Abstract
Vascular endothelial growth factor (VEGF) and β-catenin both act broadly in embryogenesis and adulthood, including in the skeletal and vascular systems. Increased or deregulated activity of these molecules has been linked to cancer and bone-related pathologies. By using novel mouse models to locally increase VEGF levels in the skeleton, we found that embryonic VEGF over-expression in osteo-chondroprogenitors and their progeny largely pheno-copied constitutive β-catenin activation. Adult induction of VEGF in these cell populations dramatically increased bone mass, associated with aberrant vascularization, bone marrow fibrosis and haematological anomalies. Genetic and pharmacological interventions showed that VEGF increased bone mass through a VEGF receptor 2- and phosphatidyl inositol 3-kinase-mediated pathway inducing β-catenin transcriptional activity in endothelial and osteoblastic cells, likely through modulation of glycogen synthase kinase 3-β phosphorylation. These insights into the actions of VEGF in the bone and marrow environment underscore its power as pleiotropic bone anabolic agent but also warn for caution in its therapeutic use. Moreover, the finding that VEGF can modulate β-catenin activity may have widespread physiological and clinical ramifications.
Keywords: β-catenin, osteoblast, skeleton, vasculature, VEGF
Introduction
Vascular endothelial growth factor (VEGF) is a positive regulator of bone development (Haigh et al, 2000; Maes et al, 2002, 2004; Zelzer et al, 2002), skeletal growth (Gerber et al, 1999) and fracture repair (Jacobsen et al, 2008). VEGF may couple angiogenesis to osteogenesis both indirectly through its effects on endothelial cells and directly by modulating chondrocytes, osteoblasts and osteoclasts that all express VEGF receptors (Dai and Rabie, 2007; Maes and Carmeliet, 2008). Accordingly, VEGF is currently tested in preclinical models as a potential therapy for stimulating fracture healing (Carano and Filvaroff, 2003). On the other hand, emerging data suggest that a disturbance in the interplay between angiogenesis and osteogenesis might be causal and/or consequential to progression of many bone and haematological pathologies (Calvi et al, 2003; Visnjic et al, 2004; Walkley et al, 2007; Larsson et al, 2008). Future therapeutic use of VEGF in bone pathology therefore warrants in-depth analyses of both the therapeutic merits as well as the potential side effects. A prime goal is to understand better the mechanisms of action of VEGF in the bone environment, including its cellular targets and downstream effectors.
A crucial regulator of bone formation is β-catenin, the main effector of canonical signalling by Wnts. In the absence of Wnts, cytoplasmic β-catenin is constitutively degraded through its phosphorylation by glycogen synthase kinase 3-β (GSK3-β) that targets it to the ubiquitin-proteasome pathway. On signalling by Wnts, β-catenin is stabilized and translocates to the nucleus, where it interacts with T-cell factor (TCF)/lymphoid enhancer factor family transcription factors to regulate the expression of its target genes (Clevers, 2006; Grigoryan et al, 2008). In addition, other pathways affecting GSK3-β (e.g. phosphatidyl inositol 3 (PI3)-kinase pathways) can also modulate β-catenin transcriptional activity; Wnt and growth factor signalling can act through convergent pathways and possibly synergistically on GSK3-β and β-catenin (Jin et al, 2008). The importance of β-catenin in skeletal biology was proven recently by studies elucidating its role as a crucial transcription factor in (i) determining osteoblast lineage commitment of early osteo-chondroprogenitors (Day et al, 2005; Hill et al, 2005; Hu et al, 2005; Rodda and McMahon, 2006) and (ii) coupling osteoblast to osteoclast activity by stimulating osteoblastic production of osteoprotegerin (OPG or TNFRSF11B), an inhibitor of osteoclast formation (Glass et al, 2005).
β-catenin signalling is also important in vascular biology. In quiescent endothelial cells of established vessels, β-catenin is concentrated at the plasmamembrane where it interacts with vascular endothelial (VE)-cadherin and mediates its linkage to the actin cytoskeleton (Dejana et al, 2008). VEGF promotes endothelial cell survival by stimulating the formation of a multi-protein transmembrane complex including VEGF receptor 2 (VEGFR-2, also known as Flk-1 or KDR), VE-cadherin and β-catenin, activating PI3-kinase/Akt (Dejana et al, 2008). In angiogenic cells, during embryogenesis, pathological angiogenesis or vascular remodelling, β-catenin may translocate to the nucleus and activate cell-cycle gene transcription (such as CyclinD1), contributing to endothelial cell proliferation. Pro-angiogenic effects of the GSK3-β/β-catenin pathway in endothelial cells have been described in vitro (Kim et al, 2002; Skurk et al, 2005) and very recently canonical Wnt signalling in endothelial cells was shown to be critical for vascularization of the developing central nervous system (Liebner et al, 2008; Stenman et al, 2008).
Thus, similar to VEGF, β-catenin acts broadly in embryogenesis and adulthood. This suggests that molecular communication involving both molecules may possibly contribute to the coupled osteogenic and angiogenic responses that are systematically seen in bone biology and pathology. In this study, using two independent conditional and/or inducible approaches to over-express VEGF164 in embryonic development, during skeletal growth and in adult bone, we provide evidence for novel mechanistic links between the VEGF and β-catenin signalling pathways. Even short-term gain-of-function of VEGF in the bone microenvironment not only stimulated vascularization and ossification, but also induced dramatic pathological changes. This phenotype correlated with VEGF-induced activation of VEGFR-2 and a PI3-kinase/GSK3-β/β-catenin pathway in both endothelial and osteoblast lineage cells, mediating its downstream responses in the bone and marrow microenvironment. These data underscore the potential of VEGF as a bone anabolic agent but also warn for caution in all therapeutic uses of this powerful molecule, pointing out the bone microenvironment as a critical locus for monitoring potential side effects.
Results
Locally increased VEGF during skeletal development leads to deformed bones
To determine the effects of local VEGF over-expression in the skeleton we targeted a conditional construct encoding the major isoform VEGF164 to the genomic ROSA26 locus (for details see Supplementary data). The expression of the VEGF164 transgene was prevented by a floxed upstream transcriptional stop cassette (Figure 1A). Only in cells expressing Cre, recombination led to the deletion of the stop cassette and activation of constitutive VEGF over-expression. Here, we used collagen type II (Col2)-Cre mice (Ovchinnikov et al, 2000) because Cre expression was reported to be activated early and broadly in the mesenchyme-derived cartilaginous condensations (including the perichondrium and postulated osteo-chondroprogenitors) that give rise to both the cartilage and the bone of the endochondral skeleton (Hilton et al, 2005; Rodda and McMahon, 2006; Ford-Hutchinson et al, 2007). As such, targeted cells and their descendants would encompass the physiological sites of VEGF production in endochondral bone (Maes et al, 2002; Zelzer et al, 2002). ROSA26R β-galactosidase (LacZ) reporter analysis (Soriano, 1999) indeed confirmed evidence of present or past Col2-Cre activity in chondrocyte and osteoblast lineage cells (Figure 1B). In agreement, compared with control littermates, mutant Col2-Cre+;ROSA26-VEGF164 (designated ‘+VEGF164') embryos had two-fold increased VEGF mRNA levels (P<0.05; real-time quantitative RT–PCR (qRT–PCR) data not shown) and three- to five-fold higher VEGF protein levels in their tibias, both in the cartilaginous epiphyses and the bony diaphyses (Figure 1C).
Figure 1.
VEGF over-expression in the developing skeleton leads to aberrant ossification and deformed bones. (A) Strategy used to conditionally target VEGF164 to the ROSA26 locus. (B) ROSA26R LacZ reporter analysis visualizing Col2-Cre targeted cells at E17.5 (femur). Blue X-Gal staining shows present or past Col2-Cre activity in cartilage and bone. PC, proliferating chondrocytes; HC, hypertrophic chondrocytes; arrows, osteoblasts; double arrows, osteocytes; arrowheads, perichondrium/periosteum. (C) VEGF protein levels measured by ELISA in E16.5 whole tibias or dissected growth cartilages (epiphyses) and bone shafts (diaphyses). Bars represent mean±s.e.m.; **P<0.01; ***P<0.001. (D) Whole-mount alcian blue (cartilage) and alizarin red (bone) skeletal stains of E16.5 control and +VEGF164 embryos. (E) Histological analysis of E16.5 hindlimbs by Safranin O, Von Kossa and PECAM-1 staining.
Mutant +VEGF164 mice died at birth by yet unknown causes and displayed marked deformities of the long bones. In particular, skeletal preparations of E16.5 embryos showed that the red stained ossified diaphyses of the limbs and ribs were abnormally short and thick, associated with kyphosis (bending) in the limbs (Figure 1D). The overall skeletal patterning and growth of the mutants were not impaired. Accordingly, histological analysis showed no striking disruption in the growth cartilage of +VEGF164 limbs other than a mild reduction of the hypertrophic chondrocyte zone (202 μm±5 in controls versus 158 μm±15 in +VEGF164 mice; P=0.047; n=3) (Supplementary Figure S1). In contrast, the primary ossification centre was drastically malformed (Figure 1E, top). As evidenced by Von Kossa staining for mineralized bone, +VEGF164 mutants had laterally expanded ossified centres filled with excessive, disorganized bone. In contrast to control mice, a proper cortex was lacking and instead aberrantly orientated trabecular-like structures extended from the widened periosteal area to the inside of the bone, obliterating the developing marrow cavity (Figure 1E, middle). In line with the strong angiogenic activity of VEGF, staining of vascularization by PECAM-1 immunohistochemistry (IHC) showed a vast increase in blood vessels throughout the mutant bones (Figure 1E, bottom). Thus, skeletal VEGF over-expression was characterized by aberrantly ossified, hypervascularized bones. A similar phenotype in the long bones was obtained using the more osteoprogenitor/osteoblast-directed gene promoters Runx2 (active in subsets of hypertrophic chondrocytes and osteoblasts (J Tuckermann, unpublished data); Supplementary Figure S2) or Osterix (Rodda and McMahon, 2006) (not shown) to drive Cre-mediated VEGF over-expression.
Generation of mice with inducible, skeletal-specific transgenic VEGF expression
As improved angiogenesis and osteogenesis is much sought after in postnatal conditions such as fracture healing and osteoporosis, we wanted to assess whether VEGF would exert bone anabolic effects later in life as well. Given the neonatal lethality associated with constitutive conditional VEGF gain-of-function, we designed a doxycycline (dox)-inducible transgenic strategy (Figure 2A). The reverse tetracycline transactivator (rtTA) was targeted to the conditional ROSA26-locus described above (as a bicistronic transcript also encoding EGFP), rendering the activation of its expression dependent on Cre activity (Belteki et al, 2005). The corresponding tetracycline-responsive element (tet(o)), induced by rtTA only in the presence of dox, regulated the expression of a VEGF164 transgene (Akeson et al, 2003). As described above, the crossing with Col2-Cre mice mediated the recombination of the conditional ROSA26-locus in embryonic osteo-chondroprogenitor cells and all progeny thereof. Consequently, the rtTA:EGFP transgene became constitutively expressed in the endochondral bones. Widespread expression of EGFP throughout the adult growth plate and metaphyseal and cortical bone areas was accordingly documented by IHC (Supplementary Figure S3A). The presence of dox would enable the produced rtTA to bind to tet(o) and switch on transgenic VEGF164 expression. Thus, VEGF over-expression was controlled both spatially (by tissue-specific Cre expression) and temporally (by dox administration). Administration of dox to triple transgenic mice (Col2-Cre+; ROSA26-Flox/Stop-rtTA-IRES-EGFP; tet(o)-VEGF164), further designated ‘+VEGF164dox mice', through the drinking water was indeed effective at inducing abundantly increased VEGF expression throughout the bone, both in chondrocytes and in osteoblast lineage cells, as detected by IHC and in situ hybridization (ISH) (Supplementary Figure S3B and C). While leaky transgene expression was not observed in the absence of dox (Supplementary Figure S3D), 2 weeks of dox supplementation, as applied throughout these studies, caused a 10-fold increase in VEGF mRNA levels in long bones of adult +VEGF164dox mice as compared with dox-treated control littermates, associated with a more moderate two- to three-fold induction at the protein level (Supplementary Figure S3E and F). Of note, the serum VEGF levels were not significantly altered in +VEGF164dox mice (Supplementary Figure S3G).
Figure 2.
VEGF-induction in adult bone causes excessive trabecular bone, cortical porosity, hypervascularization and bone marrow fibrosis. (A) Breeding scheme to generate +VEGF164dox mice expressing the inducible transcriptional activator rtTA and EGFP constitutively in chondrocyte and osteoblast cell lineages. Only on dox administration VEGF over-expression is induced in these cells through the activation of tet(o) by rtTA. Control mice were littermates lacking the tet(o)-VEGF164 or the Col2-Cre transgene, receiving dox. (B) H&E stained tibias showing excessive trabecular bone in the metaphysis (boxed areas magnified in middle) surrounded by abundant stromal cells (magnification in bottom panels) in +VEGF164dox mice after 2 weeks on dox. (C, D) Von Kossa staining of control and +VEGF164dox tibias (C) and histomorphometric analysis (D) performed in three fixed areas (red boxes). Arrows indicate cortical remodelling. BV/TV, bone volume relative to total volume. (E) PECAM-1 immunohistochemistry (IHC) visualizing vascularization. gp, growth plate. (F) Reticulin staining revealing massive fibrosis in +VEGF164dox bones. Boxes, magnified on the right.
Short-term increases of VEGF levels disrupt the architecture of juvenile and adult bone
Induction of VEGF over-expression in the endochondral skeleton of juvenile mice, by supplying dox to the nursing mother during the first 2 weeks of life, profoundly affected the shape and morphology of the growing long bones. The aberrant bone was associated with abundant stromal cells and blood vessels surrounding the numerous trabeculae (Supplementary Figure S4).
In adult mice with normally developed bones, dox supplementation for 14 days also completely disrupted the bone architecture, as seen in +VEGF164dox mice at the age of 3–4 months (Figure 2B). The induced VEGF over-expression characteristically led to excessive, aberrant bone structures and abundant peritrabecular mesenchymal stromal cells in the metaphyseal and epiphyseal regions (primary and secondary centres of ossification) (Figure 2B). Von Kossa staining and histomorphometry indeed showed a dramatically increased metaphyseal trabecular bone mass in +VEGF164dox mice (Figure 2C and D), as did pQCT analysis (70% increased trabecular density, n=12, P<0.05). The adjacent lamellar cortical bone, however, was replaced by trabecular-like porous bone structures (red arrows in Figure 2C). This manifest porosity of the cortex was characterized by abundant intercalating mesenchymal tissue components and osteoclast-rich remodelling units (Supplementary Figure S5A and B).
As during development, the morphology of the cartilage was not manifestly disrupted by VEGF over-expression in the adult, but a mild decrease in the growth plate thickness was documented (95 μm±5 in controls versus 79 μm±5 in +VEGF164dox mice; P<0.05; n=7). As well, the growth plate showed increased mineralization as quantified on Von Kossa stained sections (percent calcification: 14.3%±0.8 in controls versus 18.8%±1.7 in +VEGF164dox mice; P<0.05; n=5). As anticipated, the increased VEGF levels again caused hypervascularization of the bones. PECAM-1 IHC visualized the strongly increased microvascular density throughout the altered metaphyseal (Figure 2E) and cortical (Supplementary Figure S5C) bone areas in +VEGF164dox mice. Adjacent to the growth plate, haemangioma-like blood vessels were often observed, indicative of localized supra-physiological VEGF levels (Ozawa et al, 2004) (Figure 2E) (also see below). These data indicate that even short-term induction of transgenic VEGF expression in adult bone dramatically stimulated osteogenesis and angiogenesis.
VEGF over-expression in the bone micro-environment causes vascular anomalies, bone marrow fibrosis and haematological alterations
In addition to the increased bone mass and hypervascularization caused by increased VEGF at all stages examined in this study, adult +VEGF164dox mice displayed several marked pathological features. Reticulin staining showed increased amounts of collagen IV-positive fibres in the regions of increased osteogenesis, indicative of massive fibrosis (Figure 2F). Furthermore, vascularity in the bone was consistently increased but in severely pathological bones several bone marrow blood vessels exhibited additionally a haemangioma-like morphology filled with erythrocytes (Figure 3A). Notably, these pathological manifestations locally replaced the haematopoietic marrow and consequently, the areas heavily occupied by new bone, marrow fibrosis and aberrant vessels displayed a drastic paucity of myeloid cells (Figure 3A). Moreover, increased spleen size was observed in 25% of the +VEGF164dox mice (n=11/43), suggesting extramedullary haematopoiesis (Figure 3B). As well, we documented evidence of extramedullary haematopoiesis in the liver in +VEGF164dox mice with haematopoietic colonies developing near some central vein regions (data not shown). These observations led us to assess whether +VEGF164dox mice suffered haematological alterations. The bone marrow of +VEGF164dox mice showed excessive PECAM-1+ megakaryocytes displaying abundant extended cellular processes, characteristic of megakaryocyte activation (Figure 3C). The number of megakaryocytes and progenitors in the spleen was also dramatically increased in the mutant mice, as shown by morphological detection, colony forming units (CFU)-MK analysis, and FACS analysis using the megakaryocyte lineage antigen CD41 (Figure 3D). In addition, we detected a three-fold increase in the numbers of haematopoietic progenitor cells (HPCs) in the peripheral blood of mutant mice by analysing the CFU-C, as well as a significant increase of CFU-Cs in the spleen (Figure 3E). No significant changes in CFU-C were documented in flushed bone marrow cultures (Figure 3E). In addition, temporal haematocrit profiling of the peripheral blood did not document significant changes in the number of circulating red or white blood cells but a small significant drop in platelet numbers at the end of the 2 weeks of induction (Supplementary Table S1). These data suggest that the +VEGF164dox phenotype was associated with enhanced mobilization of HPCs from the bone marrow into the circulation and are suggestive of altered megakaryocyte maturation/function.
Figure 3.
Haematological changes in +VEGF164dox mice. (A) H&E staining showing tortuous, erythrocyte-enriched blood vessels that locally replace the haematopoietic marrow in +VEGF164dox mice. (B) Spleens of control and severely affected +VEGF164dox mice. (C) PECAM-1 IHC showing increased abundance of megakaryocytes (arrows) in the bone marrow of +VEGF164dox mice. Box, magnified on the right. (D) Number of PECAM-1+ megakaryocytes counted in spleen sections (left), number of spleen-derived CFU-MK colonies (middle) and % increase in CD41+ megakaryocyte lineage cells (right) (E) CFU-C analysis on peripheral blood samples (left), spleens (middle) and bone marrow (right) after 2 weeks on dox. *P<0.05; n=6.
VEGF over-expression alters osteoblast proliferation, differentiation and activity
The manifestly enhanced bone mass observed at all stages upon VEGF over-expression mandated a detailed study of the osteoblast characteristics. BrdU IHC of +VEGF164 embryonic bones showed increased proliferating cells in the perichondrium/periosteum where osteogenic progenitors reside (BrdU+ cells per 100 μm2: 34±5 in controls versus 75±5 in +VEGF164 mice; P<0.001; n=6), as well as throughout the primary ossification centre (Figure 4A). In adult +VEGF164dox bones, we observed markedly increased BrdU incorporation in mesenchymal cells in the metaphysis (Figure 4B) and epiphysis (not shown) already after 4 days of dox supplementation. Strongly proliferative cell clusters presenting as mesenchymal islands intercalating the aberrant bone were still observed at 10 days on dox (Figure 4B). Increased cellular proliferation was also evident in the periosteum and the aberrant cortical remodelling regions (not shown). This excessive proliferative response of osteoprogenitors and/or osteoblasts likely contributed to the increased bone (osteosclerosis) seen on VEGF over-expression.
Figure 4.
Altered proliferation, differentiation and activity of osteoblast lineage cells. (A) IHC detecting incorporated BrdU in control and +VEGF164 hindlimbs at E16.5, showing increased proliferation in the mutant perichondrium (pc)/periosteum (po) (dotted lines in magnified view of boxed area) and in the primary ossification centre (POC). Arrows, proliferating osteoblasts. (B) BrdU incorporation in adult control and +VEGF164dox tibias at day 4 (d4)and d10 of dox administration. Dotted line encompasses a cluster of proliferating mesenchymal cells. (C) In situ hybridization for the osteoblast differentiation markers Runx2, Col1a1, osteopontin and osteocalcin on tibias (ti) of control and +VEGF164 embryos; fi, fibula. Arrows highlight aberrant osteocalcin expression domains in the mutant. (D) Runx2, Col1a1, osteopontin and osteocalcin mRNA levels measured by qRT–PCR in +VEGF164dox bones relative to controls (2 weeks dox). (E) Goldner staining showing normal bone-associated osteoid (arrows) and orderly arranged cuboidal osteoblasts (arrowheads) lining mineralized bone surfaces (green) in control bones, contrasting the aberrant osteoid deposition (asterisks) and disorganized peri-trabecular stroma (magnified, right) in +VEGF164dox bones. (F) Dual calcein incorporation showing delineated double labelling in control mice and aberrant labelling in +VEGF164dox mice.
Osteoblast differentiation was altered as well in both the developmental and adult systems. ISH on E17.5 +VEGF164 tibia sections showed enlarged expression domains of the stage-dependent differentiation markers Runx2, type I collagen (Col1a1), osteopontin and osteocalcin (Figure 4C). Particularly, for the mature osteoblast marker osteocalcin, ectopic signal was detected in the perichondrial regions, and staining intensity was increased throughout the widened ossification centre (Figure 4C, bottom). In agreement, qRT–PCR showed more than three-fold increased levels of osteocalcin mRNA in +VEGF164 tibias compared to control bones (P<0.001; n=10–12), indicative of an increase in differentiated osteoblasts. In adult +VEGF164dox mice osteoblast differentiation was altered as well after 14 days on dox, although not entirely in similar ways as in the developmental system. Indeed, qRT–PCR showed increased mRNA levels of the early osteogenic marker Runx2 and of osteopontin, whereas Col1a1 expression was not significantly increased and osteocalcin mRNA was significantly reduced (Figure 4D). Western Blot and IHC analysis confirmed the increased osteopontin expression at the protein level (Supplementary Figure S6). The matrix formation versus mineralization activity of osteoblasts was unbalanced in +VEGF164dox mice, as Goldner staining showed extensive and ectopic osteoid deposition (Figure 4E). Dual calcein incorporation led to the expected delineated double labelling in control mice, but showed aberrant, excessive and disorganized labelling in +VEGF164dox mice (Figure 4F).
Increased VEGF alters osteoclast abundance and activity
We next investigated the potential contribution of altered osteoclastogenesis to the VEGF-induced phenotypes. Embryonic +VEGF164 hindlimb sections showed excessive and premature invasion of osteoclastic cells (TRAP+) together with blood vessels (PECAM-1+) into the cartilaginous anlagen (not shown), yet in the subsequently established primary ossification centre only few, small TRAP+ cells were found in the regions of excessive bone and vascularization (Figure 5A). Impaired osteoclastic resorption was suggested by the frequent persistence of unresorbed cartilage remnants in +VEGF164 diaphyses (Supplementary Figure S7A). Similarly, detailed temporal analysis of adult +VEGF164dox mice showed progressively increased vascular density (PECAM-1 IHC; top panels in Figure 5B), whereas TRAP staining on adjacent sections showed an initial abundance of osteoclasts at day 4 of dox administration followed by a dramatic decrease by day 10 and near absence of osteoclasts by day 14 in the regions of high vascular and bone density (Figure 5B). Again impaired resorptive activity was suggested, given the continued presence of residual cartilage in the aberrant bone regions of +VEGF164dox bones observed by Safranin O staining (Supplementary Figure S7B). Of note, the drop in metaphyseal osteoclasts in +VEGF164dox mice was unlikely to be explained by cell-autonomous effects, given that in vitro osteoclastogenesis from haematopoietic precursors isolated from bone marrow was not impaired (data not shown). We therefore analysed the expression of OPG, a prime secreted inhibitor of osteoclastogenesis. IHC showed increased OPG staining in the osteosclerotic/osteopetrotic metaphyseal regions of +VEGF164dox bones at day 10 of dox supplementation. Interestingly, OPG abundantly localized to the endothelium and mesenchyme in these regions of enhanced vessel and bone density (Figure 5C). In contrast to the metaphyseal osteopetrosis, cortical regions exhibited extensive TRAP+ cell coverage and remodelling in +VEGF164dox mice throughout the dox administration period (see Figure 2C; Supplementary Figure S5B). This region-specific stimulation of osteoclastogenesis was associated with significantly increased mRNA levels of RANK and resorptive enzymes (MMP-9, MMP-13, CathepsinK), specifically in the cortical bone compartment (Supplementary Table S2, showing qRT–PCR analysis of separated diaphyseal cortices and epiphyseal/metaphyseal spongiosa). Compared with controls, the +VEGF164dox cortical bone shafts showed a drastic upregulation of RANKL (eight-fold), whereas OPG levels were unaltered, unlike the mutant spongiosa (see above, Figure 5C; Supplementary Table S2).
Figure 5.
Altered osteoclastogenesis and increased OPG expression associated with VEGF induction. (A) TRAP staining of embryonic hindlimbs showing only few, small TRAP+ cells (arrows) associated with the +VEGF164 excessive bone phenotype. Asterisks, vessels. (B) PECAM-1 IHC and TRAP staining on adjacent tibia sections of control and +VEGF164dox mice at d4, d10 and d14 of dox administration, showing progressive increase in metaphyseal (m) vascular density and eventual loss of TRAP+ cells from these regions. TRAP+ cells remain prevalent at the growth plate (gp) but are absent from the metaphysis at d14 (bottom panels, higher magnification). *Indicates fibrotic regions devoid of TRAP-positive cells. Bottom right panel, temporal quantification of TRAP+ cells in the metaphysis. ***P<0.001. (C) OPG IHC at d10 of dox. Magnified view (right) showing stromal- (asterisk) and endothelial cell- (arrows)-associated OPG in +VEGF164dox mice.
Endothelial and mesenchymal stromal cells both respond to VEGF signalling
Next, we wanted to assess whether other cell types besides endothelial cells were responding to VEGF and relaying the VEGF-induced effects on the skeleton. We therefore analysed the expression of activated (phosphorylated) (P)-VEGFR-2 in adult +VEGF164dox bones using antibodies specific for the P-VEGFR-2-1175 phosphotyrosine residue. As expected, combined IHC for PECAM-1 and P-VEGFR-2-1175 showed increased staining of activated VEGFR-2 in the endothelium of +VEGF164dox bones (Figure 6A). Interestingly, co-staining of alkaline phoshatase (ALP) activity and P-VEGFR-2-1175 also showed abundant double-positive cells in the mutants (Figure 6B), suggesting a role for VEGFR-2 signalling in subsets of mesenchymal progenitors and/or osteoblasts. To investigate the possibility of direct VEGF effects, we isolated bone stromal cells from +VEGF164dox and control mice. Cultures from +VEGF164dox mice showed increased VEGF and VEGFR-2 expression, regardless of whether dox was administered in vivo (Figure 6C) or in vitro (Supplementary Figure S8). Concomitantly, we noted strongly upregulated expression of Runx2 and osteocalcin (Figure 6C), indicating enhanced osteoblastic differentiation as a direct consequence of VEGF over-expression.
Figure 6.
VEGFR-2 in osteoblast lineage cells contributes to VEGF-induced high bone mass. (A) IHC for PECAM-1 (purple) and phospho(P)-VEGFR-2 (brown) in control and +VEGF164dox samples. Right panel, magnified view. Arrows, endothelial co-localization of the signals; asterisks, vessel lumens. (B) Staining for ALP activity (blue, arrowheads) and P-VEGFR-2 (brown), often co-localizing in +VEGF164dox bones (arrows in magnification). (C) qRT–PCR for VEGF, VEGFR-2, Runx2 and osteocalcin in osteogenic cultures of bone stromal cells from adult control and +VEGF164dox mice. (D) Alcian blue/alizarin red staining and histology by H&E, PECAM-1 and BrdU IHC of E17.5 hindlimbs of control, +VEGF164, and Col2-Cre+;+VEGF164;VEGFR-2null/lox mice. Asterisks highlight rescued tibia kyphosis; histology is at × 200 (upper) or × 400 (two bottom rows) magnification.
Direct effects of VEGF/VEGFR signalling in osteoblast lineage cells in vivo have not been univocally proven to date and are still a matter of debate. To genetically address the hypothesis that such effects are involved in mediating the VEGF over-expression phenotype, we conditionally deleted VEGFR-2 (Haigh et al, 2003) from the osteo-chondrogenic lineages in +VEGF164 embryos by using Col2-Cre (Figure 6D) or Runx2-Cre mice (Supplementary Figure S9), with similar results. Although we detected no noticeable consequences of introducing one null (Shalaby et al, 1995) or a conditional allele of VEGFR-2 in embryos at E17.5 (not shown), +VEGF164;VEGFR-2null/lox embryos were rescued from the severe bone kyphosis as seen on skeletal preparations (Figure 6D, top) or by micro-CT (Supplementary Figure S9A and B). Quantification of the micro-CT scans showed that the excessive amount of mineralized bone formed in the +VEGF164 mutants was significantly reduced in the +VEGF164;VEGFR-2null/lox embryos, but did not reverse completely to the level measured in control bones (Supplementary Figure S9A). The bone shaft regularly remained widened (Supplementary Figure S9B). A pronounced hypervascularization was observed in the +VEGF164;VEGFR-2null/lox bones, both in the expanded perichondrial/periosteal region (Supplementary Figure S9C) and inside bone shaft (Figure 6D). In contrast, the mesenchymal hyperproliferation that was documented in +VEGF164 bones was notably reduced by the additional conditional deletion of VEGFR-2 in osteo-chondroprogenitors (Figure 6D, bottom). Thus, in this partial rescue of the +VEGF164 phenotype, the osteogenic response was significantly modulated whereas the angiogenic effects of increased VEGF persisted.
Enhanced β-catenin stabilization and nuclear activity in VEGF over-expressing mice
Recently, the expression of a stabilized form of β-catenin in osteoprogenitors and/or osteoblasts was shown to cause high bone mass phenotypes with very similar characteristics as our VEGF over-expression models (Glass et al, 2005; Rodda and McMahon, 2006). This striking resemblance prompted us to examine β-catenin as a potential molecular mediator of the VEGF-induced phenotype. IHC for β-catenin showed excessive staining in +VEGF164 embryos at E16.5. More specifically, control bone sections showed ample β-catenin staining in endothelial cells and osteoblasts (as defined by their cuboidal morphology and localization on the bone surface) mostly localized to the cell membrane (Figure 7A). In contrast, +VEGF164 samples displayed more intense and abundant staining throughout endothelial and osteoblast lineage cells, in the cytoplasm and the nucleus (Figure 7A). The increased nuclear localization of β-catenin in osteoblasts over-expressing VEGF was confirmed by culturing primary calvaria-derived osteoblasts from Runx2-Cre;ROSA26-VEGF164 and Runx2-Cre;Z/EG (Novak et al, 2000) control mice (Figure 7B). In control cultures, β-catenin was mostly confined to the cell membranes, whereas a pronounced increase in nuclear localization of β-catenin was monitored in VEGF over-expressing osteoblasts, as quantified based on immunofluorescence confocal imaging (correlation with DAPI nuclear staining) (Figure 7B). The relevance of this nuclear localization of β-catenin in vivo was next assessed by crossing the BAT-Gal β-catenin reporter mouse line (Maretto et al, 2003) with the +VEGF164 model. Analysis of BAT-Gal-derived LacZ expression by X-Gal staining at E16.5 showed dramatically increased β-catenin transcriptional activity in +VEGF164 embryos compared with control littermates. Increased X-Gal staining was most evident in the perichondrium/periosteum where pre-osteoblasts differentiate, and in mature osteoblasts positioned on the excessive bone fragments (Figure 7C).
Figure 7.
Enhanced β-catenin activity in VEGF over-expressing mice. (A) β-catenin IHC on E16.5 control and +VEGF164 bone sections. Note predominant membranous localization in control endothelial (black arrows) and osteoblast lineage cells (red arrowheads), and cytoplasmic and nuclear staining in the mutants. Right panels, magnified views. (B) Immunofluorescent and confocal analysis of β-catenin localization in primary calvarial osteoblast cultures of control and Runx2-Cre+;ROSA26-VEGF164 mice. Nuclear β-catenin (arrows) was quantified based on correlations with DAPI staining (bottom, ***P<0.001). (C) X-Gal and Sirius red staining of control and +VEGF164 embryos carrying the BAT-Gal reporter reflecting β-catenin transcriptional activity. Arrows denote excessive signal in the mutant; dashed line, plane of the rib cross-section shown on the right; boxed area is magnified below to show abundant BAT-Gal+ osteoblasts (blue) on the bone surfaces (dark red). (D) β-catenin IHC on adult femurs is restricted to vessels invading the growth plate (gp) in control mice (left, double arrows) but enhanced throughout the +VEGF164dox metaphysis (right, including magnifications lower panels). Middle and right higher power magnifcations of indicated regions (1,2) of +VEGF164dox metaphysis showing cytoplasmic (arrows) and nuclear (arrowheads) β-catenin localization in the endothelium and in mesenchymal stroma are seen. (E, F) Western blot of bone lysates detecting active non-phosphorylated β-catenin (β-ctn) in (E) (showing a 3.9-fold increase in +VEGF164dox samples compared to controls) and increased phosphorylated (P)-GSK3-β in (F) in +VEGF164dox samples (around a 3.5-fold increase). (G) qRT–PCR for the β-catenin target genes Axin-2, c-Jun, Fra-1, CyclinD1, BMP4 and OPG in +VEGF164dox bone samples relative to controls.
IHC on adult +VEGF164dox bones also pointed at an involvement of excessive β-catenin in both endothelial cells and mesenchymal/osteogenic stromal cells. In control mice, β-catenin was remarkably restricted to the angiogenic blood vessels invading the growth plate (Figure 7D). In contrast, +VEGF164dox mice showed dispersed and enhanced cytoplasmic and nuclear β-catenin in the haemangioma-like endothelium near the growth plate, in blood vessels throughout the metaphyseal high bone mass regions, and in the abundant mesenchymal stroma (Figure 7D). Western blot analysis using an anti-active-β-catenin antibody (van Noort et al, 2002) that specifically recognizes a transcriptionally active non-phosphorylated form of β-catenin (lacking the phosphorylated amino acids Ser-33, Ser-37 and Thr-41 that are responsible for β-catenin proteosomal degradation) (Liu et al, 2002; van Noort et al, 2002) demonstrated increased levels of active β-catenin protein in +VEGF164dox bone lysates compared to control samples (Figure 7E). Using two separate antibodies that recognize all post-translational modified forms of β-catenin (Sigma polyclonal Ab C2206, and Transduction Laboratories monoclonal Ab clone 14) we detected increased overall levels of β-catenin immunoreactive protein in +VEGF164dox bone lysates compared to control samples (data not shown). We next conducted western blot analysis of GSK3-β, a prime upstream mediator of these negative β-catenin phosphorylation events. +VEGF164dox bone lysates contained increased GSK3-β in its phosphorylated form (Figure 7F; Supplementary Figure S10A), which is known to mediate its inactivation and thus likely contributed to the enhanced stabilization and activation of β-catenin (Jin et al, 2008). Finally, we assessed whether the enhanced β-catenin stabilization and nuclear localization were associated with increased transcriptional activity. The mRNA expression levels of a panel of known β-catenin target genes including those encoding Axin-2, c-Jun, Fra-1, CyclinD1, BMP4, OPG and Von Hipel-Lindau (VHL) (for regularly updated list of targets, see http://www.stanford.edu/~rnusse/wntwindow.html) (Glass et al, 2005; Grigoryan et al, 2008) were all significantly upregulated in +VEGF164dox bones compared to controls (Figure 7G). Consistent with increased VHL expression, hypoxia-inducible factor (HIF)-1α protein levels and expression of the HIF-1α target CXCL12 (SDF-1) were decreased but no changes in SDF-1 receptor CXCR4 expression were observed (Supplementary Figure S11A–D).
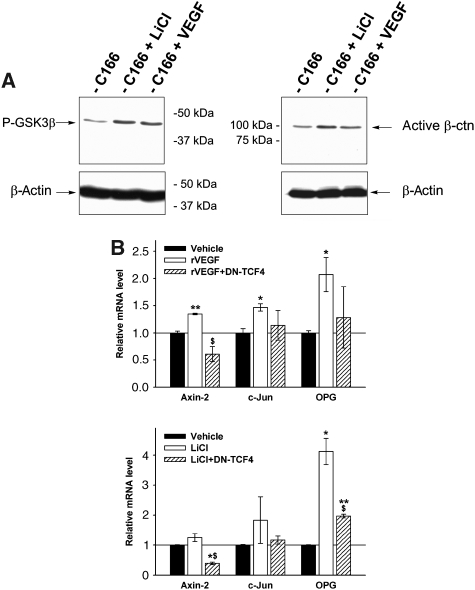
Possibly, β-catenin stabilization may represent a direct action of VEGF signalling in the endothelial and mesenchymal/osteoblastic progenitor cell populations. Consistent with this hypothesis, addition of exogenous VEGF to cultures of the haemangio-endothelial cell lines C166 (Figure 8; Supplementary Figure S10B and C) and Eoma (not shown) resulted in increased amounts of active β-catenin and phosphorylation of GSK3-β. These effects were comparable to those of the positive control LiCl, a known inhibitor of GSK-β activity causing β-catenin stabilization (Figure 8A; Supplementary Figure S10B and C) (Zhang et al, 2003). Concomitantly, the mRNA expression levels of Axin-2, c-Jun and OPG were increased by VEGF addition, to a similar extent as by LiCl, and this was partially blocked by infecting the cells with a dominant negative TCF4 (DN-TCF4) virus preventing β-catenin transcriptional activity (Figure 8B). Furthermore, primary bone stromal cells from our adult triple transgenic mice induced in vitro by dox to over-express VEGF, showed upregulated mRNA expression of the β-catenin targets c-Jun and OPG (Supplementary Figure S11E).
Figure 8.
VEGF induces β-catenin activation and transcriptional activity in endothelial cells. (A) Western blot analysis of P-GSK3-β (showing a 2.8-fold increase in LiCl-treated samples and a 2.4-fold increase with VEGF compared to untreated cells) and active β-catenin in c166 endothelial cells treated with vehicle, LiCl or rcVEGF (showing a two-fold increase in LiCl-treated samples and a 1.4-fold increase with VEGF compared to untreated cells). (B) qRT–PCR analysis of Axin-2, c-Jun and OPG mRNA levels in c166 cells treated with rcVEGF for 4 h or with LiCl for 6 h, in the presence or absence of a DN-TCF4 virus. *P<0.05; **P<0.01 versus Vehicle; $P<0.05 for effect of added DN-TCF4 to the treatment with VEGF or LiCl.
VEGF-induced high bone mass depends on β-catenin in osteochondro-lineage cells
To determine the importance of β-catenin in osteo-chondroprogenitor-derived cells for the VEGF-induced increased bone mass we bred one conditional loss-of-function Catnb (β-catenin) allele (Brault et al, 2001) into our triple transgenic +VEGF164dox system. In the absence of VEGF induction, CatnbΔcol2-cre/+ mice were indistinguishable from controls and showed similar metaphyseal bone densities (BV/TV) and trabecular number in 3D micro-CT analysis (Figure 9A; Supplementary Figure S12A). Interestingly, the heterozygous conditional inactivation of β-catenin in +VEGF164dox mutants abolished the VEGF-induced high bone density phenotype (Figure 9A; Supplementary Figure S12A). Histological analysis and PECAM-1 staining showed that the bones of these +VEGF164dox; β-cateninΔCol2-Cre/+ mice contained massively enlarged vascular structures (haemangiomas), replacing the local bone and marrow tissues (Figure 9A; Supplementary Figure S12). Osteoclastic bone remodelling and degradation was sustained in these bones (while blocked in the +VEGF164dox mice as fibrosis develops, see above) (Supplementary Figure S12C), likely mediating the reduction in the bone mass. Given the drastic nature of the modulation of the +VEGF164dox phenotype by the conditional removal of one allele of β-catenin, we performed a short-term induction experiment by giving dox for only 4 days. Already at this time, the vasculature in +VEGF164dox; β-cateninΔCol2-Cre/+ bones was increased, as in +VEGF164dox mice (Supplementary Figure S13). However, the response in the stromal/osteoblastic cells, which was associated with increased proliferation (BrdU incorporation) in +VEGF164dox mice, was blunted by deleting β-catenin from these cell types (Supplementary Figure S13). Of note, heterozygous deletion of one conditional β-catenin allele in osteochondro-linage cells did not affect the observed extramedullary haematopoiesis associated with enhanced VEGF expression (not shown).
Figure 9.
VEGF-induced activation of β-catenin occurs through a wortmannin-sensitive PI3-kinase pathway. (A) Upper panels showing representative micro-CT 3D scans and average bone density (BV/TV) of control, CatnbΔcol2-Cre/+, +VEGF164dox and +VEGF164dox;CatnbΔcol2-Cre/+ tibias. Data represent mean±s.e.; ANOVA. Lower panels showing H&E analysis of control, +VEGF164dox and +VEGF164dox;CatnbΔcol2-Cre/+ tibias (left) and PECAM vessel analysis of indicated regions (1,2) of endothelial lined haemangiomas in +VEGF164dox;CatnbΔcol2-Cre/+ tibias (right) (B) Western blot for active β-catenin on bone lysates of the respective genotypes (with equal amounts in control and CatnbΔcol2-Cre/+ samples, 5.6-fold increase in +VEGF164doxsamples and a reduced but three-fold increase in +VEGF164dox;CatnbΔcol2-Cre/+ tibias) (C) qRT–PCR for Axin-2, c-Jun, CyclinD1, BMP4 and OPG in bone samples of control, CatnbΔcol2-Cre/+, +VEGF164dox and +VEGF164dox;CatnbΔcol2-Cre/+ mice (mean±s.e.; ANOVA). No significant differences were found between control and CatnbΔcol2-Cre/+ mice. *P<0.05 versus control; $P<0.05; comparison of +VEGF164dox and +VEGF164dox;CatnbΔcol2-Cre/+ mice. (D–F) 3D micro-CT analysis (D), representative reconstructed micro-CT images (E) and histology (F) of tibias from control and +VEGF164dox mice treated or not with the PI3-kinase inhibitor wortmannin. (G) Western blot analysis of P-GSK3-β and β-catenin in bones from control and +VEGF164dox mice treated or not with wortmannin. (H) qRT–PCR for Axin-2, c-Jun, Fra-1, BMP4 and OPG in control and +VEGF164dox bone samples with or without wortmannin. *P<0.05; **P<0.01; t-test control versus wortmannin-treated controls and controls versus +VEGF164dox mice $P<0.05; comparison of +VEGF164dox and +VEGF164dox mice with wortmannin treatment.
Western blot analysis confirmed that the VEGF-induced activation of β-catenin decreased in +VEGF164dox;CatnbΔcol2-cre/+ bones, whereas the increase in phosporylated GSK3-β was largely unaffected (Figure 9B; Supplementary Figure S12B). Concomitantly, the β-catenin targets that we showed earlier to be upregulated by VEGF over-expression were completely (BMP4) or partially (Axin-2, CyclinD1 and OPG) normalized in +VEGF164dox;CatnbΔcol2-cre/+ bones (Figure 9C).
VEGF enhances β-catenin activity through a wortmannin-sensitive PI3-kinase pathway
Earlier studies have showed that signalling from growth factors/hormones (e.g. insulin) to GSK3-β is wortmannin-sensitive involving PI3-kinase, whereas Wnt signalling to GSK-3β is largely insensitive to this pharmacological PI3-kinase inhibitor (Cross et al, 1995; Cook et al, 1996; Jin et al, 2008). To determine which signalling pathway may be predominantly responsible for the observed GSK3-β phosphorylation and β-catenin stabilization, we administrated wortmannin to +VEGF164dox and control mice during the 2-week dox period. Analysis by 3D micro-CT demonstrated that the increased bone volume and trabecular number of +VEGF164dox mice were completely prevented by wortmannin (Figure 9D and E). Moreover, histological examination showed that the bone and marrow morphology were rescued (Figure 9F). Interestingly, this normalization of the bone architecture correlated with decreased P-GSK3-β (Figure 9G). As well, the total β-catenin levels were restored to normal (data not shown) and the increased expression of β-catenin target genes in +VEGF164dox mice was blunted by wortmannin treatment (Figure 9H). Similar effects of wortmannin treatment on c166 cells were also shown to decrease levels of VEGF-induced GSK3-β phosphorylation and the amount of activated β-catenin (Supplementary Figure S10B and C).
Discussion
VEGF: pleiotropic effects in bone during development, growth, and adulthood
Earlier findings established that VEGF functions as a key inducer of bone vascularization but also indicated that the various cell types involved in bone metabolism, such as perichondrial progenitor cells, osteoblasts and osteoclasts can respond to VEGF signalling (Dai and Rabie, 2007; Maes and Carmeliet, 2008). The crucial importance for both angiogenesis and osteogenesis in for instance fracture repair has consequently evoked a large interest in VEGF therapeutic applications. Our present data support the anabolic role of VEGF in bone by stimulating in a coupled way both expansion of the vascular bed and bone formation. However, they also warn for caution, as the ability of VEGF to impinge on the skeleton proved to be unexpectedly strong; developmentally increased VEGF caused severe bone malformations, and even brief induction of VEGF over-expression in normally developed, adult bones caused dramatic pathological changes that resemble the secondary manifestations seen in bones of patients suffering from myelofibrotic disorders (Tefferi, 2005). Interestingly, these VEGF-induced effects were found to be mediated by enhanced stabilization and transcriptional activity of β-catenin in endothelial and stromal/osteoblastic cells. The wide spectrum of upregulated β-catenin target genes may, on their turn, have influenced the development and/or activity of osteoblasts, osteoclasts and endothelial cells as well as haematopoietic stem/progenitor cells (HSC/HPC) in the bone environment.
Increased VEGF modulates β-catenin activity in osteoblast lineage cells in vivo
VEGF164 over-expression in bone enhanced the proliferation of stromal/osteoblast lineage cells and altered their differentiation, characterized by abundant matrix secretion and unbalanced mineralization. A direct effect of VEGF on osteogenic cells was suggested earlier by the increased proliferation, migration, differentiation and survival observed in human mesenchymal stem cells in vitro (Fiedler et al, 2005; Street and Lenehan, 2009) and is confirmed in this study. The importance of VEGF signalling through VEGFR-2 in osteogenic cells in our model is underscored by the increased staining for phosphorylated VEGFR-2 in vivo and by the upregulated expression of both VEGF and VEGFR-2 in cultured primary osteoblasts derived from adult +VEGF164dox mice, even when dox was supplemented only in vitro (see Supplementary Figure S8). This is in line with reports indicating that VEGF upregulates its own receptors in endothelial and leukaemic cells (Shen et al, 1998; Dias et al, 2000). Genetic ablation of VEGFR-2 in osteo-chondroprogenitors results in a partial rescue of the aberrant bone development in +VEGF164 mice characterized by a partial reversal of the high bone mass ossification phenotype, whereas the vascular phenotype remains abnormal (see below). To our knowledge, this is the first evidence for direct effects of VEGF on osteogenic cells in vivo.
Stabilization of β-catenin in osteo-chondroprogenitors favours osteoblast differentiation (Day et al, 2005; Hill et al, 2005; Hu et al, 2005) but continued expression of a stabilized form of β-catenin leads to a phenotype of aberrant mineralization strikingly similar to our developmental VEGF over-expression model (Glass et al, 2005; Rodda and McMahon, 2006). Accordingly, we documented enhanced nuclear localization and stabilization of β-catenin in osteoblast lineage cells from VEGF over-expressing mice in vivo and in vitro, with consequently upregulated expression of several β-catenin target genes. A genetic interaction was shown by the modulation of the VEGF-induced osteosclerotic phenotype through heterozygous deletion of β-catenin in osteo-chondroprogenitors. Although VEGF over-expression creates a coupled stimulatory response in the vascular and osteogenic cell pools, this balance became shifted dramatically in favour of the vasculature when the osteogenic cells were partially deprived of β-catenin. A similar uncoupling was observed in the embryonic model on conditional deletion of VEGFR-2, indicating that the osteogenic response is both VEGFR-2 and β-catenin dependent and suggesting a mutual pathway of action. Pharmacological intervention furthermore implicated a PI3-kinase-dependent pathway in the GSK3-β inhibition and β-catenin stabilization. Intriguingly, inactivation of the negative PI3-kinase regulator PTEN in osteo-chondroprogenitors and osteoblasts also led to increased bone mass, but the potential involvement of β-catenin was not addressed (Ford-Hutchinson et al, 2007; Liu et al, 2007). As well, the ability of LiCl to activate β-catenin through its negative effects on GSK3-β has been shown to enhance bone formation and repair (Clement-Lacroix et al, 2005; Chen et al, 2007) as well as reduce tauopathy and neurodegeneration in mouse models (Noble et al, 2005) thereby further underscoring the importance of this clinically relevant pathway.
VEGF induces β-catenin transcriptional activity in endothelial cells
When endothelial cells are quiescent, β-catenin functions as a component of transmembrane junctional complexes between endothelial cells by binding to the intracellular domain of VE-cadherin. In these complexes, VE-cadherin associates with VEGFR-2 and partially inhibits VEGFR-2 phosphorylation and its proliferative signal (Carmeliet et al, 1999; Dejana et al, 2008). Angiogenic activation by VEGF signalling induces VEGFR-2 phosphorylation at several key sites including tyrosine residue 1175 (Tyr1173 in mice), which activates the PLC-γ and PI3-kinase pathways (Olsson et al, 2006). In our model increased VEGF levels in the bone microenvironment led to hypervascularization, associated with endothelial activation of VEGFR-2 at Tyr1173. As mentioned above, the PI3-kinase pathway was essential for the establishment of this phenotype. Although this pharmacological experiment ruled out cell specificity, the central role of the PI3-kinase pathway in VEGF/VEGFR-2 signal transduction in endothelial cells—mediating primarily cell survival and migration—is very well established (Olsson et al, 2006). One of the prime mechanisms downstream of PI3-kinase is phosphorylation and inactivation of GSK3-β, a pathway that has been linked to endothelial cell migration towards VEGF and angiogenesis (Kim et al, 2002). In vitro, we found that VEGF treatment of haemangioma-like endothelial cells induced GSK3-β phosphorylation and β-catenin stabilization, as well as target gene activation, consistent with the results of an earlier study (Ilan et al, 2003). It is possible that this pathway contributed to the vascular phenotype in vivo. Interestingly, altered VEGF signalling has been shown to be causal in haemangioma formation in humans (Jinnin et al, 2008). As well, β-catenin has recently been implicated in physiological angiogenesis in the regulation of vascular patterning and development of the blood–brain barrier (Cattelino et al, 2003; Liebner et al, 2008; Stenman et al, 2008), which also requires VEGF (Zhang et al, 2000). It will be interesting to see how much of VEGF's physiological and pathological effects on the endothelium may be mediated through modulation of β-catenin.
Deregulated osteoclast differentiation and activity in bones with increased VEGF
VEGF directly enhances osteoclast recruitment, differentiation, activity and survival (Aldridge et al, 2005; Niida et al, 2005; Yang et al, 2008). In agreement, VEGF164 over-expression was associated with increased osteoclasts as an early manifestation in both our embryonic and adult models. However, at later time points, the osteoclast phenotype became more complex, likely reflecting secondary mechanisms. Sustained VEGF upregulation was associated with a dramatic drop in the number of osteoclasts in both models and led to incomplete tissue resorption. We suggest that this effect is consequential to the excessive vasculature and stromal/osteoblastic cell pool as it correlated with increased OPG expression by these cells, most likely in response to enhanced β-catenin activity. Stabilization of β-catenin in mature osteoblasts impairs osteoclast formation indirectly by increasing OPG expression (Glass et al, 2005). Of all the β-catenin target genes analysed in our study, the largest and most consistent upregulation was documented for OPG. Moreover, decreasing stabilized β-catenin by genetic intervention prevented the VEGF-induced expansion of the osteoblastic cell pool, and concomitantly OPG upregulation was reduced and osteoclast activity remained high (not shown). Increased OPG has also been shown to contribute to the bone pathology observed in mouse models and human patients with myelofibrosis (Chagraoui et al, 2003; Bock et al, 2005), much resembling the +VEGF164dox-induced phenotype. Added to this is the fact that the metaphysis of +VEGF164dox bones became devoid of haematopoietic progenitors as fibrosis developed; this would be expected to preclude the local differentiation of osteoclasts from their monocyte precursors. These data thus indicate that the late and local drop in osteoclasts in the mutant mice in vivo is due to secondary effects of the altered bone environment and β-catenin signalling. Of note, the osteoclast alterations in +VEGF164dox mice were region specific, as cortical remodelling was associated with increased osteoclasts and excessively high expression levels of RANKL (eight-fold increase over normal levels) that were not compensated by increased OPG as in the spongiosa regions.
Increased VEGF in the bone environment leads to haematological alterations
Interestingly, our adult VEGF164 over-expression mice also displayed haematopoietic alterations including increased megakaryocytes in the bone marrow and spleen and enhanced mobilization of HPCs. These effects may have contributed to the increased spleen size observed in 25% of the mutant mice, suggesting potential induction of extramedullary haematopoiesis by only a short exposure to increased VEGF in the bone environment. Of note, Hattori et al (2001) showed that increased serum VEGF164 can cause mobilization of HSCs and endothelial precursors and result in extramedullary haematopoiesis and splenomegaly (Hattori et al, 2001). The normal serum VEGF levels in our model argue against systemic VEGF eliciting the observed haematological alterations and support the notion that local increased VEGF production by chondro-osteoblast lineage cells can also induce these effects. Although beyond the scope of this study, an in-depth characterization of the haematological responses to increased VEGF secreted in bone is warranted because the current observations suggest novel ways by which VEGF may control HSC behaviour and haematopoiesis. First, VEGF profoundly affected the stromal/osteoblastic cell populations and the vasculature, constituting the two key bone marrow niches. Second, increased VEGF potently induced remodelling at the cortex and endosteum, a process that promotes mobilization of HPCs (Kollet et al, 2006). Finally, VEGF may directly or indirectly modulate signals mediating HSC/HPC maintenance and mobilization, as the bones of our mice had increased osteopontin mRNA and protein levels (see Figure 4D; Supplementary Figure S6) (Stier et al, 2005), increased expression of several proteases including MMP-9, MMP-13, MT1-MMP, Cathepsin K (Supplementary Table S2; data not shown) and alterations in HIF-1α and SDF-1 (but not its receptor CXCR4; see Supplementary Figure S8) (Hattori et al, 2003; Ceradini et al, 2004), all events that have been shown to lead to haematological alterations in the bone marrow environment.
In conclusion, our data underscore the powerful effects of VEGF in stimulating angiogenesis and osteogenesis and shed light on the downstream mediators by evidencing that VEGF influences the activity of β-catenin in both osteoblast lineage cells and endothelial cells (Figure 10). Our observations also touch on an important field of interest related to bone pathology and haematopoiesis, by showing the potential causal role of VEGF-induced activation of the PI3-kinase/β-catenin pathway in bone marrow fibrosis, osteosclerosis/petrosis, vascular anomalies and haematological disturbances in the bone and marrow environment. These features are typical secondary manifestations seen in human myelofibrosis-related diseases, in which VEGF upregulation has been documented earlier (Tefferi, 2005; Giles et al, 2007; Panteli et al, 2007; Steurer et al, 2007). Even more generally, our findings raise the possibility that increased levels of VEGF, as common to a wide range of patho-physiological and neoplastic conditions, may mediate some of the documented deregulated activity of β-catenin (Clevers, 2006). Thus, the interaction between VEGF signalling and β-catenin activity documented here in the bone environment may represent an important accomplice in the fields of osteoimmunology, haematology as well stem cell and cancer biology. As well, our data underscore the need for caution in the use of VEGF as a pro-angiogenic therapy, calling for particular attention to possible side effects within the bone and marrow niche.
Figure 10.
Model summarizing the effects of increased VEGF in bone. VEGF164, over-expressed by osteo-chondroprogenitor/-derived cells (Col2-Cre+ cells and chondrocyte (CH) and osteoblast (OB) lineage descendants) and secreted in excess in the endochondral skeleton, activates VEGFR-2 signalling in osteoblast lineage cells and endothelial cells, inducing proliferation and increased microvascular density (MVD) as well as abnormal blood vessels. VEGFR-2 mediated activation of PI3-kinase (PI3K/AKT), resulting in GSK3-β inactivation, leads to enhanced stabilization and transcriptional activity of β-catenin in both cell types. β-catenin-induced target genes can cause or contribute to the phenotype of VEGF-induced expansion of the stromal cell population, abundant matrix production (fibrosis), and osteosclerosis/osteopetrosis with increased osteoblastogenesis and reduced osteoclastogenesis. Additionally, increased VEGF in bone alters the bone marrow environment by causing extensive fibrosis and increased MVD, and is associated with excessive megakaryocytes (MK) and mobilization of haematopoietic stem and progenitor cells (HSC/HSPCs).
Materials and methods
Generation of transgenic mice and experimental set-up
Mice carrying the conditional ROSA26-VEGF164 allele were generated as described in detail in Supplementary data. The Col2-Cre, tet(o)-VEGF164 and ROSA26-rtTA mouse lines, combined here to generate triple transgenic +VEGF164dox mice, and the BAT-Gal, VEGFR-2(null), VEGFR-2(flox) and Catnb(flox) mice have all been described (Shalaby et al, 1995; Ovchinnikov et al, 2000; Brault et al, 2001; Akeson et al, 2003; Haigh et al, 2003; Maretto et al, 2003; Belteki et al, 2005). All genotyping primers are listed in Supplementary Table S3. Doxycyclin (Sigma) was supplemented at 1 mg/ml drinking water (changed daily) for up to 2 weeks. Controls for +VEGF164dox mice were sex-/age-matched double transgenic littermates (Col2-Cre+;ROSA26-Flox/Stop-rtTA-EGFP or ROSA26-Flox/Stop-rtTA-EGFP;tet(o)-VEGF164) receiving the same dox-regime. Wortmannin (Sigma) was administered daily at 5 μg i.p./mouse. BrdU was administered at 100 μg/g body weight 2–4 h before killing, depending on the age. Experiments were approved by the ethical committees of Ghent University and KU Leuven.
Bone analysis by skeletal preparation, histology and micro-CT
Skeletal preparation, X-Gal staining, histology and histomorphometry were performed as before (Haigh et al, 2000; Maes et al, 2002, 2004). Brief descriptions are in Supplementary data and details are available on request. IHC was performed according to the Cell Signaling Technology protocol or by the Renaissance® TSA™ Biotin System (Perkin Elmer) using the antibodies and dilutions listed in Supplementary data. We used staining kits for accustain-reticulin (Sigma) and ALP activity (TaKaRa). ISH used digoxigenin- (VEGF) or radioactive-labelled (osteoblast and chondrocyte markers) probes. BrdU quantification involved counting the stained cells in the perichondrium adjacent to the columnar chondrocyte zone (expressed as number of cells per area) or in a defined zone (200 × 300 μm) of the columnar chondrocytes (BrdU labelling index: BrdU+ cells as % of total cell number). Micro-CT analysis was performed on the high resolution Skyscan 1172 system at 50 kV/200 μA, 3 μm (E17.5) or 5 μm pixel size and 0.5 mm Al filters. Reconstructions of serial tomographs used a cone beam filtered back projection algorithm; 3D analysis included a standardized (600 μm) region comprising most of the diaphyseal bone shaft in the embryo, and a 500 μm metaphyseal bone segment in adult bones.
Molecular analysis
For RNA or protein extraction, bones were carefully dissected and snap-frozen in liquid nitrogen, and processed according to standard protocols. For details on qRT–PCR and list of primers and probes, see Supplementary Table S4. Primary antibodies used for western blotting were directed against HIF-1α (R&D Systems; 1:1000), β-catenin: non-phosphorylated active β-catenin (Millipore; 1:1000) (Sigma; 1:4000; or BD Transduction Lab.; 1:1000), P-GSK3-β(Ser9) (Cell Signaling; 1:1000), total GSK3-β (Cell Signaling; 1:1000), osteopontin (R&D Systems; 1:500) or β-actin (MP Biomedicals; 1:10 000). Western blot signals were quantified using ImageJ (National Institute of Health) and normalized to β-actin signals or to total GSK3-β (for P-GSK3-β). VEGF levels were determined by the Quantikine ELISA kit (R&D) and corrected for total protein levels in bone samples (Bio-Rad DC kit).
Cell culture, colony formation assays and FACS analsysis
Adult primary osteoblastic progenitors were isolated by digesting dissected femoral bone fragments in 0.1% collagenase for 2 h. At confluency, osteogenic differentiation medium was applied (αMEM with 10% FCS, 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate and 10−8 M dexamethasone) with dox added when indicated. RNA was extracted using Qiagen RNA isolation kit. Primary calvarial osteoblasts were isolated from E17.5 +VEGF164ΔRunx2-Cre and Z/EGΔRunx2-Cre mice as control (Novak et al, 2000), by sequential 10-min digests in 0.1% collagenase, 0.2% dispase. Digests 2–5 were filtered, FACS sorted for GFP (Epics Altra Beckman Coulter), and cultured in αMEM with 15% FCS. Immunofluorescence with anti-β-catenin (1:2000; Sigma) and goat anti-rabbit Alexa Fluor 568 antibodies (1:1000; Sigma) and DAPI nuclear staining was analysed using a Zeiss 710 multi-photon confocal microscope. Z sections were collected through the cell monolayers and the nuclear localization of β-catenin was quantified in three random fields per sample using Volocity 5 software. Briefly, staining intensity in the β-catenin and DAPI signal channels was determined for each voxel of the image and the Pearson's correlation between the signals and the Ky value (an index of how much of the β-catenin signal overlaps with DAPI) was calculated. CFU-C assays of peripheral blood or spleen and bone marrow mononuclear cells were performed in MethoCult (Stem Cell Technologies) or Megacult-C medium (Stem Cell Technologies) supplemented with 50 ng/ml Thrombopoietin, 10 ng/ml IL-3, 20 ng/ml IL-6 and 50 ng/ml IL-11. The haemangio-endothelial cell lines were treated with 50 ng/ml recombinant canine rcVEGF164 or 40 mM LiCl and infected with DN-TCF4 virus, as described in Supplementary data. To quantify the megakaryocyte cells, FACS analysis (Calibur Flow Cytometer, Becton Dickinson) was performed on isolated spleen cells using a PE-conjugated anti-mouse CD41 antibody (eBioscience).
Statistical analysis
Comparison between two data groups was done by two-sided Student's t-test and expressed as mean±s.e.m. Multiple group experiments were analysed by ANOVA followed by Fisher's LSD and Tukey–Kramer multiple-comparison tests for all pair-wise differences between the means, indicated±s.e. Marks * (versus control) or § (as specified) indicate P<0.05, **P<0.01 or ***P<0.001.
Supplementary Material
Supplementary Figures S1–S13
Supplementary Information
Review Process File
Acknowledgments
We are grateful to Dr Kurt Ballmer-Hofer for supplying rcVEGF, to Dr Bram De Craene, Dr Geert Berx and Dr Garry Nolan for supplying materials for DN-TCF4 retroviral experiments, Dr Chris Guerin, Dr Hamida Hammad and Dr Bart Lambrechts for assistance on confocal microscopy, to Daisy Ginneberge for sorting osteoblasts, to Nico Smets, Sophie Torrekens and Riet Van Looveren for skillful technical assistance, and to Leander Huyghe and Dr Peter Brouckaert for constructive advice. We thank Dr Henry Kronenberg for critically reading the paper. This work was partially funded by FWO grants G.0229.04 and G.0569.07 to GC and JH, KU Leuven Excellentie Financiering EF/05/08, and by a Gideon and Sevgi Rodan Fellowship of the International Bone and Mineral Society to CM. CM and MT are postdoctoral fellows of the Fund for Scientific Research Flanders (FWO-Vlaanderen).
Footnotes
The authors declare that they have no conflict of interest.
References
- Akeson AL, Greenberg JM, Cameron JE, Thompson FY, Brooks SK, Wiginton D, Whitsett JA (2003) Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev Biol 264: 443–455 [DOI] [PubMed] [Google Scholar]
- Aldridge SE, Lennard TW, Williams JR, Birch MA (2005) Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem Biophys Res Commun 335: 793–798 [DOI] [PubMed] [Google Scholar]
- Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A (2005) Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res 33: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Loch G, Schade U, Busche G, Wasielewski R, Wiese B, Kreipe H (2005) Osteosclerosis in advanced chronic idiopathic myelofibrosis is associated with endothelial overexpression of osteoprotegerin. Br J Haematol 130: 76–82 [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R (2001) Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128: 1253–1264 [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846 [DOI] [PubMed] [Google Scholar]
- Carano RA, Filvaroff EH (2003) Angiogenesis and bone repair. Drug Discov Today 8: 980–989 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F et al. (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147–157 [DOI] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E (2003) The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol 162: 1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864 [DOI] [PubMed] [Google Scholar]
- Chagraoui H, Tulliez M, Smayra T, Komura E, Giraudier S, Yun T, Lassau N, Vainchenker W, Wendling F (2003) Stimulation of osteoprotegerin production is responsible for osteosclerosis in mice overexpressing TPO. Blood 101: 2983–2989 [DOI] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA (2007) Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med 4: e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G (2005) Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA 102: 17406–17411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC (1996) Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J 15: 4526–4536 [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789 [DOI] [PubMed] [Google Scholar]
- Dai J, Rabie AB (2007) VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res 86: 937–950 [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y (2005) Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8: 739–750 [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121: 2115–2122 [DOI] [PubMed] [Google Scholar]
- Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest 106: 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler J, Leucht F, Waltenberger J, Dehio C, Brenner RE (2005) VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun 334: 561–568 [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson AF, Ali Z, Lines SE, Hallgrimsson B, Boyd SK, Jirik FR (2007) Inactivation of Pten in osteo-chondroprogenitor cells leads to epiphyseal growth plate abnormalities and skeletal overgrowth. J Bone Miner Res 22: 1245–1259 [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N (1999) VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5: 623–628 [DOI] [PubMed] [Google Scholar]
- Giles FJ, List AF, Carroll M, Cortes JE, Valickas J, Chen BL, Masson E, Jacques C, Laurent D, Albitar M, Feldman EJ, Roboz GJ (2007) PTK787/ZK 222584, a small molecule tyrosine kinase receptor inhibitor of vascular endothelial growth factor (VEGF), has modest activity in myelofibrosis with myeloid metaplasia. Leuk Res 31: 891–897 [DOI] [PubMed] [Google Scholar]
- Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764 [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22: 2308–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh JJ, Gerber HP, Ferrara N, Wagner EF (2000) Conditional inactivation of VEGF-A in areas of collagen2a1 expression results in embryonic lethality in the heterozygous state. Development 127: 1445–1453 [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A (2003) Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol 262: 225–241 [DOI] [PubMed] [Google Scholar]
- Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S (2001) Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med 193: 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Rafii S (2003) The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma 44: 575–582 [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C (2005) Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8: 727–738 [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Tu X, Cook J, Hu H, Long F (2005) Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development 132: 4339–4351 [DOI] [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F (2005) Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 132: 49–60 [DOI] [PubMed] [Google Scholar]
- Ilan N, Tucker A, Madri JA (2003) Vascular endothelial growth factor expression, beta-catenin tyrosine phosphorylation, and endothelial proliferative behavior: a pathway for transformation? Lab Invest 83: 1105–1115 [DOI] [PubMed] [Google Scholar]
- Jacobsen KA, Al Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC (2008) Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res 23: 596–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, George FI, Sun J (2008) Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal 20: 1697–1704 [DOI] [PubMed] [Google Scholar]
- Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR (2008) Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med 14: 1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Skurk C, Thomas SR, Bialik A, Suhara T, Kureishi Y, Birnbaum M, Keaney JF Jr, Walsh K (2002) Regulation of angiogenesis by glycogen synthase kinase-3beta. J Biol Chem 277: 41888–41896 [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T (2006) Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 12: 657–664 [DOI] [PubMed] [Google Scholar]
- Larsson J, Ohishi M, Garrison B, Aspling M, Janzen V, Adams GB, Curto M, McClatchey AI, Schipani E, Scadden DT (2008) Nf2/merlin regulates hematopoietic stem cell behavior by altering microenvironmental architecture. Cell Stem Cell 3: 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E (2008) Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183: 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Liu X, Bruxvoort KJ, Zylstra CR, Liu J, Cichowski R, Faugere MC, Bouxsein ML, Wan C, Williams BO, Clemens TL (2007) Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc Natl Acad Sci USA 104: 2259–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Carmeliet G (2008) Vascular and nonvascular roles of VEGF in bone development. In VEGF in Development, Ruhrberg C (ed), pp 79–90. Austin: Springer [Google Scholar]
- Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G (2002) Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev 111: 61–73 [DOI] [PubMed] [Google Scholar]
- Maes C, Stockmans I, Moermans K, Van Looveren R, Smets N, Carmeliet P, Bouillon R, Carmeliet G (2004) Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest 113: 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S (2003) Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA 100: 3299–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niida S, Kondo T, Hiratsuka S, Hayashi S, Amizuka N, Noda T, Ikeda K, Shibuya M (2005) VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proc Natl Acad Sci USA 102: 14016–14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA 102: 6990–6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG (2000) Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28: 147–155 [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371 [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR (2000) Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis 26: 145–146 [PubMed] [Google Scholar]
- Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM (2004) Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest 113: 516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteli K, Bai M, Hatzimichael E, Zagorianakou N, Agnantis NJ, Bourantas K (2007) Serum levels, and bone marrow immunohistochemical expression of, vascular endothelial growth factor in patients with chronic myeloproliferative diseases. Hematology 12: 481–486 [DOI] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133: 3231–3244 [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66 [DOI] [PubMed] [Google Scholar]
- Shen BQ, Lee DY, Gerber HP, Keyt BA, Ferrara N, Zioncheck TF (1998) Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J Biol Chem 273: 29979–29985 [DOI] [PubMed] [Google Scholar]
- Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71 [DOI] [PubMed] [Google Scholar]
- Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K (2005) Glycogen-synthase kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res 96: 308–318 [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP (2008) Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Steurer M, Zoller H, Augustin F, Fong D, Heiss S, Strasser-Weippl K, Gastl G, Tzankov A (2007) Increased angiogenesis in chronic idiopathic myelofibrosis: vascular endothelial growth factor as a prominent angiogenic factor. Hum Pathol 38: 1057–1064 [DOI] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, Scadden DT (2005) Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 201: 1781–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street J, Lenehan B (2009) Vascular endothelial growth factor regulates osteoblast survival—evidence for an autocrine feedback mechanism. J Orthop Surg Res 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A (2005) Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol 23: 8520–8530 [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H (2002) Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem 277: 17901–17905 [DOI] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL (2004) Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103: 3258–3264 [DOI] [PubMed] [Google Scholar]
- Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE (2007) A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 129: 1097–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, McHugh KP, Patntirapong S, Gu X, Wunderlich L, Hauschka PV (2008) VEGF enhancement of osteoclast survival and bone resorption involves VEGF receptor-2 signaling and beta(3)-integrin. Matrix Biol 27: 589–599 [DOI] [PubMed] [Google Scholar]
- Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, D'Amore PA, Olsen BR (2002) Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development 129: 1893–1904 [DOI] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS (2003) Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem 278: 33067–33077 [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S13
Supplementary Information
Review Process File