Abstract
The human cytomegalovirus glycoprotein US2 induces dislocation of MHC class I heavy chains from the endoplasmic reticulum (ER) into the cytosol and targets them for proteasomal degradation. Signal peptide peptidase (SPP) has been shown to be integral for US2-induced dislocation of MHC class I heavy chains although its mechanism of action remains poorly understood. Here, we show that knockdown of protein disulphide isomerase (PDI) by RNA-mediated interference inhibited the degradation of MHC class I molecules catalysed by US2 but not by its functional homolog US11. Overexpression of the substrate-binding mutant of PDI, but not the catalytically inactive mutant, dominant-negatively inhibited US2-mediated dislocation of MHC class I molecules by preventing their release from US2. Furthermore, PDI associated with SPP independently of US2 and knockdown of PDI inhibited SPP-mediated degradation of CD3δ but not Derlin-1-dependent degradation of CFTR DeltaF508. Together, our data suggest that PDI is a component of the SPP-mediated ER-associated degradation machinery.
Keywords: ER-associated degradation (ERAD), immune evasion, quality control, retrotranslocation
Introduction
Newly synthesized polypeptides in the endoplasmic reticulum (ER) are monitored by a quality control process that ensures that only correctly folded or assembled proteins exit the ER and are trafficked to their destination. Terminally misfolded or unassembled proteins are transferred from the ER back to the cytosol (retrotranslocation or dislocation), where they are degraded by the ubiquitin-proteasome system, a process known as ER-associated degradation (ERAD) (Tsai et al, 2002; Ellgaard and Helenius, 2003; McCracken and Brodsky, 2003; Taxis et al, 2003). Although EDEM (Htm1p in yeast) and OS-9/XTP3-B (Yos9p in yeast) have been shown to recognize some ERAD substrates and deliver them to the dislocation machinery (Oda et al, 2003; Eriksson et al, 2004; Christianson et al, 2008; Clerc et al, 2009; Cormier et al, 2009), how cells distinguish misfolded proteins from folding intermediates is not fully understood. Misfolded proteins delivered to dislocation channels are ubiquitinated by an ER-associated ubiquitin conjugation system. The ubiquitinated substrates are extracted by the p97/cdc48-Ufd1p-Np14p complex, which couples ATP hydrolysis with substrate dislocation (Ye et al, 2001; Rabinovich et al, 2002) and are subsequently degraded by the 26S proteasome.
MHC class I molecules consist of a heavy chain (HC), β2 microglobulin, and an 8–10mer peptide, and function on the cell surface to present antigenic peptides to cytotoxic T lymphocytes. The human cytomegalovirus (HCMV) evades cytotoxic T lymphocytes through binding of HCMV glycoproteins US2 and US11 to newly synthesized MHC class I HC, inducing their dislocation into the cytosol for subsequent degradation (Wiertz et al, 1996a, 1996b; Barel et al, 2006a). As this process resembles the ERAD pathway, deciphering the mechanism of MHC class I HC dislocation by US2 and US11 may provide insight into the ERAD pathway. US2 and US11 show similar general modes of action but differ in their requirements for MHC class I alleles and the folding and ubiquitination status of MHC class I (Machold et al, 1997; Gewurz et al, 2001b; Barel et al, 2003, 2006b; Furman et al, 2003; Hassink et al, 2006). Furthermore, US2 and US11 use different machinery for the dislocation of MHC class I HC. Signal peptide peptidase (SPP) was identified as a specific interacting partner of US2, and a decrease in SPP levels by RNA interference inhibits dislocation of MHC class I HC by US2 but not by US11 (Loureiro et al, 2006). Sec61α, the main component of the protein-conducting channel for translocation into the ER, coimmunoprecipitates with US2 and partially dislocated MHC class I HC (Wiertz et al, 1996b), suggesting that the Sec61 complex may serve as a dislocation channel. In contrast, Derlin-1, another candidate channel protein, is involved in the dislocation of MHC class I HC by US11 but not by US2 (Lilley and Ploegh, 2004; Ye et al, 2004), and a dominant-negative mutant of Derlin-1 impedes dislocation of MHC class I HC by US11 but not by US2.
US2 is a short-lived type I membrane protein that exists in two forms, a cytosolic non-glycosylated form (US2-CHO) and an ER-inserted glycosylated form (US2+CHO). The non-glycosylated form of US2 arises from failure of part of the newly synthesized US2 to insert into the ER (Gewurz et al, 2002; Lilley and Ploegh, 2004). The glycosylated form of US2 has a short half-life and represents one of the ERAD substrates. In cells coexpressing US2 and dominant-negative Derlin-1, the degradation of MHC class I HC continues but degradation of glycosylated US2 is inhibited, indicating that degradation of MHC class I HC by US2 is independent of Derlin-1, whereas degradation of US2 is dependent on Derlin-1 (Lilley and Ploegh, 2004). Thus, degradation of MHC class I HC by US2 and degradation of US2 itself appear to be independent processes, and the MHC class I HC must be dissociated from US2 for dislocation. However, the events of the US2 pathway leading to dislocation of MHC class I HC are poorly defined.
In this study, we identified protein disulphide isomerase (PDI) as an essential player in SPP-mediated degradation of MHC class I HC by US2. PDI knockdown by siRNA inhibited degradation of MHC class I HC by US2 but not by US11. Overexpression of wild-type PDI or catalytic activity mutants of PDI accelerated the degradation of MHC class I HC by US2, whereas overexpression of substrate-binding mutants of PDI dominant-negatively inhibited MHC class I HC dislocation by preventing the release of MHC class I HC from US2. Furthermore, we showed that PDI associates with SPP independent of US2 and that PDI is involved in the SPP-dependent degradation of CD3δ, a well-characterized ERAD substrate (Fang et al, 2001) but not in the degradation of CFTR DeltaF508, a Derlin-1-dependent ERAD substrate (Wang et al, 2008). These findings indicate that the protein folding catalyst PDI (Goldberger et al, 1964) is a component of the SPP-mediated ERAD machinery.
Results
PDI is required for degradation of MHC class I HC by US2
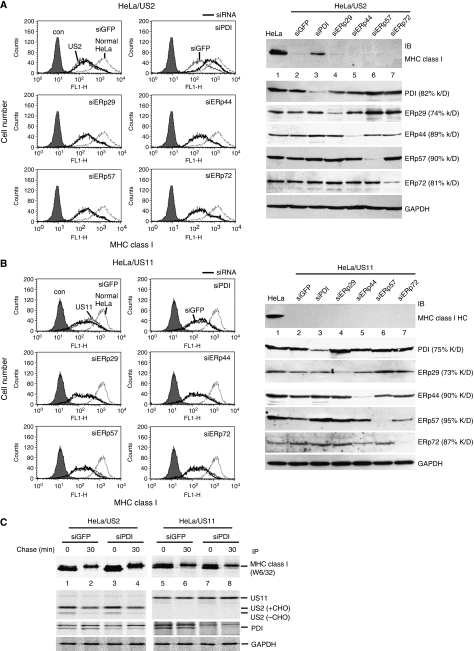
PDI has been implicated in the degradation of misfolded proteins (Gillece et al, 1999; Molinari et al, 2002) and was identified as an US2-associated protein through large-scale affinity purification (Loureiro et al, 2006). Therefore, we hypothesized that PDI might be involved in MHC class I HC dislocation by US2. To test this possibility, we reduced PDI levels using an RNA-mediated interference approach. siRNA targeting GFP (siGFP) was used as a non-specific control. As prolonged PDI knockdown (>3 weeks) induces cell death, we transiently transfected siRNAs into HeLa cells that stably expressed US2. After puromycin selection, surface MHC class I expression levels were analysed.
Consistent with earlier studies (Wiertz et al, 1996b), the cell surface expression of MHC class I was downregulated in US2-expressing cells (Figure 1A, left, compare US2 cells and normal HeLa cells). Expression of siGFP in US2 cells did not alter MHC class I expression levels (Figure 1A, left, siGFP panel), whereas depletion of PDI by siPDI restored the surface MHC class I level to closer to that of wild-type HeLa cells (Figure 1A, siPDI panel), suggesting that PDI might have a function in US2-mediated downregulation of MHC class I. We also determined the effect of PDI homologues (ERp29, ERp44, ERp57, and ERp72) on the degradation of MHC class I by US2. Knockdown of these proteins did not affect the surface expression of MHC class I in US2-expressing cells (Figure 1A, left, middle and bottom panels).
Figure 1.
PDI is essential for the degradation of MHC class I molecules by US2 but not US11. (A) HeLa cells stably expressing US2 were transfected with control siRNA (siGFP), siPDI, siERp29, siERp44, siERp57, or siERp72. After 24 h, transfectants were enriched by selection with puromycin for 2 days. The surface expression of MHC class I molecules was analysed by flow cytometry after staining with W6/32 antibody (left). Gray-filled histogram, isotype control staining; dotted line, HeLa cells; thin line, siGFP-expressing cells; thick line, PDI family member siRNA-transfected cells. To quantify the total amount of cellular MHC class I HC, a parallel set of cells were lysed, resolved by SDS–PAGE, and immunoblotted with anti-MHC class I antibody (H300) (right). Knockdown of each endogenous PDI family protein was monitored by immunoblot analysis. (B) The experimental details were essentially the same as in (A) except that US11-expressing cells were used. (C) PDI knockdown US2 cells or US11 cells were prepared as in (A). Cells were labelled for 10 min, chased for 30 min, lysed in 1% NP-40, and immunoprecipitated with antibodies against MHC class I molecules, US2, US11, PDI, or GAPDH.
To confirm whether the restoration of cell surface expression by knockdown of PDI correlates with a failure in intracellular degradation of MHC class I HC by US2, a parallel set of cells were analysed by western blot using an anti-MHC class I antibody (H300). In US2 cells that express siPDI, MHC class I HCs were markedly stabilized compared with cells expressing control siGFP or siRNAs against ERp29, ERp44, ERp57, and ERp72 (Figure 1A, right, top panel). The knockdown efficiency for each direct siRNA target was 60–90% (Figure 1A, right, bottom panel). These results imply that PDI is involved in US2-mediated degradation of MHC class I HC.
To determine whether this effect is linked to degradation per se, we labelled cells, chased and immunoprecipitated proteins with the indicated antibodies. As expected, in US2 control cells, most of the labelled MHC class I HC was degraded after a 30-min chase (Figure 1C, lanes 1 and 2), whereas ∼50% of synthesized MHC class I was remained stable after a 30-min chase in PDI-depleted US2 cells (Figure 1C, lanes 3 and 4). In contrast, PDI knockdown did not affect US2 synthesis and stability.
Next, we examined whether PDI is also involved in US11-mediated degradation of MHC class I HC. Unlike US2 cells, knockdown of PDI did not affect the cell surface expression of MHC class I molecules in US11 cells (Figure 1B, left, siPDI panel) or prevent the degradation of MHC class I HC in the same cells (Figure 1B, right, top panel and Figure 1C, lanes 5–8). Knockdown of PDI homologues ERp29, ERp44, ERp57, and ERp72 did not affect the downregulation of MHC class I by US11 (Figure 1B). Thus, these data confirm that PDI is essential for the degradation of MHC class I HC by US2, but not by US11.
Oxidative folding of US2 is required for US2 function in inducing degradation of MHC class I molecules
US2 and US11 have two conserved cysteine residues that are predicted to form an intradisulphide bond (Gewurz et al, 2001a). As the major function of PDI is to oxidize or isomerize protein substrates and knockdown of PDI inhibits US2-mediated degradation of MHC class I HC, we questioned whether PDI influences disulphide bond formation and folding of US2. We initially determined whether US2 does indeed form an intradisulphide linkage. We substituted a cysteine residue at position 52 of US2 with alanine (US2 C52A) and a cysteine residue at position 133 with serine (US2 C133S). Replacement of the cysteine residue at position 52 by alanine was specifically designed to avoid creation of a new N-linked glycosylation motif (NX(S/T)X, where X represents any amino acid other than proline). We also constructed US11 mutants in which each cysteine residue was substituted by serine.
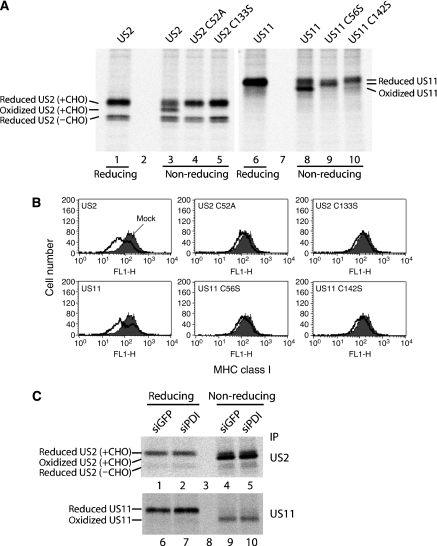
Under reducing conditions, we detected two different forms of US2, most likely the glycosylated (slower migrating) and cytosolic non-glycosylated (faster migrating) forms (Figure 2A, lane 1). Under non-reducing conditions, we detected an additional form of US2 besides the two forms of US2 observed under the reducing condition (Figure 2A, compare lanes 1 and 3). This additional band was absent in US2 C52A and US2 C133S (Figure 2A, lanes 4 and 5), suggesting that it represents the oxidized form of US2. The cytosolic glycosylated US2 showed same mobility shift under reducing and non-reducing conditions, implying that this form is never oxidized (Figure 2A, compare lanes 3–5 and 1). Wild-type US11 displayed both the oxidized and the reduced forms under the non-reducing condition (Figure 2A, lane 8), whereas US11 cysteine mutants showed only the reduced form under the non-reducing condition (Figure 2A, lanes 9 and 10). Thus, we concluded that both US2 and US11 form intradisulphide bonds through conserved cysteine residues.
Figure 2.
Oxidative folding of US2 is required for US2 function in inducing degradation of MHC class I molecules. (A) HeLa cells transiently transfected with wild-type US2, wild-type US11, or different cysteine mutants of US2 and US11 were labelled for 5 min, lysed in 1% NP-40 with 20 mM NEM, and immunoprecipitated with anti-US2 or anti-US11 antibodies. The eluates were resolved by SDS–PAGE under reducing and non-reducing conditions. (B) HeLa cells were individually transfected with the indicated US2- or US11-plasmids. Cell surface expression of MHC class I was measured by staining with mAb W6/32, followed by staining with FITC-conjugated goat anti-mouse antibodies. Filled histogram indicates mock-transfected cells; solid line indicates viral gene-transfected cells. (C) HeLa cells stably expressing US2 or US11 were transfected with siRNA targeting GFP (siGFP) or PDI (siPDI). After puromycin selection, cells were labelled for 10 min, lysed in 1% NP-40 with 20 mM NEM, and immunoprecipitated with anti-US2 or anti-US11 antibodies. The eluates were resolved by SDS–PAGE under reducing and non-reducing conditions.
We next examined whether intradisulphide bond formation of US2 is important for its function. We transiently expressed US2, US11, or their cysteine mutants in cells and measured the surface expression level of MHC class I molecules by FACS analysis. Whereas US2 and US11 downregulated the surface expression of MHC class I, cysteine mutants of US2 and US11 did not induce downregulation of MHC class I at the cell surface (Figure 2B). Pulse-chase experiments further confirmed that these cysteine mutants failed to induce degradation of MHC class I molecules (Supplementary Figure S1). These results indicate that the formation of intradisulphide bonds in US2 and US11 is essential for their function.
We further questioned whether PDI is responsible for catalysing intradisulphide linkage of US2. Knockdown of PDI did not influence the redox states of US2 and US11 compared with those in control cells (Figure 2C). Under the non-reducing condition, the ratio of oxidized to reduced forms of US2 in PDI-depleted cells was similar to that in control cells (Figure 2C, lanes 4 and 5). Similarly, oxidation of US11 was not affected by PDI knockdown (Figure 2C, lanes 9 and 10). Almost all of the US11 was oxidized under the non-reducing condition because of the relatively long pulse duration. These data indicate that PDI is not involved in the catalysis of intradisulphide bond formation of US2 and US11.
PDI specifically interacts with SPP but not with Derlin-1
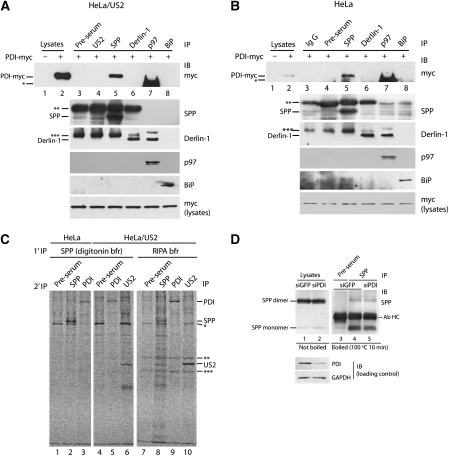
To investigate the precise function of PDI in the US2 pathway, we looked for a physical interaction between PDI and components of the ERAD machinery by serial immunoprecipitation and immunoblotting. As immunoprecipitation with rabbit polyclonal antibodies followed by immunoblotting with rabbit anti-PDI antibody yielded a high background, we expressed wild-type PDI conjugated with myc-tag at a position before the C-terminal KDEL sequence (PDI-myc) in US2 cells. Aliquots of digitonin lysates were immunoprecipitated with the indicated antibodies and immunoblotted with anti-myc antibody. PDI-myc was coimmunoprecipitated with SPP (Figure 3A, lane 5), but not with pre-immune serum or US2 (Figure 3A, lanes 3 and 4). We did not observe an association of PDI-myc with Derlin-1, an important component in the US11 pathway (Figure 3A, lane 6), or with p97 or BiP, which are known to be involved in the US2 pathway (Chevalier and Johnson, 2003; Hegde et al, 2006) (Figure 3A, lanes 7 and 8). Specific precipitation of proteins (SPP, Derlin-1, p97, and BiP) is shown in the lower panel (Figure 3A). As a positive control, we confirmed that Derlin-1 was coimmunoprecipitated with p97, consistent with an earlier report (Lilley and Ploegh, 2005) (Figure 3A, Derlin-1 panel, lane 7). Anti-US2 antibody for immunoblotting was not available. On the basis of these observations, we concluded that PDI associates with SPP.
Figure 3.
PDI associates with SPP but not with Derlin-1. (A) HeLa cells stably expressing US2 were transfected with myc-tagged PDI and lysed in 1% digitonin. Equal amounts of cell lysate were immunoprecipitated with the indicated antibodies. The immunoprecipitates were then analysed by SDS–PAGE, followed by immunoblotting with anti-myc antibody. Immunoblots of the specific precipitates with each antibody are shown as IP controls. The asterisks represent antibody heavy chain (*, **) or light chain (***). The variation between lanes in the bands for antibody heavy chain or light chain originates from variation in antibody host. PDI-myc was used as a loading control. (B) The experiment described in (A) was performed using normal HeLa cells instead of US2-expressing HeLa cells. (C) HeLa cells and US2-expressing HeLa cells were labelled for 30 min, lysed in 1% digitonin with 20 mM NEM or RIPA buffer, and immunoprecipitated with SPP antiserum or the indicated antibodies. Immunoprecipitated proteins from digitonin lysates were stripped in 1% SDS and reprecipitated with the indicated antibodies. The asterisks represent antibody background. (D) HeLa cells stably expressing US2 were transfected with control siRNA (siGFP) or PDI siRNA (siPDI). The SPP immunoprecipitates from 1% NP-40 lysates of cells were resuspended in reducing sample buffer, boiled for 10 min, and analysed by SDS–PAGE, followed by immunoblotting with anti-SPP antibody (lanes 3–5). Input lysate samples were incubated at 37°C for 10 min and subjected to direct immunoblot analysis (lanes 1 and 2).
As PDI associates with SPP but not with US2, we proceeded to examine whether US2 is required for the interaction of PDI with SPP. Analysis of normal HeLa cells by immunoprecipitation and immunoblotting revealed an interaction of PDI with SPP (Figure 3B, lane 5), but not with Derlin-1, p97, or BiP (Figure 3B, lanes 6–8). Endogenous PDI was also coimmunoprecipitated with SPP in HeLa cells (Figure 3C, lane 3) and in US2 cells (Figure 3C, lane 5). As US2 is known to be an interacting protein of SPP (Loureiro et al, 2006), we used this as a positive control and confirmed the interaction between SPP and US2 (Figure 3C, lane 6). These results suggest that physical association of PDI with SPP occurs independently of US2 and that PDI may be a bona fide component of the SPP-involving ERAD pathway.
As the majority of SPP exists as a dimeric form in vivo (Nyborg et al, 2006), we examined whether knockdown of PDI affects formation of the SPP dimer, even though the functional implications of dimer formation have not been clarified. The immunoprecipitates were resuspended in sample buffer, boiled for 10 min, and analysed by sodium dodecyl sulphate (SDS)–PAGE. As the SPP dimer is heat labile (Loureiro et al, 2006), the input lysates were loaded on a gel without boiling to aid detection of the dimer. Without boiling, most of the SPP existed in the dimeric form (Figure 3D, lanes 1 and 2), but dissociated into monomers on boiling (Figure 3D, lanes 4 and 5). Depletion of PDI did not influence the steady-state levels of SPP dimer or monomer (Figure 3D, compare lanes 4 and 5). Our data indicate that SPP is not a substrate for PDI but rather a binding partner. Conversely, PDI is probably not a substrate for SPP because SPP is a member of the presenilin (PS)/SPP-like (SPPL) superfamily of intramembrane-cleaving aspartic proteases and PDI is a soluble ER-resident protein.
The substrate binding, but not catalytic, activity of PDI is essential for US2-mediated degradation of MHC class I HC
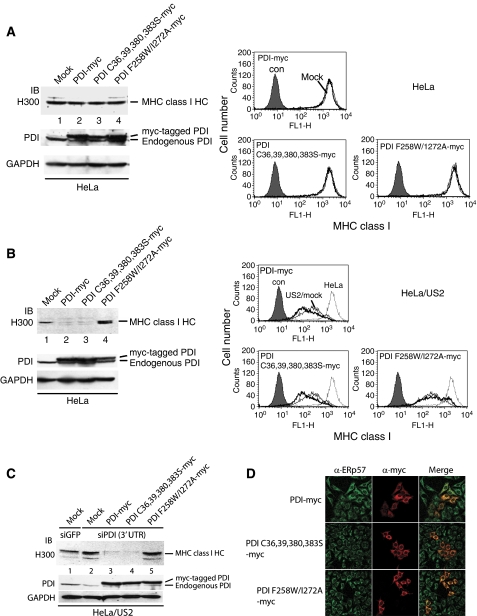
PDI consists of four distinct domains arranged in the order a-b-b′-a′ with a C-terminal acidic extension. a and a′ are thioredoxin domains containing active dithiol oxidoreductase sites (CXXC), whereas b and b′ contain thioredoxin-like domains but lack the active site motif. The b′ domain is essential for non-covalent binding of substrate (Pirneskoski et al, 2004). To determine which functional activity of PDI is vital for the US2 pathway, we constructed specific PDI mutants. PDI C36,39,380,383S-myc, in which all four cysteine residues in the a and a′ catalytic active sites are replaced by serine, is catalytically inactive. Both a phenylalanine-to-tryptophan substitution at position 258 (F258W) and an isoleucine-to-alanine substitution at position 272 (I272A) in the b′ domain greatly reduce the binding affinity for peptide substrates (Pirneskoski et al, 2004), thus PDI F258W/I272A has decreased substrate-binding activity. In normal HeLa cells, the total amount of cellular MHC class I HC did not change (Figure 4A, left, top panel) despite overexpression of ectopic PDI-myc constructs to two- to fivefold more than endogenous levels (Figure 4A, left, middle panel). Analysis of a parallel set of transfectants showed that overexpression of the PDI constructs did not affect the surface expression level of MHC class I molecules (Figure 4A, right).
Figure 4.
The peptide binding, but not the catalytic, function of PDI is essential for US2-mediated degradation of MHC class I molecules. (A) HeLa cells were transfected with mock, PDI-myc, PDI C36,39,380,383C-myc, or PDI F258W/I272A-myc. At 48 h post-transfection, aliquots of the transfectants were lysed and subjected to immunoblot analysis with anti-MHC class I antibody (H300) and anti-PDI antibody. Anti-PDI antibody recognizes both endogenous PDI and myc-tagged PDI. GAPDH was used as a loading control. Surface expression of MHC class I molecules was measured by FACS analysis after staining with MHC class I-specific W6/32 mAb. The filled histogram indicates isotype control staining; the thin line indicates mock-transfected cells; and the thick line indicates PDI construct-transfected cells. (B) The experiment described in (A) was performed using US2-expressing HeLa instead of normal HeLa cells. (C) US2-expressing HeLa cells were transfected with siRNA targeting 3′ UTR of PDI, selected with puromycin, and then transfected with mock, PDI-myc, PDI C36,39,380,383C-myc, or PDI F258W/I272A-myc. The transfectants were lysed and subjected to immunoblot analysis with anti-MHC class I antibody (H300) and anti-PDI antibody. (D) HeLa cells were transfected with the indicated PDI constructs and stained with the indicated antibodies. Bound antibodies were labelled with FITC- or Texas red-conjugated secondary antibodies.
In US2 cells, however, overexpression of PDI-myc or PDI C36,39,380,383S-myc facilitated degradation of MHC class I HC (Figure 4B, left, top panel, compare lanes 1 and 2, 3, right), suggesting that the catalytic activity of PDI is not required for US2 function. In contrast, overexpression of PDI F258W/I272A-myc inhibited degradation of MHC class I HC by US2 (Figure 4B, compare lanes 1 and 4), indicating that the substrate-binding activity of PDI is essential for US2 pathway. Accordingly, surface expression of MHC class I was significantly recovered in US2 cells expressing PDI F258W/I272A-myc (Figure 4B, right). In US11 cells, overexpression of PDI-myc constructs did not affect MHC class I expression level (data not shown).
To confirm the above results, we reduced PDI expression by siRNA targeting the PDI 3′ UTR in US2 cells and transfected these cells with mock or myc-tagged PDI constructs. Consistent with earlier data (Figure 1A), PDI knockdown inhibited degradation of MHC class I by US2 (Figure 4C, compare lanes 1 and 2). Overexpression of PDI-myc or PDI C36,39,380,383S-myc in PDI knockdown US2 cells promoted degradation of MHC class I HC (Figure 4C, lanes 3 and 4), suggesting that PDI-myc or PDI C36,39,380,383S-myc could replace endogenous PDI in this process. However, overexpression of PDI F258W/I272A-myc in PDI knockdown US2 cells did not alter MHC class I HC expression level compared with the control (Figure 4C, compare lanes 2 and 5), indicating that PDI F258W/I272A-myc could not replace endogenous PDI. Myc-tagging did not affect ER localization of PDI-myc (Figure 4D; Supplementary Figure S2A). Overexpression of PDI-myc is unlikely to perturb redox homeostasis of the ER as the surface expression of several disulphide-bonded glycoproteins appeared normal (Supplementary Figure S2B and C). Collectively, we concluded that PDI F258W/I272A-myc operates dominant-negatively and substrate-binding activity of PDI is essential for US2-mediated degradation of MHC class I HC.
PDI catalyses the release of MHC class I molecules from US2
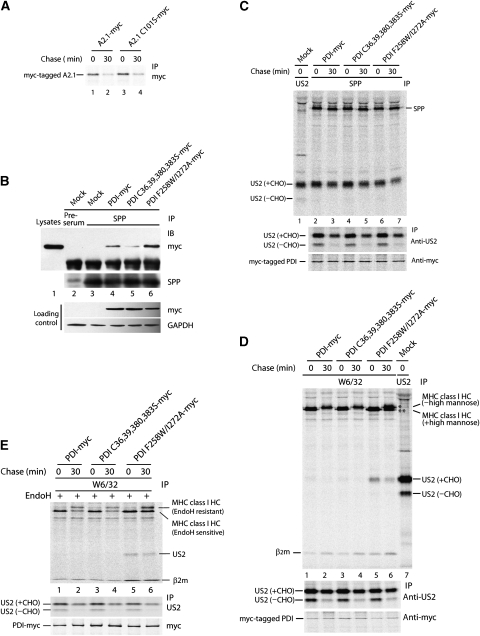
We have reported earlier that PDI catalyses oxidation of MHC class I HC (Park et al, 2006). Although the above results suggested that US2-mediated degradation of MHC class I HC does not require the catalytic activity of PDI, we wanted to investigate whether inhibition of degradation of MHC class I HC by US2 in PDI-depleted cells was due to an intrinsic inability of the reduced MHC class I HC to dislocate into the cytosol. To test this possibility, we constructed the HLA-A2.1 C101S-myc mutant in which cysteine at position 101 is substituted with serine. This mutant is unable to form the α2 intradisulphide bond (Bjorkman et al, 2005). Both wild-type A2.1-myc and A2.1 C101S-myc proteins were efficiently degraded after a 30-min chase (Figure 5A, lanes 1–4), suggesting that reduced MHC class I species are efficient targets for the US2 pathway. At the same time, this observation supports the notion that oxidoreductase activity of PDI is not involved in US2-mediated degradation of MHC class I.
Figure 5.
PDI facilitates the dissociation of MHC class I molecule from US2 for dislocation. (A) US2-expressing cells were transfected with HLA-A2.1-myc and HLA-A2.1 C101S-myc. At 48 h post-transfection, the cells were labelled and chased. 1% NP-40 lysates were immunoprecipitated with anti-myc antibody and resolved by SDS–PAGE. (B) US2-expressing cells were transfected with PDI constructs and lysed in 1% digitonin. The lysates were immunoprecipitated with anti-SPP antibody. The immunoprecipitates were then analysed by SDS–PAGE, followed by immunoblotting with anti-myc antibody or anti-SPP antibody. (C–E) US2-expressing cells were transfected with mock, PDI-myc, PDI C36,39,380,383C-myc, or PDI F258W/I272A-myc, pulse labelled, chased for 30 min, and lysed in 1% digitonin. The lysates were immunoprecipitated with the indicated antibodies. Immunoprecipitated proteins were resolved by SDS–PAGE. Expression of US2 and myc-tagged PDI proteins were confirmed by immunoprecipitation of aliquots of the lysates with the respective antibodies (bottom panels). (D) MHC class I HC marked by an asterisk is the ER-exported form and MHC class I HC marked by two asterisks is the ER-retained form. (E) W6/32 immunoprecipitates were digested with Endo H before SDS–PAGE analysis.
To gain insight into the mechanism of inhibition of MHC class I HC degradation by a dominant-negative substrate-binding mutant of PDI, we examined physical interactions between participants of the US2 pathway. First, because of the demonstrated association of PDI with SPP (Figure 3), we examined the interaction between PDI F258W/I272A-myc and SPP and observed stronger association between SPP and PDI F258W/I272A-myc than between SPP and PDI-myc (Figure 5B, compare lanes 6 and 4). This suggests that PDI F258W/I272A-myc competes with endogenous PDI for SPP and remains attached to SPP, leading to a reduced pool of SPP available for US2-mediated degradation of MHC class I. Catalytically inactive PDI C36,39,380,383S-myc was weakly associated with SPP compared with PDI-myc (Figure 4B, compare lanes 4 and 5), suggesting that the association of PDI with SPP is not required for US2-mediated degradation. Overexpression of PDI-myc constructs did not affect the interaction between SPP and glycosylated US2 (Figure 5C, lanes 2–7).
As the dominant-negative PDI F258W/I272A-myc associated strongly with SPP but did not affect the association of US2 and SPP (Figure 5B and C), the PDI F258W/I272A-myc/SPP complex might exert its effect by binding US2, thus impeding the association of functional PDI/SPP complex with US2. To determine how SPP bound PDI functions in US2-mediated degradation of MHC class I, US2 cells expressing PDI-myc or its mutants were labelled, chased for 30 min, lysed in 1% digitonin, and the lysates immunoprecipitated with either W6/32 antibody or anti-US2 serum. Consistent with the earlier observation (Figure 4B), MHC class I HC was efficiently degraded after a 30-min chase in US2 cells overexpressing PDI-myc or PDI C36,39,380,383S-myc (Figure 5D, lanes 1–4), whereas overexpression of PDI F258W/I272A-myc significantly inhibited degradation of MHC class I HC at the same chase time (Figure 5D, lanes 5 and 6). The two bands for MHC class I HC observed after a 30-min chase in cells expressing PDI F258W/I272A-myc represent differentially N-glycosylated forms (Figure 5D, lane 6). The upper band, which is also detectable in cells expressing PDI-myc or PDI C36,39,380,383S-myc, presumably represents a form that escaped the US2 pathway because of an insufficient expression level of US2. As judged by the EndoH test (Figure 5E), the lower band detected after chase in cells overexpressing PDI F258W/I272A-myc indicates an ER-retained MHC class I HC form.
The most notable observation was a strong association between US2 and MHC class I in US2 cells overexpressing PDI F258W/I272A-myc (Figure 5D, lanes 5 and 6 and Figure 5E, lanes 5 and 6). Only a very weak association of US2 with MHC class I could be observed, even at the 0-min chase time point, in cells overexpressing either PDI-myc or PDI C36,39,380,383S-myc under the same experimental conditions (Figure 5D, lanes 1 and 3), suggesting that the interaction of US2 with MHC class I is transient and unstable in the steady state. Considering these results and the requirement for dissociation of MHC class I from US2 before dislocation (Lilley and Ploegh, 2004), our data suggest that PDI has an important function in dissociating MHC class I from US2 before the dislocation of MHC class I HC.
A general function for PDI and SPP in the ERAD of misfolded proteins
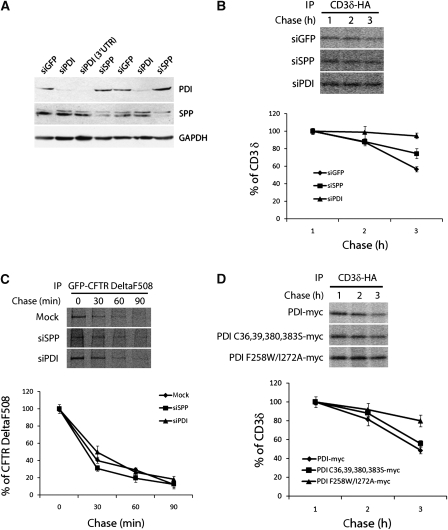
We next determined whether PDI has a general function in the SPP-dependent ERAD pathway. As it is not known whether SPP is involved in ERAD for substrates other than US2-associated MHC class I HC, we initially tested this possibility using two representative ERAD substrates, CD3δ and CFTR DeltaF508 (a mutant of the cystic fibrosis transmembrane conductance regulator). Quantification of band intensity by densitometry showed that degradation of CD3δ-HA was significantly delayed in SPP-depleted cells compared with the control knockdown cells (Figure 6B). The knockdown efficiency of SPP was 80–90% (Figure 6A). As the effect of SPP knockdown on the degradation of CD3δ-HA was modest but highly reproducible, we concluded that SPP is involved in the degradation of CD3δ-HA. In contrast, degradation of GFP-CFTR DeltaF508 was not affected by SPP knockdown (Figure 6C). As Derlin-1 has been reported to associate with CFTR DeltaF508 and facilitate its degradation similar to US11-mediated degradation of MHC class I (Sun et al, 2006; Wang et al, 2008), we concluded that degradation of CFTR DeltaF508 is independent of SPP.
Figure 6.
PDI and SPP are involved in the degradation of CD3δ-HA, a classical ERAD substrate. (A–D) To improve knockdown efficiency, we used a retroviral infection system. After enrichment of transfectants by selection in puromycine for 2 weeks, cells expressing siRNAs specific for GFP, SPP, PDI, or PDI 3′UTR were used for experiments. (A) Knockdown efficiency of each protein was verified by immunoblotting with anti-PDI and anti-SPP antibodies. GAPDH was used as a loading control. (B) The above cells were transfected with CD3δ-HA, labelled for 30 min, and chased for the indicated times. Cell lysates of 1% NP-40 were immunoprecipitated with anti-HA antibody. The intensity of CD3δ-HA bands was quantified and plotted. (C) The experimental details are the same as in (B), except that GFP-CFTR DeltaF508 and HeLa transfected with empty vector were used instead of CD3δ-HA and siGFP cells, respectively. (D) The experiment was performed as described in (B) except that the PDI construct and CD3δ-HA were cotransfected. Error bars represent standard deviations of results from triplicate wells (B–D).
On the basis of the association of PDI with SPP and its function in the SPP-dependent degradation of MHC class I HC by US2, we tested whether PDI is involved in the degradation of CD3δ-HA. Knockdown of PDI led to a delayed degradation of CD3δ-HA (Figure 6B), suggesting involvement of PDI in SPP-dependent CD3δ-HA degradation. In contrast, knockdown of PDI did not inhibit degradation of GFP-CFTR DeltaF508 (Figure 6C).
Next, we investigated whether the oxidoreductase or the substrate-binding function of PDI is essential for the degradation of CD3δ-HA. We reduced the PDI expression level in HeLa cells by siRNA targeting the PDI 3′ UTR and cotransfected the cells with CD3δ-HA and PDI constructs. In HeLa cells expressing PDI F258W/I272A-myc, CD3δ-HA was degraded significantly more slowly than in cells expressing PDI-myc or PDI C36,39,380,383S-myc (Figure 6D). These results indicate that the substrate-binding activity of PDI is essential for the degradation of CD3δ-HA, as shown for the degradation of MHC class I HC by US2.
Discussion
PDI acts both as an enzyme and as a chaperone (Noiva and Lennarz, 1992). Several recent studies have implicated a function of PDI in ERAD through its catalytic and/or chaperone activity. A mutational study of Pdi1p (a PDI homologue in yeast) showed that a catalytically inactive mutant, but not a substrate-binding site-deleted mutant, could fully support dislocation of a cysteine-free misfolded protein (Gillece et al, 1999). Recently, it has been reported that Htm1p/Mnl1p (EDEM in mammals, ER degradation enhancing α-mammosidase-like protein) physically associates with Pdi1p (Clerc et al, 2009; Sakoh-Nakatogawa et al, 2009). Htm1p/Mnl1p was identified as a candidate for lectins that recognize ERAD substrates with modified mannose moieties (Hosokawa et al, 2001). The interaction between Htm1p and Pdi1p involves intermolecular disulphide bonds, and stable interaction with Pdi1p introduces a functionally essential intradisulphide bond into Htm1p (Sakoh-Nakatogawa et al, 2009). The pro-α factor, which lacks disulphide bonds, also requires PDI for dislocation in an in vitro system (Wahlman et al, 2007). Similarly, a chaperone function for PDI in ERAD has been suggested in the dislocation of BACE457 (Molinari et al, 2002) and the unfolding of cholera toxin before dislocation (Tsai et al, 2001). On the basis of in vitro experiments, the oxidoreductase activity of PDI does not seem to be important in the dislocation of cholera toxin (Tsai et al, 2001; Forster et al, 2006).
The reduction of disulphide bonds in ERAD substrates has generally been thought to be required for the unfolding and dislocation of misfolded proteins. Recently, a PDI homologue, ERdj5, was identified as a reductase that breaks the disulphide bonds of misfolded proteins before dislocation (Ushioda et al, 2008). MHC class I HC contains two intradisulphide bonds (Park et al, 2006), and it is not known whether reduction of these disulphide bonds must precede dislocation of MHC class I HC in the US2 pathway. Agents that affect intracellular redox potential and/or free thiol status such as diamide and N-ethylmaleimide inhibit the dislocation of MHC class I HC by US2 and US11 (Tortorella et al, 1998), supporting the idea that reduction of MHC class I HC is a prerequisite for dislocation. However, our results show that the reductase activities of PDI or its homologues tested in this study are not essential for the dislocation of MHC class I HC by the US2 and US11 pathways. Overexpression of catalytically inactive PDI mutant promoted degradation of MHC class I HC by the US2 pathway, as did overexpression of wild-type PDI (Figures 4 and 5). Knockdown of ERp72, ERp57, or ERp29, which have been implicated in protein dislocation (Forster et al, 2006) or ER-to-cytosol penetration of viruses (Magnuson et al, 2005; Schelhaas et al, 2007) and knockdown of ERp44 required for ER retention of Ero1, which transfers oxidative equivalents to PDI (Otsu et al, 2006) did not influence degradation of MHC class I HC.
In this study, we have identified PDI as an important component for the ER dislocation machinery involving SPP. We show that knockdown of PDI by RNA interference inhibits dislocation of MHC class I HC by US2 but not by US11. PDI coimmunoprecipitated with SPP, an essential component of the US2 pathway (Loureiro et al, 2006), but not with Derlin-1, an important component of the US11 pathway (Lilley and Ploegh, 2004). We also show that PDI is a natural binding partner for SPP and that both PDI and SPP are vital for degradation of CD3δ but not for degradation of CFTR DeltaF508, a Derlin-1-dependent ERAD substrate, suggesting that involvement of PDI in ERAD is not limited to the US2 pathway, but rather that PDI has a general function in SPP-mediated ERAD of certain misfolded substrates.
There is no SPP in yeast although a PDI homologue has been shown to have a decisive function in ERAD in yeast as well (Gillece et al, 1999). It is therefore possible that the evolutionary expansion of the number and complexity of secretory and membrane proteins necessitated the simultaneous expansion of ERAD pathways in higher eukaryotes. The multiple functional homologues and ERAD E3 ubiquitin ligase membrane complexes found in the mammal seem to support that view (Yoshida, 2007; Christianson et al, 2008; Wang and Ng, 2008).
SPP is an ER-resident protein and a member of the PS/SPPL superfamily of intramembrane-cleaving aspartic proteases, identified by cleavage of several type II membrane signal peptides (Weihofen et al, 2002). Although the original function assigned to SPP was the removal of signal peptide remnants from substrates that were cleaved earlier by signal peptidase in animals and plants, there is no SPP in yeast. Therefore, another function of SPP in mammals other than signal peptide processing was suggested and a function for SPP in protein dislocation from the ER was shown. On the basis of the interaction of a truncated opsin chain with SPP in vitro, SPP was implicated in the recognition of misassembled transmembrane domains during membrane protein quality control in the ER (Crawshaw et al, 2004), and Ploegh and colleagues provided the first evidence of a functional role for SPP in ERAD (Loureiro et al, 2006). It is known that SPP associates with the cytosolic tail of US2, and that knockdown of SPP inhibits dislocation of MHC class I HC by US2 but not by US11 (Loureiro et al, 2006), underscoring the specificity of SPP in the US2 pathway. However, the detailed mechanism of its involvement remains uncharacterized.
We found that PDI is essential for dislocation of MHC class I HC by US2 but not by US11. In addition, our data indicate that both PDI and SPP are essential for degradation of CD3δ, suggesting that PDI and SPP are members of distinct ERAD machinery. What is the function of PDI in SPP-mediated ERAD? A clue is provided from observations that overexpression of a dominant-negative substrate-binding mutant of PDI, PDI F258W/I272A-myc, inhibits the dislocation of MHC class I HC by US2 (Figure 4). The most distinct phenotype on overexpression of this mutant was accumulation of MHC class I-US2 complexes (Figure 5D and E). We do not know how the point mutations at positions 258 and 272 of PDI inhibit the dissociation of MHC class I HC from US2. These mutations were recently shown to be in a part of PDI outside the substrate-binding site and freeze PDI in one particular conformation that inhibits substrate binding (Nguyen et al, 2008). In US2 cells overexpressing wild-type PDI-myc or PDI C36,39,380,383S-myc, we could detect only a trace amount of the MHC class I-US2 complexes.
As the association of SPP with US2 was not affected by overexpression of the PDI mutants, it is unlikely that PDI is involved in recruitment of MHC class I-US2 complex to SPP. Given that US2 is recruited to SPP (Loureiro et al, 2006) and that dissociation of MHC class I from US2 precedes dislocation of MHC class I HC (Ye et al, 2004), we envisage that the dissociation of MHC class I from US2 occurs rapidly during the steady state because of the substrate-binding activity of PDI bound to SPP. On the basis of an earlier study (Loureiro et al, 2006) and on our results obtained using US2-modified MHC class I HC and CD3δ as model substrates, we present the following model for the function of PDI in the SPP-mediated ERAD pathway: misfolded proteins are recruited to SPP where PDI bound to SPP unfolds the proteins into a dislocation-competent structure through its substrate-binding function, allowing the misfolded substrates to be escorted to the dislocation channel.
Materials and methods
Constructs
GFP-specific siRNA and PDI-specific siRNA were described earlier (Park et al, 2006). ERp29-specific (5′-AAATTTGCAGAATCTACGGAA-3′) siRNA, ERp44-specific (5′-AAGTAGTGTTTGCCAGAGTTG-3′) siRNA (Higo et al, 2005), ERp57-specific (5′-GGAATTGTCAGCCACTTGA-3′) siRNA (Kienast et al, 2007), ERp72-specific (5′-AAGCGTTCTCCTCCAATTCCC-3′) (Forster et al, 2006), SPP-specific (5′-AAGAATGCTTCAGACATGCCT-3′) siRNA and PDI 3′ UTR-specific (5′-GATGAACTGTAATACGCAA-3′) siRNA were synthesized by Bioneer (Daejeon, Korea). Synthesized siRNA oligomers were subcloned to pSUPER.retro vector. The cDNAs encoding human PDI were inserted into the mammalian expression vector pcDNA3.1 (Invitrogen, San Diego, CA, USA) and were myc-tagged at a position before the C-terminal KDEL sequence. The cysteine-to-serine (C36, 39, 380, 383S) mutation in the a/a′ domain of PDI and isoleucine-to-alanine (I272A) and phenylalanine-to-tryptophan (F258W) replacement mutations within the b′ domain of PDI were made by site-directed mutagenesis with Pfu DNA Polymerase and a myc-tag was added at a position before the C-terminal KDEL sequence.
Cell culture and transfection
To establish stable cell lines expressing US2 or US11, we subcloned each cDNA into the pcDNA3.1 mammalian expression vector (Invitrogen, Carlsbad, CA) and transfected the constructs into HeLa cells using the calcium phosphate precipitation method. Stable clones were selected by adding 0.5 mg/ml G418 (Life Technologies). The siRNAs were transfected into cells using lipofectamine2000 (Invitrogen) according to the manufacturer's protocol. After 24 h, transfectants were selected by 1 μg/ml puromycin for 2 days and then used for experiments.
Antibodies
The H300 (Santa-cruz) rabbit polyclonal antibody recognizes the epitope corresponding to amino acids 63–362 of MHC class I of human origin and monoclonal antibody W6/32 recognizes only the complex of MHC class I HC and β2m. Polyclonal antiserums for detecting US2 or US11 were raised against the synthetic peptides corresponding to the luminal N-terminal portion of the each protein (Park et al, 2002). Anti-PDI serum was raised against the PDI protein purified from Escherichia coli. Anti-ERp29 antibody and anti-SPP antibody were purchased from Abcam and anti-GAPDH antibody was purchased from LabFrontier (Seoul, Korea). Anti-ERp57 and anti-BiP antibodies were purchased from Santa Cruz Biotechnology, Inc. Anti-p97 ATPase antibody was purchased from Research Diagnostics and anti-Derlin-1 antibody was purchased from Medical and Biological Laboratories. Anti-ERp44 antibody and anti-ERp72 antibody were purchased from Cell Signaling and BD Bioscience. Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) was purchased from the Jackson ImmunoResearch Laboratories (West Grove, PA).
Pulse-chases, immunoprecipitation, and immunoblotting
Cells were starved for 30 min in medium lacking methionine and cysteine, labelled with 0.1 mCi/ml [35S]methionine (TranS-label; NEN Life Science, Boston, MA) and chased in regular medium for the indicated time. After being washed twice with cold PBS, the cells were lysed. Immunoprecipitation and immunoblotting were performed as described earlier. For endoglycosidase H treatment, the immunoprecipitates were digested with 3 mU endoglycosidase H (Roche, Indianapolis, IN) for 16 h at 37°C in 50 mM NaOAc (pH 5.6), 0.3% SDS, and 150 mM β-mercaptoethanol.
Flow cytometry
Cells were washed twice with PBS containing 1% BSA and incubated for 1 h at 4°C with W6/32. Normal mouse IgG was used as a negative control. The cells were washed twice with cold PBS containing 1% BSA and then stained with FITC-conjugated goat anti-mouse IgG for 1 h. A total of 10 000-gated events were collected by the FACSCalibur cytometer (BD Biosciences, San Jose, CA) and analysed with CellQuestPro software (BD Biosciences).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Information
Review Process File
Acknowledgments
We are grateful to Dr Allan M Weissman (National Institutes of Health, Maryland, USA) for CD3δ-HA and Dr Ryan Tyler (Stanford university, CA, USA) for CFTR DeltaF508. This work was supported by the Creative Research Initiatives Center for Antigen Presentation of MOST/KOSEF. SL, SC, IK, and CO were recipients of BK21 fellowships. SL and SC were recipients of a Seoul Science Fellowship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barel MT, Hassink GC, van Voorden S, Wiertz EJ (2006a) Human cytomegalovirus-encoded US2 and US11 target unassembled MHC class I heavy chains for degradation. Mol Immunol 43: 1258–1266 [DOI] [PubMed] [Google Scholar]
- Barel MT, Pizzato N, Le Bouteiller P, Wiertz EJ, Lenfant F (2006b) Subtle sequence variation among MHC class I locus products greatly influences sensitivity to HCMV US2- and US11-mediated degradation. Int Immunol 18: 173–182 [DOI] [PubMed] [Google Scholar]
- Barel MT, Ressing M, Pizzato N, van Leeuwen D, Le Bouteiller P, Lenfant F, Wiertz EJ (2003) Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation. J Immunol 171: 6757–6765 [DOI] [PubMed] [Google Scholar]
- Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC (2005) Structure of the human class I histocompatibility antigen, HLA-A2. J Immunol 174: 6–19 [PubMed] [Google Scholar]
- Chevalier MS, Johnson DC (2003) Human cytomegalovirus US3 chimeras containing US2 cytosolic residues acquire major histocompatibility class I and II protein degradation properties. J Virol 77: 4731–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JC, Shaler TA, Tyler RE, Kopito RR (2008) OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol 10: 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc S, Hirsch C, Oggier DM, Deprez P, Jakob C, Sommer T, Aebi M (2009) Htm1 protein generates the N-glycan signal for glycoprotein degradation in the endoplasmic reticulum. J Cell Biol 184: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier JH, Tamura T, Sunryd JC, Hebert DN (2009) EDEM1 recognition and delivery of misfolded proteins to the SEL1L-containing ERAD complex. Mol Cell 34: 627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawshaw SG, Martoglio B, Meacock SL, High S (2004) A misassembled transmembrane domain of a polytopic protein associates with signal peptide peptidase. Biochem J 384 (Pt 1): 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191 [DOI] [PubMed] [Google Scholar]
- Eriksson KK, Vago R, Calanca V, Galli C, Paganetti P, Molinari M (2004) EDEM contributes to maintenance of protein folding efficiency and secretory capacity. J Biol Chem 279: 44600–44605 [DOI] [PubMed] [Google Scholar]
- Fang S, Ferrone M, Yang C, Jensen JP, Tiwari S, Weissman AM (2001) The tumor autocrine motility factor receptor, gp78, is a ubiquitin protein ligase implicated in degradation from the endoplasmic reticulum. Proc Natl Acad Sci USA 98: 14422–14427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster ML, Sivick K, Park YN, Arvan P, Lencer WI, Tsai B (2006) Protein disulfide isomerase-like proteins play opposing roles during retrotranslocation. J Cell Biol 173: 853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman MH, Loureiro J, Ploegh HL, Tortorella D (2003) Ubiquitinylation of the cytosolic domain of a type I membrane protein is not required to initiate its dislocation from the endoplasmic reticulum. J Biol Chem 278: 34804–34811 [DOI] [PubMed] [Google Scholar]
- Gewurz BE, Gaudet R, Tortorella D, Wang EW, Ploegh HL, Wiley DC (2001a) Antigen presentation subverted: Structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc Natl Acad Sci USA 98: 6794–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz BE, Ploegh HL, Tortorella D (2002) US2, a human cytomegalovirus-encoded type I membrane protein, contains a non-cleavable amino-terminal signal peptide. J Biol Chem 277: 11306–11313 [DOI] [PubMed] [Google Scholar]
- Gewurz BE, Wang EW, Tortorella D, Schust DJ, Ploegh HL (2001b) Human cytomegalovirus US2 endoplasmic reticulum-lumenal domain dictates association with major histocompatibility complex class I in a locus-specific manner. J Virol 75: 5197–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillece P, Luz JM, Lennarz WJ, de La Cruz FJ, Romisch K (1999) Export of a cysteine-free misfolded secretory protein from the endoplasmic reticulum for degradation requires interaction with protein disulfide isomerase. J Cell Biol 147: 1443–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger RF, Epstein CJ, Anfinsen CB (1964) Purification and properties of a microsomal enzyme system catalyzing the reactivation of reduced ribonuclease and lysozyme. J Biol Chem 239: 1406–1410 [PubMed] [Google Scholar]
- Hassink GC, Barel MT, Van Voorden SB, Kikkert M, Wiertz EJ (2006) Ubiquitination of MHC class I heavy chains is essential for dislocation by human cytomegalovirus-encoded US2 but not US11. J Biol Chem 281: 30063–30071 [DOI] [PubMed] [Google Scholar]
- Hegde NR, Chevalier MS, Wisner TW, Denton MC, Shire K, Frappier L, Johnson DC (2006) The role of BiP in endoplasmic reticulum-associated degradation of major histocompatibility complex class I heavy chain induced by cytomegalovirus proteins. J Biol Chem 281: 20910–20919 [DOI] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120: 85–98 [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Wada I, Hasegawa K, Yorihuzi T, Tremblay LO, Herscovics A, Nagata K (2001) A novel ER alpha-mannosidase-like protein accelerates ER-associated degradation. EMBO Rep 2: 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast A, Preuss M, Winkler M, Dick TP (2007) Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat Immunol 8: 864–872 [DOI] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL (2004) A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429: 834–840 [DOI] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL (2005) Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc Natl Acad Sci USA 102: 14296–14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro J, Lilley BN, Spooner E, Noriega V, Tortorella D, Ploegh HL (2006) Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature 441: 894–897 [DOI] [PubMed] [Google Scholar]
- Machold RP, Wiertz EJ, Jones TR, Ploegh HL (1997) The HCMV gene products US11 and US2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J Exp Med 185: 363–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson B, Rainey EK, Benjamin T, Baryshev M, Mkrtchian S, Tsai B (2005) ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol Cell 20: 289–300 [DOI] [PubMed] [Google Scholar]
- McCracken AA, Brodsky JL (2003) Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD). Bioessays 25: 868–877 [DOI] [PubMed] [Google Scholar]
- Molinari M, Galli C, Piccaluga V, Pieren M, Paganetti P (2002) Sequential assistance of molecular chaperones and transient formation of covalent complexes during protein degradation from the ER. J Cell Biol 158: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VD, Wallis K, Howard MJ, Haapalainen AM, Salo KE, Saaranen MJ, Sidhu A, Wierenga RK, Freedman RB, Ruddock LW, Williamson RA (2008) Alternative conformations of the x region of human protein disulphide-isomerase modulate exposure of the substrate binding b′ domain. J Mol Biol 383: 1144–1155 [DOI] [PubMed] [Google Scholar]
- Noiva R, Lennarz WJ (1992) Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem 267: 3553–3556 [PubMed] [Google Scholar]
- Nyborg AC, Herl L, Berezovska O, Thomas AV, Ladd TB, Jansen K, Hyman BT, Golde TE (2006) Signal peptide peptidase (SPP) dimer formation as assessed by fluorescence lifetime imaging microscopy (FLIM) in intact cells. Mol Neurodegener 1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Hosokawa N, Wada I, Nagata K (2003) EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science 299: 1394–1397 [DOI] [PubMed] [Google Scholar]
- Otsu M, Bertoli G, Fagioli C, Guerini-Rocco E, Nerini-Molteni S, Ruffato E, Sitia R (2006) Dynamic retention of Ero1alpha and Ero1beta in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid Redox Signal 8: 274–282 [DOI] [PubMed] [Google Scholar]
- Park B, Lee S, Kim E, Cho K, Riddell SR, Cho S, Ahn K (2006) Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell 127: 369–382 [DOI] [PubMed] [Google Scholar]
- Park B, Oh H, Lee S, Song Y, Shin J, Sung YC, Hwang SY, Ahn K (2002) The MHC class I homolog of human cytomegalovirus is resistant to down-regulation mediated by the unique short region protein (US)2, US3, US6, and US11 gene products. J Immunol 168: 3464–3469 [DOI] [PubMed] [Google Scholar]
- Pirneskoski A, Klappa P, Lobell M, Williamson RA, Byrne L, Alanen HI, Salo KE, Kivirikko KI, Freedman RB, Ruddock LW (2004) Molecular characterization of the principal substrate binding site of the ubiquitous folding catalyst protein disulfide isomerase. J Biol Chem 279: 10374–10381 [DOI] [PubMed] [Google Scholar]
- Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S (2002) AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol 22: 626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoh-Nakatogawa M, Nishikawa S, Endo T (2009) Roles of protein-disulfide isomerase-mediated disulfide bond formation of yeast Mnl1p in endoplasmic reticulum-associated degradation. J Biol Chem 284: 11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K, Helenius A (2007) Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 131: 516–529 [DOI] [PubMed] [Google Scholar]
- Sun F, Zhang R, Gong X, Geng X, Drain PF, Frizzell RA (2006) Derlin-1 promotes the efficient degradation of the cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR folding mutants. J Biol Chem 281: 36856–36863 [DOI] [PubMed] [Google Scholar]
- Taxis C, Hitt R, Park SH, Deak PM, Kostova Z, Wolf DH (2003) Use of modular substrates demonstrates mechanistic diversity and reveals differences in chaperone requirement of ERAD. J Biol Chem 278: 35903–35913 [DOI] [PubMed] [Google Scholar]
- Tortorella D, Story CM, Huppa JB, Wiertz EJ, Jones TR, Bacik I, Bennink JR, Yewdell JW, Ploegh HL (1998) Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J Cell Biol 142: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B, Rodighiero C, Lencer WI, Rapoport TA (2001) Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 104: 937–948 [DOI] [PubMed] [Google Scholar]
- Tsai B, Ye Y, Rapoport TA (2002) Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol 3: 246–255 [DOI] [PubMed] [Google Scholar]
- Ushioda R, Hoseki J, Araki K, Jansen G, Thomas DY, Nagata K (2008) ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science 321: 569–572 [DOI] [PubMed] [Google Scholar]
- Wahlman J, DeMartino GN, Skach WR, Bulleid NJ, Brodsky JL, Johnson AE (2007) Real-time fluorescence detection of ERAD substrate retrotranslocation in a mammalian in vitro system. Cell 129: 943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Heath-Engel H, Zhang D, Nguyen N, Thomas DY, Hanrahan JW, Shore GC (2008) BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell 133: 1080–1092 [DOI] [PubMed] [Google Scholar]
- Wang S, Ng DT (2008) Lectins sweet-talk proteins into ERAD. Nat Cell Biol 10: 251–253 [DOI] [PubMed] [Google Scholar]
- Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B (2002) Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296: 2215–2218 [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL (1996a) The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84: 769–779 [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL (1996b) Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384: 432–438 [DOI] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414: 652–656 [DOI] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429: 841–847 [DOI] [PubMed] [Google Scholar]
- Yoshida H (2007) ER stress and diseases. FEBS J 274: 630–658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Information
Review Process File