Abstract
The cytoskeleton has a key function in the temporal and spatial organization of both prokaryotic and eukaryotic cells. Here, we report the identification of a new class of polymer-forming proteins, termed bactofilins, that are widely conserved among bacteria. In Caulobacter crescentus, two bactofilin paralogues cooperate to form a sheet-like structure lining the cytoplasmic membrane in proximity of the stalked cell pole. These assemblies mediate polar localization of a peptidoglycan synthase involved in stalk morphogenesis, thus complementing the function of the actin-like cytoskeleton and the cell division machinery in the regulation of cell wall biogenesis. In other bacteria, bactofilins can establish rod-shaped filaments or associate with the cell division apparatus, indicating considerable structural and functional flexibility. Bactofilins polymerize spontaneously in the absence of additional cofactors in vitro, forming stable ribbon- or rod-like filament bundles. Our results suggest that these structures have evolved as an alternative to intermediate filaments, serving as versatile molecular scaffolds in a variety of cellular pathways.
Keywords: cytoskeleton, Myxococcus xanthus, penicillin-binding protein, peptidoglycan, stalk
Introduction
The cytoskeleton is an integral part of the machinery that controls the temporal and spatial organization of a cell. It is composed of a dynamic network of protein filaments, forming a scaffold that provides structural support to the cell and recruits macromolecular complexes to defined subcellular locations. In recent years, a variety of polymer-forming proteins have been identified in bacteria, acting in cell polarity, morphogenesis, DNA segregation, and cell division. Although many of them are only found in a limited group of organisms, the tubulin homologue FtsZ, the actin homologue MreB, and, to a lesser extent, intermediate filament proteins have emerged to be conserved across a variety of evolutionary lineages (Graumann, 2007; Pogliano, 2008; Thanbichler and Shapiro, 2008).
FtsZ polymerizes into a ring-shaped complex at the future division site, acting as a platform for the assembly of the cell division apparatus (Bi and Lutkenhaus, 1991; Goehring and Beckwith, 2005). Dynamic reorganization of this structure is thought to provide a major driving force for cytokinesis (Li et al, 2007; Osawa et al, 2008). Furthermore, the FtsZ ring contributes to normal cell elongation by recruiting the morphogenetic machinery to midcell and thus stimulating medial cell wall growth before the onset of constriction in the α-proteobacterium Caulobacter crescentus (Aaron et al, 2007). In most rod-shaped bacteria, general cell morphology is regulated by an additional cytoskeletal system, based on MreB and its various paralogues. These proteins polymerize into helical cables that line the cytoplasmic membrane and serve to control the spatial distribution of cell wall biosynthetic enzymes, but they have also been implicated in chromosome segregation and cell polarity (Jones et al, 2001; Carballido-Lopez, 2006). A third class of factors involved in bacterial morphogenesis are intermediate filament-like proteins (Izard et al, 1999; Ausmees et al, 2003; Bagchi et al, 2008). The best-characterized member of this class, crescentin, assembles into a polymeric structure at the inner curvature of C. crescentus, mediating the characteristic crescent shape of this bacterium (Ausmees et al, 2003). Unlike MreB, it appears to act mechanically by constraining longitudinal extension of the cell wall, resulting in uneven cell growth and bending (Cabeen et al, 2009). Whereas polymerization of FtsZ and MreB is a highly dynamic process, dependent on the presence of nucleotide cofactors (Mukherjee and Lutkenhaus, 1998; Carballido-Lopez and Errington, 2003; Anderson et al, 2004; Esue et al, 2005), intermediate filament proteins assemble in a spontaneous manner, forming stable filamentous structures both in vitro and in vivo (Ausmees et al, 2003; Bagchi et al, 2008; Charbon et al, 2009).
In addition to cytoskeletal filaments, many bacteria contain proteins that polymerize into membrane-associated, lattice-like structures (Stahlberg et al, 2004; Bowman et al, 2008), providing scaffolds for the assembly and localization of other proteins. The first representative of these non-canonical cytoskeletal proteins, DivIVA, was identified in Bacillus subtilis, where it assembles at the cell poles and the division septum and acts as a recruitment factor for the cell division regulator MinCD (Edwards and Errington, 1997). Moreover, during sporulation, it associates with the DNA-binding protein RacA, thereby mediating polar attachment of the chromosomal origin region (Ben-Yehuda et al, 2003; Wu and Errington, 2003). In actinomycetes, DivIVA has adopted a different function, serving as a key regulator of polar growth and development (Flärdh, 2003; Letek et al, 2008). Although DivIVA homologues are restricted to Gram-positive bacteria, C. crescentus and its relatives contain a functionally analogous protein, designated PopZ, which is involved in anchoring of the chromosomal origin regions to the cell poles, polar morphogenesis, and cell polarity (Bowman et al, 2008; Ebersbach et al, 2008).
Here, we describe a new class of polymer-forming proteins, designated bactofilins, that are almost universially conserved among bacteria. In C. crescentus, two bactofilins assemble into a membrane-associated laminar structure that shows cell-cycle-dependent polar localization and acts as a platform for the recruitment of a cell wall biosynthetic enzyme involved in polar morphogenesis. Bactofilins display distinct subcellular distributions and dynamics in different bacterial species, suggesting that they are versatile structural elements that have adopted a range of different cellular functions.
Results
Identification and localization of bactofilin homologues in C. crescentus
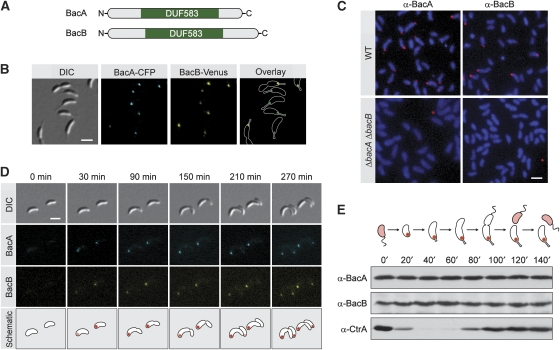
C. crescentus is characterized by its asymmetric cell division, which gives rise to two morphologically and physiologically distinct daughter cells. The swarmer sibling carries a single polar flagellum and is mobile. The stalked sibling, by constrast, is immobile and displays a long polar protrusion, called the ‘stalk', which carries an adhesive organelle at its tip. Whereas the stalked offspring can immediately enter a new round of cell division, swarmer cells first have to differentiate into a stalked cell to continue their cell cycle. To identify factors mediating polar morphogenesis and development in C. crescentus, open reading frames whose transcription is upregulated during the swarmer-to-stalked-cell transition (McGrath et al, 2007) were fused to a gene encoding the yellow fluorescent protein Venus and expressed ectopically under the control of a xylose-inducible promoter (Meisenzahl et al, 1997). Screening the resulting strains for polar fluorescent signals, we identified the thus-far uncharacterized proteins CC1873 and CC3022 (Nierman et al, 2001), now designated bactofilin A (BacA) and bactofilin B (BacB), respectively. BacA has a molecular mass of 16.8 kDa. BacB was originally annotated as a 24.3 kDa protein, but our analyses showed that translation of open reading frame CC3022 initiates at a downstream ATG codon, resulting in a product with a molecular mass of 18.8 kDa (data not shown). BacA and BacB are paralogous proteins (67% sequence similarity) that mainly consist of a conserved domain of unknown function (DUF583), flanked by short, proline-rich terminal regions (Figure 1A; Supplementary Figure S1).
Figure 1.
Cell-cycle-dependent localization and abundance of BacA and BacB. (A) Schematic representation of BacA and BacB. The position of the conserved DUF583 domain is indicated in green. (B) Localization of BacA and BacB in live cells. Cells of strain JK34 (bacA-ecfp bacB-venus) were grown in PYE-rich medium and visualized by DIC and fluorescence microscopy (bar: 2 μm). (C) Localization of BacA and BacB by immunofluorescence microscopy. Cells of strains CB15N (wild type) and JK5 (ΔbacAB) were probed with anti-BacA and anti-BacB antibodies. Immunocomplexes were detected with a Alexa-Fluor 555-conjugated secondary antibody. To visualize the cells, chromosomal DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). The micrographs shown were created by overlaying the Alexa-Fluor 555 and DAPI signals (bar: 2 μm). (D) Cell-cycle-dependent subcellular localization of BacA and BacB. Swarmer cells of strain JK34 (bacA-ecfp bacB-venus) were transferred onto an agarose pad (t=0 min) and observed as they progressed through the cell cycle, using DIC and fluorescence microscopy (bar: 2 μm). (E) Cell-cycle-dependent abundance of BacA and BacB. Swarmer cells of wild-type strain CB15N were transferred into M2G minimal medium and incubated for the duration of one cell cycle. At the indicated timepoints, samples were taken from the culture and analysed by immunoblotting using anti-BacA, anti-BacB, and anti-CtrA antiserum. The schematic illustrates the morphology of C. crescentus and the subcellular distribution of BacA and BacB at the different cell-cycle stages.
To investigate the subcellular localization of BacA and BacB when produced under the control of their native promoters, we constructed a strain (JK34) in which the endogenous bacA and bacB genes were replaced by bacA-ecfp and bacB-venus fusions, respectively. Fluorescent microscopic analysis of JK34 cells showed that the corresponding fusion proteins localized consistently to the stalked pole of the cell (Figure 1B), frequently spreading into the stalk base, whereas no foci were detectable in swarmer cells (data not shown). To verify that the fluorescent tags had no influence on the positioning of BacA and BacB, the localization of the two proteins was further analysed by immunofluorescence microscopy, using affinity purified anti-BacA and anti-BacB antibodies (Figure 1C). In agreement with the in vivo results, both antibodies yielded polar fluorescent signals in wild-type CB15N cells. By contrast, no such signals were detectable in a ΔbacAB double mutant (JK5). As the absence of foci in swarmer cells suggested that BacA and BacB localize dynamically within the cell, time-lapse microscopy was used to follow the subcellular distribution of the two proteins over the course of the cell cycle in strain JK34 (bacA-ecfp bacB-venus). Both bactofilin paralogues were found to accumulate at the stalked pole specifically during the swarmer-to-stalked-cell transition (Figure 1D). The resulting complexes were maintained throughout the subsequent developmental stages and passed on to the stalked progeny on cell division.
Earlier work has shown that bacA and bacB mRNA is detectable throughout the cell cycle, although transcription of the two genes peaks during the swarmer-to-stalked-cell transition. To determine whether the absence of bactofilin complexes in swarmer cells is a result of protein degradation, we monitored the cellular abundance of BacA and BacB in wild-type strain CB15N at different developmental stages (Figure 1E). Both proteins were detectable during the swarmer as well as the stalked phase, with their levels remaining constant throughout. Thus, BacA and BacB are present but delocalized in the swarmer progeny and recruited to the stalked pole on transition to the stalked phase. Using quantitative immunoblot analysis, the copy number of BacA and BacB was estimated to about 200 and 20 molecules per cell, respectively (data not shown).
BacA and BacB assemble into membrane-associated polymeric sheets
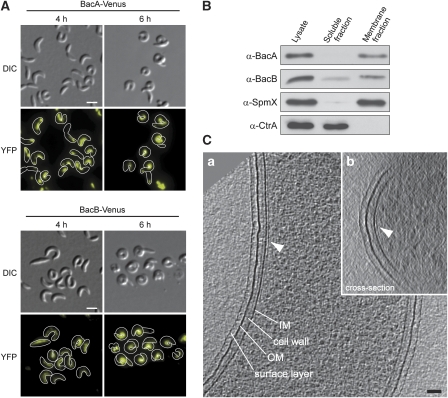
As a first approach to determine the function of BacA and BacB, fluorescently labelled derivatives of the two proteins were overproduced in wild-type strain CB15N under the control of a xylose-inducible promoter. Accumulation of the bactofilin homologues was in each case accompanied by distinct morphological changes (Figure 2A). The cells initially became noticeably swollen, with many of them showing unusually high curvature (4 h). Later on, they developed into tightly curled filaments (6 h), which lysed on further incubation. Under these conditions, both fusion proteins formed elongated structures that localized to the inner curvature of the cell. This pattern was strikingly similar to that observed for the intermediate filament-like protein crescentin (CreS) (Ausmees et al, 2003), suggesting that crescentin could act as a scaffold for the assembly of BacA and BacB. However, deletion of creS had no effect on the phenotype induced by overproduction of the bactofilin fusion proteins (Supplementary Figure S2), which indicates that BacA and BacB have an intrinsic propensity to assemble into polymeric complexes. The same morphological defects were also observed on overproduction of the wild-type proteins (data not shown).
Figure 2.
Assembly of BacA and BacB into membrane-bound polymeric sheets. (A) Filamentous structures and cell shape defects induced by overproduction of BacA and BacB. Cells of wild-type strain CB15N carrying the overexpression plasmid pJK13 (Pxyl-bacA-venus) or pJK14 (Pxyl-bacB-venus), respectively, were grown in PYE-rich medium. Xylose was added to a final concentration of 0.3% to induce synthesis of BacA–Venus or BacB–Venus (t=0 h). At the indicated timepoints, cells were withdrawn from the cultures and visualized by DIC and fluorescence microscopy (bars: 2 μm). The concentrations of BacA–Venus and BacB–Venus were increased about 130- and 360-fold, respectively, over the corresponding wild-type levels (data not shown). (B) Membrane association of BacA and BacB. Whole-cell lysate of wild-type strain CB15N was fractionated by ultracentrifugation. Samples from the lysate, the soluble fraction, and the insoluble membrane fraction were analysed by immunoblotting using anti-BacA, anti-BacB, anti-SpmX, and anti-CtrA antiserum. The intregral membrane protein SpmX (Radhakrishnan et al, 2008) and the cytoplasmic response regulator CtrA (Quon et al, 1996) serve as controls for the fractionation efficiency. (C) Cryo-electron tomography of cells overproducing BacA. Cells of wild-type strain CB15N bearing overexpression plasmid pJK4 (Pxyl-bacA) were grown in PYE medium, induced for 4 h with 0.03% xylose, and analysed by cryo-electron tomography. Shown are a longitudinal section (13-nm slice, panel a) and a cross-section (38-nm slice, panel b) through a reconstructed cell, with the arrow heads pointing to the membrane-associated BacA polymer (bar: 50 nm).
Although BacA and BacB are predicted to be soluble, their subcellular localization points to an association with the cell envelope. Cell fractionation studies indeed showed that BacA and the bulk of BacB co-sediment with the cell membranes, even when synthesized at wild-type levels (Figure 2B). Both bactofilin homologues were released in soluble form under alkaline conditions (Supplementary Figure S3), supporting the notion that they are peripheral membrane proteins (Fujiki et al, 1982). To clarify whether BacA and BacB assemble into membrane-bound polymers, the ultrastructure of a strain overproducing BacA was investigated by cryo-electron tomography (Figure 2C). In agreement with the in vivo localization data, all misshaped cells analysed (n=18) showed an extensive sheet-like structure that lined the inner face of the cytoplasmic membrane at a centre-to-centre distance of 9 nm, correlating with the broad bands of fluorescence observed with the tagged proteins. Similar to the fluorescence signals, these polymeric assemblies were consistently localized to the inner cell curvature and generally restricted to regions that displayed unusually strong bending. Analogous structures were observed on overproduction of BacB (Supplementary Figure S4), suggesting that both bactofilin homologues are capable of polymerizing into membrane-associated sheets. It is difficult to acertain whether the polar BacAB clusters formed under normal conditions have a similar architecture, as the surface of the neck region joining the stalk with the cell body is small and highly curved, thus decreasing sensitivity and resolution. However, analysing wild-type cells, we could detect distinct patches of density at the base of the stalk that displayed the same general appearance and distance from the membrane as the sheets observed on bactofilin overproduction (Supplementary Figure S5). This finding supports the hypothesis that BacA and BacB do in fact assemble on the membrane in proximity of the stalked pole.
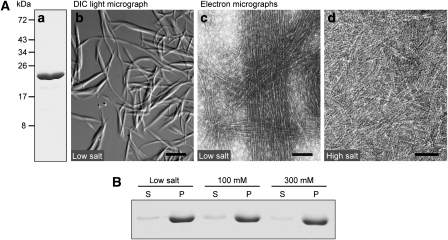
Consistent with these ultrastructural analyses, BacA and BacB spontaneously assembled into filaments on overproduction in Escherichia coli. Both proteins could be isolated in the polymeric state using standard chromatographic methods. Under low-salt conditions, BacA formed large rods and ribbons that were readily visualized by light microscopy (Figure 3A, panel b). Electron microscopic analyses showed that these structures were composed of numerous loosely packed protofilaments, each measuring ∼3 nm in diameter (Figure 3A, panel c). Individual bundles were interconnected by a dense filament network, rending the solution highly viscous. At physiological salt concentrations, the total amount of BacA incorporated into high-molecular weight structures was unchanged (Figure 3B). However, lateral contacts between polymers were reduced, resulting in the accumulation of ribbon-like assemblies comprising two or several protofilaments (Figure 3A, panel d). Polymerization of BacA was still observed at protein concentrations as low as 250 nM (Supplementary Figure S6), indicating that bactofilin monomers interact in a highly efficient manner. Unlike its paralogue, BacB could not be purified in sufficient quantities for detailed biochemical analyses. However, when analysed by electron microscopy, its polymerization behaviour was comparable to that of BacA (Supplementary Figure S7). In addition, we found that BacA and BacB assembled into the same sheet-like structures when overproduced individually in a bactofilin-deficient strain (Supplementary Figure S8), supporting the idea that they share similar biochemical characteristics. Together, our results strongly suggest that the two bactofilin homologues form membrane-associated polymeric clusters, which interfere with normal cell shape when expanding beyond their wild-type dimensions because of overproduction of their constituents.
Figure 3.
Polymerization of BacA. (A) Polymers formed by BacA. (a) Purified BacA, applied to an 11% SDS–polyacrylamide gel and stained with Coomassie Blue (5 μg of total protein). (b) DIC micrograph of polymers observed after dialysis of BacA (1.6 mg/ml) against 10 mM Tris/HCl (pH 7.5) (bar: 10 μM). (c) Transmission electron micrograph of BacA protofilament bundles formed in a low-salt buffer (buffer LS; see Materials and methods) (bar: 75 nm). (d) Pairs and ribbons of protofilaments obtained by dialysis of BacA against buffer LS containing 300 mM KCl (bar: 50 nm). The same polymerization behaviour was observed in the presence of 100 mM KCl (data not shown). (B) Polymerization efficiency of BacA at different salt concentrations. BacA (0.9 mg/ml) was dialysed against buffer LS containing 0 mM (low salt), 100 mM, or 300 mM KCl. After ultracentrifugation, samples from the supernatant (S) and the pellet (P) were applied to an SDS–gel, and proteins were detected by Coomassie blue staining. Densitometric analysis showed that, in all three conditions, more than 95% of BacA was found in the pellet fraction (data not shown).
As the two C. crescentus bactofilin homologues showed similar localization patterns under all conditions tested, it was conceivable that they interacted with each other. To investigate this possibility, we generated strains producing either a BacA–HA (KL7) or a BacB–HA fusion (KL8) in place of the respective wild-type protein. When co-immunoprecipitation analysis was performed on lysates from these strains using anti-HA-affinity beads, BacB co-purified with BacA–HA and vice versa, indicating close association of the two proteins (Figure 4A). In support of this conclusion, a chromosomally encoded BacA–eCFP fusion lost its typical polar localization on overproduction of BacB–Venus, adopting the same filament-like subcellular distribution as its paralogue instead (Figure 4B). Thus, bactofilin sheets appear to represent mixed polymers composed of both BacA and BacB subunits.
Figure 4.
Assembly of BacA and BacB into co-polymers. (A) Co-immunoprecipitation analysis. HA-tagged derivatives of BacA and BacB were precipitated from cell lysates of strains KL7 (bacA-HA) and KL8 (bacB-HA), respectively, using anti-HA-affinity beads. Proteins co-precipitating with BacA-HA and BacB-HA were probed with anti-BacB and anti-BacA antibodies, respectively. As a control, the same analyses were performed with lysates of wild-type (WT) strain CB15N. (B) Detection of an interaction between BacA and BacB in vivo. Cells of strain MT260 (bacA-ecfp) bearing overexpression plasmid pJK17 (Pxyl-bacB-venus) were grown in PYE-rich medium, induced for 6 h with 0.3% xylose, and visualized by DIC and fluorescence microscopy (bar: 2 μm).
Bactofilins mediate polar localization of a peptidoglycan synthase
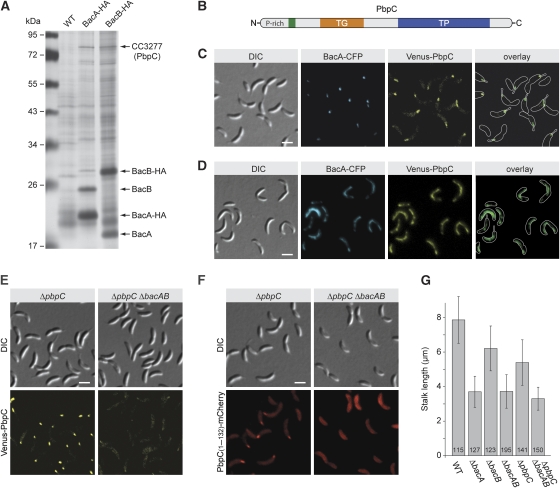
Despite the lack of evolutionary relationship, BacA and BacB resemble the protein localization factors DivIVA from B. subtilis (Edwards et al, 2000; Stahlberg et al, 2004) and PopZ from C. crescentus (Bowman et al, 2008; Ebersbach et al, 2008) in their ability to form polarly localized, membrane-associated polymers. It was, therefore, conceivable that the two bactofilin homologues could have a role in targeting other proteins to the stalked cell pole. To identify possible interaction partners, we generated strains that produced HA-tagged derivatives of BacA or BacB in place of the respective wild-type proteins. After crosslinking with formaldehyde, the hybrid proteins were immunoprecipitated, and co-purifying proteins were identified by mass spectrometry (Figure 5A). Apart from interacting with their respective paralogue, both BacA and BacB associated specifically with one of the five bifunctional penicillin-binding proteins (CC3277) encoded in the C. crescentus genome. CC3277, now designated PbpC (Penicillin-binding protein C), is a 733 amino acid protein, composed of a proline-rich cytoplasmic tail, a single transmembrane helix, and a large periplasmic portion containing a transglycosylase and a transpeptidase domain (Figure 5B). Similar to BacA and BacB, the protein is detectable at equal levels throughout the course of the cell cycle (data not shown).
Figure 5.
Interaction of BacA and BacB with the penicillin-binding protein PbpC. (A) Identification of proteins interacting with BacA and BacB. Lysates prepared from formaldehyde-treated cells of strains CB15N (wild type), KL7 (bacA-HA), and KL8 (bacB-HA) were subjected to co-immunoprecipitation analysis using anti-HA-affinity beads. Precipitated proteins were resolved in an 11% SDS–polyacrylamide gel and detected by silver staining. Bands specific for the samples from strains KL7 or KL8 were analysed by mass spectrometry. Owing to the limited amount of starting material, only the five most abundant proteins could be identified. (B) Schematic representation of PbpC. The transmembrane helix (residues 86–108) is indicated in green, the transglycosylase domain (TG; residues 133–300) in orange, and the transpeptidase domain (TP; residues 401–668) in blue. (C) Colocalization of BacA and PbpC. Cells of strain JK271 (bacA-ecfp xylX::Pxyl-venus-pbpC) were grown in PYE-rich medium, induced for 1 h with 0.03% xylose, and visualized by DIC and fluorescence microscopy (bar: 2 μm). (D) Detection of an interaction between BacA and PbpC in vivo. Cells of strain MT279 (xylX::Pxyl-venus-pbpC) carrying overexpression plasmid pJK53 (Pxyl-bacA-ecfp) were grown in PYE-rich medium, induced for 4 h with 0.3% xylose, and visualized by DIC and fluorescence microscopy (bar: 2 μm). Note: For strain MT279 lacking an overexpression plasmid, only polar signals were observed when grown under the same conditions (data not shown). (E) Loss of polar PbpC localization on deletion of bacA and bacB. Strains JK308 (ΔpbpC xylX::Pxyl-venus-pbpC) and JK310 (ΔbacAB ΔpbpC xylX::Pxyl-venus-pbpC) were grown in PYE-rich medium, induced for 1 h with 0.03% xylose, and visualized by DIC and fluorescence microscopy (bar: 2 μm). (F) Determination of the PbpC region responsible for interaction with BacA and BacB. Strains JK308 (ΔpbpC xylX::Pxyl-pbpC[AA 1−132]-mCherry) and JK289 (ΔbacAB ΔpbpC xylX::Pxyl-pbpC[AA 1−132]-mCherry) were grown in PYE-rich medium, induced for 2 h with 0.03% xylose, and visualized by DIC and fluorescence microscopy (bar: 2 μm). (G) Reduced stalk length of strains lacking bactofilin homologues and/or PbpC. Strains CB15N (wild type (WT)), MT257 (ΔbacA), MT259 (ΔbacB), JK5 (ΔbacAB), MT304 (ΔpbpC), and JK281 (ΔbacAB ΔpbpC) were grown in PYE-rich medium, diluted 1:20 in minimal medium lacking phosphate (M2G−P), and cultivated for another 24 h. The graph gives the average stalk length (±s.d.) reached under these conditions and the number of cells analysed.
In support of an interaction between PbpC and the two bactofilin homologues, localization studies showed that PbpC is recruited to the stalked cell pole, where it colocalizes with BacA and thus, by implication, also with BacB (Figure 5C). Moreover, time-lapse analyses indicate that a Venus–PbpC fusion shows the same cell-cycle-dependent localization pattern as BacA–eCFP, with a transition from diffuse to polar localization at the onset of the stalked phase (Supplementary Figure S9). Finally, overproduction of a BacA–eCFP fusion caused Venus–PbpC to adopt an elongated localization pattern identical to that observed for BacA–eCFP (Figure 5D). As PbpC colocalizes with BacA and BacB under all conditions investigated, bactofilin clusters might serve as assembly platforms that recruit PbpC molecules to the stalked cell pole. Consistent with this hypothesis, Venus–PbpC no longer formed polar foci in a ΔbacAB double mutant (JK310) (Figure 5E). In most cells, the fusion protein was evenly distributed in the cytoplasmic membrane. Sporadiacally, slightly brighter clusters were observed, but the subcellular position of these assemblies was random. By contrast, disruption of MreB filaments using the antibiotic A22 (Iwai et al, 2002; Gitai et al, 2005) did not have any effect on the localization of PbpC (Supplementary Figure S10), indicating that BacA and BacB act independently of the actin-like cytoskeleton. Given that BacA and BacB are cytoplasmic membrane-associated proteins, the cytoplasmic tail and the transmembrane helix of PbpC should contain all determinants necessary for interaction with bactofilin clusters. Indeed, a fusion protein generated by replacing the periplasmic portion of PbpC with the red fluorescent protein mCherry showed the same BacAB-dependent localization pattern as the wild-type protein (Figure 5F). Moreover, the cytoplasmic tail of PbpC alone was sufficient to target a membrane-integral derivative of mCherry to the stalked pole in a BacAB-dependent manner (Supplementary Figure S11).
Our results indicate that bactofilin clusters serve as polar localization factors that recruit the peptidoglycan synthase PbpC to the stalked pole on transition of C. crescentus to a sessile life style, suggesting a role for BacA and BacB in stalk biogenesis. To test this possibility, we investigated the effects of in-frame deletions in bacA, bacB, and pbpC on stalk formation. These analyses were facilitated by growing the cells under phosphate-limiting conditions, which strongly stimulate stalk elongation (Gonin et al, 2000), thus accentuating mutant phenotypes. None of the strains constructed displayed significant changes in general cell morphology. However, stalk length was reduced to ∼45% of the wild-type value in ΔbacA cells, supporting a role for bactofilin clusters in polar development (Figure 5G). Cryo-electron tomographic analyses did not show defects in the fine structure of the mutant stalks (data not shown), suggesting that deletion of bacA mainly affects longitudinal extension. Very similar results were obtained for a ΔbacAB double and a ΔbacAB ΔpbpC triple mutant. Given that lesions in bacB or pbpC alone caused a less pronounced phenotype, BacA might be functionally dominant over BacB and involved in processes that go beyond targeting PbpC to the stalked pole.
Bactofilin homologues are widely conserved among bacteria
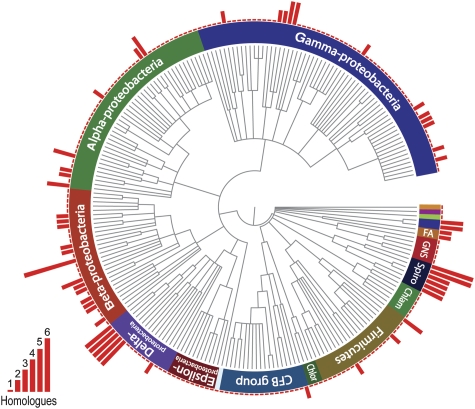
Searching the Pfam database (Finn et al, 2008) for proteins that show a DUF583 signature, we retrieved a total of 391 sequences from 287 different bacterial species, covering essentially all phyla for which complete genomes are available (Figure 6). With one exception, all these proteins share the same architecture as BacA and BacB, consisting of a single DUF583 domain that is flanked by short, often disordered and highly charged, terminal segments. Although the majority of bactofilin homologues are predicted to be soluble, all enterobacterial proteins exhibit N-terminal transmembrane regions, oriented such that the DUF583 domain is located in the cytoplasm. Interestingly, many species possess two or more bactofilin alleles, which are either organized in operons or scattered throughout the genome. The ubiquity of bactofilins and the high frequency of gene duplication events suggest that these proteins perform a significant cellular function.
Figure 6.
Conservation of bactofilin among bacteria. The PFAM database (Finn et al, 2008) was used to search for bacterial species possessing DUF583-containing proteins. Where sequence information was available for more than one strain per species, only a single strain was chosen for further analysis. After retrieving the corresponding taxonomy IDs from the National Center for Biotechnology Information (NCBI) website, a phylogenetic tree of the species identified was created using the iTOL server (Letunic and Bork, 2007). For each species shown, the number of bactofilin homologues encoded in the genome is indicated by a red bar. Chlor, green sulphur bacteria; Chlam, chlamydias; Spiro, spirochaetes; GNS, green non-sulphur bacteria; FA, fibrobacteres-acidobacteria group.
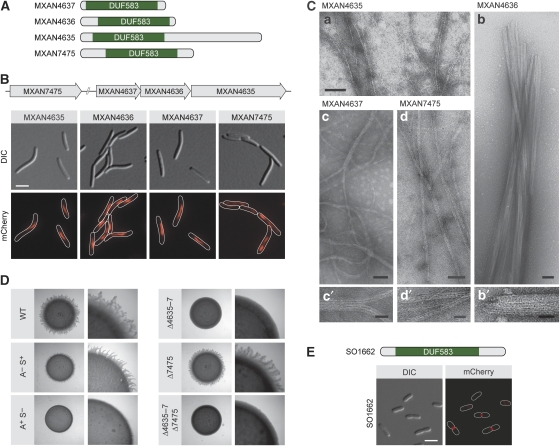
To determine whether the ability to polymerize is a general feature of bactofilins, we set out to investigate the subcellular localization and biochemical properties of DUF583-containing proteins from other bacterial lineages. We first analysed four thus-far uncharacterized bactofilin paralogues encoded in the genome of Myxococcus xanthus (Figure 7A), a δ-proteobacterium that is genetically amenable, thus facilitating molecular biological studies (Ueki et al, 1996). On replacement of the respective endogenous genes with alleles encoding fluorescent protein fusions, conspicuous filamentous structures were detectable in all of the resulting strains (Figure 7B). MXAN4635, MXAN4636, and MXAN4637 showed very similar localization patterns, forming slender filaments that were restricted to the medial parts of the cells. This finding suggests that the three proteins interact with each other, consistent with the fact that their genes are overlapping and thus presumably coexpressed (Goldman et al, 2006). The structures formed by MXAN7475 were slightly more irregular and typically stretched throughout the whole cell. In agreement with their filament-like localization, all four bactofilins polymerized spontanously in vitro (Figure 7C). Whereas MXAN4636 formed massive, rod-shaped filament bundles, all other proteins assembled into extensive networks of long, predominantly straight polymers composed of only a few collateral protofilaments. These results suggest that bactofilins have a general propensity to form filamentous structures both in vivo and in vitro. Our analyses showed that an M. xanthus mutant lacking all four bactofilin genes still displayed wild-type cell morphology (data not shown). Prompted by the observation that MXAN4635-7 are located in the immediate vicinity of genes implicated in gliding motility (Youderian et al, 2003; Youderian and Hartzell, 2006), we went on to investigate the involvement of these three paralogues in cell movement. M. xanthus shows two distinct modes of locomotion, both of which are central to the developmental programme that coordinates aggregation of cells into fruiting bodies (Sogaard-Andersen, 2004). Adventurous motility uses polar slime secretion and focal adhesion complexes to propel individual cells across a solid surface. Social motiliy, on the other hand, facilitates the coordinated movement of cells by means of pili-mediated cell–cell contacts. We found that a strain lacking MXAN4635-7 (MT295) was indeed severely impaired in social motility, as reflected by the formation of smooth colony edges on low-percentage agar plates (Figure 7D). By contrast, microscopic analysis did not show any defects in locomotion on the single-cell level, indicating that adventurous motility was still functional (data not shown). Unlike its paralogues, MXAN7475 appeared to be dispensable for normal cell movement, suggesting that this gene is part of a different cellular pathway (Figure 7D).
Figure 7.
Conservation of the filament-forming properties of bactofilin across species. (A) Schematic representation of the bactofilin homologues from Myxococcus xanthus. The DUF583 domain is indicated in green. (B) Subcellular localization of bactofilin homologues in M. xanthus. Cells of strains MT296 (MXAN4635-mCherry), MT297 (MXAN4636-mCherry), MT298 (MXAN4637-mCherry), and MT299 (mCherry-MXAN7475) were grown in CTT-rich medium and visualized by DIC and fluorescence microscopy (bar: 3 μm). The schematic shows the chromosomal organization of the four M. xanthus bactofilin genes investigated. (C) Polymers formed by M. xanthus bactofilin homologues in vitro. Shown are elecron micrographs of polymers formed by purified MXAN4635 (panel a), MXAN4636 (panels b and b′), MXAN4637 (panels c and c′), and MXAN7475 (panels d and d′). Bars: 75 nm (panels a, b, c, d) or 25 nm (panels b′, c′, d′). (D) Involvement of MXAN4635-7 in social gliding motility. Strains DK1622 (WT), DK1217 (aglB1; A− S+) (Hodgkin and Kaiser, 1979), DK10416 (pilB; A+ S−) (Wu et al, 1997), MT295 (ΔMXAN4635-7), MT300 (ΔMXAN7475), and JK328 (ΔMXAN4635-7 ΔMXAN7475) were grown in CTT medium, spotted on low-percentage agar plates, and analysed for motility. Flare-like structures projecting from the rim of the colony represent rafts of cells moving by means of social motility. (E) Subcellular localization of the S. oneidensis bactofilin homologue. Cells of strain MT288 (SO1662-mCherry) were grown in LB-rich medium and visualized by DIC and fluorescence microscopy (bar: 3 μm). The schematic indicates the position of the DUF583 domain within SO1662.
To extend our study, we aimed to investigate a γ-proteobacterial BacAB homologue. As E. coli lacks a chromosomal bactofilin gene, this analysis was performed in a related species, Shewanella oneidensis, which produces a single DUF583-containing protein (SO1662). It was not possible to isolate this bactofilin in soluble form, thus precluding polymerization assays. However, studying the subcellular distribution of a fluorescently tagged derivative, we found that SO1662 has the typical localization pattern of a cell division protein (Figure 7E). Whereas early pre-divisional cells only showed diffuse fluorescence or random puncta, cells that were noticeably constricted consistently displayed a bright fluorescent band at the division septum. Thus, the subcellular positioning of bactofilins appears to vary significantly depending on the host organism.
Discussion
In recent years, a number of polymeric proteins have been identified in bacteria, but only the tubulin homologue FtsZ and the actin homologue MreB turned out to be widely conserved across species (Graumann, 2007; Pogliano, 2008). In addition, although less widespread, intermediate filament proteins have been discovered in several different bacterial lineages (Ausmees et al, 2003; Bagchi et al, 2008). These findings suggest that bacterial and eukaryotic cells are organized by a similar core set of cytoskeletal elements, even though the functions of individual elements often differ considerably. Our work has now identified bactofilins as a new class of polymeric proteins with almost universal distribution among bacteria. Bactofilin genes are absent from eukaryotic and archaeal genomes, indicating that they either represent a bacterial invention or were lost from the other kingdoms soon after separation of the three domains of life. Consistent with an early origin, the proteins have adopted a wide range of localization patterns and, presumably, functions during the course of evolution. Thus, the characteristic DUF583 domain is likely to serve as a general polymerization module, whose precise function is determined by its short flanking regions. In support of this notion, the N- and C-terminal segments of bactofilins frequently feature repetitive, proline-rich sequences, which are known to mediate protein–protein interactions (Williamson, 1994). As observed for bacterial actin-like proteins (Carballido-Lopez et al, 2006; Defeu Soufo and Graumann, 2006), many species contain several paralogues, which can assemble into a single superstructure. The presence of multiple related subunits might increase the number of possible interaction partners and possibly serve regulatory purposes.
Function of bactofilins
The two C. crescentus bactofilin homologues BacA and BacB form membrane-associated clusters that serve to recruit the peptidoglycan synthase PbpC to the stalked cell pole, thus complementing the global function of the actin-like cytoskeleton and the cell division apparatus in the positioning of cell wall biosynthetic enzymes (Cabeen and Jacobs-Wagner, 2007). However, the precise role of PbpC still remains to be clarified. Earlier studies have shown that stalk elongation is achieved by insertion of new cell wall material in a conical growth zone around the stalk base (Schmidt and Stanier, 1966; Aaron et al, 2007). Thus, PbpC could directly contribute to longitudinal extension of the stalk by supporting the activity of other cell wall synthases that are targeted to the stalked pole as part of the generic MreB/RodZ-dependent morphogenetic system (Alyahya et al, 2009). Alternatively, the recruitment of PbpC to polar bactofilin clusters could promote reorganization of the cell wall in the vicinity of the stalk base such as to render it more resistant against mechanical stress. Interestingly, the composition of peptidoglycan was proposed to differ between the stalk and the cell body (Poindexter and Hagenzieker, 1982), consistent with the observation that stalks are more resistant to lysozyme treatment than the remaining parts of the cell (Schmidt and Stanier, 1966). Therefore, PbpC could also serve to enhance the rigidity of the stalk by modulating the composition or structure of stalk peptidoglycan.
The reason for the cell shape defects induced by the ectopic polymerization of bactofilins is unclear. We found that deletion of pbpC could not attenuate the effects of BacA overproduction (data not shown), indicating that the deformations observed do not originate from the spatial deregulation of PbpC activity. Given that heterologous expression of bacA and bacB can also effect abnormal cell shapes in E. coli (Supplementary Figure S12), bactofilin sheets might rather perturb cell wall biosynthesis indirectly, for example by sterically hindering access of the morphogenetic machinery to certain regions of the cell envelope. Alternatively, they could exert a mechanical force that constrains longitudinal extension of the cell and thus induces uneven growth of the peptidoglycan sacculus—a mechanisms that likely underlies the morphogenetic role of crescentin (Cabeen et al, 2009) as well as the cell shape defects resulting from overproduction of a mutant version of the cell division protein FtsA in E. coli (Gayda et al, 1992). It remains to be clarified whether the potential of BacAB clusters to modulate cell morphology contributes to their function under normal conditions.
The functions of the M. xanthus and S. oneidensis bactofilin homologues have not been investigated extensively in the context of this study. Interestingly, MXAN4635-7 assemble into filamentous structures (Figure 7B) that are strikingly reminiscent of submembrane filament bundles discovered earlier in M. xanthus by electron microscopy (Burchard et al, 1977). The three proteins are encoded in a putative operon located immediately downstream of sgmTS, two genes involved in social motility (Youderian and Hartzell, 2006), and agmH, a gene implicated in adventurous motility (Youderian et al, 2003). Cells lacking MXAN4635-7 were indeed deficient in social motility, suggesting a role for bactofilins in cell movement. MXAN7475, by contrast, lies adjacent to genes encoding the ParAB chromosome partitioning system and could therefore participate in DNA segregation. Whereas the precise function of the M. xanthus proteins remains speculative, the localization pattern of S. oneidensis SO1662 clearly points to a role in cell division. The protein could serve to stabilize the cell division apparatus or recruit other proteins to the division site.
Consistent with our results, a bactofilin homologue (CcmA) has been associated earlier with the maintenance of proper cell shape and swarming in Proteus mirabilis, even though the underlying mechanism has remained obscure (Hay et al, 1999). Moreover, the bactofilins YhbE and YhbF were shown to be required for efficient swimming motility in B. subtilis (Rajagopala et al, 2007). Collectively, these findings suggest that DUF583-containing proteins might function preferentially in morphogenesis and locomotion.
Dynamics of bactofilin cluster assembly
Our analyses showed BacA as the major constituent of bactofilin clusters in C. crescentus. Its copy number was estimated to ∼200 molecules per cell, a value that is considerably lower than that determined for other cytoskeletal proteins (Quardokus et al, 2001). However, in wild-type cells, bactofilin clusters are confined to the vicinity of the stalked pole, an exceedingly thin part of the cell with an inner diameter of less than 50 nm (compare Supplementary Figure S5). Furthermore, the high stability of bactofilin filaments suggests that most of the BacA and BacB molecules synthesized are present as polymers. The amount of protein required to establish a functional cytoskeletal structure might thus be significantly lower for bactofilins than for other cytoskeletal proteins, which are highly dynamic and have to span much larger regions within the cell.
It was possible to enlarge the polar BacAB clusters into extensive polymeric sheets by overproducing either of the two subunits, thereby facilitating high-resolution ultrastructural analyses. Given their small diameter, these sheets likely represent a single layer of bactofilin protofilaments. Consistent with this notion, BacA was found to assemble into ribbon-like structures under physiological conditions in vitro. Attachment of these units to the membrane surface might promote additional lateral interactions, thus allowing the formation of larger polymeric arrays. Our analyses indicate a centre-to-centre distance of 9 nm between the inner membrane and the BacAB sheets, corresponding to a gap of ∼5 nm when assuming a thickness of 5 nm for a lipid bilayer and 3 nm for the bactofilin polymer. The reason for this pronounced spacing is unclear. However, we suggest that the disordered regions flanking the DUF583 polymerization domain serve as flexible linkers connecting bactofilin sheets to the cell envelope. Comprising between 29 and 44 residues, they can easily bridge a distance of 5 nm when adopting an extended conformation. Moreover, their terminal regions are highly enriched in basic amino acids and thus ideally suited to interact with the negatively charged phospholipid membrane. Similarly, the 85-residue cytoplasmic tail of PbpC is sufficiently long to reach the polymeric BacAB layer. Bactofilin sheets are clearly distinguishable from the ‘inner curvature bundles' described earlier in C. crescentus (Briegel et al, 2006). Unlike the laminar structures formed by BacA and BacB, inner curvature bundles have an elongated, rod-shaped appearance and consist of multiple layers, which are thicker and significantly farther away from the membrane than BacAB polymers.
In vitro, all bactofilin homologues analysed polymerized spontaneously without the need of additional cofactors. BacA filaments were resistant to treatment with chelating agents and exposure to high salt concentrations, and they did not show any large-scale dynamics when observed by DIC microscopy (data not shown), suggesting that individual subunits are exchanged very slowly under these conditions. In the cell, however, the assembly of BacA and BacB is a highly regulated and dynamic process, triggered specifically during the swarmer-to-stalked-cell transition. The underlying regulatory mechanism still remains to be clarified. Although bactofilins have a high intrinsic propensity to polymerize, their cellular concentration might not reach the threshold necessary for spontaneous assembly, thus potentially allowing temporal control of the polymerization process by the cell-cycle-regulated accumulation of a nucleation factor. Alternatively, swarmer cells could actively prevent cluster formation by synthesizing an inhibitory protein that masks the interaction determinants necessary for bactofilin assembly. Similar to the temporal regulators, the determinants responsible for targeting BacAB clusters to the stalked cell pole are unknown. It is conceivable that the two proteins interact with a localization factor that marks the site of polymerization. The only C. crescentus proteins known to have the same localization pattern as BacA and BacB are the histidine kinase DivJ (Wheeler and Shapiro, 1999) and its targeting factor SpmX (Radhakrishnan et al, 2008). However, bactofilin clusters were still positioned normally after disruption of the divJ and spmX genes (data not shown). In a different scenario, the localization of BacAB clusters could be controlled by membrane curvature, similar to the situation seen for eukaryotic BAR-domain proteins (Frost et al, 2009) and B. subtilis DivIVA (Lenarcic et al, 2009; Ramamurthi and Losick, 2009). Establishment of the stalk structure involves remodelling of the cell envelope, creating areas of high positive curvature around the junction between the stalk and the cell body (compare Supplementary Figure S5). Assuming that BacAB sheets have a domed shape of similar curvature, their assembly should occur preferentially in this transition region. Under conditions of excess BacA or BacB, further expansion of the sheets is likely to proceed along the concave side of the cell, which is also positively curved but may normally be avoided because of its larger and thus suboptimal radius. Comparable to BAR-domain proteins (Frost et al, 2009), BacAB sheets might be able to impose their intrinsic curvature on the interacting membrane. In this way, they could create a bending stress on the cell envelope, inducing the hypercurved morphology that is characteristic for bactofilin-overproducing cells.
Similar to their C. crescentus homologues, bactofilins from other bacteria show apparent dynamic behaviour. In M. xanthus, the structures formed by MXAN4635-7 consistently occupy the midcell region, implying the existence of a regulatory mechanism that controls the length and subcellular position of the polymers and facilitates their division during cytokinesis. Likewise, transition of S. oneidensis SO1662 from diffuse to midcell localization is tightly synchronized with the cell cycle, probably by coupling its recruitment to the maturation state of the division apparatus. Overall, bactofilins share several prominent features with intermediate filament proteins, such as slow large-scale dynamics and the ability to polymerize in a nucleotide-independent manner (Herrmann and Aebi, 2004). This similarity might be based on convergent evolution, driven by the requirement for proteins capable of forming stable cytoskeletal scaffolds. It will be interesting to analyse the polymerization dynamics of bactofilins in detail and to investigate the full range of functions they perform.
Materials and methods
Media and growth conditions
All C. crescentus strains used in this study were derived from the synchronizable wild-type strain CB15N (NA1000) (Evinger and Agabian, 1977). They were grown at 28°C in peptone-yeast extract (PYE) medium or M2-glucose (M2G) minimal medium (Ely, 1991), which was supplemented with antibiotics at the following concentration when appropriate (μg/ml; liquid/solid medium): spectinomycin (25/50), streptomycin (−/5), gentamicin (0.5/5), kanamycin (5/25), chloramphenicol (1/1). Phosphate depletion experiments were performed in M2G medium containing 20 mM Tris/HCl (pH 7.0) instead of phosphate salts (M2G−P). S. oneidensis MR-1 (Venkateswaran et al, 1999) and mutants derived from this strain were grown at 30°C in Luria-Bertani (LB) broth (SigmaAldrich) supplemented with 30 μg/ml kanamycin when appropriate. M. xanthus DK1622 (Kaiser, 1979) and derivatives of this strain were grown in CTT medium (Hodgkin and Kaiser, 1977) at 32°C. When necessary, kanamycin was added at a concentration of 50 μg/ml. In order to analyse M. xanthus for motility, cells were grown in CTT medium to an OD550 of 0.5–0.9, collected, and resuspended in CTT medium to a density of 5.0 × 109 cells/ml. 5-μl aliquots were withdrawn from the suspensions and spotted on 0.5% casitone-CTT plates containing 0.5% (for S-motility) or 1.5% (for A-motility) agar. After incubation of the plates for 24 h at 32°C, colony morphology was visualized using a Leica MZ8 stereomicroscope.
Plasmid and strain construction
Details on the construction of plasmids and strains are given in the Supplementary data. C. crescentus and M. xanthus were transformed by electroporation (Ely, 1991; Kashefi and Hartzell, 1995). Transfer of plasmids into S. oneidensis was achieved by conjugation using the dap− helper strain E. coli WM3064 (W Metcalf, unpublished). Proper chromosomal integration of non-replicating vectors was verified by colony PCR. Gene replacement in C. crescentus and M. xanthus was achieved by a two-step procedure using selection for sucrose (Thanbichler et al, 2007) or galactose (Ueki et al, 1996) resistance, respectively, to identify cells that have undergone double homologous recombination.
Light and immunofluorescence microscopy
Cells were grown to mid-exponential phase and induced by addition of D-xylose or L-arabinose as indicated. They were then transferred onto a pad made of 1% agarose in M2 salts (Ely, 1991) and visualized using an Axio Imager.M1 microscope (Zeiss) equipped with a Zeiss Plan Apochromat × 100/1.40 Oil DIC objective and a Cascade:1K CCD camera (Photometrics). Images were processed with Metamorph 7.1.2 (Universal Imaging Group) and Adobe Photoshop CS2 (Adobe Systems). Immunofluorescence microscopy was performed as described (Domian et al, 1997), using affinity purified anti-BacA and anti-BacB antibodies at a dilution of 1:50 and Alexa-Fluor 555 Goat Anti-Rabbit (Molecular Probes) secondary antibody at a dilution of 1:300.
Electron microscopy
For cryo-electron tomography, 2 ml of cell suspension was centrifuged for 5 min at 1500 g and the pellet was resuspended in 30–50 μl of supernatant. A solution of 10-nm colloidal gold (Ted Pella, Redlands, CA) was added to the cells immediately before plunge freezing and after treatment with BSA to avoid aggregation of the gold particles (Iancu et al, 2006). A 4 μl droplet of the sample solution was applied to a glow-discharged R2/2 copper/rhodium grid, then automatically blotted and plunge-frozen in liquid ethane or a liquid ethane/propane mixture (Tivol et al, 2008) using a Vitrobot (FEI Company, Hillsboro, OR). The grids were stored under liquid nitrogen until data collection. Images were collected using the FEI Polara TM (FEI Company) 300 kV FEG transmission electron microscope, equipped with a Gatan energy filter (slit width 20 eV) on a lens-coupled, cooled 4k × 4k Ultracam (Gatan, Pleasanton, CA). The pixel size on the specimen plane was 0.961 nm. Using the predictive UCSF-Tomo package (Zheng et al, 2004) or Leginon (Suloway et al, 2009), single axis tilt series were recorded from −60° to 60° with an increment of 1° and an underfocus of 12 μm. The cumulative dose was limited to 200 e/A2. Three-dimensional reconstructions were calculated using the IMOD software package (Mastronarde, 1997).
For transmission electron microscopy, BacA and MXAN4636 were dialysed against buffer LS (10 mM HEPES/NaOH, pH 7.2, 10 mM NaCl, 5 mM MgCl2, 0.1 mM EDTA, 1 mM β-mercaptoethanol), containing 300 mM KCl when appropriate. MXAN4635, MXAN4637, and MXAN7475 were refolded to their native state and analysed directly in the renaturation buffer. The proteins were applied to glow-discharged carbon-coated grids and stained with 2% uranyl acetate. Images were taken with a Philips 301G electron microscope at 80 kV, recorded on negative film, scanned, and processed digitally to enhance contrast. Alternatively, samples were analysed using a JEOL 2100 electron microscope, operated at 80 kV and equipped with a 1024 × 1024 pixel CCD camera.
Protein purification and polymerization
Proteins were fused to a hexahistidine affinity tag and synthesized in E. coli Rosetta2(DE3)/pLysS (Invitrogen). Polymers of BacA, BacB, and MXAN4636 formed spontaneously during overproduction. BacA and BacB were isolated in the native state using affinity and cation exchange chromatrography. MXAN4636 filaments were separated from the crude cell extract by low-speed centrifugation. All other bactofilins were purified under denaturing conditions using Ni-NTA-affinity beads. A detailed description of the purification protocols is given in the Supplementary data. MXAN4635 (2.5 mg/ml) and MXAN4637 (2.3 mg/ml) were refolded to their native state by gradual removal of urea. To this end, solutions of denatured protein were dialysed successively against batches of buffer B2 (100 mM NaH2PO4, 10 mM Tris, 0.5% Triton X-100, pH 8.0, adjusted with NaOH) containing 4, 2, 1 M, and no urea, respectively, at room temperature. Refolding of MXAN7475 (0.6 mg/ml) was achieved in a similar manner using buffers that lacked detergent. In all cases, renaturation was accompanied by polymer formation.
Protein concentrations were determined using a modified Bradford assay (Roti-Nanoquant reagent, Carl Roth, Germany) with bovine serum albumin (New England Biolabs) as standard. For BacA, the validity of the measurements was verified by quantitative amino acid analysis (Alphalyse A/S, Denmark).
Sedimentation assays
BacA was dialysed overnight against buffer LS (10 mM HEPES/NaOH, pH 7.2, 10 mM NaCl, 5 mM MgCl2, 0.1 mM EDTA, 1 mM β-mercaptoethanol), supplemented with 100 mM or 300 mM KCl when appropriate. The protein was centrifuged for 1 h at 100 000 r.p.m. in a Beckman TLA 120.1 rotor. Samples of the pellet and supernatant were analysed by SDS–PAGE.
Co-immunoprecipitation and mass spectrometry
Exponentially growing cells (M2G medium, OD600=0.6) were incubated for 20 min at 37°C in the presence of 0.6% formaldehyde. After quenching of the crosslinking reaction by addition of glycine (prepared as a 1.25 M stock solution in PBS) to a final concentration of 125 mM and 5 min incubation at room temperature, the cells were collected by centrifugation, washed twice with 200 ml buffer C1 (50 mM sodium phosphate, pH 7.4, 5 mM MgCl2), flash-frozen in liquid nitrogen, and stored at −80°C until further use. Cells (1.3 g) were thawed on ice, washed with 100 ml buffer C2 (20 mM HEPES/NaOH, pH 7.4, 100 mM NaCl, 20% glycerol, 0.5% Triton X-100) and resuspended in 10 ml buffer C2. The suspension was supplemented with 10 mM MgCl2, 10 mg/ml lysozyme, 5 μg/ml DNase I, and 100 μg/ml phenylmethylsulfonyl fluoride, and incubated on ice for 30 min. After disruption of the cells using a French press, cell debris was removed by centrifugation for 5 min at 13 000 g. A 2-ml aliquot of the cleared solution was mixed with 20 μl (BacA–HA) or 80 μl (BacB–HA) Ezview Red Anti-HA Affinity Gel (Sigma), which had been equilibrated with buffer C2, and was incubated for 2 h at 4°C with gently agitation. The beads were recovered by centrifugation for 2 min at 8000 g, washed twice for 5 min with 750 μl buffer C2, three times for 5 min with 750 μl buffer C3 (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100), and three times for 5 min with 750 μl buffer C4 (100 mM Tris/HCl, pH 8.0, 750 mM NaCl, 1 mM EDTA, 0.05% Triton X-100). Subsequently, they were resuspended in 100 μl SDS sample buffer lacking reducing agents and incubated for 20 min at 95°C to reverse the crosslinks. After centrifugation, the supernatant was applied to an 11% SDS–polyacrylamide gel. Proteins were visualized by silver staining and identified after excision from the gel by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry(Kahnt et al, 2007).
In case of subsequent immunoblot analysis, co-immunoprecipitation was performed in a similar manner but without prior crosslinking, using buffer C5 (20 mM HEPES/NaOH, pH 7.2, 100 mM NaCl, 20% glycerol, 0.05% Triton X-100) instead of buffer C2 and washing the beads three times with buffer C5 and three times with buffer C6 (50 mM Tris/HCl, pH 7.5, 150 mM NaCl) before elution.
Immunoblot analysis
Immunoblotting was performed as described (Thanbichler and Shapiro, 2006). Anti-CtrA (Domian et al, 1997) and anti-SpmX (Radhakrishnan et al, 2008) antisera were used at dilutions of 1:10000 and 1:50000, respectively. Antibodies against BacA and BacB were raised by immunization of rabbits with a mixture of the peptides CATPAEPARRAPPKV and CGRSLKFQRPAPAPSQ (anti-BacA) or with the peptide CPQPQPAPAPARPKPA (anti-BacB) (Eurogentec). They were affinity purified and applied at dilutions of 1:10000 and 1:5000, respectively.
Supplementary Material
Supplemental Material
Review Process File
Acknowledgments
We thank Lotte Søgaard-Andersen for access to the Department of Ecophysiology mass spectrometry facility and Stefanie Reiβmann, Susan Schlimpert, and Daniela Kiekebusch for critical reading of the manuscript. This work was supported by funds from the Max Planck Society, a Young Investigator Grant (RGY 69/2008) from the Human Frontier Science Program to MT, and National Institutes of Health (NIH) grant R01 AI067548 to GJJ.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aaron M, Charbon G, Lam H, Schwarz H, Vollmer W, Jacobs-Wagner C (2007) The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol Microbiol 64: 938–952 [DOI] [PubMed] [Google Scholar]
- Alyahya SA, Alexander R, Costa T, Henriques AO, Emonet T, Jacobs-Wagner C (2009) RodZ, a component of the bacterial core morphogenic apparatus. Proc Natl Acad Sci USA 106: 1239–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DE, Gueiros-Filho FJ, Erickson HP (2004) Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J Bacteriol 186: 5775–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausmees N, Kuhn JR, Jacobs-Wagner C (2003) The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell 115: 705–713 [DOI] [PubMed] [Google Scholar]
- Bagchi S, Tomenius H, Belova LM, Ausmees N (2008) Intermediate filament-like proteins in bacteria and a cytoskeletal function in Streptomyces. Mol Microbiol 70: 1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehuda S, Rudner DZ, Losick R (2003) RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299: 532–536 [DOI] [PubMed] [Google Scholar]
- Bi EF, Lutkenhaus J (1991) FtsZ ring structure associated with division in Escherichia coli. Nature 354: 161–164 [DOI] [PubMed] [Google Scholar]
- Bowman GR, Comolli LR, Zhu J, Eckart M, Koenig M, Downing KH, Moerner WE, Earnest T, Shapiro L (2008) A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole. Cell 134: 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel A, Dias DP, Li Z, Jensen RB, Frangakis AS, Jensen GJ (2006) Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography. Mol Microbiol 62: 5–14 [DOI] [PubMed] [Google Scholar]
- Burchard AC, Burchard RP, Kloetzel JA (1977) Intracellular, periodic structures in the gliding bacterium Myxococcus xanthus. J Bacteriol 132: 666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeen MT, Charbon G, Vollmer W, Born P, Ausmees N, Weibel DB, Jacobs-Wagner C (2009) Bacterial cell curvature through mechanical control of cell growth. EMBO J 28: 1208–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeen MT, Jacobs-Wagner C (2007) Skin and bones: the bacterial cytoskeleton, cell wall, and cell morphogenesis. J Cell Biol 179: 381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballido-Lopez R (2006) The bacterial actin-like cytoskeleton. Microbiol Mol Biol Rev 70: 888–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballido-Lopez R, Errington J (2003) The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev Cell 4: 19–28 [DOI] [PubMed] [Google Scholar]
- Carballido-Lopez R, Formstone A, Li Y, Ehrlich SD, Noirot P, Errington J (2006) Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev Cell 11: 399–409 [DOI] [PubMed] [Google Scholar]
- Charbon G, Cabeen MT, Jacobs-Wagner C (2009) Bacterial intermediate filaments: in vivo assembly, organization, and dynamics of crescentin. Genes Dev 23: 1131–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeu Soufo HJ, Graumann PL (2006) Dynamic localization and interaction with other Bacillus subtilis actin-like proteins are important for the function of MreB. Mol Microbiol 62: 1340–1356 [DOI] [PubMed] [Google Scholar]
- Domian IJ, Quon KC, Shapiro L (1997) Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell 90: 415–424 [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Briegel A, Jensen GJ, Jacobs-Wagner C (2008) A self-associating protein critical for chromosome attachment, division, and polar organization in Caulobacter. Cell 134: 956–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DH, Errington J (1997) The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol 24: 905–915 [DOI] [PubMed] [Google Scholar]
- Edwards DH, Thomaides HB, Errington J (2000) Promiscuous targeting of Bacillus subtilis cell division protein DivIVA to division sites in Escherichia coli and fission yeast. EMBO J 19: 2719–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B (1991) Genetics of Caulobacter crescentus. Methods Enzymol 204: 372–384 [DOI] [PubMed] [Google Scholar]
- Esue O, Cordero M, Wirtz D, Tseng Y (2005) The assembly of MreB, a prokaryotic homolog of actin. J Biol Chem 280: 2628–2635 [DOI] [PubMed] [Google Scholar]
- Evinger M, Agabian N (1977) Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol 132: 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL, Bateman A (2008) The Pfam protein families database. Nucleic Acids Res 36: D281–D288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flärdh K (2003) Essential role of DivIVA in polar growth and morphogenesis in Streptomyces coelicolor A3(2). Mol Microbiol 49: 1523–1536 [DOI] [PubMed] [Google Scholar]
- Frost A, Unger VM, De Camilli P (2009) The BAR domain superfamily: membrane-molding macromolecules. Cell 137: 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB (1982) Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda RC, Henk MC, Leong D (1992) C-shaped cells caused by expression of an ftsA mutation in Escherichia coli. J Bacteriol 174: 5362–5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitai Z, Dye NA, Reisenauer A, Wachi M, Shapiro L (2005) MreB actin-mediated segregation of a specific region of a bacterial chromosome. Cell 120: 329–341 [DOI] [PubMed] [Google Scholar]
- Goehring NW, Beckwith J (2005) Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr Biol 15: R514–R526 [DOI] [PubMed] [Google Scholar]
- Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS, Eisen JA, Ronning CM, Barbazuk WB, Blanchard M, Field C, Halling C, Hinkle G, Iartchuk O, Kim HS, Mackenzie C, Madupu R, Miller N, Shvartsbeyn A, Sullivan SA, Vaudin M et al. (2006) Evolution of sensory complexity recorded in a myxobacterial genome. Proc Natl Acad Sci USA 103: 15200–15205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonin M, Quardokus EM, O'Donnol D, Maddock J, Brun YV (2000) Regulation of stalk elongation by phosphate in Caulobacter crescentus. J Bacteriol 182: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann PL (2007) Cytoskeletal elements in bacteria. Annu Rev Microbiol 61: 589–618 [DOI] [PubMed] [Google Scholar]
- Hay NA, Tipper DJ, Gygi D, Hughes C (1999) A novel membrane protein influencing cell shape and multicellular swarming of Proteus mirabilis. J Bacteriol 181: 2008–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H, Aebi U (2004) Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem 73: 749–789 [DOI] [PubMed] [Google Scholar]
- Hodgkin J, Kaiser D (1977) Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci USA 74: 2938–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Kaiser D (1979) Genetics of gliding motility in Myxococcus xanthus (Myxobacterales)—2 gene systems control movement. Mol Gen Genet 171: 177–191 [Google Scholar]
- Iancu CV, Tivol WF, Schooler JB, Dias DP, Henderson GP, Murphy GE, Wright ER, Li Z, Yu Z, Briegel A, Gan L, He Y, Jensen GJ (2006) Electron cryotomography sample preparation using the Vitrobot. Nat Protoc 1: 2813–2819 [DOI] [PubMed] [Google Scholar]
- Iwai N, Nagai K, Wachi M (2002) Novel S-benzylisothiourea compound that induces spherical cells in Escherichia coli probably by acting on a rod-shape-determining protein(s) other than penicillin-binding protein 2. Biosci Biotechnol Biochem 66: 2658–2662 [DOI] [PubMed] [Google Scholar]
- Izard J, Samsonoff WA, Kinoshita MB, Limberger RJ (1999) Genetic and structural analyses of cytoplasmic filaments of wild-type Treponema phagedenis and a flagellar filament-deficient mutant. J Bacteriol 181: 6739–6746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LJ, Carballido-Lopez R, Errington J (2001) Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104: 913–922 [DOI] [PubMed] [Google Scholar]
- Kahnt J, Buchenau B, Mahlert F, Kruger M, Shima S, Thauer RK (2007) Post-translational modifications in the active site region of methyl-coenzyme M reductase from methanogenic and methanotrophic archaea. FEBS J 274: 4913–4921 [DOI] [PubMed] [Google Scholar]
- Kaiser D (1979) Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA 76: 5952–5956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashefi K, Hartzell PL (1995) Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF-defect. Mol Microbiol 15: 483–494 [DOI] [PubMed] [Google Scholar]
- Lenarcic R, Halbedel S, Visser L, Shaw M, Wu LJ, Errington J, Marenduzzo D, Hamoen LW (2009) Localisation of DivIVA by targeting to negatively curved membranes. EMBO J 28: 2272–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letek M, Ordonez E, Vaquera J, Margolin W, Flardh K, Mateos LM, Gil JA (2008) DivIVA is required for polar growth in the MreB-lacking rod-shaped actinomycete Corynebacterium glutamicum. J Bacteriol 190: 3283–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P (2007) Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23: 127–128 [DOI] [PubMed] [Google Scholar]
- Li Z, Trimble MJ, Brun YV, Jensen GJ (2007) The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J 26: 4694–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN (1997) Dual-axis tomography: an approach with alignment methods that preserve resolution. J Struct Biol 120: 343–352 [DOI] [PubMed] [Google Scholar]
- McGrath PT, Lee H, Zhang L, Iniesta AA, Hottes AK, Tan MH, Hillson NJ, Hu P, Shapiro L, McAdams HH (2007) High-throughput identification of transcription start sites, conserved promoter motifs and predicted regulons. Nat Biotechnol 25: 584–592 [DOI] [PubMed] [Google Scholar]
- Meisenzahl AC, Shapiro L, Jenal U (1997) Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J Bacteriol 179: 592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Lutkenhaus J (1998) Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J 17: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierman WC, Feldblyum TV, Laub MT, Paulsen IT, Nelson KE, Eisen JA, Heidelberg JF, Alley MR, Ohta N, Maddock JR, Potocka I, Nelson WC, Newton A, Stephens C, Phadke ND, Ely B, DeBoy RT, Dodson RJ, Durkin AS, Gwinn ML et al. (2001) Complete genome sequence of Caulobacter crescentus. Proc Natl Acad Sci USA 98: 4136–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Anderson DE, Erickson HP (2008) Reconstitution of contractile FtsZ rings in liposomes. Science 320: 792–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J (2008) The bacterial cytoskeleton. Curr Opin Cell Biol 20: 19–27 [DOI] [PubMed] [Google Scholar]
- Poindexter JS, Hagenzieker JG (1982) Novel peptidoglycans in Caulobacter and Asticcacaulis spp. J Bacteriol 150: 332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quardokus EM, Din N, Brun YV (2001) Cell cycle and positional constraints on FtsZ localization and the initiation of cell division in Caulobacter crescentus. Mol Microbiol 39: 949–959 [DOI] [PubMed] [Google Scholar]
- Quon KC, Marczynski GT, Shapiro L (1996) Cell cycle control by an essential bacterial two-component signal transduction protein. Cell 84: 83–93 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Thanbichler M, Viollier PH (2008) The dynamic interplay between a cell fate determinant and a lysozyme homolog drives the asymmetric division cycle of Caulobacter crescentus. Genes Dev 22: 212–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopala SV, Titz B, Goll J, Parrish JR, Wohlbold K, McKevitt MT, Palzkill T, Mori H, Finley RL Jr, Uetz P (2007) The protein network of bacterial motility. Mol Syst Biol 3: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Losick R (2009) Negative membrane curvature as a cue for subcellular localization of a bacterial protein. Proc Natl Acad Sci USA 106: 13541–13545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JM, Stanier RY (1966) The development of cellular stalks in bacteria. J Cell Biol 28: 423–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard-Andersen L (2004) Cell polarity, intercellular signalling and morphogenetic cell movements in Myxococcus xanthus. Curr Opin Microbiol 7: 587–593 [DOI] [PubMed] [Google Scholar]
- Stahlberg H, Kutejova E, Muchova K, Gregorini M, Lustig A, Muller SA, Olivieri V, Engel A, Wilkinson AJ, Barak I (2004) Oligomeric structure of the Bacillus subtilis cell division protein DivIVA determined by transmission electron microscopy. Mol Microbiol 52: 1281–1290 [DOI] [PubMed] [Google Scholar]
- Suloway C, Shi J, Cheng A, Pulokas J, Carragher B, Potter CS, Zheng SQ, Agard DA, Jensen GJ (2009) Fully automated, sequential tilt-series acquisition with Leginon. J Struct Biol 167: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M, Iniesta AA, Shapiro L (2007) A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res 35: e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M, Shapiro L (2006) MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell 126: 147–162 [DOI] [PubMed] [Google Scholar]
- Thanbichler M, Shapiro L (2008) Getting organized—how bacterial cells move proteins and DNA. Nat Rev Microbiol 6: 28–40 [DOI] [PubMed] [Google Scholar]
- Tivol WF, Briegel A, Jensen GJ (2008) An improved cryogen for plunge freezing. Microsc Microanal 14: 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T, Inouye S, Inouye M (1996) Positive-negative KG cassettes for construction of multi-gene deletions using a single drug marker. Gene 183: 153–157 [DOI] [PubMed] [Google Scholar]
- Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, MacGregor BJ, Ringelberg DB, White DC, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealson KH (1999) Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol 49: 705–724 [DOI] [PubMed] [Google Scholar]
- Wheeler RT, Shapiro L (1999) Differential localization of two histidine kinases controlling bacterial cell differentiation. Mol Cell 4: 683–694 [DOI] [PubMed] [Google Scholar]
- Williamson MP (1994) The structure and function of proline-rich regions in proteins. Biochem J 297: 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Errington J (2003) RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol Microbiol 49: 1463–1475 [DOI] [PubMed] [Google Scholar]
- Wu SS, Wu J, Kaiser D (1997) The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol Microbiol 23: 109–121 [DOI] [PubMed] [Google Scholar]
- Youderian P, Burke N, White DJ, Hartzell PL (2003) Identification of genes required for adventurous gliding motility in Myxococcus xanthus with the transposable element mariner. Mol Microbiol 49: 555–570 [DOI] [PubMed] [Google Scholar]
- Youderian P, Hartzell PL (2006) Transposon insertions of magellan-4 that impair social gliding motility in Myxococcus xanthus. Genetics 172: 1397–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QS, Braunfeld MB, Sedat JW, Agard DA (2004) An improved strategy for automated electron microscopic tomography. J Struct Biol 147: 91–101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material
Review Process File