol transport involve the use of a synthetic LXR agonist, which robustly transcriptionally upregulates heterodimer expression in the liver and intestine [54,110–112]. However, LXR activation elicits the unwanted side effect of increased de novo lipogenesis, resulting in pronounced hepatic steatosis [122,123], ruling out synthetic LXR agonists as a safe way to promote ABCG5/ABCG8 function. Therefore, alternative strategies for ABCG5/ABCG8 modulation need to be pursued. It remains possible that the ABCG5/ABCG8 heterodimer may be more directly targeted by small molecule activators. With in vitro and cell-based functional assays for ABCG5/ABCG8-dependent sterol transport in place, it may now be possible to screen for such compounds. Based on the limited data generated in mice transgenically overexpressing ABCG5 and ABCG8, pharmacologic activators of this pathway could serve as powerful promoters of cholesterol removal from the body, and may provide additional protection against atherosclerosis when given in combination with statin therapy.
CONCLUSION AND FUTURE PERSPECTIVES
In the search for cholesterol-lowering therapies in the post-statin era, inhibitors of NPC1L1 and activators of ACBG5/ABCG8 hold great promise as future synergistic therapies. This is true not only for atherosclerosis prevention, but could potentially hold promise for many other prevalent metabolic diseases including hepatic steatosis, cholelithiasis, type II diabetes, and obesity. Acting as opposing gatekeepers at the apical membrane of enterocytes and hepatocytes, NPC1L1 serves as cholesterol’s way in, and ABCG5/ABCG8 as cholesterol’s way out of the body (Fig. 1). Over the last decade we have gained enormous insight into the enterohepatic recirculation of cholesterol by studying these proteins, and future research will no doubt shed light on the connections between cholesterol metabolism and other metabolic diseases. In regards to NPC1L1 and ABCG5/ABCG8, several important questions remain to be resolved by future studies: Do sterols physically interact with NPC1L1 during its endocytic recycling itinerary? What determine the metabolic fate of cholesterol delivered to a cell by NPC1L1-mediated uptake? What is the relationship between NPC1L1 and other membrane proteins implicated in intestinal sterol transport such as CD36, SR-BI [124,125]? How does NPC1L1 regulate hepatic steatosis, obesity and other metabolic disorders? Does ABCG5/ABCG8 actively transport sterols across membranes, or simply move sterols between membrane leaflets? Can a dual therapy, such as inhibition of both cholesterol absorption and de novo cholesterol synthesis (ezetimibe plus statin), or stimulation of ABCG5/ABCG8-mediated cholesterol excretion and simultaneous inhibition of cholesterol synthesis (potential ABCG5/ABCG8 activator plus statin), in humans result in improved protection against atherosclerosis? In answering these and many other questions, we will assuredly strengthen our chances of developing statin-synergistic therapies to help further decrease atherosclerosis risk in future generations.
Acknowledgments
Dr. Liqing Yu is supported by a Scientist Development Grant #0635261N, and Dr. Mark Brown is supported by a Postdoctoral Fellowship #0625400U, both generously provided by the American Heart Association.
Abbreviations used
- ABC
ATP-binding cassette transporter
- ASCVD
atherosclerotic cardiovascular disease
- CHD
coronary heart disease
- FH
familial hypercholesterolemia
- HDL-C
high density lipoprotein cholesterol
- HNF4α
hepatocyte nuclear factor 4 alpha
- LXR
liver X receptor
- MβCD
methyl-β-cyclodextrin
- LDL-C
low density lipoprotein cholesterol
- NPC1
Niemann-Pick C1
- NPC1L1
Niemann-Pick C1-Like 1
- SREBP
sterol regulatory element-binding protein
- SR-BI
scavenger receptor class B type I
- STSL
sitosterolemia locus
References
- 1.Rosamond WD, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 3.Van Heek M, France C, Compton DS, Mcleod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HRJ. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. Pharmacol Exp Ther. 1997;283:157–163. [PubMed] [Google Scholar]
- 4.Schulthess G, Compassi S, Boffelli D, Werder M, Weber FE, Hauser H. A comparative study of sterol absorption in different small-intestinal brush border membrane models. J Lipid Res. 1996;37(11):2405–2419. [PubMed] [Google Scholar]
- 5.Schoenheimer R. New contributions in sterol metabolism. Science. 1931;74:579–584. doi: 10.1126/science.74.1928.579. [DOI] [PubMed] [Google Scholar]
- 6.Glover J, Morton RA. The absorption and metabolism of sterols. Br Med Bull. 1958;14(3):226–233. doi: 10.1093/oxfordjournals.bmb.a069688. [DOI] [PubMed] [Google Scholar]
- 7.Rosenblum SB, Huynh T, Afonso A, Davis HR, Jr, Yumibe N, Clader JW, Burnett DA. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4 -hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41(6):973–980. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- 8.Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65(2):137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 9.Altmann SW, Davis HR, Jr, Zhu L, Yao X, Hoos LM, Tetzloff G, Iyer M, Maguire A, Golovko M, Zheng L, Wang N, Murgolo N, Graziano M. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Niemann-Pick C1- like 1 is required for an LXR agonist to raise plasma HDL cholesterol in mice. Arterioscler Thromb Vasc Biol. 2008;28(3):448–454. doi: 10.1161/ATVBAHA.107.160465. [DOI] [PubMed] [Google Scholar]
- 11.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J Lipid Res. 2008 doi: 10.1194/jlr.M800439-JLR200. (In Press) [DOI] [PubMed] [Google Scholar]
- 12.Davis HR, Jr, Zhu L, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam M, Lund EG, Detmers PA, Graziano MP, Altmann S. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 13.Davis HR, Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2007;27(4):841–849. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 14.Garcio-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, MacIntyre DE, Ogawa A, O’Neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci USA. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinglass AB, Kohler M, Schulte U, Liu J, Nketiah EO, Thomas A, Schmalhofer W, Williams B, Bildl W, McMasters DR, Dai K, Beers L, McCann ME, Kaczorowski GJ, Garcia ML. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc Natl Acad Sci USA. 2008;105(32):11140–11145. doi: 10.1073/pnas.0800936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawes BE, O’neill KA, Yao X, Crona JH, Davis HR, Jr, Graziano MP, Altmann SW. In vivo responsiveness to ezetimibe correlates with niemann-pick C1 like-1 (NPC1L1) binding affinity: Comparison of multiple species NPC1L1 orthologs. Mol Pharmacol. 2007;71(1):19–29. doi: 10.1124/mol.106.027896. [DOI] [PubMed] [Google Scholar]
- 17.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280:12710–12720. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 18.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Heek M, Compton DS, Davis HR. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J Pharmacol. 2001;415(1):79–84. doi: 10.1016/s0014-2999(01)00825-1. [DOI] [PubMed] [Google Scholar]
- 20.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106(15):1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 21.Clarenbach JJ, Reber M, Lutjohann D, von Bergmann K, Sudhop T. The lipid-lowering effect of ezetimibe in pure vegetarians. J Lipid Res. 2006;47(12):2820–2824. doi: 10.1194/jlr.P600009-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Dujovne CA, Ettinger MP, McNeer JF, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP Ezetimibe Study Group. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90(10):1092–1097. doi: 10.1016/s0002-9149(02)02798-4. [DOI] [PubMed] [Google Scholar]
- 23.Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, LeBeaut AP, Suresh R, Yang B, Veltri EP Ezetimibe Study Group. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24(8):729–741. doi: 10.1016/s0195-668x(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 24.Knopp RH, Dojovne CA, LeBeaut A, Lipka LJ, Suresh R, Veltri EP Ezetimimbe Study Group. Evaluation of the efficacy, safety, and tolerability of ezetimibe in primary hypercholesterolaemia: a pooled analysis from two controlled phase III clinical studies. Int J Clin Pract. 2003;57(5):363–368. [PubMed] [Google Scholar]
- 25.Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005;80(5):587–595. doi: 10.4065/80.5.587. [DOI] [PubMed] [Google Scholar]
- 26.Pisciotta L, Fasano T, Bellocchio A, Bocchi L, Sallo R, Fresa R, Colangeli I, Cantafora A, Calandra S, Bertolini S. Effect of ezetimibe coadministered with statins in genotype-confirmed heterozygous FH patients. Atherosclerosis. 2007;194(2):e116–e122. doi: 10.1016/j.atherosclerosis.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, LeBeaut AP, Suresh R, Sun S, Veltri EP. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40(12):2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 28.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP Ezetimibe Study Group. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 29.Kerzner B, Corbelli J, Sharp S, Lipka LJ, Melani L, LeBeaut A, Suresh R, Mukhopadhyay P, Veltri EP Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol. 2003;91(4):418–424. doi: 10.1016/s0002-9149(02)03236-8. [DOI] [PubMed] [Google Scholar]
- 30.Melani L, Mills R, Hassman D, Lipetz R, Lipka L, LeBeaut A, Suresh R, Mukhopadyay P, Veltri E Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with pravastatin in patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Eur Heart J. 2003;24(8):717–728. doi: 10.1016/s0195-668x(02)00803-5. [DOI] [PubMed] [Google Scholar]
- 31.Bays HE, Ose L, Fraser N, Tribble DL, Quinto K, Reyes R, Johnson-Levonas AO, Sapre A, Donahue SR Ezetimibe Study Group. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26(11):1758–1773. doi: 10.1016/j.clinthera.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol. 2004;93(12):1487–1494. doi: 10.1016/j.amjcard.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 33.Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am Heart J. 2005;149(3):464–473. doi: 10.1016/j.ahj.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RB, Guyton JR, Mazzone T, Weinstock RS, Polis A, Edwards P, Tomassini JE, Tershakovec AM. Ezetimibe/simvastatin vs atorvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia: the VYTAL study. Mayo Clin Proc. 2006;81(12):1579–1588. doi: 10.4065/81.12.1579. [DOI] [PubMed] [Google Scholar]
- 35.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E ENHANCE Investgators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358(14):1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 36.Rossebo AB, Pederson TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 37.Couzin J. Clinical trials and tribulations. Cholesterol veers off script. Science. 2008;322(5899):220–223. doi: 10.1126/science.322.5899.220. [DOI] [PubMed] [Google Scholar]
- 38.Nissen SE. ENHANCE and ACCORD: controversy over surrogate end points. Curr Cardiol Rep. 2008;10(3):159–161. doi: 10.1007/s11886-008-0027-z. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Bharadway S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem. 2006;281:6616–6624. doi: 10.1074/jbc.M511123200. [DOI] [PubMed] [Google Scholar]
- 40.Iyer SP, Yao X, Crona JH, Hoos LM, Tetzloff G, Davis HR, Jr, Graziano MP, Altmann SW. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochem Biophys Acta. 2005;1722(3):282–292. doi: 10.1016/j.bbagen.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of non-esterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem J. 2007;406(2):273–283. doi: 10.1042/BJ20070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinglass AB, Kohler MG, Nketiah EO, Liu J, Schmalhofer W, Thomas A, Williams B, Beers L, Smith L, Hafey M, Bleasby K, Leone J, Tang YS, Braun M, Ujjainwalla F, McCann ME, Kaczorowski GJ, Garcia ML. Madin-Darby canine kidney II cells: a pharmacologically validated system for NPC1L1-mediated cholesterol uptake. Mol Pharmacol. 2008;73(4):1072–1084. doi: 10.1124/mol.107.043844. [DOI] [PubMed] [Google Scholar]
- 43.Yamanashi Y, Takada T, Suzuki H. Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and beta-sitosterol uptake in CaCo-2 cells. J Pharmacol Exp Ther. 2007;320(2):559–564. doi: 10.1124/jpet.106.114181. [DOI] [PubMed] [Google Scholar]
- 44.Petersen NH, Faegeman NJ, Yu L, Wustner D. J. Kinetic imaging of NPC1L1 and sterol trafficking between plasma membrane and recycling endosomes in hepatoma cells. Lipid Res. 2008;49(9):2023–2037. doi: 10.1194/jlr.M800145-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7(6):508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 47.Connelly MA, Williams DL. SR-BI and HDL cholesteryl ester metabolism. Endocr Res. 2004;30(4):697–703. doi: 10.1081/erc-200043979. [DOI] [PubMed] [Google Scholar]
- 48.Schoenheimer RZ. Uber die bedeutung der pflanzensterine fur den tierschen organismus. Hoppe-Seyler’s. Fur Physiol Chem. 1929;180:1–5. [Google Scholar]
- 49.Schoenheimer R, Breusch F. Synthesis and destruction of cholesterol in the organism. J Biol Chem. 1933;103:439–448. [Google Scholar]
- 50.Borgstrom B. Quantitative aspects of the intestinal absorption and metabolism of cholesterol and beta-sitosterol in the rat. J Lipid Res. 1968;9(4):473–481. [PubMed] [Google Scholar]
- 51.Gould RG, Jones RJ, LeRoy GV, Wissler RW, Taylor CB. Absorbability of beta-sitosterol in humans. Metabolism. 1969;18(8):652–662. doi: 10.1016/0026-0495(69)90078-x. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez HH, Chaikoff IL, Dauben WG, Abraham S. The absorption of C14-labeled epicholesterol in the rat. J Biol Chem. 1954;206(2):757–765. [PubMed] [Google Scholar]
- 53.Kuksis A, Huang TC. Lymphatic absorption of cholesterol in the dog following corn oil and butterfat feeding. Can J of Biochem Physiol. 1962;40(11):1493–1504. doi: 10.1139/y65-029. [DOI] [PubMed] [Google Scholar]
- 54.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290(5497):1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 55.Lee MH, Lu K, Hazard S, Yu H, Sulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27(1):79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu L, Hammer RE, Li-Hawkins J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99(25):16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B Multicenter Sitosterolemia Study Group. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109(8):966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J Lipid Res. 2005;46(8):1739–1744. doi: 10.1194/jlr.M500124-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116(4):1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Shah JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res. 2005;46(11):2423–2431. doi: 10.1194/jlr.M500232-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Field FJ, Born E, Mathur SN. LXR/RXR ligand activation enhances basolateral efflux of beta-sitosterol in CaCo-2 cells. J Lipid Res. 2004;45(5):905–913. doi: 10.1194/jlr.M300473-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, Staels B, Lestavel S. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun. 2006;340(4):1259–1263. doi: 10.1016/j.bbrc.2005.12.137. [DOI] [PubMed] [Google Scholar]
- 63.Mathur SN, Watt KR, Field FJ. Regulation of intestinal NPC1L1 expression by dietary fish oil and docosahexaenoic acid. J Lipid Res. 2007;48(2):395–404. doi: 10.1194/jlr.M600325-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Liu R, Iqbal J, Yeang C, Wang DQ, Hussain MM, Jiang XC. Phospholipid transfer protein-deficient mice absorb less cholesterol. Arterioscler Thromb Vasc Biol. 2007;27(9):2014–2021. doi: 10.1161/ATVBAHA.107.149914. [DOI] [PubMed] [Google Scholar]
- 65.Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, Singla A, Dudeja PK, Gill RK. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: Role of sterol regulatory element binding protein 2. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G369–G376. doi: 10.1152/ajpgi.00306.2006. [DOI] [PubMed] [Google Scholar]
- 66.Iwayanagi Y, Takada T, Suzuki H. HNF4alpha is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm Res. 2008;25(5):1134–1141. doi: 10.1007/s11095-007-9496-9. [DOI] [PubMed] [Google Scholar]
- 67.Valasek MA, Repa JJ, Quan G, Dietschy JM, Turley SD. Inhibiting intestinal NPC1L1 activity prevents diet-induced increase in biliary cholesterol in Golden Syrian hamsters. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G813–G822. doi: 10.1152/ajpgi.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134(7):2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuniga S, Molina H, Azocar L, Amigo L, Nervi F, Pimentel F, Jarufe N, Arrese M, Lammert F, Miquel JF. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28(7):935–947. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 70.Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, Ishii H, Yoshida M. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 2007;581(29):5664–5670. doi: 10.1016/j.febslet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 71.Yamagishi S, Nakamura K, Matsui T, Sato T, Takeuchi M. Inhibition of intestinal cholesterol absorption by ezetimibe is a novel therapeutic target for fatty liver. Med Hypotheses. 2006;66(4):844–846. doi: 10.1016/j.mehy.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 72.Lobonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, Howles PN. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G776–G783. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53(4):1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miettinen TA. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur J Clin Invest. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 75.Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36:1319–1330. [PubMed] [Google Scholar]
- 76.Gregg RE, Connor WE, Lin DS, Brewer HB. Abnormal metabolism of shellfish sterols in a patient with sitosterolemia and xanthomatosis. J Clin Invest. 1986;77:1864–1872. doi: 10.1172/JCI112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salen G, Horak I, Rothkopf M, Cohen JL, Speck J, Tint GS, Shore V, Dayal B, Chen T. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J Lipid Res. 1985;26:1126–1133. [PubMed] [Google Scholar]
- 78.Kolovou G, Voudris B, Drogari E, Palantianos G, Cokkinos DV. Coronary bypass grafts in a young girl with sitosterolemia. Eur Heart J. 1996;17:965–966. doi: 10.1093/oxfordjournals.eurheartj.a014983. [DOI] [PubMed] [Google Scholar]
- 79.Mymin D, Wang J, Frohlich J, Hegele RA. Phenotypic heterogeneity of sitosterolemia. Circulation. 2003;107:791. [Google Scholar]
- 80.Bjorkhem I, Boberg K, Leitersdorf E. In: The Metabolic and Molecualr Basis of Inherited Disease. Scriver A, Beaudet A, Sly W, Valle, editors. McGraw-Hill; New York: 2001. pp. 2961–2988. [Google Scholar]
- 81.Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Mietinen TA, Grundy SM, Lee MH, Rubenstein JS, Polymeropoulos MH, Brownstein MJ. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J Clin Invest. 1998;102(5):1041–1044. doi: 10.1172/JCI3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu K, Lee MH, Hazard S, Brooks-Wilson A, Hidaka H, Kojima H, Ose L, Stalenhoef AF, Mietinnen T, Bjorkhem I, Bruckert E, Pandya A, Brewer HB, Jr, Salen G, Dean M, Srivastava A, Patel SB. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Human Genet. 2001;69(2):278–290. doi: 10.1086/321294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004;4:21. doi: 10.1186/1471-230X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 85.Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler Thromb. 1992;12(5):563–568. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- 86.Bhattacharyya AK, Connor WE, Lin DS, McMurry MM, Shulman RS. Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler Thromb. 1991;11(5):1287–1294. doi: 10.1161/01.atv.11.5.1287. [DOI] [PubMed] [Google Scholar]
- 87.Salen G, Shore V, Tint GS, Forte T, Shefer S, Horak I, Horak E, Dayal B, Nguyen L, Batta AK, et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res. 1989;30(9):1319–1330. [PubMed] [Google Scholar]
- 88.Plosch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, Kema IP, Groen AK, Shan B, Kuipers F, Schwarz M. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126(1):290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 89.Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, Shefer S, Batta AK, Yu H, Chen J, Klein R, Looije N, Oude-Elferink R, Groen AK, Maeda N, Salen G, Patel SB. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2004;24:1–21. doi: 10.1186/1741-7015-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang HH, Patel SB, Carey MC, Wang DQ. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8(−/−) mice. Hepatology. 2007;45(4):998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110(5):671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kosters A, Frijters RJ, Schaap FG, Vink E, Plosch T, Ottenhoff R, Jirsa M, DeCuyper IM, Kuipers F, Groen AK. Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J Hepatol. 2003;38(6):710–716. doi: 10.1016/s0168-8278(03)00093-x. [DOI] [PubMed] [Google Scholar]
- 93.Plosch T, van der Veen JN, Havinga R, Huijkman NC, Bloks VW, Kuipers F. Abcg5/Abcg8-independent pathways contribute to hepatobiliary cholesterol secretion in mice. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G414–G423. doi: 10.1152/ajpgi.00557.2005. [DOI] [PubMed] [Google Scholar]
- 94.Groen A, Kunne C, Jongsma G, van den Oever K, Mok KS, Petruzzelli M, Vrins CL, Bull L, Paulusma CC, Oude Elferink RP. Abcg5/8 independent biliary cholesterol excretion in Atp8b1-deficient mice. Gastroenterology. 2008;134(7):2091–2100. doi: 10.1053/j.gastro.2008.02.097. [DOI] [PubMed] [Google Scholar]
- 95.Wilund KR, Yu L, Xu F, Hobbs HH, Cohen JC. High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr−/− mice. J Lipid Res. 2004;45(8):1429–1436. doi: 10.1194/jlr.M400167-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Wu JE, Basso F, Shamburek RD, Amar MJ, Vaisman B, Szakacs G, Joyce C, Tansey T, Freeman L, Paigen BJ, Thomas F, Brewer HB, Jr, Santamarina-Fojo S. Hepatic ABCG5 and ABCG8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J Biol Chem. 2004;279(22):22913–22925. doi: 10.1074/jbc.M402838200. [DOI] [PubMed] [Google Scholar]
- 97.Basso F, Freeman LA, Ko C, Joyce C, Amar MJ, Shamburek RD, Tansey T, Thomas F, Wu J, Paigen B, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J Lipid Res. 2007;48(1):114–126. doi: 10.1194/jlr.M600353-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J Clin Invest. 2004;114(6):813–822. doi: 10.1172/JCI22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bao L, Li Y, Deng SX, Landry D, Tabas I. Sitosterol-containing lipoproteins trigger free sterol-induced caspase-independent death in ACAT-competent macrophages. J Biol Chem. 2006;281(44):33635–33649. doi: 10.1074/jbc.M606339200. [DOI] [PubMed] [Google Scholar]
- 100.Wilund KR, Yu L, Xu F, Vega GL, Grundy SM, Cohen JC, Hobbs HH. No association between plasma levels of plant sterols and atherosclerosis in mice and man. Arterioscler Thromb Vasc Biol. 2004;24(12):2326–2332. doi: 10.1161/01.ATV.0000149140.00499.92. [DOI] [PubMed] [Google Scholar]
- 101.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110(5):659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Graf GA, Cohen JC, Hobbs HH. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J Biol Chem. 2004;279(23):24881–24888. doi: 10.1074/jbc.M402634200. [DOI] [PubMed] [Google Scholar]
- 103.Okiyoneda T, Kono T, Niibori A, Harada K, Kusuhara H, Takada T, Shuto T, Suico MA, Sugiyama Y, Kai H. Calreticulin facilitates the cell surface expression of ABCG5/G8. Biochem Biophys Res Commun. 2006;347(1):67–75. doi: 10.1016/j.bbrc.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 104.Vrins C, Vink E, Vandenberghe KE, Frijters R, Seppen J, Groen AK. The sterol transporting heterodimer ABCG5/ABCG8 requires bile salts to mediate cholesterol efflux. FEBS Lett. 2007;581(24):4616–4620. doi: 10.1016/j.febslet.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 105.Tachibana S, Hirano M, Hirata T, Matsuo M, Ikeda I, Ueda K, Sato R. Cholesterol and plant sterol efflux from cultured intestinal epithelial cells is mediated by ATP-binding cassette transporters. Biosci Biotechnol Biochem. 2007;71(8):1886–1895. doi: 10.1271/bbb.70109. [DOI] [PubMed] [Google Scholar]
- 106.Wang J, Sun F, Zhang DW, Ma Y, Xu F, Belani JD, Cohen JC, Hobbs HH, Xie XS. Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J Biol Chem. 2006;281(38):27894–27904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J, Zhang DW, Lei Y, Xu F, Cohen JC, Hobbs HH, Xie XS. Purification and reconstitution of sterol transfer by native mouse ABCG5 and ABCG. Biochemistry. 2008;47(18):5194–5204. doi: 10.1021/bi800292v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamada T, Egashira N, Nishizono S, Tomoyori H, Nakagiri H, Imaizumi K, Ikeda I. Lymphatic absorption and deposition of various plant sterols in stroke-prone spontaneously hypertensive rats, a strain having a mutation in ATP binding cassette transporter G5. Lipids. 2007;42(3):241–248. doi: 10.1007/s11745-006-3015-3. [DOI] [PubMed] [Google Scholar]
- 109.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277(21):18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 110.Plosch T, Kok T, Bloks VW, Smit MJ, Havinga R, Chimini G, Groen AK, Kuipers F. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J Biol Chem. 2002;277(37):33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- 111.Kaneko E, Matsuda M, Yamada Y, Tachibana Y, Shimomura I, Makishima M. Induction of intestinal ATP-binding cassette transporters by a phytosterol-derived liver X receptor agonist. J Biol Chem. 2003;278(38):36091–36098. doi: 10.1074/jbc.M304153200. [DOI] [PubMed] [Google Scholar]
- 112.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–15570. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 113.Calpe-Berdiel L, Rotlan N, Fievet C, Roig R, Blanco-Vaca F, Escola-Gil JC. Liver X receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J Lipid Res. 2008;49(9):1904–1911. doi: 10.1194/jlr.M700470-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Freeman LA, Kennedy A, Wu J, Bark S, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J Lipid Res. 2004;45(7):1197–1206. doi: 10.1194/jlr.C400002-JLR200. [DOI] [PubMed] [Google Scholar]
- 115.Sumi K, Tanaka T, Uchida A, Magoori K, Urashima Y, Ohashi R, Ohguchi H, Okamura M, Kudo H, Daigo K, Maejima T, Kojima N, Sakakibara I, Jiang S, Hasegawa G, Kim I, Osborne TF, Naito M, Gonzalez FJ, Hamakubo T, Kodama T, Sakai J. Cooperative interaction between hepatocyte nuclear factor 4 alpha and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Mol Cell Biol. 2007;27(12):4248–4260. doi: 10.1128/MCB.01894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J Lipid Res. 2003;44(9):1728–1736. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- 117.Kajinami K, Takekoshi N, Brousseau ME, Schaefer EJ. Pharmacogenetics of HMG-CoA reductase inhibitors: exploring the potential for genotype-based individualization of coronary heart disease management. Athersclerosis. 2004;177(2):219–234. doi: 10.1016/j.atherosclerosis.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Miettinen TA, Strandberg TE, Gylling H. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler Thromb Vasc Biol. 2000;20(5):1340–1346. doi: 10.1161/01.atv.20.5.1340. [DOI] [PubMed] [Google Scholar]
- 119.Gylling H, Miettinen TA. Baseline intestinal absorption and synthesis of cholesterol regulate its response to hypolipidaemic treatments in coronary patients. Atherosclerosis. 2002;160(2):477–481. doi: 10.1016/s0021-9150(01)00608-6. [DOI] [PubMed] [Google Scholar]
- 120.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110(5):671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang W, Ma Y, Yu L. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 2006;44(5):1259–1266. doi: 10.1002/hep.21380. [DOI] [PubMed] [Google Scholar]
- 122.Rader DJ. Liver X receptor and farnesoid X receptor as therapeutic targets. Am J Cardiol. 2007;100(11):15–19. doi: 10.1016/j.amjcard.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 123.Cao G, Liang Y, Jiang XC, Eacho PI. Liver X receptors as potential therapeutic targets for multiple diseases. Drug News Perspect. 2004;17(1):35–41. doi: 10.1358/dnp.2004.17.1.829024. [DOI] [PubMed] [Google Scholar]
- 124.Hui DY, Labonte ED, Howles PN. Development and physiological regulation of intestinal lipid absorption. III. Intestinal transporters and cholesterol absorption. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G839–G843. doi: 10.1152/ajpgi.00061.2008. [DOI] [PubMed] [Google Scholar]
- 125.Knopfel M, Davies JP, Duong PT, Kvaerno L, Carreira EM, Phillips MC, Ioannou YA, Hauser H. Multiple plasma membrane receptors but not NPC1L1 mediate high-affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim Biophys Acta. 2007;1771(9):1140–1147. doi: 10.1016/j.bbalip.2007.05.011. [DOI] [PubMed] [Google Scholar]