Abstract
GDP-bound prenylated Rabs, sequestered by GDI (GDP dissociation inhibitor) in the cytosol, are delivered to destined sub-cellular compartment and subsequently activated by GEFs (guanine nucleotide exchange factors) catalysing GDP-to-GTP exchange. The dissociation of GDI from Rabs is believed to require a GDF (GDI displacement factor). Only two RabGDFs, human PRA-1 and Legionella pneumophila SidM/DrrA, have been identified so far and the molecular mechanism of GDF is elusive. Here, we present the structure of a SidM/DrrA fragment possessing dual GEF and GDF activity in complex with Rab1. SidM/DrrA reconfigures the Switch regions of the GTPase domain of Rab1, as eukaryotic GEFs do toward cognate Rabs. Structure-based mutational analyses show that the surface of SidM/DrrA, catalysing nucleotide exchange, is involved in GDI1 displacement from prenylated Rab1:GDP. In comparison with an eukaryotic GEF TRAPP I, this bacterial GEF/GDF exhibits high binding affinity for Rab1 with GDP retained at the active site, which appears as the key feature for the GDF activity of the protein.
Keywords: GDF, GDI, GEF, p-Rab1, SidM/DrrA
Introduction
Rab proteins comprise the largest sub-group of the Ras superfamily of small GTPases and regulate all aspects of secretory vesicle trafficking. They are able to reversibly associate with membranes through the hydrophobic prenyl group(s) covalently linked to cysteine residue(s) at the C-terminal extreme (Pfeffer and Aivazian, 2004). They undergo conformational transition between the GTP-bound active state and the GDP-bound inactive state. The transition between the two states is a slow process by itself, because their intrinsic GTPase activity is low, and GDP release from the active site is slow (Vetter and Wittinghofer, 2001). The catalytic activity is enhanced by GTPase-activating proteins, whereas GDP release and the following GTP replacement are accelerated by GEFs. The conformational transition is coupled with recycling of Rabs between the cytosol and membranes, which is mediated by RabGDIs that deliver GDP-bound prenylated Rabs to membranes, and also function to extract inactive Rabs from membranes. RabGDI sequesters Rabs in the inactive form in the cytosol by interacting with their Switch loops near the nucleotide-binding site and also with their C-terminal prenyl groups. As the interaction between RabGDI and a prenylated Rab is tight (Shapiro and Pfeffer, 1995; Wu et al, 2007), the dissociation between the two proteins is presumed to require the activity of GDF in the vicinity of membranes (Pfeffer and Aivazian, 2004). An integral membrane protein human PRA-1 (Yip3 in yeast) was identified as a GDF, catalysing dissociation of RabGDI from endosomal Rab9 (Sivars et al, 2003). So far, this is the only known eukaryotic protein with GDF activity distinct from a GEF. Consequently, the molecular mechanism of GDI displacement from Rabs remains elusive, whereas the other processes for the conformational interconversion and the recycling of Rabs are extensively studied and well characterized.
The intracellular human pathogen Legionella pneumophila translocates a large number of bacterial proteins into the host cytosol through the Dot/Icm type IV secretion system (Segal et al, 1998; Derre and Isberg, 2004; Kagan et al, 2004; Ninio and Roy, 2007). One of the translocated proteins, SidM/DrrA, has been identified to have dual GEF and GDF activity specifically toward Rab1 (Machner and Isberg, 2006, 2007; Murata et al, 2006; Ingmundson et al, 2007), which is an essential regulator for endoplasmic reticulum (ER)-to-Golgi vesicle trafficking (Nuoffer et al, 1994; Allan et al, 2000; Moyer et al, 2001). This activity is required for hijacking ER-derived vesicles to the L. pneumophila-containing vacuole (LCV), within which the bacteria reside and replicate (Ingmundson et al, 2007; Machner and Isberg, 2007). Through deletion mutational analysis, it was further shown that a central region of SidM/DrrA, comprising residues 317–545, is sufficient for binding Rab1 and for the dual GEF/GDF activity (Machner and Isberg, 2007). SidM/DrrA is a soluble protein in contrast to PRA-1, which is a very unstable integral membrane protein (Sivars et al, 2003, 2005). This feature of SidM/DrrA facilitates structural and biochemical studies for probing into the molecular mechanism underlying the dual GEF and GDF activity.
In this study, we determined the structure of a SidM/DrrA fragment, containing residues 317–533, in complex with Rab1, revealing that the GEF/GDF domain is structurally distinct from all the known eukaryotic GEFs. In conjunction with extensive physicobiochemical experiments, this study indicates that the GDF activity of SidM/DrrA is mediated by the Rab1-binding interface responsible for its GEF activity, and that the dual activity is likely to arise from tight binding affinity of the protein for GDP-bound Rab1.
Results and discussion
Structure of the central domain of SidM/DrrA bound to Rab1
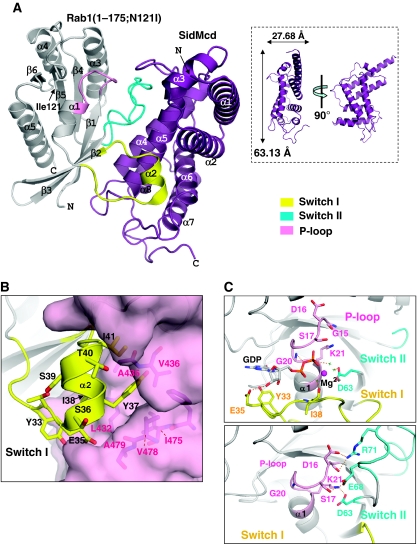
Full-length SidM/DrrA and four different truncated versions of the protein, including its GEF/GDF domain (residues 317–545), were produced as tight complexes with full-length Rab1 or with a C-terminally truncated Rab1 variant, which is defective in nucleotide binding due to an Asn121Ile substitution near the active site (Weide et al, 1999). Well-diffracting crystals were obtained only with the central domain of SidM/DrrA containing residues 317–533, referred to as SidMcd, in complex with the core GTPase domain of the Rab1 variant, referred to as Rab1(1-175;N121I). Subsequently, the structure of the complex was determined to 1.5 Å resolution (Supplementary Table 1). SidMcd is an α-helix-only protein containing eight α-helices, which together organize a flat, elongated single-domain structure (Figure 1A). The central α-helix pair (α4 and α5) lies on a surface formed by the rest six α-helices. Database search using the Dali server (Holm and Sander, 1996) showed that the GEF/GDF domain of SidM/DrrA is not significantly similar to any known three-dimensional structures.
Figure 1.
Interaction between SidM/DrrA and Rab1. (A) Structure of SidMcd–Rab1(1-175;N121I). SidM/DrrA interacts with the Switch I, Switch II and P-loop regions of Rab1. The mutated residue Ile121 (in sticks) of Rab1 is not involved in the intermolecular interaction. The inset shows that SidMcd is a flat molecule. (B) Interaction between SidM/DrrA and α2 of Rab1. The largely dislocated central region of Switch I forms α2 that is stabilized by mixed hydrophobic and hydrophilic interactions with a cleft of SidMcd. Tyr37 of Rab1 interacts tightly with multiple hydrophobic residues of SidMcd, including A435 that is mutated in this study (Figure 3A). An arrow pointing Ile38 is shown to emphasize that this residue is also involved in tight hydrophobic interaction with SidMcd, and thus is an important residue conferring recognition specificity (see text). (C) Stabilization of the nucleotide-free P-loop. The structures of isolated Rab1:GDP (PDB code: ) and Rab1 in complex with SidMcd are shown in the same orientation. The dotted lines, red sphere and green crosses denote polar interactions, Mg2+ and Mg2+-chelating water molecules. P-loop is in close contact with bound Mg2+-GDP, which is also involved in direct interactions with Switch I (top). On SidMcd binding, P-loop moves into the GDP-binding site and its conformation is stabilized by Switch II involved in multiple interactions with the loop, including the Lys21–Asp63 interaction (bottom).
SidMcd interacts with Rab1 extensively, burying a solvent-accessible area of 1771 Å2. Its central α-helix pair is the primary interface contacting various parts of Rab1: the segment between α1 and β2 (the Switch I region), loop β3–β4 (the Switch II region) and the strands β2, β3. By these and other interactions, Switch I, Switch II and P-loop of Rab1 undergo conformational changes compared with the structure of Rab1 bound to GDP (Rab1:GDP), with the conformational change of Switch I being the most significant. The Switch I loop is snatched significantly away from the nucleotide-binding site by SidMcd (Figure 1A). This pronounced conformational change of Switch I involves translocation of its residues to as far as 28 Å and transformation of its central loop segment (residues 36–41) into an one-and-half-turn α-helix that fits into a cleft formed by α5, α6 and loop α6–α7 of SidMcd (Figure 1B). The Switch II region in the complex is in an ordered conformation whereas that in free Rab1:GDP is largely disordered. In addition, the phosphate-binding P-loop motif (loop β1–α1) interacts with loop α5–α6 of SidMcd and is slightly dislocated to partly overlap with the space for binding the guanine nucleotide (Figure 1C; Supplementary Figure S1). In many GEFs, the nucleotide-free P-loop is stabilized by an ionic interaction between a conserved lysine residue on this loop and a glutamate or aspartate residue on Switch II (Vetter and Wittinghofer, 2001). The corresponding residues: Lys21 and Asp63 of Rab1 are engaged in an ionic interaction in the presented structure (Figure 1C), showing a recurring theme of stabilizing the disturbed P-loop. The conformations of the Switch I, Switch II and P-loop are also quite different from those of the equivalent loops in GPPNHP-bound Ypt1 (Eathiraj et al, 2005), yeast Rab1 sharing 69% sequence identity with human Rab1. The reconfiguration of these segments by SidMcd conforms to the general mechanism by which GEFs reduce the nucleotide-binding affinity of small GTPases (Vetter and Wittinghofer, 2001).
Although we used the nucleotide binding-defective mutant Rab1, virtually the same intermolecular interaction should be observed with wild-type Rab1. This is because the N121I mutation site is remote from the SidMcd–Rab1 binding interface (Figure 1A) and because SidMcd binding to Rab11:GDP results in the eviction of the bound nucleotide (Supplementary Figure S2A). A quantitative binding analysis by isothermal titration calorimetry (ITC) showed that full-length SidM/DrrA interacts with full-length Rab1:GDP with an apparent dissociation constant (Kd) of 84 nM, which is close to the Kd value of 79 nM measured for the interaction between SidMcd and Rab1(1-175):GDP (Supplementary Figure S2B). Therefore, the structure of the complex between the two truncated proteins represents the interaction between their full-length versions.
Substrate binding and destabilization of Mg2+-GDP by SidM/DrrA
The interaction between SidMcd and Rab1 is distinct from the four other structurally characterized RabGEF–Rab interactions in that Switch I and Switch II in Rab1 exhibit the most extensive intermolecular interactions in comparison with those in the other RabGEF–Rab complexes. As a result, the conformations of the two switch regions in Rab1 are obviously different from those in any of the four other Rabs (Supplementary Figure S3). It was previously reported that SidM/DrrA binds Rab1 specifically among eight different Rabs tested (Machner and Isberg, 2006). A sequence alignment of these Rabs shows that the SidMcd-interacting residues on Switch I region are quite variable, whereas those on Switch II are highly conserved (Supplementary Figure S4). Especially, Tyr37 and Ile38 on Switch I of Rab1, the two key residues tightly interacting with SidMcd (Figure 1B), are not conserved or singly conserved in other Rabs. The presented structure indicates that other amino acids at these two positions should affect the tight interaction between Switch I and SidMcd. These observations suggest that the Switch I–SidMcd interaction is critical for the binding affinity and recognition specificity of SidM/DrrA for Rab1. The importance of this interaction in the binding affinity is supported by site-directed mutagenetic analyses described below.
As a GEF, SidMcd should weaken the binding between GDP and Rab1. We suggest that SidMcd achieves this goal primarily by dislocating Switch I (Figure 1B), residues of which interact with the bound Mg2+-GDP directly (Tyr33, Glu35) or indirectly (Ile38) through a Mg2+-coordinating water molecule (Figure 1C). SidMcd does not seem to destabilize the Mg2+-GDP binding by a so-called ‘glutamate finger' or by a protein ‘wedge' that sterically interferes with Mg2+ binding, the strategies observed for other GEFs (Beraud-Dufour et al, 1998; Thomas et al, 2007). This is because none of the residues of the SidMcd bound to Rab(1-175; N121I) is close to the position of the Mg2+-GDP bound to Rab1 (>4 Å), although we cannot rule out a possibility of a transient intrusion of SidMcd into the Mg2+-GDP site along the reaction path.
GDF activity of SidM/DrrA
A comparison of the presented structure with that of the yeast RabGDI bound to prenylated Ypt1:GDP (p-Ypt1:GDP) reveals that the SidM/DrrA-interacting residues of Rab1 mostly overlap with the RabGDI-interacting residues of the GTPase domain of Ypt1 (Figure 2A), pointing that the GTPase domain has to be dissociated from RabGDI to interact with SidM/DrrA. Given the dual GEF and GDF activity of SidMcd, it can be imagined that an unidentified surface of SidMcd mediates full displacement of RabGDI from p-Rab1:GDP, and then the crystallographically defined Rab1-interacting surface of SidMcd catalyses the GDP-to-GTP exchange on Rab1. The presented structure, however, does not hint at how such dissociation might be mediated by SidM/DrrA. To understand the biochemical basis for the GDF activity of SidM/DrrA, we produced human GDI1 in complex with prenylated Rab1:GDP (p-Rab1:GDP) using Sf9 insect cells and Escherichia coli cells (see Materials and methods section). This complex was then reacted with full-length SidM/DrrA and the reaction mixture was analysed by native gel electrophoresis. This assay showed that SidM/DrrA is able to displace GDI1 from p-Rab1:GDP, but inefficiently; notable but incomplete dissociation of GDI1 was achieved by SidM/DrrA at 32-fold molar excess over GDI1–p-Rab1:GDP (Figure 2B). We also noted that SidMcd has GDF activity indistinguishable from that of the full-length protein (data not shown). The observed SidM/DrrA-mediated GDI1 displacement is consistent with the reported capacity of a central SidM/DrrA fragment, containing residues 317–545, to dissociate GDI2–p-Rab1:GDP in an assay involving ∼7 pM of the complex and affinity resins saturated with the SidM/DrrA fragment, which is likely to correspond to micromolar concentration of the protein (Machner and Isberg, 2007).
Figure 2.
Basis for the GDF activity of SidM/DrrA. (A) SidM/DrrA and RabGDI bind to the same surface of Rab1/Ypt1. The residues of Rab1 and Ypt1 involved in the intermolecular interaction (<4.0 Å) with SidMcd or RabGDI were identified from the presented structure and the RabGDI–p-Ypt1:GDP structure (PDB entry: ). These residues are indicated on the sequence alignment (top) by the triangles. They are mapped on Rab1 bound to SidMcd or on Ypt1:GDP bound to RabGDI (bottom). They are located on the same surface of Rab1/Ypt1 and largely overlap with each other. (B) Native-gel based GDF activity assay involving SidM/DrrA and GDI1–p-Rab1:GDP only. GDI1–p-Rab1:GDP (5 μM) was incubated with SidM/DrrA at 5, 20, 80 and 160 μM for 5 h at 25°C. Large molar excess of SidM/DrrA is required for considerable GDI1 displacement from p-Rab1. The GDI1–p-Rab1:GDP sample contained residual GDI1 (first lane) because supplemented GDI1 was not completely isolated from the complex. (C) A putative model for simultaneous interaction of p-Rab1 with SidM/DrrA and GDI1. The partly dissociated GDI1–p-Rab1:GDP complex is constructed based on the structures of Ypt1:GDP alone or bound to RabGDI. The flexible C-terminal segment of Ypt1 in the structure of Rab1GDI–p-Ypt1:GDP is indicated by a dotted line. NTD and CTD stand for the N- and C-terminal domain of SidM/DrrA, respectively. The Kd value for the interaction between RabGDI and unprenylated Ypt1:GDP (Pylypenko et al, 2006) is shown to indicate that the interaction between GDI1 and the GTPase domain of Rab1:GDP would be similarly weak. (D) Detection of the SidM/DrrA–p-Rab1 binary complex. In the presence of 0.1 mM EDTA, GDI1–p-Rab1:GDP (15 μM) was incubated for 5 h at 25°C with SidM/DrrA (WT) or a SidM/DrrA mutant (A435E), both at 60 μM. The top band was identified as the binary complex by mass spectrometry and immunoblotting using antibodies against the FLAG tag (linked to SidM/DrrA), GDI1 or Rab1 (data not shown). EDTA does not seem to affect the stability of the GDI1–p-Rab1GDP complex (lanes 1 and 2). Lane 5 represents a negative control using a SidM/DrrA mutant containing A435E mutation that results in reduced affinity for Rab1 as described in Figure 3.
How could the ‘inefficient' GDF activity of SidM/DrrA be explained? In the structure of RabGDI–p-Ypt1:GDP, the intermolecular interaction is roughly two-sited in that the core part (the GTPase domain plus a portion of the C-terminal loop) and the prenylated C-terminal tail of Ypt1 are flexibly linked and interact with two separate regions of RabGDI (Rak et al, 2003; Pylypenko et al, 2006). Notably, although RabGDI interacts with prenylated Rabs with significantly high affinity (Kd⩽23 nM), its affinity for unprenylated Rabs is about 103-fold weaker (Shapiro and Pfeffer, 1995; Pylypenko et al, 2006; Wu et al, 2007). Therefore, it is possible that the GTPase domain of p-Rab1 often dissociates from the Rab-binding platform of GDI1 spontaneously, whereas the prenylated C-terminus of Rab1 remains attached to the inhibitor. SidM/DrrA may be able to capture the dissociated GTPase domain to form a triple complex between SidM/DrrA, p-Rab1:GDP and GDI1 (Figure 2C). This three-way interaction might result in the eviction of GDP from p-Rab1, but could hardly drive full dissociation of GDI1, because the apparent binding affinity between SidM/DrrA and Rab1:GDP (Kd=84 nM) is weaker than that between RabGDI and prenylated Rabs. However, large amount of SidM/DrrA should drive equilibrium toward full dissociation of GDI1. A calculation based on dissociation constants, assuming a Kd=20 nM for the interaction between GDI1 and p-Rab1:GDP, tells that 985 μM SidM/DrrA is required to achieve 95% dissociation of 5 μM GDI1–p-Rab1:GDP, which is consistent with our experimental observation. The putative triple complex of GDI1–p-Rab1–SidM/DrrA should be undetectable under normal conditions, according to the reported observations that GDI did not precipitate with SidM/DrrA-bound p-Rab1 in a pull-down assay involving SidM/DrrA and the GDI–p-Rab1:GDP complex (Ingmundson et al, 2007; Machner and Isberg, 2007). In an attempt to detect the putative triple complex, we used ethylenediaminetetraacetic acid (EDTA) in the reaction mixture. The binding affinity between SidM/DrrA and Rab1:GDP should be increased by EDTA, because it prevents evicted Mg2+-GDP from rebinding nucleotide-free Rab1. Accordingly, we expected to detect the triple complex as well as a reaction product SidM/DrrA–p-Rab1 on the native gel with less amount of SidM/DrrA, which otherwise masked a large portion of the gel. Under these conditions, the binary complex was detected, which migrated slowly on the gel (Figure 2D), but the triple complex was not. Nonetheless, we posited that the triple complex can be formed but transiently, and that GDI1 displacement by SidM/DrrA is achieved by the crystallographically determined interaction of the protein with Rab1.
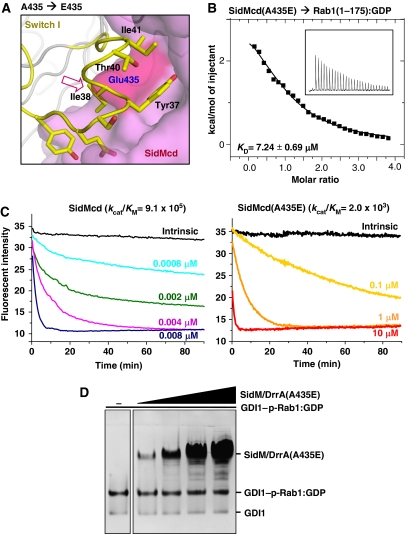
Mutation on SidM/DrrA affecting GEF activity also affects GDF activity
To confirm that the same surface of SidM/DrrA exerts both GEF and GDF activities, we generated a SidM/DrrA mutant containing an A435E substitution based on the presented structure, which indicated that presence of a hydrophilic and bulky residue at the Ala435 position would sterically interfere with the SidM/DrrA's interaction with Rab1 (Figure 3A), and thus affect the GEF activity of the protein. Consistently, the SidMcd mutant containing the A435E substitution bound to Rab1:GDP with a Kd of ∼7 μM (Figure 3B), which is a significantly reduced binding affinity as compared with the binding affinity (79 nM) of the wild-type SidMcd. As a result, the mutant SidMcd exhibited a 450-fold reduced catalytic efficiency (kcat/KM) in the GEF activity compared with the wild-type SidMcd in a nucleotide exchange assay using 1′(3)-bis-O-(N-methylanthraniloyl) GDP (mant-GDP; Figure 3C). Notably, the SidM/DrrA(A435E) mutant retained barely detectable GDF activity towards the GDI1–p-Rab1:GDP complex (Figure 3D), demonstrating that the SidM/DrrA's surface for binding the GTPase domain of Rab1 mediates both GEF and GDF activities.
Figure 3.
Both GEF and GDF activities of SidM/DrrA are affected by a single mutation on its Rab1-interacting interface. (A) Structure-based design of a SidM/DrrA mutant. SidMcd is in surface representation and the Rab1 residues are represented by sticks. The red arrow indicates that glutamic acid at the position of A435 of SidM/DrrA is sterically incompatible for interacting with Rab1. (B) The A435E mutation reduces the binding affinity between SidMcd and Rab1(1-175):GDP. The ITC run and the deduced Kd are shown. (C) The A435E mutation reduces the GEF activity of SidMcd. In the presence of 0.2 mM GTP, Rab1:mant-GDP was incubated with wild-type SidMcd or SidMcd(A435E) at the indicated concentrations. The decreased fluorescence as a result of the mant-GDP-to-GTP exchange was continuously monitored and used to deduce the kcat/KM values (M−1 s−1), as reported previously (Murata et al, 2006). (D) On the native gel, the SidM/DrrA(A435E) mutant exhibits barely detectable GDF activity.
Effects of liposomes and GTP on the GDF activity of SidM/DrrA
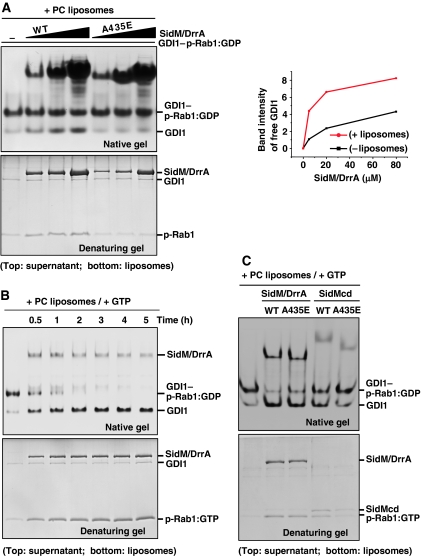
The GDF activity assay involving no hydrophobic milieu is in fact unusual, because SidM/DrrA has to carry out a thermodynamically unfavourable reaction of exposing the hydrophobic prenyl groups to the polar solvent. As SidM/DrrA works on LCV membranes, we next examined the effect of liposomes on the GDF activity of the protein. In the presence of phosphatidylcholine (PC) liposomes, GDI1 displacement by SidM/DrrA was slightly but noticeably enhanced (Figure 4A; lanes 1–4), compared with that in its absence (Figure 2B; lanes 1–4). As expected, dissociated p-Rab1 was enriched in the liposome fraction (Figure 4A). This stimulatory effect of the liposomes on GDI1 displacement conceivably arises from the effect of sequestering prenyl groups in the hydrophobic lipid environment, and thus shifts the equilibrium toward dissociation of GDI1 and p-Rab1. Nonetheless, SidM/DrrA present even at 16-fold molar excess over GDI1–p-Rab1:GDP could not dissociate this complex completely. We also examined the GDF activity of the SidM/DrrA(A435E) mutant in the presence of the liposomes. This mutant was barely active compared with the wild-type protein, as observed in the absence of the liposomes, indicating that the Rab1-binding interface of SidM/DrrA is responsible for the GDI1 displacement regardless of the presence or absence of the liposomes. Our observations support a possibility that SidM/DrrA is able to interact with p-Rab1:GDP, spontaneously and partly dissociated from GDI1, and catalyse the nucleotide exchange on it in the presence of GTP. We therefore examined the effect of GTP on GDI1 displacement by SidM/DrrA. In the presence of both PC liposomes and GTP, GDI1 release was greatly enhanced; a substoichiometric amount of SidM/DrrA dissociated the GDI1–p-Rab1:GDP complex completely (Figure 4B). The drastic enhancement of the GDF activity conceivably arises from two effects: (i) SidM/DrrA, interacting weakly to Rab1 in the presence of GTP (Supplementary Figure S5), should be easily released from partly dissociated p-Rab1 after nucleotide exchange on it and become available for binding another GDI1–p-Rab1:GDP complex and (ii) the prenyl group of partly dissociated p-Rab1:GTP should be more easily released from GDI1 and would not rebind to it, because RabGDI selectively interacts with GDP-bound p-Rabs (Araki et al, 1990; Pfeffer et al, 1995). In addition, GTP-bound p-Rabs are not extracted from membranes by RabGDI (Pfeffer et al, 1995). These observations suggest that SidM/DrrA located on the LCV membranes in host cells would be able to greatly facilitate complete dissociation of GDI1 and p-Rab1. Our observations are consistent with previously reported vesicle-floating experiments showing that significant accumulation of p-Rab1 on PC vesicles occurred only in the presence of both SidM/DrrA and GTP-γ-S (Machner and Isberg, 2007). As an extension, we carried out the GDF activity assay with SidMcd in the presence of both PC liposomes and GTP. SidMcd was less active than SidM/DrrA (Figure 4C; lanes 2 and 4). This may be explained by the membrane-targeting capacity of the full-length protein (Murata et al, 2006; Brombacher et al, 2009), allowing the protein to place incoming GDI1–p-Rab1:GDP in the vicinity of the membranes. Under these conditions, both SidM/DrrA(A435E) and SidMcd(A435E), having considerable GEF activity, although lower than that of wild-type proteins (Figure 3C), exhibited notable GDF activity (Figure 4C; lanes 3 and 5). These observations support our idea that the GDF activity of SidM/DrrA is intimately linked to its GEF activity.
Figure 4.
Effects of PC liposomes and GTP on GDI displacement by SidM/DrrA. (A) Effect of PC liposomes. GDI1–p-Rab1:GDP (5 μM) was incubated for 5 h with wild-type or A435E mutant SidM/DrrA at 5, 20 or 80 μM in the presence of 2 mM PC liposomes. After centrifugation, the supernatant and liposome fractions were visualized on a native or denaturing gel. The A435E mutant exhibits barely detectable GDI1 displacement. The right panel shows quantification of the band intensities of released GDI1 (lanes 1–4 in Figures 2B and 4A). Wild-type SidM/DrrA exhibits higher GDF activity compared with its activity in the absence of the liposomes. (B) Effect of the presence of both PC liposomes and GTP. GDI1–p-Rab1:GDP (5 μM) was incubated with wild-type SidM/DrrA (3.5 μM) up to 5 h in the presence of 2 mM PC liposomes and 1 mM GTP. The native gel shows complete displacement of GDI1 from p-Rab1 in 4 h with concomitant enrichment of p-Rab1 in the liposome fraction (denaturing gel). Lane 1 is for control showing GDI1–p-Rab1:GDP complex incubated for 5 h without added SidM/DrrA. (C) GDF activity assay involving the SidM/DrrA(A435E) and SidMcd(A435E) mutants. GDI1–p-Rab1:GDP (5 μM) was incubated with 5 μM of wild-type SidM/DrrA, SidM/DrrA(A435E), wild-type SidMcd or SidMcd(A435E) for 30 min in the presence of 2 mM PC liposomes and 1 mM GTP. Displacement of GDI1 is observable for the two mutants. Regardless of the mutation, full-length protein is more active than the central domain.
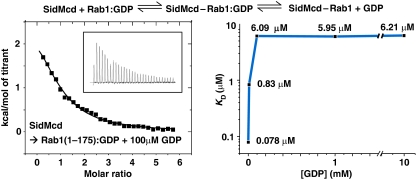
SidM/DrrA has high binding affinity for Rab1 with GDP retained at the active site
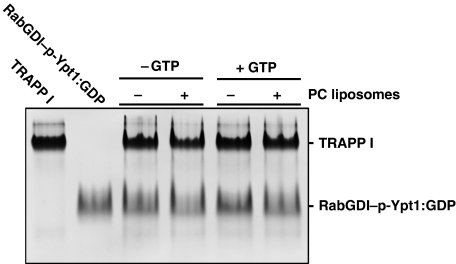
The robust enhancement of GDI1 release by SidM/DrrA in the presence of both PC liposomes and GTP raises a question of whether any GEF may act as a GDF in the presence of the two. This is not the case because the yeast TRAPP I complex, which is a GEF for Ypt1 (Jones et al, 2000; Wang et al, 2000; Kim et al, 2006), was unable to dissociate the yeast RabGDI–p-Ypt1:GDP complex in the presence of both PC liposomes and GTP (Figure 5). Then, by what feature is SidM/DrrA distinguished from TRAPP I and possibly other GEFs? The postulation of SidM/DrrA's capacity to capture p-Rab1:GDP partly dissociated from GDI1 implies that SidM/DrrA has high binding affinity for Rab1 with GDP retained at the active site, which is the form initially recognized by SidM/DrrA. To quantify this binding affinity, we performed ITC by titrating SidMcd into the sample chamber containing Rab1 and increasing the amount of GDP, which should have increasingly suppressed GDP eviction from Rab1 bound to SidMcd. In this experiment, the binding affinity reduced progressively with increased GDP concentration and reached a plateau Kd=∼6 μM at 0.1 mM of GDP (Figure 6). This quantified interaction is considerably tighter than the interaction between yeast RabGDI and Ypt1:GDP (Kd=36 μM; Pylypenko et al, 2006), providing a physical basis for our postulation. In the light of these data, the lack of GDF activity of TRAPP I may be due to its low binding affinity for Ypt1:GDP, which was too low to be measured by ITC in the presence of GDP (data not shown).
Figure 5.
TRAPP I has no GDF activity toward RabGDI–p-Ypt1:GDP. Yeast RabGDI–p-Ypt1:GDP (5 μM) was incubated with yeast TRAPP I (5 μM) for 4 h in a buffer solution containing 20 mM Tris−HCl (pH 7.5), 150 mM NaCl in the absence or presence of PC liposomes (2 mM) and GTP (1 mM). On the native gel, RabGDI release is unobservable.
Figure 6.
Measurement of the binding affinity between SidMcd and Rab1(1-175) with GDP retained. ITC was performed with increasing concentration of GDP, which should suppress the release of GDP in the two-step reaction shown at the top. The protein samples were prepared in a buffer solution containing 20 mM Tris−HCl (pH 7.5), 100 mM NaCl and 1 mM MgCl2. A representative ITC run is shown and the Kd values measured at the different GDP concentrations are shown on the plot.
Concluding remarks
This study suggests that the GEF and GDF activities of SidM/DrrA are mediated by the same surface of the protein. Although more kinetic study is needed to further investigate or dissect the intimately linked GDF and GEF activities of SidM/DrrA, we speculate that the bacterial protein has evolved to have the two activities in a single polypeptide by increasing the binding affinity for GDP-bound Rab1. Similar to SidM/DrrA, eukaryotic GEFs are recruited to target membranes (Ortiz et al, 2002; Ali and Seabra, 2005). It would be of great interest to investigate whether some of them may have high affinity for their cognate Rab:GDP and function as a GDF.
Materials and methods
Plasmid construction and protein production
DNA fragments encoding full-length SidM/DrrA, SidM/DrrA(317–533), human Rab1A (denoted as Rab1 throughout the text), human GDI1 or yeast RabGDI were subcloned into the pPROExHTa (Invitrogen) or pGEX-4T-3 plasmid (GE Healthcare). Each protein was produced in E. coli and purified using metal affinity, size-exclusion and ion exchange chromatography. The SidMcd–Rab1(1-175;N121I) complex was obtained by co-expression of the two proteins in E. coli and purified using Ni2+–NTA affinity column, HiTrapQ ion-exchange column and size-exclusion chromatography. Each of the GDI1–p-Rab1:GDP and RabGDI–p-Ypt1:GDP complexes was produced by co-expression of the two constituent proteins in the Sf9 insect cells based on the Baculovirus expression vector system. To increase the yield of the two complexes, the insect cell lysates were incubated with additional GDI1 or RabGDI produced from E. coli before purification. TRAPP I complex was produced and purified as described previously (Kim et al, 2006).
Crystallization and structure determination
The crystal form of the SidMcd–Rab1(1-175;N121I) complex was obtained by the sitting-drop vapour-diffusion method at 22°C by mixing and equilibrating the protein solution (1 μl) and precipitant solution (1 μl) containing 22% polyethyleneglycol 300, 3% polyethyleneglycol 8000, 8% glycerol, 1 mM L-cysteine and 0.1 M Tris−HCl (pH 8.5). The single-wavelength anomalous dispersion data set was collected using a crystal of the selenomethionine-substituted complex on beamline 4A of the Pohang Accelerator Laboratory. The structure of the complex was determined by single isomorphous replacement with anomalous scattering using a crystal of the selenomethionine-substituted complex. The final model does not include residues 317–333 of SidMcd, electron densities of which were not observed or were very weak. Crystallographic data statistics are summarized in Supplementary Table 1.
Native gel-based GDF activity assay
The native gel-based GDF activity assay was carried out at 25°C, involving a reaction buffer containing 20 mM Tris−HCl (pH 7.5) and 100 mM NaCl. The GDI1 displacement from p-Rab1 was visualized by Coomassie staining after native gel electrophoresis. The PC liposomes were prepared according to a previously reported procedure (Dumenil et al, 2004). When PC liposomes were included in the reaction buffer, the liposomal fraction was separated by centrifugation. The lipid fraction was analysed by denaturing gel electrophoresis. The intensity of protein bands was analysed using the Multi Gauge v3.0 program supplied with LAS-3000 (Fuji Film).
GEF activity assay
GEF activity assay was performed using Rab1 proteins charged with mant-GDP (Invitrogen) in a reaction buffer containing 200 μM GTP, 30 mM Tris−HCl (pH 8.0), 150 mM NaCl and 0.5 mM MgCl2, as reported in the literature (Murata et al, 2006). Data were collected on Cary Eclipse fluorescence spectrophotometer (Varian) with the excitation wavelength set to 360 nm and the emission monitored at 440 nm.
Isothermal titration calorimetry
All measurements were carried out at 25°C on a MicroCalorimetry System (MicroCal). The protein samples were prepared in a buffer solution containing 20 mM Tris−HCl (pH 7.5) and 100 mM NaCl. The samples were degassed for 20 min and centrifuged to remove any residuals before the measurements. The experiments were carried out by titrating 0.1–0.2 mM SidM/DrrA or SidMcd into 5–10 μM Rab1:GDP or Rab1(1-175):GDP. Dilution enthalpies were determined in separate experiments (titrant into buffer) and subtracted from the enthalpies of the binding between the proteins. Data were analysed using the Origin software (OriginLab). The Kd values were deduced from curve fittings of the integrated heat per mol of added titrants.
Coordinates: The coordinates of the structure and the structure factors have been deposited in the Protein Data Bank with the accession code .
Supplementary Material
Supplementary data
Supplementary Table 1 and Supplementary Figures S1–S5
Review Process File
Acknowledgments
We thank Dr Y-K Shin for the help with the preparation of liposomes and Dr M Sacher for providing plasmids necessary for producing TRAPP I. This study was supported by the GRL Program (K20815000001) from National Research Foundation of Korea. H-YS, D-WL and S-JC were supported by the Brain Korea 21 Project. This study made use of the beamline 4A at the Pohang Accelerator Laboratory in Korea.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ali BR, Seabra MC (2005) Targeting of Rab GTPases to cellular membranes. Biochem Soc Trans 33(Part 4): 652–656 [DOI] [PubMed] [Google Scholar]
- Allan BB, Moyer BD, Balch WE (2000) Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289: 444–448 [DOI] [PubMed] [Google Scholar]
- Araki S, Kikuchi A, Hata Y, Isomura M, Takai Y (1990) Regulation of reversible binding of smg p25A, a ras p21-like GTP-binding protein, to synaptic plasma membranes and vesicles by its specific regulatory protein, GDP dissociation inhibitor. J Biol Chem 265: 13007–13015 [PubMed] [Google Scholar]
- Beraud-Dufour S, Robineau S, Chardin P, Paris S, Chabre M, Cherfils J, Antonny B (1998) A glutamic finger in the guanine nucleotide exchange factor ARNO displaces Mg2+ and the beta-phosphate to destabilize GDP on ARF1. EMBO J 17: 3651–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brombacher E, Urwyler S, Ragaz C, Weber SS, Kami K, Overduin M, Hilbi H (2009) Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J Biol Chem 284: 4846–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derre I, Isberg RR (2004) Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun 72: 3048–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumenil G, Montminy TP, Tang M, Isberg RR (2004) IcmR-regulated membrane insertion and efflux by the Legionella pneumophila IcmQ protein. J Biol Chem 279: 4686–4695 [DOI] [PubMed] [Google Scholar]
- Eathiraj S, Pan X, Ritacco C, Lambright DG (2005) Structural basis of family-wide Rab GTPase recognition by rabenosyn-5. Nature 436: 415–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Sander C (1996) Mapping the protein universe. Science 273: 595–603 [DOI] [PubMed] [Google Scholar]
- Ingmundson A, Delprato A, Lambright DG, Roy CR (2007) Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 450: 365–369 [DOI] [PubMed] [Google Scholar]
- Jones S, Newman C, Liu F, Segev N (2000) The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol Biol Cell 11: 4403–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Stein MP, Pypaert M, Roy CR (2004) Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J Exp Med 199: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Raunser S, Munger C, Wagner J, Song YL, Cygler M, Walz T, Oh BH, Sacher M (2006) The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell 127: 817–830 [DOI] [PubMed] [Google Scholar]
- Machner MP, Isberg RR (2006) Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell 11: 47–56 [DOI] [PubMed] [Google Scholar]
- Machner MP, Isberg RR (2007) A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science 318: 974–977 [DOI] [PubMed] [Google Scholar]
- Moyer BD, Allan BB, Balch WE (2001) Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic 2: 268–276 [DOI] [PubMed] [Google Scholar]
- Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR (2006) The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol 8: 971–977 [DOI] [PubMed] [Google Scholar]
- Ninio S, Roy CR (2007) Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol 15: 372–380 [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Davidson HW, Matteson J, Meinkoth J, Balch WE (1994) A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol 125: 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P (2002) Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 157: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Aivazian D (2004) Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol 5: 886–896 [DOI] [PubMed] [Google Scholar]
- Pfeffer SR, Dirac-Svejstrup AB, Soldati T (1995) Rab GDP dissociation inhibitor: putting Rab GTPases in the right place. J Biol Chem 270: 17057–17059 [DOI] [PubMed] [Google Scholar]
- Pylypenko O, Rak A, Durek T, Kushnir S, Dursina BE, Thomae NH, Constantinescu AT, Brunsveld L, Watzke A, Waldmann H, Goody RS, Alexandrov K (2006) Structure of doubly prenylated Ypt1:GDI complex and the mechanism of GDI-mediated Rab recycling. EMBO J 25: 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak A, Pylypenko O, Durek T, Watzke A, Kushnir S, Brunsveld L, Waldmann H, Goody RS, Alexandrov K (2003) Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science 302: 646–650 [DOI] [PubMed] [Google Scholar]
- Segal G, Purcell M, Shuman HA (1998) Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA 95: 1669–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AD, Pfeffer SR (1995) Quantitative analysis of the interactions between prenyl Rab9, GDP dissociation inhibitor-alpha, and guanine nucleotides. J Biol Chem 270: 11085–11090 [DOI] [PubMed] [Google Scholar]
- Sivars U, Aivazian D, Pfeffer S (2005) Purification and properties of Yip3/PRA1 as a Rab GDI displacement factor. Meth Enzymol 403: 348–356 [DOI] [PubMed] [Google Scholar]
- Sivars U, Aivazian D, Pfeffer SR (2003) Yip3 catalyses the dissociation of endosomal Rab–GDI complexes. Nature 425: 856–859 [DOI] [PubMed] [Google Scholar]
- Thomas C, Fricke I, Scrima A, Berken A, Wittinghofer A (2007) Structural evidence for a common intermediate in small G protein-GEF reactions. Mol Cell 25: 141–149 [DOI] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294: 1299–1304 [DOI] [PubMed] [Google Scholar]
- Wang W, Sacher M, Ferro-Novick S (2000) TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol 151: 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide T, Koster M, Barnekow A (1999) Inactive and active mutants of rab1b are not tightly integrated into target membranes. Int J Oncol 15: 727–736 [DOI] [PubMed] [Google Scholar]
- Wu YW, Tan KT, Waldmann H, Goody RS, Alexandrov K (2007) Interaction analysis of prenylated Rab GTPase with Rab escort protein and GDP dissociation inhibitor explains the need for both regulators. Proc Natl Acad Sci USA 104: 12294–12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary Table 1 and Supplementary Figures S1–S5
Review Process File