Abstract
Nanoparticles have been extensively used for a variety of biomedical applications and there is a growing need for highly specific and efficient delivery of the nanoparticles into target cells and subcellular location. We attempted to accomplish this goal by modifying gold particles with peptide motif’s that are known to deliver a ‘cargo’ into chosen cellular location specifically, we intended to deliver nanogold particles into cells through chemical cross-linking with different peptides known to carry protein into cells. Our results suggest that specific sequence of such ‘carrier peptides’ can efficiently deliver gold nanoparticles into cells when chemically cross-linked with the metal particles.
Introduction
The diagnosis and treatment of disease at the cellular level would be greatly enhanced by the ability to deliver therapeutic agents into specific cells and cellular compartments. The nucleus is a desirable target because the genetic information of the cell and transcription machinery resides there. The therapeutic agents are often prevented from reaching the cell nucleus because of size and charge. Metal, semiconductor, polymer, and magnetic particles have been previously used to target cells [1–7]. Utilizing nanoparticles for nuclear targeting has not been proved very successful due to the impermeable nature of the plasma and nuclear membranes. Particles mostly localize into the cytoplasm. To address this issue, for last few years, enormous attention has been paid in the self-assembly fabrication of hybrid materials from inorganic nanoparticles and biomolecules [8]. Nanoparticles are comparable in size range to many biomolecules and thus appear to be natural companions in hybrid systems. At present, it is simple to control and modify the properties of nanostructures to better suit their integration with biological systems, for example, controlling their size and modifying their surface layer for enhanced aqueous solubility, biocompatibility, or biorecognition [9, 10].
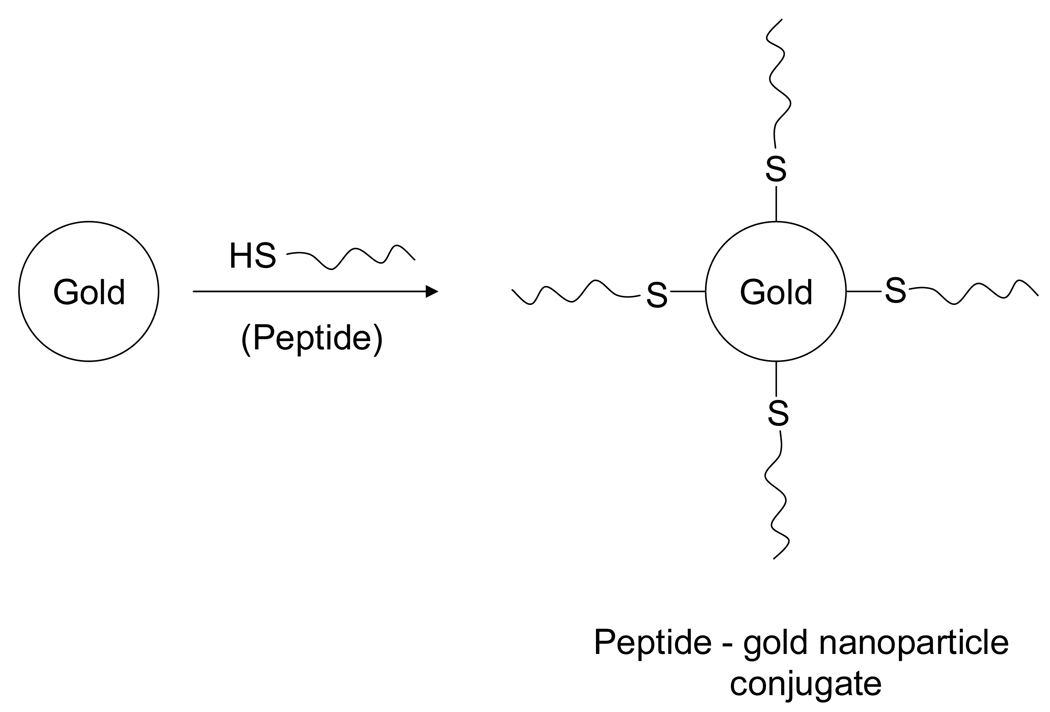
Gold particles modified with the nuclear localization signal (NLS) from SV-40 virus have been used extensively for nuclear transport in the pioneering work of Feldherr et al [11–15]. However, in those cases the nanoparticle-peptide complex was injected directly into the cytoplasm near the nucleus avoiding cellular membrane entry and endosomal/lysosomal pathways. Tat peptide has been found as an efficient molecule to translocate gold nanoparticles into the cell nucleus [16]. Cationic and hydrophilic Cell-penetrating peptides (CPP) of natural and synthetic origin were first suggested to cross cellular membrane [17–19]. Very recently, cellular delivery of proteins by transportan peptides has been studied by Padari et al [20]. In this study the cellular uptake of noncovalent complexes of biotin-transportan was followed with streptavidin-gold by electron microscopy. Gold nanoparticles coated with Mycobacterium cell entry protein, a cell penetrating peptide induced plasma membrane invagination and entered membrane-bound compartments inside HeLa cells [21]. However, current methods have shown that gold nanoparticles are attached with peptides via a linking agent, e.g., BSA. In this paper, we have demonstrated that gold nanoparticles can be directly attached with the ‘cystein terminus’ of the designed peptide through chemical cross-linking (Fig. 1). Peptide modified nanoparticles were subsequently tested in vitro with osteosarcoma cell line by transmission electron microscopy to determine the biocompatibility of these nanoparticles and their ability to cross the cell membrane. In this study, we focus on three peptides, a cell penetrating peptide sequence AAVA LLPA VLLA LLAP (CPPs), nuclear localization signal PKKKRKV (NLS) derived from the Large T antigen of the SV40 virus, as well as combination of the above mentioned peptides PKKKRKV AAVA LLPA VLLA LLAP (CPPsNLS). Each peptide was designed with a terminal cystein to permit conjugation to the gold surface.
Fig. 1.
Illustration of the synthesis of peptide modified goldn anoparticle conjugates for cellular targeting.
Materials and Methods
Materials
Tetrachloroauric acid trihydrate and sodium borohydride were purchased from Sigma-Aldrich, St. Louis, Missouri. Trump fixative (1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer, pH 7.2) was obtained from Electron Microscopy Sciences, Hatfield, PA. House distilled water was further purified using a Milli-Q reagent grade water system (Millipore). Purified and lyophilized peptides were obtained from the Mayo Protein Core Facility.
Preparation of peptide-gold nanoparticles bioconjugate
Gold nanoparticles were prepared by the reduction of aqueous chloroaurate ions with sodium borohydride. In a typical experiment 50 ml of aqueous solution containing 4 mg of solid sodium borohydride was added to 100 ml of 100 uM aqueous solution of tetrachloroauric acid under vigorous stirring that was continued overnight. Gold nanoparticles thus formed were filtered through 0.22 uM filter syringe and used for experiments. Nanoparticle size and morphology were determined using a transmission electron microscope (TEM) after drop coating the gold nanoparticles on a TEM grid. The peptide (200 nmoles) was conjugated with 100 ml aqueous solution of gold nanoparticles under stirring at room temperature for 24 hrs. The gold-peptide bioconjugate was concentrated by centrifugation at 14, 000 rpm for 45 min.
Cell culture and particle delivery
Osteosarcoma cell line TE85 was obtained from Dr. T. C. Spelsberg’s laboratory at Mayo Clinic, Rochester, MN. TE85 was cultured in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 1% (vol/vol) 200 mM glutamine, 1% (vol/vol) antibiotics (streptomycin, 10,000 ug/ml; penicillin, 10,000 IU/ml), and 10% (wt/vol) Fetal Bovine Serum, at 37° C in a humidified atmosphere containing 5% CO2. For investigating the cellular localization of nanoparticles, cells were plated in 100 mm plates and grown to 75–80% confluency and then incubated with nanoparticle conjugates for 3 hrs with serum containing media.
Electron microscopy
On removal of excess nanoparticles, cell samples were washed with PBS and fixed in trump fixative. Cells were then rinsed for 30 min in 3 changes of 0.1 M phosphate buffer, pH 7.2, followed by a one hour postfix in phosphate –buffered 1% OsO4. After rinsing in 3 changes of distilled water for 30 min the tissue was en bloc stained with 2 % uranyl acetate for 30 min at 60° C. After en bloc staining, the tissue was rinsed in three changes of distilled water, dehydrated in progressive concentrations of ethanol and 100% propylene oxide and embedded in spurr’s resin. Thin sections were cut on a Reichert Ultracut E ultramocrotome, placed on 200 mesh copper grids and stained with lead citrate. TEM analysis was carried out in a JEM-1200EX electron microscope working at 80 KV.
Results and Discussion
The TEM image of the gold nanoparticles clearly shows ~5 nm spherical gold particles with a nearly uniform particle size distribution (Fig. 2). We compared intracellular delivery with only gold, gold-CPPs, gold-NLS and gold-CPPsNLS conjugates. Nanoparticles modified with each of the peptides were observed inside the cells by transmission electron microscopy (TEM).
Fig. 2.
TEM micrograph of borohydride reduced gold nanoparticles. (Scale bar 50 nm.)
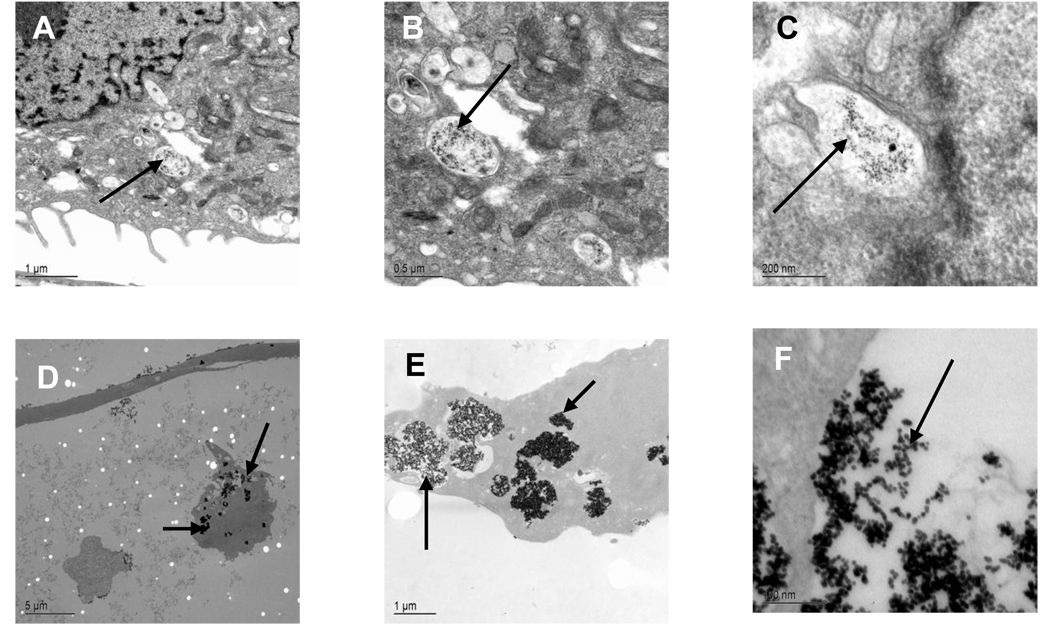
In case of bare gold nanoparticles, particles were rarely observed inside cells. Whereas, peptides like CPPs or NLS coated nanoparticles efficiently entered inside the cells. But exceptionally high uptake of conjugates by cells was observed with a peptide consisting of the CPPs coupled with NLS (Fig. 3). After 3h of incubation with the cells, most of the internalized peptide-nanoparticle conjugates were found clustered together inside membrane-bound cellular compartments that appear to be endosomes as observed by TEM. No visible damage to the cells was observed. The fate of nanoparticles inside the cells depended upon the targeting peptide complexed to the gold surface.
Fig. 3.
Transmision electron micrographs of TE85 cell line incubated with gold-CPPs (A–C) and gold-CPPsNLS (D–F) bioconjugate for 3hrs at different magnifications.
Entry into the cytoplasm of TE85 cells was strongly dependent on the peptide sequence attached to nanoparticles. Here, the peptide modified gold nanoparticles were able to enter the cytoplasm membrane or close to the nuclear membrane, which suggests that these nanoparticles were unable to escape from endosomes. The gold particles were clearly detectable attached at the plasma membrane and also in the vesicular structures of cortical cytoplasm. The pinocytosis and receptor-mediated endocytosis are the potential cellular uptake mechanisms of large molecular structures. Endocytosis occurs usually within minutes to several hours and is cellular-energy-dependent. In this study, the absence of nuclear translocation of nanoparticles could be due to the selective nature of the cells.
Our gold-peptide bioconjugation method has advantage over the other current protocol which involves extra and /or complicated step to make the conjugation. In contrast, our method involves easy and a single step to make the conjugation. The chemisorption of organosulfur compounds onto gold has been reported to be a powerful method for the preparation of well-defined surfaces [22]. Using this concept we have designed peptides having an N-terminal cystein and were conjugated with gold nanoparticles.
Conclusion
In summary, a new and simple approach for efficient cellular uptake of the gold nanoparticles has been demonstrated where particles are directly attached with peptide through chemical cross-linking. This research has many potential applications in cell imaging, but also extends to wider biomedical applications, such as gene therapy and drug delivery. Future work will focus on nuclear targeting using different cells and different size of gold nanoparticles. In addition, detailed biocompatibility experiments for the peptide-conjugated nanogold particles will need to be carried out to realize full potential of the engineered particles for eventual biological applications.
Acknowledgement
This work has been supported by NIH grant #AR47974.
References
- 1.West J, Halas N. Curr. Opin. Biotechnol. 2000;11:215. doi: 10.1016/s0958-1669(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 2.Hogemann D, Ntziachristos V, Josephson L, Weissleder R. Bioconjugate Chem. 2002;13:116. doi: 10.1021/bc015549h. [DOI] [PubMed] [Google Scholar]
- 3.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos A. Science. 1998;281:2013. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Zhang Q, Remsen E, Wooley K. Biomacromolecules. 2001;2:362. doi: 10.1021/bm015515c. [DOI] [PubMed] [Google Scholar]
- 5.Marikanos SM, Anderson MF, Ryan J, Martin LD, Feldheim DL. J. Phys.Chem. B. 2001;105:8872. [Google Scholar]
- 6.Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Frazen S, Feldheim DL. J. Am. Chem. Soc. 2003;125:4700. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 7.Tkachenko AG, Xie H, Liu Y, Coleman D, Ryan J, Glomm W, Shipton MK, Frazen S, Feldheim DL. Bioconjugate Chem. 2004;15:482. doi: 10.1021/bc034189q. [DOI] [PubMed] [Google Scholar]
- 8.Niemeyer CM. Angew. Chem., Int. Ed. 2001;40:4128. doi: 10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Katz E, Willner I. Angew. Chem., Int. Ed. 2004;43:6042. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 10.Barrientos AG, De La Fuente JM, Rojas TC, Fernandez A, Penades S. Chem.-Eur. J. 2003;9:1909. doi: 10.1002/chem.200204544. [DOI] [PubMed] [Google Scholar]
- 11.Feldherr CM, Akin D. J. Cell. Biol. 1990;111:1. doi: 10.1083/jcb.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldherr C, Lanford R, Akin D. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11002. doi: 10.1073/pnas.89.22.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldherr C, Akin D. Exp. Cell. Res. 1993;205:179. doi: 10.1006/excr.1993.1073. [DOI] [PubMed] [Google Scholar]
- 14.Feldherr C, Akin D. Exp. Cell. Res. 1994;215:206. doi: 10.1006/excr.1994.1333. [DOI] [PubMed] [Google Scholar]
- 15.Feldherr C, Akin D. J. Cell. Sci. 1999;112:2043. doi: 10.1242/jcs.112.12.2043. [DOI] [PubMed] [Google Scholar]
- 16.Fuente JM, Berry CC. Bioconjugate Chem. 2005;16:1176. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- 17.Derossi D, Joliot AH, Chassaing G, Prochiantz A. J. Biol. Chem. 1994;269:10444. [PubMed] [Google Scholar]
- 18.Futaki S, Goto S, Sugiura Y. J. Mol. Recognit. 2003;16:260. doi: 10.1002/jmr.635. [DOI] [PubMed] [Google Scholar]
- 19.Vives E, Brodin P, Lebleu B. J. Biol. Chem. 1997;272:16010. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 20.Padari K, Saalik P, Hansen M, Koppel K, Raid R, Langel U, Pooga M. Bioconjugate Chem. 2005;16:1399. doi: 10.1021/bc050125z. [DOI] [PubMed] [Google Scholar]
- 21.Lu S, Tager LA, Chitale S, Riley LW. Anal. Biochem. 2006;353:7. doi: 10.1016/j.ab.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 22.Bain CD, Whitesides GM. Science. 1988;240:62. doi: 10.1126/science.240.4848.62. [DOI] [PubMed] [Google Scholar]