Abstract
The electroencephalogram (EEG) is the most common tool used in sleep research. This unit describes the methods for recording and analyzing the EEG. Detailed protocols describe recorder calibration, electrode application, EEG recording, and computer EEG analysis with power spectral analysis. Computer digitization of an analog EEG signal is discussed, along with EEG filtering and the parameters of fast Fourier transform (FFT) power spectral analysis. Sample data are provided for a typical night's analysis of EEG during NREM (non-REM) and REM sleep.
Keywords: sleep, spectral analysis, EEG
EEG Recording and Analysis for Sleep Research
This unit presents methods for recording and analyzing the human electroencephalogram (EEG). Although the focus is on use for sleep research, the methods can be adapted for other fields of neuroscience investigation. The protocol provides instruction on EEG recorder calibration, electrode application, EEG recording, and spectral analysis of the EEG. EEG amplifiers and recording instruments have changed greatly in the past 20 years. Computer digitization and recording have replaced paper recording, and handheld ambulatory recorders now can replace whole racks of amplifiers. The following protocol describes calibration and EEG recording on an ambulatory recorder, but a clinical recorder in a laboratory could substitute for an ambulatory recorder. The EEG recorded on a study night depends strongly on the subject's history prior to recording. The subject's sleep schedule in the days prior to recording can greatly affect the sleep and EEG on the recording night, as can various illegal, prescription, and over the counter drugs. This protocol assumes that the subjects have been appropriately screened and are suitable for study.
Note: Research involving human subjects should adhere to all local and national regulations that ensure protection of human subjects.
Basic Protocol
Materials (Rather than metric units, materials are listed in commonly available dimensions.)
Subject who has provided informed consent and is dressed in sleeping attire
A well ventilated room for electrode application
Tape measure
Grease pencil
Hair clips or hairpins
70% isopropyl alcohol
Gauze pads 2” × 2”
Cotton-tipped applicators
Abrading cream, such as NuPrep, SkinPure, or Lemon Prep from a supplier such as Integra NeuroSupplies or MVAP.
Electrode conductive paste, such as Ten20 (MVAP and Integra Neurosupplies)
Electrode conductive gel, such as Signa gel (MVAP and Integra Neurosupplies)
EEG electrodes – gold or silver/silver chloride10 mm diameter cup electrodes with a 48” wire lead with safety connector (Grass, MVAP and Integra Neurosupplies)
Gauze pad separated from four layer thickness to two layer thickness and trimmed to 3.5 cm × 2.5 cm.
Petri dish
Collodion (produced by Mavidon Medical available from MVAP and Integra Neurosupplies)
Air compressor (20 PSI output) with foot pedal switch and ~4 m of tubing attached to an electrode applicator tip. (MVAP and Integra Neurosupplies)
1” wide adhesive type tape such as 3M Durapore or 3M Transpore White (MVAP and Integra Neurosupplies)
1/2” wide adhesive type tape
Connection from electrodes to EEG recorder such as a head-box or electrode connector box, typically supplied with the EEG recorder.
Impedance meter, such as Grass EZM
Acetone
Non-acetone collodion remover such as Mavidon Collodion Remover (MVAP and Integra Neurosupplies)
Adhesive tape remover pads (produced by Dynarex supplied by (MVAP and Integra Neurosupplies)
Function generator that can produce a sine wave approximately the same amplitude of an EEG signal, i.e. about 200 microvolt. Grass-Technologies, Neurotronics, and Falk Minnow Services produce devices that generate signals in this range with about 98% accuracy. (Alternatively a function generator that produces a higher amplitude sine wave can be attached to a voltage divider such as the Grass Instruments SWC Square Wave Oscillator (no longer in production) to achieve a signal similar in amplitude to the EEG. A multimeter or oscilloscope can be used to measure the higher amplitude sine waves prior to voltage reduction).
Cables and connectors to connect calibration equipment to EEG recorder.
Ambulatory EEG recorder. The recorder used for this protocol was a Grass Aura. Other ambulatory recorders include Lifelines Trackit, and Embla.
Battery power source for recorder
Computer for recorder set-up
Computer for data download
Connection between computer and recorder
EEG analysis software such as PassPlus from Delta Software.
EEG Recorder Calibration
This section provides details on calibrating the recorder with an external signal of known amplitude.
Most amplifiers have some form of internal calibration. Follow the manufacturer's instructions as to how often the amplifier should be internally calibrated. As discussed in the background, this form of calibration is not sufficient to ensure the stability of the recordings.
Human EEG slow waves are typically 100 to 500 μV in amplitude; therefore, use a signal such as a 200-μV, 3.5-Hz sine wave for calibration. The 3.5 Hz frequency is high enough that the amplifier's low frequency filter does not affect the amplitude of the wave. If the function generator can produce a signal similar in amplitude to human EEG, skip to step 4. Otherwise, follow the instructions in step 3 on how to use a voltage divider to decrease the output of a function generator to the approximate amplitude of slow wave EEG.
Grass square wave calibrators work nicely as voltage dividers. An external 1.73 V sine wave can be decreased to a wide range of smaller amplitude sine waves including 200 μV. Connect the function generator output to the voltage divider with the voltmeter or oscilloscope connected in parallel to determine the output amplitude of the function generator signal. Most AC voltmeters report root mean square (RMS) voltage. To determine peak-to-peak sine wave amplitude, multiply the RMS voltage by 2√2. Many AC voltmeters are most accurate for a 60-Hz sine wave. It may be necessary to measure AC voltage at 60 Hz before setting the appropriate calibration frequency. Use the oscilloscope to ensure that sine wave amplitude at 60 Hz is the same as the amplitude at the calibration frequency, but confirm that the oscilloscope is not using a low frequency filter (i.e., use the DC rather than AC setting on the oscilloscope).
Connect the output of the sine wave generator or voltage divider (if using one) to the recorder. This is not always a simple task as demonstrated by the following example for connecting the output of a Grass Square Wave Calibrator to a Grass Aura recorder. The output from the calibrator is carried on a cable with positive, negative and ground leads. Connect the ground lead to the amplifier ground. Typically the positive oscillates and the negative is a flat line. The Aura recorder needs a reference electrode. It is possible to connect the negative lead to the reference and the positive lead to all 10 electrode inputs. However, this wiring arrangement will produce a flat line on signals of electrode pairs such as C3-A2. C3 vs. reference will be a 200-μV sine wave and A2 vs. reference will be a 200-μV sine wave, but subtracting out the references will produce a flat line on C3-A2. Instead, connect the negative to A1, A2, FH, and Reference, and connect all other electrodes to positive. The arrangement produces 200-μV sine waves on all electrode pair channels and flat lines on A1, A2, and FH vs. reference.
Connect the EEG recorder to the computer.
Launch the EEG recording software, and view the calibration signal.
Record the calibration signal for 5 minutes.
Analyze this signal with the spectral analysis EEG analysis software. To avoid problems of leakage (described in Background), sum spectral energy (power*time) in a wide band around 3.5 Hz, e.g 2.5-4.5 Hz. As described below in the EEG analysis section of the protocol, this calibration recording will be used to scale the EEG recordings.
Electrode application for human EEG studies
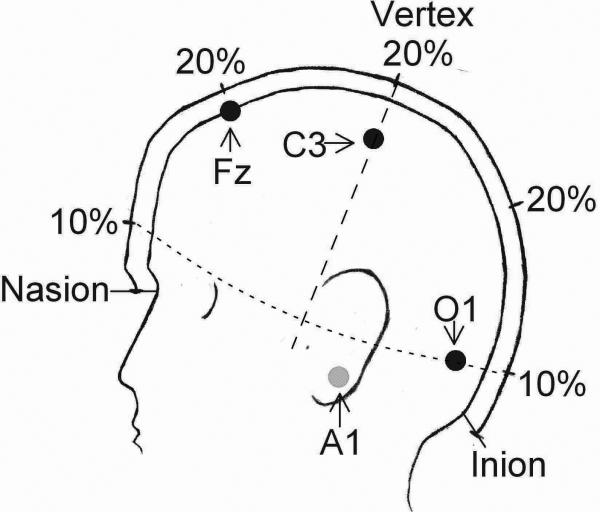
This protocol describes EEG electrode application using a 10-20 electrode system (Jasper, 1958). Distinct landmarks are identified on the head and electrodes are placed at 10% or 20% intervals of the distance between the landmarks (Fig. 1). The number of electrodes used will depend on the purpose of the study. The 2007 AASM visual scoring rules recommend a frontal electrode for best detecting K-complexes, a central electrode for spindles, and an occipital electrode for alpha waves (Silber, et al., 2007). This section describes placement of a frontal electrode Fz, a central electrode C4, and an occipital electrode O1. Backup electrodes at F4, C3, Cz, and O2 are recommended in case an electrode falls off. Signals from the EEG electrodes are referred to an electrode placed over the contralateral mastoid. Many recording systems use a separate reference electrode to which all signals are initially referred. Electrode pairs such as C3-A2 are obtained by subtraction, i.e. C3-ref minus A2-ref = C3-A2. The protocol also describes application of ground and reference electrodes, electro-occulogram (EOG; used to monitor eye movements) electrodes, and chin electromyogram (EMG; electrical activity of muscles) electrodes.
Figure 1.
Head measurements and electrode placement according to the 10-20 electrode placement system (Jasper, 1958). The longitudinal line from the nasion to inion is divided into 10% and 20% segments. Distances along the transverse line (dashed) and the circumference (dotted) are not to scale because the 3-dimensional head is drawn in 2-dimensional profile. Approximate locations of Fz, C3, O1, and A1 (behind the ear) are indicated.
Caution: This protocol describes EEG electrode application using collodion, a nitrocellulose glue dissolved in ether. Nitrocellulose is flammable, and ether is explosive. Electrodes must be applied in a well ventilated room free of ignition sources. Buying collodion in the small 2-ounce tubes, although more expensive, avoids the potential of large volume spills and the associated increased fire risks.
Measure the head and mark EEG electrode sites (left refers to the subject's left)
-
9
Identify the nasion (the depression between the eyes where the frontal bone meets the nasal bones) and the inion (the highest point of the projection of the occipital bone at the lower rear portion of the skull). Figure 1 here.
-
10
Measuring across the crown of the skull (along the longitudinal line), record the distance between the nasion and inion. In adults this distance is typically 32-38 cm.
-
11
Calculate 50%, 20%, and 10% of this distance.
-
12
Using a grease pencil, mark the scalp at the point 50% of the distance between the nasion and inion.
-
13
Measuring across the crown of the head (along the transverse line, passing over the grease mark made in step 4, record the distance between the left and right preauricular points (directly in front of the external acoustic meatus, i.e., ear canal). In adults this distance is typically 34-38 cm.
-
14
Calculate 20% of this distance.
-
15
Using the grease pencil, mark the scalp half way between the two ears. This mark will be close to or on top of the mark made in step 12 depending on how close the tape was to the midline of the skull in step 10. This is labeled as the vertex on Fig 1.
-
16
Measure and record the circumference of the skull starting at the forehead and wrapping around just above the inion and returning to the forehead.
-
17
Calculate 5% of the circumference.
-
18
Mark the location of electrode C4 by measuring down from the vertex along the transverse line toward the right preauricular point, the 20% distance calculated in step 14.
-
19
Mark the location of electrode O1 by measuring up from the inion along the longitudinal line the 10% distance calculated in step 11 and to the left the 5% distance calculated in step 17.
-
20
Mark the location of electrode Fz by measuring toward the face along the longitudinal line the 20% distance calculated in step 11.
-
21
If desired, measure and mark additional sites according to the 10-20 measuring system.
Prepare the electrodes sites and apply the EEG electrodes
Follow the steps below for each of the EEG electrode sites marked in steps 9-21.
-
22
Using a hair clip or hairpin, clip the hair back to expose the scalp where the grease pencil marks the location of the electrode.
-
23
Wipe the area with a 70% isopropyl alcohol soaked gauze pad.
-
24
With a cotton-tipped applicator rub abrading cream in small circles at the electrode site for 20 to 30 seconds. If the subject feels any pain, use less pressure. Dead skin and skin oils interfere with recording noise free signals. The alcohol wiping and abrading allow the electrode to make good contact with the skin for better signal quality.
-
25
Wipe off the abrading cream and immediately (so as not to lose sight of the abrasion site) rub electrode paste into the scalp at the abrasion site.
-
26
Fill the cup of the EEG electrode with electrode paste and press it firmly against the scalp at the abrasion site. The paste contains electrolytes that provide good electrical contact between the skin and the electrode. Place the electrode over scalp, not over hair. The thick paste will hold the electrode in place while you prepare the collodion-soaked gauze.
-
27
Place a trimmed gauze pad in the Petri dish and pour on collodion so that the entire pad is wet but not dripping. With the air pump electrode applicator tip in your dominant hand, use the other hand to place the collodion-soaked gauze over the electrode. Hold the gauze in place with one finger directly over the electrode.
-
28
With the air compressor switched on, use the applicator tip to press down firmly on the gauze while moving the tip repeatedly from the edge of the covered electrode to the edges of the gauze pad. The applicator tip should be oriented such that the fumes are directed away from the subject's face. Continue until the glue dries and the gauze patch is firmly attached to the hair and scalp. Drying duration depends on how much glue is poured on the gauze but is typically less than a minute.
-
29
To avoid gluing a finger to the subject's head, frequently change the finger holding down the gauze over the electrode. When finished drying, the gauze should form a small cast tightly over the electrode. Ensure that the corners of the gauze pad are glued down firmly by adding additional collodion, if necessary, with a cotton-tipped applicator.
-
30
If the recorder uses a separate reference electrode, choose a site on the scalp away from other electrodes and prepare the site and attach the electrode in the same manner as for the EEG electrodes.
-
31
Ground electrodes can be placed anywhere on the body. Since, collodion securely holds electrodes in place, another electrode applied to an open spot on the scalp could serve as a reliable ground.
Apply the EOG, EMG, and mastoid electrodes
-
32
With the subject's eyes closed, wipe an alcohol soaked gauze pad on the skin lateral to the left eye.
-
33
At a spot about 1 cm lateral to the and 1cm below the left outer canthus, abrade the skin as described in step 24.
-
34
Wipe off the abrasive cream and immediately place a small dot of electrode conductive gel at the abrasion site.
-
35
Tear off a 4 cm strip of 1” wide tape. Place the back of the electrode on the tape so that the edge of the cup is about 1 cm from one end of the tape and the wire extends from the other end.
-
36
Fill, but do not overfill, the cup of the electrode with conductive gel. If the cup gets overfilled, the gel will emerge and prevent the tape from sticking to the skin.
-
37
At the spot marked with a dot of gel in step 34, place the electrode on the face so that the wire runs up toward to the temple. This will be the left EOG electrode, commonly called LOC.
-
38
Press firmly on the tape being careful not to get any hair caught under the tape.
-
39
Reinforce with a 5 cm strip of 1/2” tape.
-
40
Repeat at a location 1 cm lateral to and 1 cm below the right outer canthus for electrode ROC
-
41
Repeat at a location in the middle of the forehead for electrode FH which will serve as a reference for the EOG electrodes.
-
42
The AASM07 standards list the above EOG electrode placements as an alternate configuration. This configuration allows determination of eye movement direction. For the highest amplitude EOG signals, the following electrode configuration is recommended: E1 at 1 cm above the left outer canthus, E2 at 1 cm below the right outer canthus, and the singal derivations be versus the right mastoid, i.e. E1-A2 and E2-A2.
-
43
One chin EMG electrode should be placed above the chin between the chin and the lower lip and the other directly under the chin. Repeat electrode application at these sites. If the subject is bearded, this site may require collodion application as was used for the EEG electrodes. Recording is bipolar, one EMG electrode versus the other.
-
44
Repeat at the skin behind the left (electrode A1) and right (electrode A2) ears overlying the mastoid bone.
Check electrode impedance and complete subject preparation
The purpose of the alcohol wiping, abrading, and the electrolyte gel and paste is to provide good conductance between the electrode and the skin. If impedance is high the signal quality will be poor and may be corrupted by electrical line noise (60 or 50 Hz noise). The following procedure ensures that electrodes were applied correctly.
-
45
Check the impedance meter's battery level and 10 KOhm calibration standard.
-
46
Attach all electrodes to a head-box or connector that attaches to the impedance meter.
-
47
Check and record the impedance for each electrode.
-
48
Alternatively, the electrode impedances can be checked with the EEG recording software. Connect the electrodes to the recorder as described below and use the recording software to check electrode impedances.
-
49
If taped-on electrodes exhibit high impedance (>5 KOhm), remove the tape from the skin and the electrode. Slide the electrode around on the skin while measuring the impedance. If the impedance drops below 5 KOhm, the electrode was likely not taped at the abrasion site. Mark the site with a dot of electrode conductive gel. Clean the skin with alcohol except at the correct electrode site. Clean the electrode. Attach the electrode to a new piece of tape, refill with gel, and apply at the correct site. If the impedance does not drop when the electrode is moved, insufficient abrading caused the high impedance. Clean the skin with alcohol. Re-abrade and reapply.
-
50
If EEG electrodes exhibit high impedance (>5 KOhm), remove the gauze patch by rubbing it with a gauze pad soaked with acetone. Do not use commercial non-acetone collodion removers. They will not evaporate and will prevent reapplication of the electrode. Slowly lift the electrode to determine if it was applied it over a patch of hair. Hair is an insulator and will greatly increase impedance. Move the electrode around while measuring impedance to determine if the electrode was applied at a site different from site abraded. If impedance does not drop, it was likely not abraded sufficiently. Once the acetone has fully evaporated, either reapply at the correct site or re-abrade and reapply.
-
51
Once impedance is <5 KOhm on all electrodes, pull the slack from all electrode leads and attach them to the side or back of the head, in a pony-tail, by gluing the leads to the head with collodion-soaked gauze.
EEG Recording
This section indicates the steps in a typical ambulatory recording, but most steps are appropriate for clinical/laboratory recording. Pros and cons of home recording as opposed to laboratory recordings are presented in Hirshkowitz and Moore (2000). The main advantages of home recording are the opportunity to study a subject in his/her natural sleeping environment and reduced costs. The main disadvantages are the loss of investigator control over the sleeping environment and the inability to correct loose electrodes. Follow the manufacturer's instructions for the particular recording device. The choices of digitization rate and filter settings are critical and are discussed in the Background Information section of the Commentary.
-
52
Prepare the calibrated recorder for all-night recording. Insert fresh batteries or recharged battery pack, and insert the storage media, e.g. compact flash card.
-
53
Insert safety leads on electrode wires into the head box.
-
54
Instruct the subject to lie down in bed without sleeping.
-
55
Connect the head box to the recorder.
-
56
Connect the recorder to the computer.
-
57
Launch the software and view the EEG signal.
-
58
Evaluate the quality of the signals. If the signal is contaminated by 60 Hz noise, locate the noise source and unplug it. Also, a break in an electrode lead can cause a channel to show signs of intermittent connection, so replace any such electrodes.
-
59
Use the recording software to begin the recording.
-
60
Disconnect the computer from the ambulatory recorder and provide subject with any specific instructions for the night's recording.
-
61The recording can begin at lights out time or earlier if the subject gives a signal to indicate when lights out occurred.
- Recorders typically include some kind of event marker that can be used to indicate lights out. Alternatively, the subject can blink in some prearranged pattern.
-
62
In the morning after the recording the subject may turn off the recorder.
Electrode removal
Most subjects are capable of removing their own electrodes but some may require assistance of a family member or laboratory personnel.
-
63
The EEG electrodes must be removed by dissolving the collodion. Use acetone-free collodion remover to soak the guaze pad covering each electrode. After about a minute the pad should slide off. Remove the electrode and wipe off the electrode paste. The subject can then wash the collodion remover out of his/her hair. Residual glue can be combed out with a fine toothed comb.
-
64
Remove the taped-on electrodes by carefully peeling off the tape starting and wiping off the electrode gel. The tapes commonly used to affix electrodes stick very securely to the skin. Adhesive tape removal pads make removal easier. Rub the pad over the edges of the tape. Wait a minute then remove the tape.
EEG Analysis
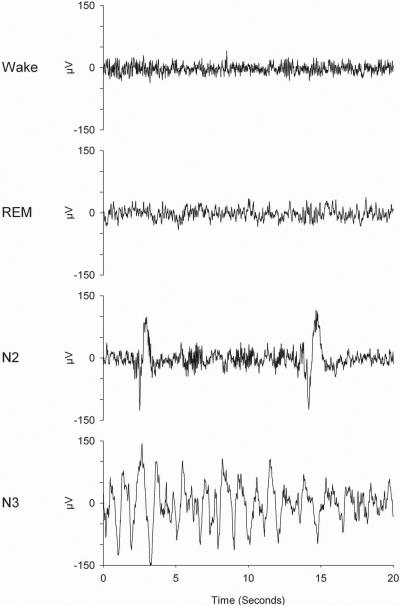
The most common analyses for sleep EEG recordings are sleep stage scoring according to AASM’07 and spectral analysis of the EEG. Detailed manuals describe scoring criteria for sleep stages (Rechtschaffen and Kales, 1968; Silber, et al., 2007). Figure 2 illustrates examples of the C3/A2 EEG in different vigilance states and sleep stages. Visual sleep stage scoring provides information such as sleep latency and time spent in various sleep stages, but it does not quantify EEG activity. Below are instructions for the most commonly used EEG quantification method, power spectral analysis using fast Fourier transform (FFT). The Background Information section of the Commentary discusses the application of FFT to the EEG and its limitations.
Figure 2.
C3/A2 EEG 20-sec samples from a 21-year-old woman. These recordings were digitized at 200 Hz. Wake EEG is characterized by high frequency low amplitude waves. High-frequency, low-amplitude EEG is also present in REM sleep. NREM stage N2 EEG contains K-complexes and sleep spindles. Large, slow waves characterize NREM stage N3.
EEG vigilance state and sleep stage scoring and artifact marking
-
65
Score each 30-sec epoch for vigilance state or sleep stage.
-
66
Separately mark each epoch for artifacts. Alternatively, mark artifacts on a smaller time scale. Rather than eliminating from analysis a whole 30-sec epoch that contains an artifact, it is possible to mark individual FFT windows (see below) and eliminate them from analysis.
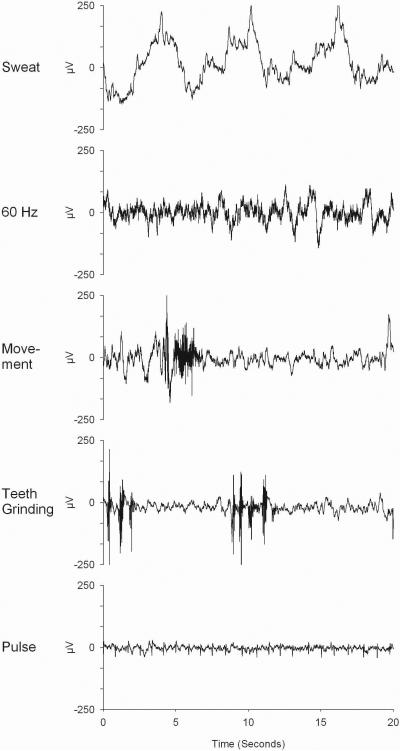
Figure 3 shows EEG samples with common artifacts
Figure 3.
Common EEG artifacts. An unstable rolling baseline is commonly called a sweat artifact whether or not it is caused by the subject sweating. The 60-Hz artifact is seen as a dark buzz riding on top of the normal EEG. When expanded (not shown), 60 waves per second can be seen. Movement artifacts are characterized by a combination of high-frequency EMG and a low-frequency component caused by cable sway. Regular periodic bursts of high-frequency EMG activity are seen on the EEG trace during teeth grinding. Pulse artifacts are typically recorded by A1 or A2 electrodes and are most apparent during low amplitude EEG in REM sleep.
-
67If the study requires analysis by sleep cycle, identify and mark each epoch with the appropriate cycle. Feinberg and Floyd (1979) contains the commonly used criteria for sleep cycle definition. In brief:
- sleep cycles 2 and later require at least 15 min of NREM and 5 min of REM sleep.
- Because the first REM period is often shorter than 5 min, cycle 1 and 2 can be separated by any duration of REM sleep.
- Even with this lax criterion for separating cycles 1 and 2, some subjects (typically children or sleep-deprived adults) have nights with a very long cycle 1. Their records often show two distinct peaks in delta power in NREM 3 sleep (stage 3 and stage 4 from Rechtschaffen and Kales) sleep separated by at least 10 min of NREM 2 sleep. In this case, it is acceptable to break the abnormally long cycle 1 into cycle 1 and 2 during the NREM 2 sleep that separates the delta peaks (Feinberg, et al., 1990; Jenni and Carskadon, 2004)
-
68
Save all the scoring data in a spreadsheet or database that will later be linked to the EEG analysis for summing within NREM sleep or REM sleep across the entire night or across sleep cycles.
FFT set-up and analysis
-
69
Many FFT analysis programs use a preconditioning filter to correct for DC offset problems. This filter will also affect the low frequency EEG. If the filter is adjustable, set it as low as possible.
-
70Decide on FFT parameters: Window duration, overlap, and taper function.
- These values differ from lab to lab, creating difficulties in comparing recordings between labs.
- Window duration must correspond to a number of samples that is a power of 2. With a 256-Hz digitization rate, 1024 (210) samples comprise a 4-sec window. With a 200-Hz digitization rate, 1024 samples comprise a 5.12-sec window.
- Hann, Hamming, Bartlett, and Welch windows are common window taper functions which produce slightly differently shaped windows (Press, et al., 2007).
- The window duration minus the window overlap is the increment from window to window. Choose an overlap duration that is approximately half the window duration, such that the window increment evenly divides the epoch length. (Each analysis epoch is the sum of successive, overlapping FFT windows.) Thus a 2-sec overlap of 4-sec windows produces a 2-sec window increment, yielding 10 windows per 20-sec epoch. A 2.62-sec overlap of 5.12-sec windows produces a 2.5-sec window increment, yielding 8 windows per 20-sec epoch.
-
71Choose frequency bands. Common frequency bands are low delta 0.3-1 Hz, delta 1-4 Hz , theta 4-8 Hz, alpha 8-12 Hz, sigma 12-15 Hz, and beta 15-30 Hz. Depending on the focus of the study, it may be desirable to break these down into narrower bands, such as 1-2, 2-3, and 3-4 Hz. The true band limits will usually be different than these nominal values.
- FFT produces a spectrum whose domain consists of discrete points between 0 Hz and the Nyquist frequency (half the digitization rate). However sleep EEG analysis traditionally reports data in frequency bands. Such bands, to be accurate, must be centered on the points of the spectrum, not delimited by them. For example, if sampling at 256 Hz and the FFT window duration is 4 sec, then the resulting spectrum contains points at 0, 0.25, 0.50, 0.75, 1.00, ... 127.75 Hz. To interpret these results as frequency bands, the frequency band limits must be taken from the midpoints of these values, i.e. from 0, 0.125, 0.375, 0.625, 0.875, 1.125 Hz...
-
72
Find the calibration analysis results from step 8.
-
73
The EEG analysis software listed in the Materials, PassPlus, uses the results of the calibration analysis to generate a scaling factor that is entered into the EEG analysis set up table. Other software packages may have a similar procedure. If using such an analysis program, enter the calibration data in the set up table and analyze the recorded EEG. If not follow steps 74-78 to calculate and apply a calibration scaling factor.
-
74
Check that the calibration signal was clean, i.e. that there was minimal power in frequency bands other than the calibration frequency band.
-
75
Average the spectral energy in the calibration frequency band in all epochs which contain a clean calibration signal.
-
76Calculate the expected energy per epoch of the calibration signal. For a sine wave of peak to peak amplitude V (μV) over an epoch of length t (seconds), the energy per epoch is V2×t/8.
- For example, the energy of a clean 200 μV peak to peak sine wave over a 30-sec epoch will be 150,000 μV2sec.
-
77
Calculate the calibration scaling factor by dividing the expected value calculated in step 76 by the recorded average calibration energy per epoch in step 75.
-
78
Analyze the all-night EEG data. Multiply the analysis results by the calibration scaling factor.
Data Standardization
Because standardization should be avoided when possible, the following information is not included as steps of the protocol. Standardization discards important information. If possible, pool data across subjects without standardizing. However, some experiment designs may require standardization. For example, standardization might be used in an experimental that tests the effect of a treatment on two age groups with very different EEG activity under control conditions. Two common methods are 1) expressing spectral energy in a portion of the night (e.g., data in an NREM period) as a percent of spectral energy in the entire night, and 2) expressing power in a frequency band as a percent of power in the entire spectrum (e.g. 1-4 Hz power in NREM sleep can be divided by 0.3-50 Hz power in NREM sleep.)
COMMENTARY
Background Information
Aserinsky and Kleitman's (1953) all-night EEG recordings showing alternation of REM and NREM sleep launched modern sleep research. More than 50 years later the EEG remains the primary tool for laboratory sleep evaluation. The increase in computing power has provided a means to quantify EEG activity. The activity in certain frequency bands provides information about the quality of the sleep beyond the information that sleep stage scoring provides. The most intensively investigated band has been the delta band (commonly 1-4 Hz) that characterizes NREM sleep. Delta EEG is thought to reflect a homeostatic process of sleep (c.f. Feinberg, 1974). Delta activity is highest at the beginning of the night when the need for recuperation is greatest and declines across the night as recuperation proceeds. Delta is increased in sleep following extended waking (c.f. Berger and Oswald, 1962; Borbely, et al., 1981) and is decreased in sleep following a daytime nap (Campbell and Feinberg, 2005; Feinberg, et al., 1985; Werth, et al., 1996). Computer quantification of delta activity reveals that delta is regulated such that, on average, the amount of delta activity accumulated in a daytime nap is precisely subtracted from the subsequent night-time sleep. Other frequency bands provide further information about the night's sleep. Sigma activity produced by organized sleep spindles dominates power in the 12-15 Hz frequency range (Uchida, et al., 1991) and can serve as an index of sleep spindle abundance (Dijk, et al., 1993). High frequency EEG such as beta (15-30 Hz) reflects within-sleep arousal level and is often increased in insomnia (c.f. Merica, et al., 1998).
Critical Parameters and Troubleshooting
Digitizing the EEG
The first step in computer analysis of the EEG is sampling the continually changing analog EEG signal. For a 200-Hz digitization rate, the amplitude of the signal is measured every 0.005 sec. This sampling rate produces a very accurate picture of a low frequency wave such as the 3.5-Hz sine wave used for calibration. A single wave of this frequency, which has a period (1/f) of 0.286 sec, is sampled 57 times. At higher frequencies the number of samples per wave decreases, and the shape of the wave is distorted. A single 40-Hz wave is sampled only five times. Movie 1 demonstrates this progressive distortion as the frequency increases. Above the Nyquist frequency, however, frequency components are impossible to detect. They appear to have a frequency less than the Nyquist frequency. For example, when sampling at 200 Hz, a 110-Hz wave is seen as a 90-Hz wave, a 125-Hz wave as a 75-Hz wave, and a 199-Hz wave as a 1-Hz wave. This misreading of frequencies, called aliasing, is shown in Movie 1. Because of aliasing, it is vital that the signal being digitized has no significant component faster than the Nyquist frequency. This is accomplished by matching the analog filters to the digitization rate. The high frequency filter should filter the signal such that waves with frequencies higher than 1/2 the digitization rate are decreased to a small fraction of their original amplitude.
The digitization rate for the EEG recorder may be preset or adjustable. If the digitization rate can be selected, it may be tempting to choose the highest rate possible. However, higher digitization rates produce larger files and can create storage problems. Counteracting the aliasing problem is the fact that the EEG is a naturally anti-aliasing waveform because its amplitude decreases as the frequency increases. Aliasing problems can arise with high-frequency artifacts or high amplitude EMG contamination of the EEG (Dumermuth, et al., 1987). Visually prescreening the data for artifacts as described in step 66 can eliminate these sources of aliased waveforms.
Filtering the EEG
Filters are commonly applied to the EEG to prevent aliasing and to remove unwanted low frequency oscillations that are not of biological origin. Filtering will alter the amplitude and shape of EEG waveforms. The degree to which the waves are affected depends on their frequencies and the characteristics of the filter. The EEG, like any complex signal, can be considered as the sum of an infinite number of components, each of a different frequency. Filtering the EEG will alter these components so that the results of FFT analysis of a filtered EEG will differ from those of an unfiltered EEG.
The language describing filters can be confusing. The same filter may be described by different authors in many different ways. A low-frequency filter attenuates low-frequency components and is also called a low-cut filter or high-pass filter. A high-frequency filter attenuates high-frequency components and is also called a high-cut filter or low-pass filter.
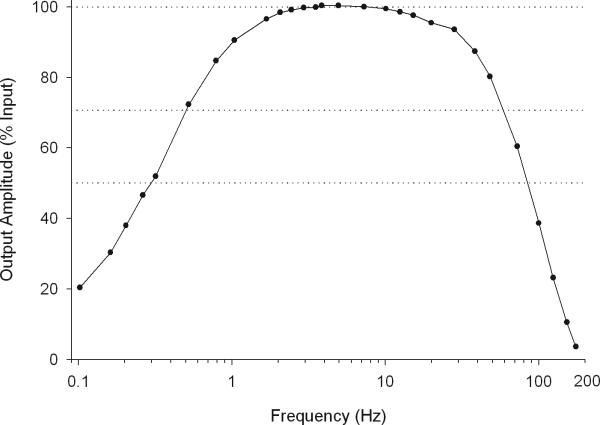
A filter's order begins to describe its electrical components. A first-order filter, or RC filter, is comprised of a resistor and a capacitor. Higher order filters are more complex. A filter may also be described by how it affects the signal it is filtering. For example, a low frequency filter with a -3 dB point at 0.5 Hz and a 6 dB per octave roll off indicates that at 0.5 Hz the recorded signal's amplitude is reduced to about 71% of its input amplitude, and that for every 50% decrease in the frequency (1 octave decrease) the signal's amplitude is reduced by 50% (-6 dB). A frequency response curve (Fig. 4) most effectively describes a filter's effect on a signal. The curve shows the output amplitude as a percent of the input amplitude over a range of frequencies. The frequency response curve in Figure 4 shows that the filter output of a 0.3 Hz wave is 50% of its input amplitude and that a 3-Hz wave is unfiltered.
Figure 4.
Frequency-response curve for the hardware low frequency and high frequency filters on the Grass-Telefactor H2O ambulatory EEG recorder. Output amplitude as a percent of input amplitude is plotted against frequency (on a logarithmic scale). Signals between 2 and 13 Hz are largely unaffected (<2% decrease) by the filters. The low-frequency filter is a 0.3 Hz ½ amplitude filter with a 6-dB per octave roll off. A 0.5-Hz signal is decreased by ~30%; a 0.3-Hz signal is decreased by ~50 %. The high frequency filter decreases a 50 Hz signal by ~20% and a 100Hz signal by ~60%.
If using a commercial EEG recorder controlled by a computer with EEG recording software, the system may contain both hardware filters in the recorder and also software filters in the computer program. Furthermore, the signal that is analyzed may be filtered differently than the signal displayed on the computer screen during recording. In order to determine the true filtering of the analyzed signal, it is recommended to create a frequency-response curve by analyzing calibrated sine waves of a fixed amplitude over a range of frequencies from 0.1 Hz up to the digitization rate. Again use a clean signal and use wide frequency bands to avoid the problem of leakage. Remember that waves above the Nyquist frequency will be aliased as lower frequency waves. If the software performs spectral analysis and the output is energy in each epoch, use the following formula to calculate peak-to-peak amplitude of a pure sine wave from energy:
units are μV for amplitude, seconds for epoch duration, and μV2sec for energy This formula applies only to pure sine waves and cannot be used to determine the amplitude of EEG waves. The frequency response curve for the Grass H2O shown in Figure 4 represents the effects of the hardware filters. Software filter effects were eliminated by using amplitude calculated from FFT with no preconditioning filter. It is informative to generate separate frequency response curves with and without contributions from software filters.
A low-frequency filter minimizes low frequency baseline oscillations that are not of biological origin but should affect the low frequency EEG as little as possible. Some systems strongly filter the low-frequency EEG so that the recorded signal appears noise-free. These filters also attenuate biologically meaningful signals. A high frequency filter should be chosen such that it eliminates aliasing. Ideally, the filter would not affect waves with frequency lower than the Nyquist frequency and would completely block waves above that frequency. Most systems have a high frequency filter that minimizes aliasing but decreases the amplitude of a wide range of higher EEG frequencies. Again, a frequency-response curve will allow determination of how filters affect the signal.
Most recording systems also include a notch filter to eliminate 60- or 50-Hz electrical noise. Many of these filters have a fairly broad “notch” that will affect EEG across a wide range of frequencies. It is sound practice to eliminate the source of the electrical noise rather than using notch filters. Proper electrode application and proper grounding deal with most 60-Hz problems, but some equipment (e.g., AC/DC transformers) are powerful broadcasters of 60-Hz noise. Such equipment should be located and unplugged or shielded prior to recording.
Calibration
Recording an external calibration signal of known amplitude provides a standard that stays with the recorded EEG. Calibration provides a means of assessing the stability of recordings. If a subject's EEG recording changed from one night to the next, without an external calibration signal it cannot be ascertained if it was the subject or the amplifier that changed. Most recorders include an internal calibration mechanism, but the internal calibration may change with the amplifier. An external calibration provides insurance against changes in the recorder over time. It allows data to be pooled across different recorders, and a recorded calibration signal enables data to be shared more easily between laboratories.
Spectral Analysis
The EEG can be mathematically decomposed into an infinite number of pure sinusoidal components, each of a different frequency, which when added together yield the original signal. Just as a digitized signal is an approximation of its original analog signal, an FFT of that digitized signal is an approximation of these frequency components. If an FFT is performed on a 4-sec window containing 1024 digitized samples, the result can be summarized as a power spectrum consisting of 512 power values, at frequencies equally spaced between 0 and 127.75 Hz. These frequencies are typically combined into wider frequency bands when reporting EEG results.
EEG analysis with FFT has problems that can be minimized by choosing appropriate FFT parameters. Power spectral analysis assumes that the EEG is composed of periodic (exactly repeating) waveforms of many different frequencies, and that none of these components change in frequency, amplitude, or shape during each window of analysis. This property is called “stationarity.” The EEG violates this assumption because transient waveforms, such as K-complexes and spindles, appear intermittently. FFT is typically computed on shorter duration windows within a 30-sec epoch, and the sum of the data from these windows comprises the data for the epoch. Choosing a short-duration window minimizes the violation of the stationarity assumption (Press, et al., 2007). However, shortening the window duration decreases the spectral analysis frequency resolution, which is the inverse of the window length. For example, FFT analysis with an 8-sec window produces 0.125-Hz bins, whereas analysis with a 1-sec window produces 1-Hz bins. Window lengths around 4 sec are commonly used in EEG analysis.
Another problem that can arise from FFT analysis of the EEG is leakage of power from one frequency bin to others (Press, et al., 2007). Tapering the shape of the window, so that the window begins and ends at 0 amplitude and rises to 100% in the middle, decreases power leakage (Press, et al., 2007). Tapering itself introduces a problem in that the data at the edge of the windows is weighted less heavily than data in the middle of the window. Overlapping tapered windows resolves this problem (Press, et al., 2007). If the overlap is half the window length, then each sample will make approximately the same contribution to the spectrum.
A problem inherent in spectral analysis that cannot be overcome with proper parameter settings is the basic assumption that complex wave forms are comprised of sine waves. FFT analysis of a 1-Hz square wave will show power across a wide range of frequencies. The brain does not produce square waves, but it does produce wave forms that are not sinusoidal. In general, this problem is outweighed by the analytic usefulness of being able to decompose the signal into component frequencies, as long as one realizes that not all components are necessarily biologically meaningful in themselves.
Other methods of signal analysis, such as the Fujimori method of PAA, and wavelet analysis, overcome some of the above limitations of FFT. However these methods have only recently begun to be applied to EEG analysis (Stam, 2005). If these methods become more widely used and are validated for EEG analysis, they may in the future replace FFT.
Anticipated Results
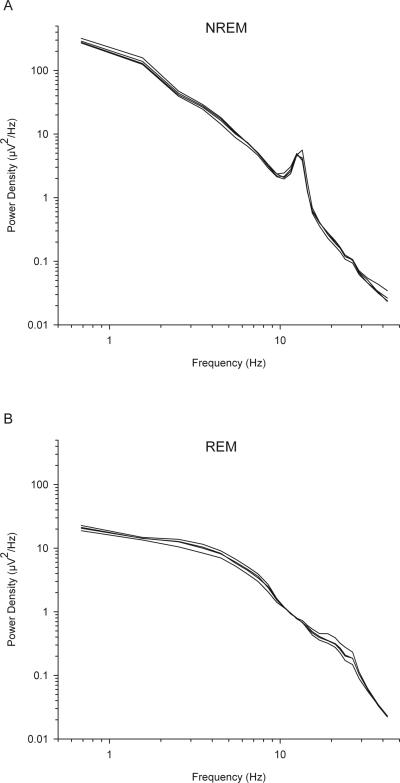
Examples of raw EEG recordings are shown in the sleep stage samples in Figure 2. Examples of all-night power density (power/Hz) spectra for NREM and REM sleep EEG are shown in Figure 5. EEG power density decreases exponentially with increasing frequency. In NREM, the power density in the 1-2 Hz frequency band is 5000 times greater than the power density in the 40-45 Hz band. Another common landmark of the NREM power spectrum is the peak in the sigma (12-15 Hz) frequency range. This landmark is less pronounced or is absent in the NREM power spectra of elderly subjects. In the REM sleep EEG power spectrum, despite having much lower slow wave activity than NREM sleep, low-frequency power density exceeds power density in higher frequency bands. Figure 5 shows 4 nights of NREM and REM power spectra from a single subject. These spectra are remarkably consistent from night to night within a subject and represent an individual trait (Tan, et al., 2001).
Figure 5.
Log-log plot of NREM (A) and REM (B) all-night power density (power/Hz) versus frequency. Each graph shows four traces representing four nights of EEG recorded from C3/A2 from a 20-year-old man. The traces are remarkably similar from night to night. In both REM and NREM, power is highest in the low frequencies and decreases as frequency increases. Low frequency power is about 10 times greater in NREM than REM. The NREM spectra show a peak in the sigma (12-15 Hz) frequency range.
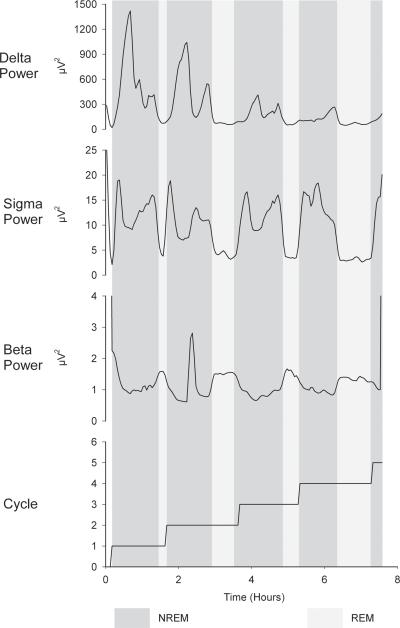
Typical FFT data for various frequency bands for an entire night of sleep are shown in Figure 6. The data are divided into clear cycles of NREM and REM sleep. NREM EEG is characterized by high power in delta and sigma frequencies and low power in the beta frequency. Within NREM sleep, delta and sigma EEG power oscillate reciprocally, i.e. when delta is high sigma is low and vice versa (Uchida, et al., 1991). Within REM sleep, both delta and sigma activities fall to very low levels. Beta behaves in the opposite manner, being low during NREM and increasing during REM (Uchida, et al., 1992).
Figure 6.
All-night smoothed traces of analyzed EEG in three frequency bands. Dark background shading indicates NREM sleep; light background shading indicates REM sleep. Sleep cycle number is plotted on the bottom panel. Delta (1-4 Hz) power is high during NREM sleep and low during REM sleep. Sigma (12-15 Hz) power is also high during NREM and low during REM, but during NREM it fluctuates reciprocally with delta, being high when delta is low and low when delta is high. Beta (23-30 Hz) power is higher in REM than in NREM, with the exception of an artifactual peak during cycle 2.
Supplementary Material
Movie 1. This 1-minute video clip demonstrates the effect of digitizing an analog signal by showing sine waves of various frequencies recorded at a 200-Hz digitization rate. The waves recorded are all 200-μV peak-to-peak amplitude. The video demonstrates distortion of high frequency waves and the problem of aliasing. Notice that, as the frequency increases, the high frequency filter decreases the amplitude of the recorded waves. This filter helps counteract the problem of aliasing.
Acknowledgments
Jonathan March of Delta Software provided valuable advice on the spectral analysis sections of this protocol. Igor Dykan and Lisa Higgins assisted with the aliasing movie.
Literature Cited
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Berger RJ, Oswald I. Effects of sleep deprivation on behaviour, subsequent sleep, and dreaming. Journal of Mental Science. 1962;108:457–465. doi: 10.1192/bjp.108.455.457. [DOI] [PubMed] [Google Scholar]
- Borbely A, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep-deprivation: effect on sleep stages and EEG power density in man. Electroencephalography and Clinical Neurophysiology. 1981;51:483–493. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Homeostatic response to naps is similar in normal elderly and young adults. Neurobiology of Aging. 2005;26:135–144. doi: 10.1016/j.neurobiolaging.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Research. 1993;626:190–199. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- Dumermuth G, Ferber G, Herrmann WM, Hinrichs H, Kunkel H. International pharmaco-EEG group (IPEG). Committee on standardization of data acquisition and analysis in pharmaco-EEG investigations. Neuropsychobiology. 1987;17:213–218. doi: 10.1159/000118367. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Changes in sleep cycle patterns with age. Journal of Psychiatric Research. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–291. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Feinberg I, March JD, Flach K, Maloney T, Chern WJ, Travis F. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (Delta) electroencephalogram of human sleep. Brain Dysfunction. 1990;3:183–192. [Google Scholar]
- Feinberg I, March JD, Floyd TC, Jimison R, Bossom-Demitrack L, Katz PH. Homeostatic changes during post-nap sleep maintain baseline levels of delta EEG. Electroencephalography and Clinical Neurophysiology. 1985;61:134–137. doi: 10.1016/0013-4694(85)91051-x. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Moore CA. Computers in sleep medicine. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Third ed. W.B. Saunders Company; Philadelphia: 2000. pp. 1302–1307. [Google Scholar]
- Jasper HCC. Report of the committee on methods of clinical examination in electroencephalography: 1957. Electroencephalography and Clinical Neurophysiology. 1958;10:370–375. [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–783. [PubMed] [Google Scholar]
- Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. European Journal of Neuroscience. 1998;10:1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes: The Art of Scientific Computing. Cambridge University Press; Cambridge: 2007. [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Public Health Services, U.S. Government Printing Office; Washington, D.C.: 1968. [Google Scholar]
- Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, Pressman MR, Iber C. The visual scoring of sleep in adults. Journal of Clinical Sleep Medicine. 2007;3:121–31. [PubMed] [Google Scholar]
- Stam CJ. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clinical Neurophysiology. 2005;116:2266–301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Tan X, Campbell IG, Feinberg I. Internight reliability and benchmark values for computer analyses of non-rapid eye movement (NREM) and REM EEG in normal young adult and elderly subjects. Clinical Neurophysiology. 2001;112:1540–1552. doi: 10.1016/s1388-2457(01)00570-3. [DOI] [PubMed] [Google Scholar]
- Uchida S, Maloney T, Feinberg I. Beta (20-28 Hz) and delta (0.3-3 Hz) EEG oscillate reciprocally across NREM and REM sleep. Sleep. 1992;15(4):352–358. doi: 10.1093/sleep/15.4.352. [DOI] [PubMed] [Google Scholar]
- Uchida S, Maloney T, March JD, Azari R, Feinberg I. Sigma (12-15 Hz) and delta (.3-3 Hz) EEG oscillate reciprocally within NREM sleep. Brain Research Bulletin. 1991;27:93–96. doi: 10.1016/0361-9230(91)90286-s. [DOI] [PubMed] [Google Scholar]
- Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. American Journal of Physiology. 1996;271:R501–R510. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. This 1-minute video clip demonstrates the effect of digitizing an analog signal by showing sine waves of various frequencies recorded at a 200-Hz digitization rate. The waves recorded are all 200-μV peak-to-peak amplitude. The video demonstrates distortion of high frequency waves and the problem of aliasing. Notice that, as the frequency increases, the high frequency filter decreases the amplitude of the recorded waves. This filter helps counteract the problem of aliasing.