Abstract
The mobilization of metabolic energy from adipocytes depends on a tightly regulated balance between hydrolysis and resynthesis of triacylglycerides (TAGs). Hydrolysis is stimulated by β-adrenergic signalling to PKA that mediates phosphorylation of lipolytic enzymes, including hormone-sensitive lipase (HSL). TAG resynthesis is associated with high-energy consumption, which when inordinate, leads to increased AMPK activity that acts to restrain hydrolysis of TAGs by inhibiting PKA-mediated activation of HSL. Here, we report that in primary mouse adipocytes, PKA associates with and phosphorylates AMPKα1 at Ser-173 to impede threonine (Thr-172) phosphorylation and thus activation of AMPKα1 by LKB1 in response to lipolytic signals. Activation of AMPKα1 by LKB1 is also blocked by PKA-mediated phosphorylation of AMPKα1 in vitro. Functional analysis of an AMPKα1 species carrying a non-phosphorylatable mutation at Ser-173 revealed a critical function of this phosphorylation for efficient release of free fatty acids and glycerol in response to PKA-activating signals. These results suggest a new mechanism of negative regulation of AMPK activity by PKA that is important for converting a lipolytic signal into an effective lipolytic response.
Keywords: adipocytes, AMPK, β-adrenergic, lipolysis, PKA
Introduction
White adipocytes have a central function in the control of whole-body energy homeostasis (Rosen and Spiegelman, 2006). These cells are able to accumulate and store dietary energy in the form of triacylglycerides (TAGs) through lipid synthesis and to mobilize the stored energy in times of caloric need by hydrolysing TAGs to generate non-esterified free fatty acids (NEFAs) and glycerol that are released into the circulation for use by other organs as energy substrates (Duncan et al, 2007). A breakdown in the regulation of adipocyte lipid storage and mobilization pathways can contribute to increased levels of NEFA in the circulation, which is an established risk factor for the development of insulin resistance in type II diabetes and related disorders (Gesta et al, 2006; Guilherme et al, 2008).
In the basal state, the TAG pool in adipocytes is in a constant state of flux in that NEFAs are continuously released from TAG stores and reesterified again to produce TAG (Duncan et al, 2007). The simultaneously ongoing breakdown or lipolysis and resynthesis of TAG creates a ‘substrate cycle' referred to as the TAG-NEFA cycle, which is characterized by energy expenditure in the absence of net conversion of substrate into product and allows adipocytes to respond rapidly to changes in peripheral requirements of NEFA (Kalderon et al, 2000; Large et al, 2004). Indeed, during periods of increased energy demands, the rate of lipolysis is enhanced through the action of lipolytic hormones that stimulate β-adrenergic signalling to activate the cAMP-PKA pathway (McKnight et al, 1998), which mediates phosphorylation and activation of lipolytic enzymes, including perilipin, adipose triglyceride lipase and hormone-sensitive lipase (HSL) (Khoo et al, 1972; Holm, 2003; Schweiger et al, 2006; Ducharme and Bickel, 2008; Granneman and Moore, 2008; Zimmermann et al, 2009).
Phosphorylation of HSL occurs on multiple sites, including Ser-660, which stimulates catalytic activity and Ser-563, which is believed to be mutually exclusive with phosphorylation of HSL at the non-PKA site Ser-565 (Anthonsen et al, 1998; Watt et al, 2006). Thus, hormonal cues that signal systemic energy induce HSL phosphorylation at Ser-563 by PKA, which contributes to adipocyte lipolysis to maintain whole-body energy homeostasis.
Existing evidence suggests that even during fasting as much as 30–40% of NEFAs released from TAG stores are reesterified (Reshef et al, 2003), providing a mechanism to limit NEFA release into the circulation. The reesterification step of the TAG-NEFA cycle is a highly energy-demanding process that consumes significant amounts of cellular ATP and also generates AMP, and when immoderate, can create a state of relative energy depletion (Gauthier et al, 2008). A key sensor of cellular energy level is AMPK (Hardie et al, 2006; Hue and Rider, 2007; Hardie, 2008), a heterotrimeric kinase complex, composed of the catalytic kinase α subunit and two associated regulatory subunits, β and γ, that maintains the balance between ATP production and consumption. AMPK is activated by phosphorylation of the critical activation loop threonine (Thr-172) in the α-subunit that is mediated by upstream kinases (Carling et al, 2008). Prominent among these is the tumour suppressor kinase LKB1 (Sakamoto et al, 2005; Shaw et al, 2005). Energy stress leads to an increase in the AMP:ATP ratio and AMP binds directly to the AMPKγ subunit, thereby stimulating the kinase activity allosterically and inducing a conformational change that inhibits the deactivation by phosphatases (Suter et al, 2006; Sanders et al, 2007). In the context of adipocytes, evidence suggests that AMPK is activated as a consequence of constitutively ongoing reesterification that consumes energy (Gauthier et al, 2008). An immediate consequence of enhanced AMPK activity is the phosphorylation of HSL at Ser-565, which precludes activation of HSL by PKA (Garton et al, 1989; Daval et al, 2005). Thus, stimulation of adipocyte lipolysis through PKA activation triggers, in turn, a negative feedback mechanism involving AMPK to match the rate of lipolysis to energy supply. The mechanism(s) underlying coordination of these apparently opposing activities under conditions of acute systemic metabolic needs remain elusive. Hence, we explored whether PKA and AMPK kinase pathways crosstalk in adipocytes as part of their lipolysis regulatory functions.
Results
PKA and AMPK associate in vivo
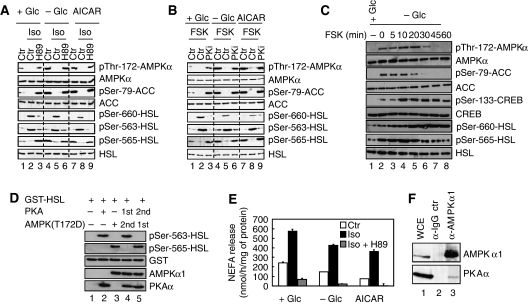
Primary mouse adipocytes were treated with isoproterenol, an epinephrine analogue and β-adrenergic agonist that simulates the physiological stimulus for lipolysis during fasting, to activate the cAMP-PKA pathway. The activation state of AMPK was monitored using an antibody specific for AMPKα that is phosphorylated on Thr-172, the critical residue in the activation loop of AMPKα phosphorylated by LKB1 (Hawley et al, 1996). As shown in Figure 1A, 30 min of 200 nM isoproterenol treatment suppressed basal AMPKα phosphorylation at Thr-172 (compare lanes 2 and 1). Simultaneous addition of 1 μM H89, a pharmacological inhibitor of PKA, prevented this (lane 3). The effect of PKA signalling on Thr-172 phosphorylation of AMPKα under AMPK-activating conditions, such as in response to glucose deprivation or addition of 1 mM of the AMP-mimicking agent aminoimidazole carboxamide ribonucleotide (AICAR), was also evaluated. As expected, these treatments induced AMPKα Thr-172 and Ser-79 AMPK substrate acetyl-CoA-carboxylase (ACC) phosphorylation (compare lanes 4, 7 and 1). Induction of AMPKα phosphorylation was, however, blocked by isoproterenol (lanes 5 and 8) and recovered in the presence of H89 inhibitor (lanes 6 and 9). Thus, signals that activate PKA reduce AMPKα phosphorylation at Thr-172. In line with these data, isoproterenol inhibits AMPK activity as assessed by the ability of AMPKα1 immunoprecipitates to phosphorylate SAMS peptide (Supplementary Figure S1A). Consistent with this finding, the phosphorylation state of an established common effector of PKA and AMPK in the regulation of lipolysis, HSL, changed accordingly. Conditions that activated PKA-induced phosphorylation of HSL at Ser-660 and Ser-563 and suppressed phosphorylation of HSL at the AMPK site Ser-565 (Figure 1A). Conversely, inhibition of PKA signalling reversed these effects, resulting in increased phosphorylation of HSL at the AMPK site Ser-565 (Figure 1A). Similar changes in the phosphorylation states of AMPKα, ACC and HSL were observed when PKA signalling was induced by 20 μM forskolin, a potent inhibitor of phosphodiesterases that leads to cAMP increase, or suppressed by 100 μM of a specific myristoylated protein kinase A inhibitor (PKi) (Figure 1B). Activation of Ca2+ signalling by ionomycin did not interfere with Thr-172 phosphorylation of AMPKα in adipocytes (Supplementary Figure S1B). The negative effect of forskolin on AMPK Thr-172 phosphorylation was first detectable around 20 min after treatment (Figure 1C) and correlated with increased phosphorylation of HSL at Ser-660 and the cAMP response element-binding protein (CREB) at Ser-133 (both PKA mediated) and decreased phosphorylation of ACC at Ser-79 and HSL at Ser-565 (both AMPK mediated) (Figure 1C). In vitro kinase assays using purified PKA and AMPK further support the notion that phosphorylation of HSL at Ser-563 and Ser-565 is mutually exclusive. Specifically, phosphorylation of purified glutathione-S-transferase (GST)-HSL fusion protein at Ser-563 by PKA renders the substrate resistant to subsequent phosphorylation at Ser-565 by AMPK complexes in which the Thr-172 residue of the AMPKα1 subunit has been mutated to an aspartic acid, referred to as AMPK(T172D) to yield constitutive active kinase complexes (Figure 1D). In contrast, when GST-HSL was first phosphorylated by AMPK(T172D), PKA failed to phosphorylate HSL at Ser-563 (Figure 1D). In keeping with these observed changes in the phosphorylation state of HSL, isoproterenol-stimulated NEFA release from adipocytes, whereas addition of H89 inhibitor blocked both basal and isoproterenol-induced NEFA release (Figure 1E), whereas glucose deprivation or treatment of cells with AICAR that activates AMPK caused reduced basal and isoproterenol-induced NEFAs release (Figure 1E). As phosphorylation of AMPK at Thr-172 is required for kinase activation, these findings imply that activation of PKA in adipocytes suppresses, directly or indirectly, AMPK activity and thus the extend to which the downstream effector of PKA, HSL, is phosphorylated. Finally, we detected PKAα in endogenous AMPKα1 immunoprecipitates derived from primary adipocytes whole-cell extracts (Figure 1F, lane 3), suggesting that PKAα and AMPKα1 physically interact in vivo. Stable complex formation of these kinases was, however, not affected by treatment of cells with either isoproterenol or ionomycin (Supplementary Figure S1C).
Figure 1.
PKA signalling inhibits Thr-172 phosphorylation of AMPKα. (A) Primary adipocytes were cultivated for 30 min either in the presence of 25 mM glucose (+Glc) (lanes 1–3 and 7–9) or the absence of glucose (−Glc) (lanes 4–6) and treated with 1 mM AICAR (lanes 7–9), 200 nM isoproterenol (Iso) (lanes 2, 5 and 8) or 200 nM isoproterenol in combination with 1 μM H89 (lanes 3, 6 and 9). Protein lysates were prepared and probed with the indicated antibodies. (B) Similar experiment as in (A) except that 20 μM Forskolin (FSK) and 100 μM myristoylated PKi were added instead of isoproterenol and H89, respectively. (C) Primary adipocytes were glucose starved for 60 min (−Glc) and incubated with 20 μM Forskolin (FSK) for indicated times. FSK was either present ab initio (lane 8), added during the last 5, 10, 20, 30 or 45 min of starvation (lanes 3–8) or omitted (lane 2). As control, cells were grown in glucose-rich medium (+Glc) (lane 1). Equal amounts of protein lysates were subjected to immunoblotting with the indicated antibodies. (D) Phosphorylation of HSL by PKA and AMPK at Ser-563 and Ser-565, respectively, is mutually exclusive. In vitro kinase assay of GST-HSL in the presence of PKA (lanes 2, 4 and 5) and constitutively active AMPK(T172D) (lanes 3, 4 and 5). PKA was either added before (lane 4) or after incubation of HSL with AMPK(T172D) (lane 5). Proteins were subjected to immunoblotting with the indicated antibodies. (E) NEFA release in response to PKA signalling. Primary adipocytes were incubated for 30 min with 200 nM isoproterenol alone (Iso) or in combination with 1 μM H89. Furthermore, this treatment was done in the presence (+Glc) or absence (−Glc) of glucose or in the presence of glucose and 1 mM AICAR. NEFA were measured in the incubation medium. Bars represent the mean NEFA release from three independent experiments. (F) Whole-cell extracts of primary mouse adipocytes (WCE, lane 1) were prepared and aliquots were subjected to immunoprecipitation with control IgG (lane 2) or anti-AMPKα1 antibodies (lane 3) and immunoblotted for AMPKα1 and PKAα. Immunoblots are representative of at least three independent experiments.
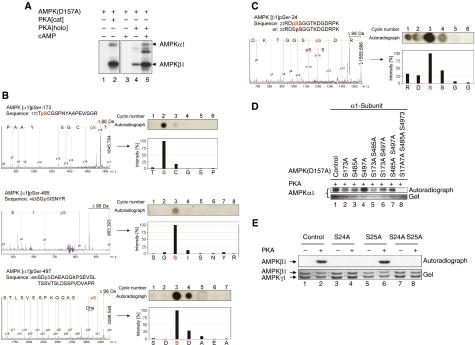
PKA phosphorylates AMPKα1 at Ser-173, Ser-485, Ser-497 and AMPKβ1 at Ser-24
Given the observation that PKA signalling inhibits AMPK activation and that these two kinases associate in vivo, we asked next whether PKA phosphorylates AMPK in vitro. For these assays, we produced heterotrimeric AMPK complexes with a catalytically inactive AMPKα1 subunit in which Asp-157 has been mutated to alanine in bacteria. We refer to this complex as AMPK(D157A). It follows that incorporation of radioactive phosphate into any of the AMPK subunits would then be solely due to the action of PKA and not due to autophosphorylation activity of AMPK. Indeed, the constitutively active catalytic subunit of PKA (PKA[cat]) or a partially purified PKA holoenzyme (PKA[holo]) that is dependent on cAMP for full activity, both phosphorylated the catalytic α1- and regulatory β1-subunits of AMPK(D157A) (Figure 2A). Phosphorylation of the AMPKγ1 subunit by PKA was not observed under these conditions. Conversely, PKAα did not serve as a substrate for active AMPK in vitro (data not shown). Phosphorylation site mapping by mass spectrometry and solid-phase sequencing revealed that PKA phosphorylated the AMPKα1 subunit at Ser-173, Ser-485 and Ser-497 and the β1-subunit at Ser-24 (Figure 2B and C, respectively). All these sites are highly conserved between human, mouse and rat AMPK subunits (Supplementary Figure S2). In addition, the amino-acid sequence encompassing the Ser-173 site in AMPKα1 is also conserved in rat AMPKα2 (Supplementary Figure S2A). It has been reported that α1-containing AMPK is the predominant complex in adipocytes accounting for >90% of total AMPK activity detectable in these cells (Lihn et al, 2004; Daval et al, 2005). Therefore, we focused our further analysis on the regulation of AMPKα1 by PKA. Mutation of each of the above-noted serine residues to alanine as well as combined mutations of these critical serines were introduced as secondary mutations in AMPKα1(D157A)- or β1-subunits and the corresponding AMPK complexes exposed to PKA. Only complexes harbouring the triple mutant of AMPKα1(S173A/S485/S497A) (Figure 2D) or the single mutant AMPKβ1(S24A) (Figure 2E) proved to be resistant to PKA phosphorylation, suggesting that each of these sites can be phosphorylated by PKA in vitro.
Figure 2.
PKA phosphorylates catalytic α- and regulatory β-subunits of AMPK at multiple sites. (A) PKA phosphorylates AMPK in vitro. Autoradiograph of α1β1γ1 catalytically inactive AMPK(D157A) complexes incubated in the presence of [γ-32P]ATP with either the cAMP-dependent PKA holoenzyme, PKA[holo], or the constitutively active, cAMP independent, catalytic domain of PKA, PKA[cat]. Arrows indicate phosphorylated AMPK(D157) at the α1- and β1-subunits. (B) Mass fingerprinting of isolated phosphopeptides derived from HPLC was performed by MALDI-ToF MS. Peptides differing by +80 Da (HPO3) from computed masses were then selected for MS/MS and phosphorylation was confirmed by a neutral loss of −98 Da (H3PO4) during fragmentation. Further verification of the phosphosites was attempted by solid-phase sequencing of the radiolabelled peptides. After each cycle of N-terminal Edman degradation, liberated single amino acids were collected and spotted onto DEAE cellulose. The respective phosphorylated residues were then detected by autoradiography. The annotated mass spectra indicates the phosphorylated residues of AMPKα1 pSer-173, pSer-485 and pSer-497. (C) As in (B) but related to AMPKβ1 pSer-24. Dha, dehydroala. (D) Analysis of AMPKα1 phosphorylation site mutants. Purified AMPK(D157A) (control), or the indicated serine to alanine mutants thereof, were incubated with PKA in the presence of [γ-32P]ATP and processed for SDS–PAGE and autoradiography. (E) Verification of in vitro AMPK β1-phosphorylation sites by mutational analysis. Catalytically inactive AMPK(D157A) complexes containing a regulatory β1-subunit mutated at Ser-24 and/or Ser-25 were incubated with [γ-32P]ATP in the presence or absence of PKA. Phosphorylation was visualized by SDS–PAGE and autoradiography. PKA was unable to phosphorylate AMPKβ1 mutated in Ser-24 (lane 4) but not Ser-25 (lane 6), showing that only Ser-24 is targeted by PKA. Control, AMPK(D157A) containing no additional mutations. Immunoblots are representative of at least three independent experiments.
Phosphorylation of AMPKα1 at Ser-173 by PKA precludes phosphorylation of AMPKα1 at Thr-172 by LKB1
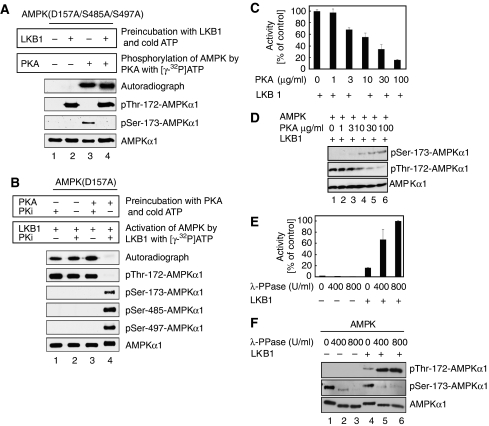
Next, we assessed whether phosphorylation of AMPKα1 by either LKB1 or PKA interferes with subsequent phosphorylation by PKA and LKB1, respectively. As shown above, PKA phosphorylates AMPK(D157A/S485A/S497A) complexes at Ser-173 as AMPK(D157A/S485A/S497A/S173A) complexes that harbour an additional mutation at Ser-173 are resistant to phosphorylation by this kinase (see Figure 2D, compare lanes 7 and 8). Consistent with these data, PKA phosphorylated AMPKα1(D157A/S485A/S497A) at Ser-173 as evidenced by 32P-incorporation and recognition by anti-phospho Ser-173 antibodies (Figure 3A, lane 3). When AMPK(D157A/S485A/S497A) complexes were incubated with LKB1 before the addition of PKA, phosphorylation of AMPKα1(D157A/S485A/S497A) by PKA still occurred to a similar extend as indicated by 32P-incorporation (Figure 3A, lane 4). However, the anti-phospho Ser-173 antibodies failed now to recognize the phospho-Ser173 epitope (Figure 3A, lane 4). These results indicate that Thr-172 phosphorylation of AMPKα1 by LKB1 does not preclude subsequent PKA phosphorylation of AMPKα1 at Ser-173 in vitro. Moreover, they further suggest that the anti-phospho Ser-173 antibodies recognize the Ser-173 epitope only when AMPKα1 is not phosphorylated at Thr-172.
Figure 3.
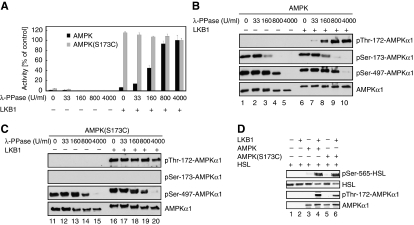
PKA-mediated phosphorylation of AMPKα1 prevents its phosphorylation by LKB1 at Thr-172 in vitro and phosphorylation of AMPK at Thr172 and Ser-173 is not mutually exclusive. (A) LKB1 was allowed to phosphorylate AMPKα1(D157S485A/S497A) in the presence of ATP before addition of PKA and [γ-32P]ATP. Signals obtained by autoradiography indicate phosphorylation at Ser-173 (lanes 3 and 4). Phosphorylation of Thr-172 and Ser-173 is coexistent, if PKA is allowed to phosphorylate AMPK after preincubation with LKB1 (lane 4). In this double-phosphorylated form, phosphorylation of Thr-172 but not Ser-173 was recognized by the corresponding antibodies. PKi, PKA inhibitor. Samples were processed for immunoblotting with the specified antibodies. (B) PKA phosphorylation of AMPKα1 prevents LKB1-mediated Thr-172 phosphorylation in vitro. AMPKα1(D157A) was preincubated with/without PKA and non-radioactive ATP as indicated, followed by LKB1 assays in the presence of [γ-32P]ATP. LKB1 was unable to phosphorylate AMPK after PKA phosphorylation. Samples were processed for immunoblotting with the specified antibodies. (C) PKA-mediated AMPKα1 phosphorylation inhibits AMPK activity. AMPK was preincubated with different amounts of PKA before in vitro kinase reactions with LKB1. AMPK activity assessed as the amount of phosphate incorporated into the synthetic peptide substrate SAMS. (D) Immunoblot corresponding to (B) probed with the indicated antibodies. (E) PKA-phosphorylated AMPK was either left untreated or dephosphorylated with λ-PPase using the indicated concentrations, then processed for in vitro kinase assays with or without LKB1. AMPK activity quantified using the SAMS assay. (F) Immunoblot corresponding to (E) probed with the indicated antibodies. All activity measurements were done in triplicates. Error bars represent±s.e.m. Immunoblots are representative of at least three independent experiments.
In reciprocal experiments, LKB1 phosphorylated AMPK (D157A) complexes on Thr-172 as evidenced by 32P-incorporation and recognition of AMPKα1(D157A) by anti-phospho Thr-172 antibodies (Figure 3B, lane 2). Importantly, when AMPK(D157A) complexes were incubated with PKA before the addition of LKB1, Ser-173 phosphorylation occurred but autoradiography of in vitro kinase assays show that subsequent phosphorylation of AMPKα1(D157A) at Thr-172 by LKB1 was blocked (Figure 3B, lane 4). Phosphorylation of AMPKα1(D157A) by LKB1 at Thr-172 was, however, observed when PKA was inhibited by PKA inhibitory peptide PKi (Figure 3B, lane 3). Addition of PKi alone did not negatively affect LKB1-mediated AMPKα1(D157A) phosphorylation at Thr-172 in the absence of PKA (Figure 3B, lane 1). These results suggest that PKA-mediated phosphorylation of AMPKα1 at Ser-173 interferes with subsequent LKB1 phosphorylation of AMPKα1 at Thr-172.
Accordingly, LKB1-mediated activation of AMPK, as measured by the ability of AMPK to phosphorylate the synthetic peptide substrate SAMS, decreased, as increasing amounts of PKA were added to the reaction mix (Figure 3C). The observed decline in AMPK activity correlated with a decrease in Thr-172 phosphorylation and an increase in Ser-173 phosphorylation of AMPKα1 (Figure 3D). Moreover, when PKA-phosphorylated AMPK was treated with λ phosphatase before the addition of LKB1, activation of AMPK was recovered (Figure 3E). Immunoblotting revealed that λ phosphatase caused dephosphorylation of AMPKα1 at Ser-173 and that LKB1 phosphorylated AMPKα1 at the adjacent Thr-172 residue (Figure 3F). These results suggest that PKA phosphorylation of AMPKα1 prohibits subsequent activation of AMPK by LKB1 at Thr-172.
Ser-173 phosphorylation of AMPKα1 antagonizes phosphorylation of AMPKα1 at Thr-172 by LKB1
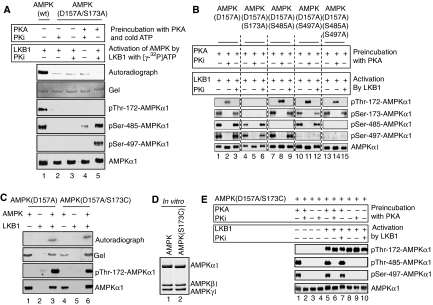
To elucidate the molecular mechanism underlying PKA-mediated inhibition of AMPK activation by LKB1, we generated a non-phosphorylatable mutant of AMPKα1 in which Ser-173 was mutated to an alanine residue. The AMPKα1(S173A) mutant protein proved to be resistant to Thr-172 phosphorylation by LKB1, but was still phosphorylated at Ser-485 and Ser-497 by PKA (Figure 4A). One possible explanation of a failure of LKB1 to phosphorylate Thr-172 in AMPKα1 in the context of a Ser-173 to alanine mutation may relate to the fact that these two sites are located within the recognition sequence of LKB1. Alternatively, the mutation may disrupt the epitope of the phospho-Thr-172 antibody. However, we also failed to observe any 32P-incorporation into the AMPKα1(S173A) mutant protein on exposure to LKB1 (Figure 4A). Therefore, it is likely that the introduction of an alanine residue at Ser-173 affects the ability of LKB1 to phosphorylate Thr-172. We note that equivalent alanine mutations of AMPKα1 at Ser-485 and/or Ser-497 (Figure 4B) or AMPKβ1 at Ser-24 (Supplementary Figure S3) did not negatively affect Thr-172 phosphorylation of AMPKα1 by LKB1. Furthermore, the ability of PKA to inhibit subsequent activation by LKB1 was preserved in AMPKα1(S485A), AMPKα1(S497A) and AMPKβ1(S24A) mutant complexes, thus suggesting that these sites are not responsible for PKA-mediated inhibition.
Figure 4.
Phosphorylation of AMPKα1 at Ser-173 mediates the inhibitory effect by PKA. (A) Mutagenesis of Ser-173 to alanine blocks phosphorylation of AMPK at Thr-172 by LKB1. AMPK(D157A/S173A) was phosphorylated by PKA with non-radioactive ATP and subsequently by LKB1 in the presence of [γ-32P]ATP in the absence or presence of PKi as indicated. Note that LKB1 is unable to phosphorylate this mutant AMPK protein, suggesting that Ser-173 is a key residue in the recognition sequence for LKB1. As control, AMPK(wt) was used in the assay (lane 1). (B) Mutation of Ser-485 and/or Ser-497 on AMPKα1 does not abolish the inhibitory effect of PKA. AMPK mutants were incubated with PKA and LKB1. Reactions were performed in the absence or presence of PKi as indicated. In vitro kinase assays were processed for immunoblotting with the indicated antibodies. Neither mutation of Ser-485 nor Ser-497 was able to nullify the inhibitory action of PKA on LKB1-mediated Thr-172 phosphorylation (lanes 7–15). Note that no phosphorylation of Thr-172 by LKB1 was observed when Ser-173 was exchanged to alanine (lanes 4–6). (C) AMPK(D157A) (lanes 1–3) and AMPK(D157A/S173C) (lanes 4–6) complexes were either incubated with LKB1 or not, in the presence of [γ-32P]ATP. AMPKα1(D157A) and AMPKα1(D157A/S173C) protein phosphorylation assessed by autoradiography (upper panel) and immunoblotting (lower panel). (D) AMPK(S173C) forms a 1:1:1 complex with the regulatory β- and γ-subunits in vitro. Histidine-tagged wild-type AMPKα1 or the (S173C) mutant thereof were coexpressed with the AMPK β- and γ-subunits, followed by affinity chromatography with Ni2+-NTA, SDS–PAGE and Coomassie blue staining. (E) AMPK(D157A/S173C) complexes were incubated with or without PKA and then treated with LKB1. Reactions were performed in the absence or presence of PKi as indicated. In vitro kinase assays were processed for immunoblotting with the indicated antibodies. Immunoblots are representative of at least three independent experiments.
Given these findings, we hypothesized that phosphorylation of Ser-173 in AMPKα1 may be critical to PKA-dependent inhibition of AMPK activation. Thus, we endeavored to construct a phosphorylation site mutant capable of phosphorylation by LKB1. We replaced the hydroxyl group of Ser-173 in AMPKα1 more conservatively with a thiol group by mutating the serine to a cysteine residue, thereby maintaining the nucleophilic nature and approximative size of the side chain. As shown in Figure 4C, AMPKα1(D157A) and AMPKα1(D157A/S173C) mutant proteins were readily phosphorylated at Thr-172 by LKB1 as evidenced by autoradiography of in vitro kinase assays (upper panel) and immunoblotting with a phospho-specific antibody (lower panel). When coexpressed with β- and γ-subunits in bacteria, the AMPKα1(S173C) single mutant also assembled as efficient as AMPKα1 wild type into trimeric AMPK complexes (Figure 4D). Importantly, however, LKB1 phosphorylated the AMPKα1(D157A/S173C) mutant at Thr-172 despite preincubation of the AMPK(D157A/S173C) complex with PKA (Figure 4E, lane 5). Notably, in this setting, PKA was active and phosphorylated Ser-485 and Ser-497 in AMPKα1(D157A/S173C) (Figure 4E). These results strongly suggest that phosphorylation of AMPKα1 at Ser-173 by PKA renders AMPKα1 refractory to the subsequent activating phosphorylation at Thr-172 by LKB1.
PKA-mediated phosphorylation of AMPKα1 at Ser-173 efficiently interferes with LKB1-dependent AMPK activation
To further assess the functional significance of Ser-173 phosphorylation, we next measured the inhibitory effect of PKA on activation of AMPK or AMPK(S173C) by LKB1. To this end, highly phosphorylated AMPK or AMPK(S173C) complexes were produced by coexpression with PKA in bacteria. After purification, the corresponding AMPK complexes were dephosphorylated with varying amounts of λ phosphatase and subsequently incubated with LKB1. AMPK activity was determined using the synthetic peptide substrate SAMS. As shown in Figure 5A, although activation of AMPK by LKB1 was dependent on prior dephosphorylation with λ phosphatase, activation of AMPK(S173C) was not. Immunoblotting with phospho-specific antibodies confirmed that the AMPKα1 wild type had been dephosphorylated on Ser-173 and that both AMPKα1 wild type and the Ser-173A mutant protein were subsequently phosphorylated by LKB1 at Thr-172 (Figure 5B and C). The AMPK(S173C) complexes phosphorylated HSL at the established Ser-565 site only on activation by LKB1 (Figure 5D), thus confirming the functional integrity of the mutant enzyme. In further support of this notion, both proteins, when individually expressed as myc-tagged species in primary adipocytes using recombinant adenoviruses, formed complexes with endogenous AMPKβ1 and γ1 subunits as well as with PKA as shown by coimmunoprecipitation assays (Supplementary Figure S4). Taken together, these results suggest that phosphorylation of AMPKα1 at Ser-173 by PKA efficiently interferes with LKB1-dependent Thr-172 phosphorylation and activation of AMPK, thus providing a mechanistic explanation for the observed suppression of AMPK activation in response to cAMP-elevating agents.
Figure 5.
PKA-mediated phosphorylation of AMPKα1 at Ser-173 efficiently interferes with LKB1-dependent activation of AMPK. (A) AMPK(S173C) complex is refractory to inhibition by PKA. PKA-phosphorylated AMPK and AMPK(S173C) complexes were dephosphorylated with λ-PPase using the indicated concentrations, then incubated with or without LKB1. Subsequently, AMPK activity was quantified using the SAMS assay. Assays were done in triplicate and errors bars represent±s.e.m. (B, C) Immunoblots corresponding to (A) probed with indicated antibodies. (D) LKB1-activated AMPK or AMPK(S173C) complexes were incubated with GST-HSL and processed for immunoblotting with the indicated antibodies. Immunoblots represent one experiment of three independent experiments.
PKA-mediated phosphorylation of AMPKα at Ser-173 is critical for efficient lipolysis
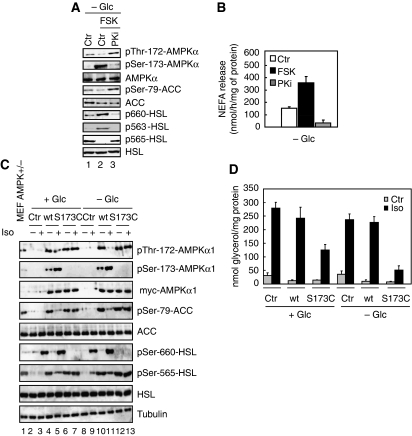
Next, we asked whether forskolin stimulation of glucose-deprived adipocytes induces phosphorylation of AMPKα at Ser-173 in vivo and if so, whether this correlates with a lack of HSL phosphorylation at the AMPK site Ser-565. Stimulation of PKA by forskolin induced downregulation of Thr-172 phosphorylation, upregulation of Ser-173 phosphorylation and a concomitant decrease in HSL phosphorylation on the AMPK site Ser-565 (Figure 6A). As expected, the phosphorylation of ACC at residue Ser-79 correlated with the level of AMPKα Thr-172 and HSL Ser-565 phosphorylation (Figure 6A). A myristoylated PKi peptide, when added to adipocytes, reversed the negative effect of forskolin on Thr-172 phosphorylation of AMPKα, indicating that this event is PKA dependent (Figure 6A). Consistent with the observed changes in kinase and substrate phosphorylation states, 20 μM forskolin-stimulated NEFA release in glucose-deprived adipocytes (Figure 6B). Addition of 100 μM of PKi inhibitor blocked both basal and forskolin-induced NEFA release from adipocytes (Figure 6B). These data suggest that Ser-173 is a bona fide phosphorylation site of AMPKα in vivo and that activation of PKA by forskolin increases phosphorylation of AMPKα at Ser-173. Importantly, this regulatory mechanism is not restricted to adipocytes alone but extends also to other cell types. First, 20 μM forskolin treatment inhibits glucose starvation-induced Thr-172 phosphorylation of AMPKα in U2-OS osteosarcoma and HepG2 hepatoma cells, while promoting AMPKα Ser-173 phosphorylation in a time-dependent manner (Supplementary Figure S5A and B, respectively). Moreover, in U2-OS cells, glucose starvation-induced Thr-172 phosphorylation of AMPKα is significantly enhanced in U2-OS cells depleted for both PKAα and β subunits (Supplementary Figure S5C). Finally, endogenous AMPKα1 immunoprecipitates derived from U2-OS cells also contain PKAα (Supplementary Figure S5D).
Figure 6.
PKA-mediated phosphorylation of AMPKα at Ser-173 is critical for efficient lipolysis. (A) Ser-173 of AMPKα is phosphorylated in vivo by PKA. Primary adipocytes were glucose starved (−Glc) for 30 min (lanes 1–3) and then treated with 20 μM forskolin (FSK) alone (lane 2) or in combination with 100 μM PKi (lane 3). Protein lysates were processed for immunoblotting with the indicated antibodies. (B) NEFA release in response to PKA signalling. Primary adipocytes were glucose starved (−Glc) for 30 min (lanes 1–3) and then treated with 20 μM forskolin (FSK) alone (lane 2) or in combination with 100 μM PKi (lane 3). NEFA were measured in the incubation medium. Bars represent the mean NEFA release from three independent experiments. (C) AMPKα1(S173C) is refractory to inhibition by PKA. Immortalized control AMPKα+/− and AMPKα−/− MEFs were differentiated into adipocytes as described in the Materials and methods section. AMPKα−/− adipocytes were infected with an adenovirus containing either an empty plasmid (lanes 2, 3, 8, 9), a plasmid encoding for myc-AMPKα1(wt) (lanes 4, 5, 10, 11) or myc-AMPKα1(S173C) (lanes 6, 7, 12, 13). Before immunoblotting experiments and lipolysis assays, adipocytes were grown for 30 min in the presence (+Glc) (lanes 2–7) or absence (−Glc) (lanes 8–13) of glucose and simultaneously treated with or without 200 nM isoproterenol (Iso). Aliquots of protein lysates were immunoblotted with the indicated antibodies. (D) Incubation medium of adipocytes described in (C) were processed for glycerol measurements. Bars represent the mean of glycerol release from three independent experiments and immunoblots are representative of at least three independent experiments.
We next sought to determine the functional consequences of PKA-mediated inhibition of AMPK activation by LKB1 on lipolysis. Differentiation of mouse embryo fibroblasts (MEFs) into adipocytes by hormonal induction is a well-established model system for the study of adipogenesis and the regulation of lipolysis (Tanaka et al, 1997). Therefore, we isolated MEFs from AMPKα1−/− and AMPKα2lox/lox mice (Vaahtomeri et al, 2008), immortalized these cells by stably expressing a mutant p53 allele and infected with adenovirus encoding Cre recombinase to obtain AMPKα null cells (referred thereafter as AMPKα−/− cells). MEFs harbouring still one allele of AMPKα1 and AMPKα2 served as control (referred thereafter to as AMPKα+/− cells). When AMPKα+/− MEFs infected with control adenoviruses expressing green fluorescence protein only were exposed to differentiation medium to induce adipogenesis, we observed efficient lipid accumulation in differentiated cells as indicated by Oil Red-O staining (Supplementary Figure S6A, E and I). AMPKα−/− MEFs infected either with control adenovirus (Supplementary Figure S6B, F and J) or recombinant adenoviruses producing myc-tagged species of AMPKα1(wt) or a (S173C) mutant derivative differentiated with similar high efficiency (Supplementary Figure S6C, G, K and D, H, L, respectively).
Treatment of the above-noted infected cells with isoproterenol in the presence or absence of glucose diminished phosphorylation of myc-AMPKα1(wt) at Thr-172 and induced Ser-173 phosphorylation (Figure 6C). The observed changes in the phosphorylation state of myc-AMPKα1(wt) were particularly pronounced on glucose starvation. Importantly, isoproterenol failed to suppress Thr-172 phosphorylation in cells expressing the myc-AMPKα1(S173C) mutant, suggesting that phosphorylation of AMPKα1 at Ser-173 is inhibitory to Thr-172 phosphorylation. Immunoblotting also revealed that both myc-AMPKα1(wt) and myc-AMPKα1(S173C) mutant are produced at similar amounts (Figure 6C). Furthermore, the failure of isoproterenol to suppress phosphorylation of myc-AMPKα1(S173C) at Thr-172 translated into a failure to induce phosphorylation of HSL at Ser-660 (a PKA site) and to suppress phosphorylation of HSL at Ser-565 (an AMPK site) (Figure 6C). As a consequence, we observed significant less release of glycerol from myc-AMPKα1(S173C) expressing adipocytes as compared with adipocytes expressing myc-AMPKα1(wt) (Figure 6D). These results suggest that PKA-mediated phosphorylation of AMPKα1 at Ser-173 is critical for efficient lipolysis in vivo.
Long-term isoproterenol treatment induces phosphorylation of AMPKα1 at Thr-172 and activates AMPKα1
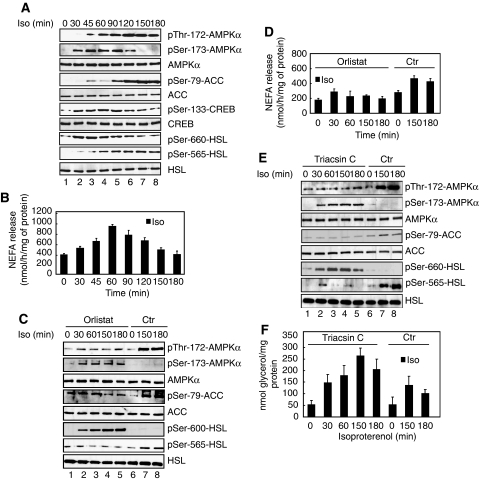
Recently, it has been proposed that AMPK is activated during lipolysis because of the high energy demand of resynthesis of TAG (Gauthier et al, 2008; Omar et al, 2009), a pathway which becomes activated as a consequence of PKA-mediated stimulation of NEFA release. Thus, we asked whether extended treatment of primary adipocytes with isoproterenol would engage such a negative feedback mechanism resulting in a decrease in Ser-173 and increase in Thr-172 phosphorylation of AMPKα at later time points. Indeed, measurements of ATP levels confirmed that long-term treatment of primary adipocytes with the lipolytic agent isoproterenol caused a significant decline in ATP levels at later time points (Supplementary Figure S7A). This was paralleled by an increase in AMPKα Thr-172 phosphorylation and of its activity as assessed by the ability of AMPKα1 immunoprecipitates to phosphorylate SAMS peptide (Supplementary Figure S7B). Consistent with these findings, there was a concomitant increase in the phosphorylation levels of the AMPK substrates ACC and HSL at Ser-79 and Ser-565, respectively (Figure 7A, lanes 5–8). At earlier times after 200 nM isoproterenol treatment, phosphorylation of PKA substrates prevailed as evidenced by high levels of HSL and CREB phosphorylation at Ser-660 and Ser-133, respectively (Figure 7A, lanes 2–4). This was also apparent for AMPKα, which became rapidly phosphorylated at Ser-173 during the early time points (Figure 7A, lanes 2–4). As the anti-phospho Ser-173 antibodies used here cannot recognize phosphorylated Ser-173 when AMPKα is phosphorylated at Thr-172 (see Figure 3A and B), we cannot formally exclude the possibility that Ser-173 remains phosphorylated despite increased phosphorylation of AMPKα at Thr-172 at later time points. Finally, NEFA release from isoproterenol-stimulated adipocytes increased in parallel to phosphorylation of the PKA substrates investigated and declined when AMPKα became increasingly phosphorylated at Thr-172 (Figure 7B). Together, these results suggest that in response to lipolytic signals such as isoproterenol, adipocytes induce, in a first phase, activation of PKA and phosphorylation of HSL and, in a second phase, activation of AMPK, which is accompanied by a decline in NEFA release.
Figure 7.
Sustained PKA signalling activates and phosphorylates AMPKα at Thr-172. (A) Long-term isoproterenol treatment activates and phosphorylates AMPKα. Primary adipocytes were cultivated in the presence of 200 nM of isoproterenol (Iso) in a time-dependent manner. Protein lysates were prepared and probed with the indicated antibodies. (B) Incubation medium of primary adipocytes described in (A) were processed for NEFA measurements. Bars represent the mean NEFA release from three independent experiments. (C) Primary adipocytes were treated with 50 μM orlistat for 180 min and incubated with 200 nM isoproterenol for indicated times, in a serum-free DMEM and presence of glucose. Isoproterenol was either present ab initio (lane 5), added during the last 30, 60 or 150 min of treatment (lanes 1–5). As control, cells were cultivated without orlistat but in the presence of 200 nM isoproterenol for 150 and 180 min in a serum-free DMEM and presence of glucose (lanes 6–8). Equal amounts of protein lysates were subjected to immunoblotting with the indicated antibodies. (D) Incubation medium of primary adipocytes described in (C) were processed for NEFA measurements. Bars represent the mean NEFA release from three independent experiments. (E) Primary adipocytes were treated with 10 μM triacsin C for 180 min and incubated with 200 nM isoproterenol for indicated times, in a serum-free DMEM and in the presence of glucose. Like in (C), isoproterenol was either present ab initio (lane 5), added during the last 30, 60 or 150 min of treatment (lanes 1–5). As control, cells were cultivated in the presence of 200 nM isoproterenol for 150 and 180 min in a serum-free DMEM and presence of glucose (lanes 6–8). Equal amounts of protein lysates were subjected to immunoblotting with the indicated antibodies. (F) Incubation medium of primary adipocytes described in (E) were processed for glycerol measurements as described in the Materials and methods section. Bars represent the mean NEFA or glycerol release from three independent experiments. Immunoblots are representative of at least three independent experiments.
In an effort to further investigate whether phosphorylation of AMPKα at Ser-173 and Thr-172 changes in a manner dependent on stimulated lipolysis, we treated adipocytes with 50 μM orlistat, a pharmacological inhibitor of lipolysis. Importantly, orlistat treatment blocked AMPK activation as evidenced by reduced Thr-172 phosphorylation of AMPKα. Phosphorylation of AMPKα at Ser-173 remained at elevated levels (Figure 7C), consistent with reduced Thr-172 phosphorylation of AMPKα. As expected, orlistat inhibited isoproterenol-induced NEFA release (Figure 7D). These findings support the view that ongoing lipolysis is important for changes in the antagonistic phosphorylation of AMPKα at Ser-173 and Thr-172 after long-term stimulation of β-adrenergic signalling. Importantly, when adipocytes were stimulated with isoproterenol in the presence of 10 μM of triacsin C, a potent inhibitor of long chain fatty acyl CoA synthetase that blocks the reesterification of NEFA, AMPKα phosphorylation at Thr-172 was also blunted, whereas phosphorylation of Ser-173 persisted as with orlistat (Figure 7E). However, release of NEFA (data not shown) or glycerol (Figure 7F) remained elevated even after long-term treatment.
Discussion
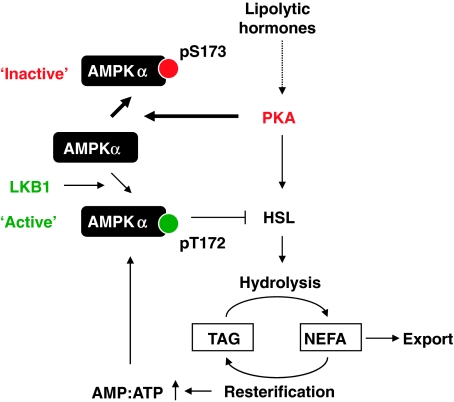
Here, we provide direct evidence that PKA phosphorylates AMPKα to antagonize activation of AMPKα by LKB1 and show that this mechanism is critically important for implementing an effective lipolytic response to lipolytic signals conveyed through the cAMP-PKA pathway. Central to this mechanism of crosstalk between a hormone stimulated and an energy-sensing pathway is the phosphorylation of AMPKα by PKA at Ser-173. This site is highly conserved and located directly adjacent to the critical activation loop Thr-172, whose phosphorylation is absolutely required for AMPK activation (Carling et al, 2008). Therefore, phosphorylation at Ser-173 may create constraints by steric hindrance or charge that are incompatible with subsequent phosphorylation at the adjacent Thr-172 residue. As such, this mechanism provides a novel means of negative regulation of AMPK activity. The fact that we were able to detect PKA/AMPKα1 complexes as well as Ser-173-phosphorylated AMPKα species also in different human cancer cell lines implies a function of this regulatory mechanism beyond regulation in the context of adipocyte lipolysis.
The physiologic relevance of PKA-mediated phosphorylation of AMPKα at Ser-173 in response to lipolytic hormones derives from the observation that AMPKα−/− MEFs differentiated into adipocytes and engineered to re-express an AMPKα1 mutant species resistant to PKA phosphorylation (AMPKα1(S173C)) released significantly less glycerol than cells expressing wild-type AMPKα1. These results suggest that PKA-mediated phosphorylation of AMPKα1 at Ser-173 represents a critical and relevant mechanism contributing to the regulation of lipolysis in vivo and that previously identified mechanisms such as the direct phosphorylation of the AMPK upstream kinases LKB1 or Ca2+/Calmodulin-dependent kinase kinase 2 by PKA (Collins et al, 2000; Sapkota et al, 2001) or the direct phosphorylation of AMPKα1 at Ser-485/497 (Hurley et al, 2006) cannot fully account for the observed effects reported here. In addition, the results also support the view that regulation of lipolysis necessitates a balancing of the activities of PKA and AMPK in a coordinate manner such that PKA-stimulated lipolysis is followed by the activation of AMPK, which acts as part of a negative feedback that dampens again the rate of lipolysis. According to our model (Figure 8), PKA activation promotes efficient mobilization of TAG, at least in part, by opposing the activity of the AMPK-mediated negative feedback loop through phosphorylation of AMPKα at Ser-173. This leads to an increased output of the PKA-stimulated lipolytic pathway and fine-tuning of the lipolytic response, at least in the short term. However, in the longer term, AMPK activity ultimately rises to establish negative feedback inhibition of lipolysis to maintain adipocyte function. Therefore, it is conceivable that in this setting, AMPK safeguards against further depletion of ATP as a result of increased rates of NEFA reesterification. Notably, there is evidence to suggest that the rate of NEFA reesterification increases proportionally with the rate of lipolysis (Reshef et al, 1970; Brooks et al, 1982).
Figure 8.
Model proposing a function of PKA in phosphorylating and inactivating AMPKα and its potential effects on lipolysis (for details see discussion).
Existing evidence suggests that in adipocytes, cAMP-PKA pathway activating signals such as isoproterenol, adrenaline or forskolin (Moule and Denton, 1998; Yin et al, 2003; Daval et al, 2005; Koh et al, 2007; Lobo et al, 2009; Omar et al, 2009) or exercise (Koh et al, 2007) induce AMPK activation within 30 min after application of the stimulus. On the basis of the response time and overexpression studies using a dominant-inhibitory mutant of AMPKα2 (Yin et al, 2003) or pharmacological inhibition of AMPK by Compound C (Koh et al, 2007), it has been proposed that AMPK stimulates lipolysis. However, a series of papers using a variety of cell systems (e.g. adipocytes derived from AMPKα1 knock-out mice) and approaches (e.g. shRNA-mediated knockdown of endogenous proteins) suggest that AMPK has anti-lipolytic activity (Daval et al, 2005; Gauthier et al, 2008; Lobo et al, 2009; Omar et al, 2009), consistent with earlier in vivo studies showing an anti-lipolytic effect of the AMPK activator AICAR (Bergeron et al, 2001). In addition, these studies provide evidence that ongoing lipolysis is critical for the activation of AMPK in response to PKA-activating signals and that activation of AMPK occurs within 15–30 min after treatment of cells with agents that activate PKA (Moule and Denton, 1998; Yin et al, 2003; Daval et al, 2005; Koh et al, 2007; Lobo et al, 2009; Omar et al, 2009). The observed activation profile of AMPK in response to PKA stimulation is, however, not mutually exclusive with a model in which PKA inhibits AMPK in the short term, since PKA activation, respectively, PKA-mediated HSL phosphorylation, has been shown to occur within seconds after treatment of adipocytes with activators of PKA signalling (Martin et al, 2009). This rapid activation of PKA after β-adrenergic stimulation is in agreement with the model proposed here that suggests an inhibitory action of PKA on AMPK to promote efficient lipolysis.
A mechanism that remains to be investigated relates to the observed downregulation of PKA activity to allow the rise of AMPK activity. One could envision the action of a specific phosphatase that removes the Ser-173 inhibitory phosphorylation on AMPKα1, which in turn allows the phosphorylation and activation of AMPK by LKB1. Indeed, the phospho-Ser173 signal of AMPKα1 diminishes at later time points after isoproterenol treatment. As our anti-phospho Ser-173 antibody does not recognize phosphorylated Ser-173 when AMPKα1 is also phosphorylated at Thr-172 (see Figure 3A and B), we cannot formally exclude the possibility that Ser-173 remains phosphorylated despite increased phosphorylation of AMPKα1 at Thr-172. However, we also observed diminished phosphorylation of the PKA substrate CREB at the PKA-site Ser-133, suggesting that PKA activity has diminished at that time. Therefore, it is conceivable to hypothesize that also Ser-173 phosphorylation of AMPKα1 diminishes during this period of time after the addition of a lipolytic signal, pointing to the operation of a negative feedback mechanism that dampens PKA activity and thus contributes to the precise regulation of the lipolytic response.
In conclusion, our data provide insight into the mechanism of how information about systemic energy requirements is fed into decisions on the metabolic fate of lipids at the cellular level. Moreover, they suggest that this mechanism may have evolved to serve, in the first place, the energy requirements of the organism and not of the individual cell. As breakdown in the regulation of adipocyte lipid storage and mobilization pathways can contribute to increased levels of NEFAs in the circulation, which is an established risk factor for the development of insulin resistance in type II diabetes (Gesta et al, 2006; Guilherme et al, 2008), it will be important to elucidate the regulation of the PKA-AMPK circuitry identified here in such disease settings.
Materials and methods
Materials
[γ-32P]ATP was purchased from Hartmann Analytic (Braunschweig, Germany). λ-PPase was obtained from New England Biolabs (Frankfurt, Germany). Tetrameric, partially purified cAMP-dependent protein kinase A holoenzyme (PKA[holo]) from bovine heart, myristoylated and non-myristoylated protein kinase A inhibitor fragment 5–24 (PKi), isoproterenol, H89 and forskolin, ionomycin, orlistat and free glycerol reagent were obtained from Sigma (St Louis, MO). Triacsin C was obtained from Biomol and rosiglitazone was obtained from Alexis Biochemicals. The SAMS peptide (HMRSAMSGLHLVKRR) was synthesized by EZBiolab (Westfield, IN). All other chemicals and reagents were from standard suppliers. U2-OS and HepG2 cell lines were purchased from the American Type Culture Collection (ATCC, Rockville, MA). Predesigned and validated small interfering RNA (siRNA) for LKB1 gene silencing (#1498210 HP) was from Qiagen (Valencia, CA). Two different sets of siRNA for co-silencing of the α- and β-subunit of PKA were synthesized by Qiagen to target the following sequences: siRNAPKA-set 1 (PKAα: ACAGAAGGTGGTGAAACTGAA, PKAβ: GGCATTAGGAGTGCTAATCTA), siRNAPKA-set 2 (PKAα: CAGTGTGCTGTTGTAAACATA, PKAβ: ACCAATGTGTTCTATATGTAA).
Antibodies
Custom-made anti-phospho-Ser-173 and anti-phospho-Ser-497 AMPKα1 antibodies were from Eurogentec (Seraing, Belgium). Antibodies were raised in rabbits against keyhole limpet hemocyanin-coupled peptides H2N-GEFLRTS(PO3H2)CGSPNY-CONH2 and H2N-CQRSDS(PO3H2)DAEAQGK-CONH2, followed by double-affinity purification on appropriate non-phosphopeptide- and phosphopeptide-coupled columns. Sheep anti-AMPKα1 and sheep anti-AMPKγ1 antibodies were a kind gift from DG Hardie (Dundee University, UK). Anti-ACC, anti-phospho-Ser-79 ACC, anti-AMPKα1, anti-phospho-Thr-172 AMPK, anti-phospho-Ser-485 AMPK, anti-phospho-Ser-563 HSL, anti-phospho-Ser-565 and anti-phospho-Ser-660 HSL antibodies were obtained from Cell Signaling Technology (Beverly, CA). Anti-HSL was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CREB and anti-phospho-Ser-133-CREB were obtained from Abcam.
Plasmids
The plasmid for expression of the hexahistidine-tagged catalytic domain of PKA (PKA[cat]) in bacteria was a kind gift of RKY Cheng and LN Johnson (Oxford, UK). GST-HSL plasmid was a gift from R Ricci (ETH Zurich, Switzerland). Tricistronic plasmids encoding all three subunits of AMPK were constructed as described elsewhere (Neumann et al, 2003; Woods et al, 2003). Mutations encoding for single amino acid changes were introduced into the α1- and β1-subunits of AMPKα1 using the QuikChange mutagenesis procedure (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Primer sequences and cloning details are available from authors on request.
Recombinant protein expression
The hexahistidine-tagged catalytic domain of PKA was purified by immobilized-metal affinity chromatography using 1 ml His-Trap FF crude columns (GE Healthcare, Otelfingen, Switzerland) according to the manufacturer's instructions. Recombinant LKB1 was expressed and purified as described earlier (Neumann et al, 2007). Expression of recombinant AMPK in Escherichia coli was performed overnight at room temperature in 1 l of auto-inducing medium as described (Studier, 2005). Proteins were purified by Ni-NTA chromatography and gel filtration as published earlier (Neumann et al, 2003).
Mass spectrometry and solid-phase sequencing
See Supplementary data.
In vitro kinase and phosphatase assays
See Supplementary data.
Cell culture and siRNA transfection
See Supplementary data.
MEF differentiation, primary adipocyte isolation and free fatty acid and glycerol release measurements
See Supplementary data.
Infection of adipocytes with adenoviruses
Adenoviruses were propagated in human embryonic kidney 293 cells and stored at −80°C. Adipocytes were infected directly in their culture DMEM medium containing, 5% fetal calf serum, 2% BSA and antibiotics. The efficiency of infection was estimated by the presence of green fluorescent protein in adipocytes, produced from an independent promoter. Adipocytes were infected for a maximum of 48 h before immunoblotting experiments and lipolysis assays.
Immunoblotting and immunoprecipitation
Primary adipocytes from a 6 cm-culture dish were washed once in ice-cold phosphate-buffered saline and lysed in 300 μl of RIPA buffer (10 mM Tris pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 0.1% (w/v) SDS, 0.5% (w/v) sodium deoxycholate, 1% (v/v) Triton-X 100, 10% (v/v) glycerol), supplemented with protease inhibitors and processed for immunoblotting and immunoprecipitation as described (Djouder et al, 2007).
ATP measurement
ATP was measured using an ATP bioluminescence assay kit ApoSENSOR™ (MBL) and a TD20/20 luminometer (Turner Designs).
Supplementary Material
Supplementary data
Review Process File
Acknowledgments
We thank all members of our laboratories for discussions. We thank DG Hardie (Dundee University, UK), RKY Cheng and LN Johnson (Oxford UK) and R Ricci (ETH, Zurich, Switzerland) for providing material. This work was supported in part by the Swiss National Science Foundation Grant to TW and DN, the EU FP6 contract LSHM-CT-2004-005272 (EXGENESIS) and graduate training fellowships from ETH Zurich (for RT, R Th, R Sch, given to TW and DN and Uwe Schlattner, Grenoble).
Footnotes
The authors declare that they have no conflict of interest.
References
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C (1998) Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273: 215–221 [DOI] [PubMed] [Google Scholar]
- Bergeron R, Previs SF, Cline GW, Perret P, Russell RR III, Young LH, Shulman GI (2001) Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes 50: 1076–1082 [DOI] [PubMed] [Google Scholar]
- Brooks B, Arch JR, Newsholme EA (1982) Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett 146: 327–330 [DOI] [PubMed] [Google Scholar]
- Carling D, Sanders MJ, Woods A (2008) The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 32(Suppl 4): S55–S59 [DOI] [PubMed] [Google Scholar]
- Collins SP, Reoma JL, Gamm DM, Uhler MD (2000) LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J 345(Pt 3): 673–680 [PMC free article] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F (2005) Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 280: 25250–25257 [DOI] [PubMed] [Google Scholar]
- Djouder N, Metzler SC, Schmidt A, Wirbelauer C, Gstaiger M, Aebersold R, Hess D, Krek W (2007) S6K1-mediated disassembly of mitochondrial URI/PP1gamma complexes activates a negative feedback program that counters S6K1 survival signaling. Mol Cell 28: 28–40 [DOI] [PubMed] [Google Scholar]
- Ducharme NA, Bickel PE (2008) Lipid droplets in lipogenesis and lipolysis. Endocrinology 149: 942–949 [DOI] [PubMed] [Google Scholar]
- Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS (2007) Regulation of lipolysis in adipocytes. Annl Rev Nutr 27: 79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ (1989) Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem 179: 249–254 [DOI] [PubMed] [Google Scholar]
- Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB (2008) AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283: 16514–16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR (2006) Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA 103: 6676–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG, Moore HP (2008) Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab 19: 3–9 [DOI] [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG (2008) AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 32(Suppl 4): S7–12 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW (2006) AMP-activated protein kinase--development of the energy sensor concept. J Physiol 574(Pt 1): 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879–27887 [DOI] [PubMed] [Google Scholar]
- Holm C (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31(Pt 6): 1120–1124 [DOI] [PubMed] [Google Scholar]
- Hue L, Rider MH (2007) The AMP-activated protein kinase: more than an energy sensor. Essays Biochem 43: 121–137 [DOI] [PubMed] [Google Scholar]
- Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281: 36662–36672 [DOI] [PubMed] [Google Scholar]
- Kalderon B, Mayorek N, Berry E, Zevit N, Bar-Tana J (2000) Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab 279: E221–E227 [DOI] [PubMed] [Google Scholar]
- Khoo JC, Fong WW, Steinberg D (1972) Activation of hormone-sensitive lipase from adipose tissue by cyclic AMP-dependent protein kinase. Biochem Biophys Res Commun 49: 407–413 [DOI] [PubMed] [Google Scholar]
- Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, Goodyear LJ (2007) Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem J 403: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large V, Peroni O, Letexier D, Ray H, Beylot M (2004) Metabolism of lipids in human white adipocyte. Diabetes Metab 30: 294–309 [DOI] [PubMed] [Google Scholar]
- Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B (2004) AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun 316: 853–858 [DOI] [PubMed] [Google Scholar]
- Lobo S, Wiczer BM, Bernlohr DA (2009) Functional analysis of long-chain Acyl-CoA synthetase 1 in 3T3-L1 adipocytes. J Biol Chem 284: 18347–18356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Okano S, Kistler C, Fernandez-Rojo MA, Hill MM, Parton RG (2009) Spatiotemporal regulation of early lipolytic signalling in adipocytes. J Biol Chem 284: 32097–32107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight GS, Cummings DE, Amieux PS, Sikorski MA, Brandon EP, Planas JV, Motamed K, Idzerda RL (1998) Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog Horm Res 53: 139–159; discussion 160–131 [PubMed] [Google Scholar]
- Moule SK, Denton RM (1998) The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett 439: 287–290 [DOI] [PubMed] [Google Scholar]
- Neumann D, Suter M, Tuerk R, Riek U, Wallimann T (2007) Co-expression of LKB1, MO25alpha and STRADalpha in bacteria yield the functional and active heterotrimeric complex. Mol Biotechnol 36: 220–231 [DOI] [PubMed] [Google Scholar]
- Neumann D, Woods A, Carling D, Wallimann T, Schlattner U (2003) Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr Purif 30: 230–237 [DOI] [PubMed] [Google Scholar]
- Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E (2009) Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal 21: 760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef L, Hanson RW, Ballard FJ (1970) A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem 245: 5979–5984 [PubMed] [Google Scholar]
- Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW (2003) Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem 278: 30413–30416 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR (2005) Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24: 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D (2007) Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR (2001) Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem 276: 19469–19482 [DOI] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R (2006) Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281: 40236–40241 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41: 207–234 [DOI] [PubMed] [Google Scholar]
- Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D (2006) Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281: 32207–32216 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S (1997) Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J 16: 7432–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaahtomeri K, Ventela E, Laajanen K, Katajisto P, Wipff PJ, Hinz B, Vallenius T, Tiainen M, Makela TP (2008) Lkb1 is required for TGFbeta-mediated myofibroblast differentiation. J Cell Sci 121(Pt 21): 3531–3540 [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA (2006) Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 290: E500–E508 [DOI] [PubMed] [Google Scholar]
- Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH (2003) Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem 278: 28434–28442 [DOI] [PubMed] [Google Scholar]
- Yin W, Mu J, Birnbaum MJ (2003) Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J Biol Chem 278: 43074–43080 [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Lass A, Haemmerle G, Zechner R (2009) Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta 1791: 494–500 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Review Process File