Abstract
Dynamic interactions of cells with their environment regulate multiple aspects of tissue morphogenesis and function. Integrins are the major class of cell surface receptors that recognize and bind extracellular matrix proteins, resulting in the engagement and organization of the cytoskeleton as well as activation of signalling pathways to regulate cell behaviour and morphogenetic processes. The ternary complex of integrin-linked kinase (ILK), PINCH, and parvin (IPP complex), which was identified more than a decade ago, interacts with the cytoplasmic tail of β integrins and couples them to the actin cytoskeleton. In addition, ILK has been shown to act as a serine/threonine kinase and to directly activate several signalling pathways downstream of integrins. However, the kinase activity of ILK and the precise functions of the IPP complex have remained elusive and controversial. This review focuses on the recent advances made towards understanding the specialized roles this complex and its individual components have acquired during evolution.
Keywords: integrin, integrin-linked kinase, parvin, PINCH, tissue morphogenesis
Introduction
The structural organization of embryos and organogenesis require individual cells to sense and process extracellular information and to respond to these cues by changing cellular programmes such as cell division, death, proliferation, migration, and cell shape (Lecuit and Lenne, 2007). The extracellular information consists of chemical and mechanical cues imparted from growth factors and the extracellular matrix (ECM). The major cell surface receptors that cells use to recognize and assemble the ECM are integrins. Integrins are α/β heterodimers that can assemble in different combinations to confer substrate and signalling specificity. Lower eukaryotes such as worms and flies possess only a few integrin subunits, whereas mammals express 18 α subunits and 8 β subunits that dimerize in 24 different combinations with cell-type-specific expression patterns (Hynes, 2002; Humphries et al, 2006). Deletion of individual subunits in both lower and higher organisms confirmed an essential role for integrins in development and tissue morphogenesis by regulating cell attachment to the ECM, cell migration, cell survival, cell cycle progression, and by modulating differentiation pathways or the structure and composition of the ECM (Legate et al, 2009).
Integrins are composed of large extracellular domains and relatively small cytoplasmic tails and exist on the plasma membrane in both inactive and active conformations, which exhibit either low or high affinity for extracellular ligands, respectively (Luo et al, 2007; Moser et al, 2009b). For integrins to become activated (inside-out signalling) non-integrin-mediated signals lead to the recruitment and binding of adaptor proteins such as talin and kindlin to the cytoplasmic tails of β integrins (Garcia-Alvarez et al, 2003; Tadokoro et al, 2003; Montanez et al, 2008; Moser et al, 2008, 2009a; Anthis et al, 2009), resulting in integrin activation through conformational changes in the cytoplasmic, transmembrane and particularly in the extracellular domains. However, for integrins to generate high-affinity adhesion or to relay intracellular signals, clustering of several integrin heterodimers is necessary to increase avidity towards the ligand. After clustering, integrins assemble multiprotein cytoplasmic adhesion complexes termed focal adhesions (FA), which enable them to induce a vast array of intracellular changes (outside-in signalling) (Ginsberg et al, 2005; Legate et al, 2009). Thus, the response of the cell to integrin ligation depends not only on the type of integrin heterodimer but also on the molecular composition of the adhesion complex. The integrin-linked kinase (ILK)/PINCH/parvin (IPP) complex is a central constituent of at least β1 and β3 integrin containing adhesion sites, from where it regulates multiple cellular processes. This review will address the recent advances made towards understanding the function of the IPP complex and its individual components both in vivo and in vitro.
Molecular architecture of the IPP complex and its components
ILK, which is ubiquitously expressed in mammalian tissues, is composed of three structurally distinct domains. The N-terminus consists of five ankyrin repeats followed by a pleckstrin homology (PH)-like domain and a C-terminal kinase-like domain. The ankyrin repeats mediate the interaction with PINCH, a family of LIM domain only containing proteins consisting of two members, PINCH-1, and PINCH-2. Both PINCH proteins contain five LIM domains, the first of which binds ILK (Tu et al, 1999, 2001; Chiswell et al, 2008). The PH domain of ILK has been shown to bind phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) (Delcommenne et al, 1998; Pasquali et al, 2007). The C-terminal kinase-like domain binds several adaptor proteins including the parvins that consist of three members; the ubiquitously expressed α-parvin (also known as actopaxin or CH-ILKBP), β-parvin (also known as affixin), which is primarily expressed in heart and skeletal muscle, and γ-parvin, which is expressed in the haematopoietic system (Nikolopoulos and Turner, 2000; Olski et al, 2001; Tu et al, 2001; Yamaji et al, 2001; Chu et al, 2006). Parvins are characterized by an N-terminal polypeptide stretch followed by two calponin homology (CH) domains, of which the second mediates the interaction with ILK (Tu et al, 2001).
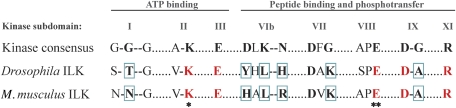
The significant sequence homology of the C-terminus of ILK to Ser/Thr protein kinases was the reason why at the time of discovery the integrin tail-binding protein was called a kinase. However, ILK lacks well-conserved motifs required for eukaryotic protein kinase activity (Hanks et al, 1988), and the putative kinase activity and its physiological relevance has remained a subject of debate and controversy. Although ILK contains the lysine residue in subdomain II required for phosphotransfer and the A/SPE motif in subdomain VIII involved in substrate recognition, the GxGxxG consensus sequence of the kinase subdomain I required for covering and anchoring the non-transferable phosphates of ATP is not conserved in ILK from different species. This suggests that if ILK is indeed a kinase, this function would have evolved late during evolution. It is, however, even more difficult to reconcile that ILK lacks the catalytic base in subdomain VIb, which accepts the proton from the hydroxyl group of the substrate during the phosphotransfer reaction, as well as the DFG motif in subdomain VII required to align the γ-phosphate of ATP. A conserved lysine, which neutralizes the charge on the γ-phosphate of ATP, and a conserved asparagine, which chelates the secondary magnesium ions, both in subdomain VIb, are also missing (Figure 1). Owing to these characteristics, ILK has also been classified as a pseudokinase, a catalytically inactive remnant of an active kinase that uses its substrate recognition motif to interact with other proteins (Boudeau et al, 2006). It should be noted that in some cases proteins with a pseudokinase-like sequence have been shown to possess catalytic activity. Ca2+/calmodulin-activated serine–threonine kinase (CASK), for example, binds ATP despite lacking two conserved residues that coordinate Mg2+ ions in most kinases, but it functions as a Mg2+-independent kinase that is actually inhibited by Mg2+ (Kannan and Taylor, 2008; Kornev and Taylor, 2009). Thus, the lack of key conserved residues has not been sufficient to exclude ILK as a kinase with an unusual catalytic mechanism.
Figure 1.
The kinase homology domain of ILK lacks several critical residues present in eukaryotic serine/threonine kinases. Alignment of highly conserved amino acids within the 12 subdomains of eukaryotic serine/threonine kinases (Hanks et al, 1988) with Drosophila and Mus musculus ILK. Conserved amino acids are marked in bold, and their counterparts in the ILK sequences are shown in red. Conserved amino acids that are not present in the ILK sequences are highlighted with blue boxes. Most importantly, ILK lacks the catalytic base in subdomain VIb. Substitution of the invariant lysine in subdomain II (ATP binding) to either an alanine or methionine (K220A/M in M. musculus ILK; marked with *) or of the invariant glutamate in subdomain VIII (substrate recognition) to a lysine (E359K in M. musculus ILK; marked with **) have been shown to render ILK catalytically inactive.
The kinase activity of ILK—the end of a controversy
As integrins themselves lack enzymatic activity, they propagate intracellular signals by recruiting signalling proteins such as tyrosine and serine/threonine kinases to their cytoplasmic tails. In the initial study identifying ILK as a direct binding partner of β1 integrin, Dedhar and co-workers showed that bacterially expressed recombinant ILK possesses kinase activity and phosphorylates serine and threonine residues in the cytoplasmic tail of β1 integrin (Hannigan et al, 1996). Since then, a large number of studies have been published on the potential kinase activity of ILK, claiming that ILK can directly phosphorylate a diverse set of substrates ranging from Akt, a kinase regulating key cellular functions such as cell cycle progression, survival, differentiation, and energy homeostasis, to myosin light chain (MLC) whose phosphorylation regulates actomyosin contractility and vascular tone (Legate et al, 2006).
Several lines of biochemical and cell biological evidence have supported the initial view that ILK might be an active kinase (Hannigan et al, 1996). It has been shown that recombinant ILK expressed in bacteria can phosphorylate the cytoplasmic tail of β1 integrin as well as the model substrate myelin basic protein (Hannigan et al, 1996; Delcommenne et al, 1998). Furthermore, purified ILK from mammalian cell extracts was shown to co-immunoprecipitate and phosphorylate Akt (Persad et al, 2001). Mutational analysis has been used to gain further insight into the catalytic activity of ILK, and several mutations have been described to abrogate the kinase activity in vitro. A serine (S) to alanine (A) substitution in the potential autophosphorylation site (S343A), an arginine (R) to A substitution in the potential PtdIns(3,4,5)P3-binding site of the PH domain (R211A), or a lysine (K) to A or to methionine (M) substitution in the putative ATP-binding site (K220A/M) have all been shown to result in a catalytically inactive ILK (Persad et al, 2001; Filipenko et al, 2005), whereas an S to aspartate (D) substitution in the autophosphorylation site (S343D) was shown to generate a hyperactive kinase (Persad et al, 2001). Importantly, however, these mutations have also been shown to disrupt the interaction of ILK with essential binding partners. The inactivating R211A mutation apparently disrupts the interaction with α-parvin and impairs the recruitment of ILK to FAs (Attwell et al, 2003), whereas the K220A mutation reduces β-parvin binding (Yamaji et al, 2001). These findings together with the observation that a combination of two inactivating mutations (S343D and K220M) can reverse the kinase dead phenotype despite abolishing the ability to bind ATP (Lynch et al, 1999), suggested that these mutations might affect the activation status of downstream substrates such as Akt phosphorylation by an indirect mechanism.
A breakthrough came with genetic studies in Caenorhabditis elegans and Drosophila melanogaster, which failed to confirm the kinase function of ILK in vivo. The reported kinase dead ILK mutants were capable of fully rescuing the severe phenotypes caused by ILK deletion in both species, indicating that ILK functions as a scaffold protein and not as a kinase in invertebrates (Zervas et al, 2001; Mackinnon et al, 2002). Controversy still remained, as it was argued that ILK might have acquired catalytic activity later in evolution. Some genetic studies in mice failed to show a critical role for ILK in phosphorylating key substrates, despite showing overt phenotypes related to impaired actin organization; ILK-deficient fibroblasts, chondrocytes, or keratinocytes do not show changes in Akt or Gsk-3β phosphorylation (Grashoff et al, 2003; Sakai et al, 2003; Lorenz et al, 2007). In addition, depletion of ILK in vascular smooth muscle cells (vSMCs) results in hyperphosphorylation of the proposed substrate MLC (Kogata et al, 2009; Montanez et al, 2009). In contrast, ablation of ILK in the heart, skeletal muscle, nervous system, or macrophages abrogated phosphorylation of Ser473 of Akt (Troussard et al, 2003; White et al, 2006; Wang et al, 2008a; Pereira et al, 2009), raising the possibility of redundancy or tissue-specific kinase function.
Owing to this controversy, it was important to determine whether the catalytic activity of ILK might be specific for mammals or certain cell types, and whether phosphorylation of key substrates was normal in ILK-null mice and cells because of compensation by another integrin-associated kinase. We established knock-in mouse strains with point mutations in ILK that were reported to affect ILK kinase activity. Surprisingly, knockin mice carrying mutations in the putative PH domain (R211A) or in the autophosphorylation site (S343A or S343D) are completely normal and do not show changes in Akt or Gsk-3β phosphorylation or actin organization downstream of integrins (Lange et al, 2009). In contrast, mice carrying point mutations in the potential ATP-binding site (K220A/M) die shortly after birth because of kidney agenesis. This phenotype does not result from impaired kinase activity, as the mutations did not alter the phosphorylation levels of reported ILK substrates in vivo. In addition, no evidence of kinase activity was detected in vitro (Lange et al, 2009). However, these mutations selectively impair the interaction of ILK and α-parvin. In line with this, similar kidney defects occur also in α-parvin-null mice (Lange et al, 2009; Montanez et al, 2009). Interestingly, the K220M mutant can fully rescue the developmental defects of ILK-null Drosophila (Zervas et al, 2001), which is likely because of major differences in organ systems of mammals and invertebrates. Flies and worms, for example, do not have kidneys.
Despite the lack of high-resolution structural data of the kinase-like domain as further proof for the pseudokinase function of ILK, it is now clear that the proposed kinase activity does not exist and, therefore, cannot have a role in mammalian development or adult life, or in the function of ILK as a mediator of the integrin–actin linkage in cells. This calls for the re-evaluation of a large number of studies published on the role of the kinase activity in numerous cellular processes. Several of the amino acid substitutions used in these studies proposed to abrogate the catalytic activity of ILK actually disrupt the function of the kinase homology domain as a critical and highly conserved mediator of protein–protein interactions at adhesion sites. Despite this conserved scaffold function it is also obvious that the complexity of mammalian tissue morphogenesis is facilitated by the assembly of more specialized adhesion complexes with distinct, tissue-specific signalling functions not present in lower organisms. This complexity is partly achieved by the different cellular functions of the PINCH and parvin isoforms, but also through interactions of ILK with various other structural and signalling proteins (Table 1). Therefore, complete characterization of the ILK interactome as well as careful analyses of the functional significance of the various interactions represent important tasks for future research.
Table 1.
The ILK interactome
| Binding partner | Proposed function | Mode of interaction | References |
|---|---|---|---|
| β1 integrin | Anchorage-independent growth | Directa | Hannigan et al (1996) |
| β3 integrin | Platelet aggregation | NA | Pasquet et al (2002) |
| PINCH-1/2 | Stabilization of IPP complex, cell spreading, migration | Directa,b,c | Chiswell et al (2008); Tu et al (2001); Tu et al (1999) |
| α/β/γ-Parvin | Stabilization of IPP complex, cell spreading, migration | Directa,c | Nikolopoulos and Turner (2000); Tu et al (2001); Yamaji et al (2001) |
| Paxillin | Recruitment of ILK to focal adhesions | Directc | Nikolopoulos and Turner (2001); Nikolopoulos and Turner (2002) |
| Kindlin-2 | Recruitment of ILK to focal adhesions | NA | Mackinnon et al (2002); Montanez et al (2008) |
| Thymosin-β4 | Actin polymerization, Akt phosphorylation | Directc | Bock-Marquette et al (2004); Fan et al (2009) |
| ILKAP | Regulation of Gsk-3β signalling | Directa | Leung-Hagesteijn et al (2001) |
| Rictor | Akt phosphorylation, cell survival | Directa | McDonald et al (2008b) |
| EphA1 | Cell shape and motility | Directa | Yamazaki et al (2009) |
| Akt1 | Akt1 phosphorylation, cell survival | NA | Persad et al (2001) |
| ELMO-2 | Cell polarity | NA | Ho et al (2009) |
| c-Src | Phosphorylation of cofilin, actin organization | NA | Kim et al (2008) |
| kAE1 | Actin linkage and membrane stability of kAE1 in kidney | NA | Keskanokwong et al (2007) |
| CNKSR3 gene chromatin | Regulation of transcription | NA | Acconcia et al (2007) |
| RUVBL1 | Spindle assembly | Indirecta | Fielding et al (2008) |
| Abbreviations: ILK, integrin-linked kinase; IPP, ILK/PINCH/parvin; ILKAP, ILK-associated phosphatase; ELMO-2, engulfment and cell motility 2; kAE1, kidney anion exchanger 1; NA, not analysed. | |||
| aDemonstrated with yeast-two-hybrid. | |||
| bDemonstrated with co-crystallization. | |||
| cDemonstrated with recombinant proteins. | |||
Assembly of distinct IPP complexes
The assembly of the mammalian IPP complex occurs before adhesion, suggesting that it assembles in the cytoplasm and is subsequently recruited to integrin adhesions (Zhang et al, 2002). ILK was identified in yeast-two-hybrid experiments to directly bind to the cytoplasmic tail of β1 integrin. Therefore, it was hypothesized that this direct interaction facilitates the recruitment of the IPP complex to FAs (Hannigan et al, 1996). However, a detailed molecular analysis of this interaction is still lacking, and the binding site on the integrin tail has not been mapped. Moreover, Drosophila ILK does not bind β integrin (βPS) indicating that a direct interaction is not required for its function in lower organisms (Zervas et al, 2001). Nevertheless, yeast-two-hybrid assays in the same study showed that Drosophila ILK can weakly bind human β1 integrin, whereas human ILK does not bind βPS, suggesting that evolutionary changes in the cytoplasmic tail of β1 integrin could have generated a binding site for ILK (Zervas et al, 2001). Recent studies, however, reported that the interaction between ILK and the integrin might be indirect also in mammals. Depletion of the FA protein kindlin-2, which directly binds to β integrin tails and also interacts with ILK, leads to the loss of ILK or PINCH from FAs, even when integrin activation and clustering was restored exogenously by the addition of manganese (Chen et al, 2008; Montanez et al, 2008). In addition, studies from our laboratory using quantitative proteomics to identify proteins that bind to the cytoplasmic tail of β1 integrin have failed to detect ILK whereas kindlin-2 and talin are readily detected (Meves and Fässler, unpublished data). It is currently unclear, however, whether the kindlin-2-mediated recruitment of ILK to integrin tails occurs only in a subset of cells. Likewise, it has not been tested whether all kindlin family members can bind ILK. It has also been shown that a point mutation in the kinase homology domain of ILK, which abolishes its interaction with the adaptor protein paxillin, prevents ILK localization to FAs (Nikolopoulos and Turner, 2001, 2002). However, paxillin binding seems not to be sufficient to recruit ILK, as paxillin localizes to integrin adhesions in the absence of kindlin-2, whereas ILK does not (Montanez et al, 2008). Therefore, it is likely that coordinated interplay between kindlin-2 and paxillin, instead of a direct interaction between ILK and the β1 integrin tail, facilitates the localization of the IPP complex to integrin adhesions. It is of course possible that later during the course of adhesion maturation the interaction of certain proteins with integrin cytoplasmic tails become displaced by ILK. An alternative possibility to such a sequential binding mode is that direct interactions of ILK with integrins may be restricted to certain cell types. Detailed molecular mapping of the interaction sites between ILK and kindlin-2 as well as between ILK and β1 integrin and analyses of the relevance of these interactions in vivo are required to obtain answers to these questions.
The stability of each individual component of the IPP complex depends on the assembly of the complex. Depletion of ILK or PINCH leads to a decrease, albeit not a total loss, in the protein levels of the other two complex members (Fukuda et al, 2003; Li et al, 2005). This has made it difficult to assess the independent functions of the IPP proteins. Interestingly, however, multiple cell types of both epithelial and mesenchymal lineages express both isoforms of PINCH as well as both α- and β-parvin. Binding of PINCH and parvin isoforms to ILK is mutually exclusive (Fukuda et al, 2003; Montanez et al, 2009), allowing cells to engineer molecularly distinct IPP complexes that have specific functions in modulating integrin signalling. Forced overexpression of PINCH-2 in PINCH-1-depleted cells can restore the assembly of the IPP complex, but this complex is unable to compensate for the functional defects caused by the loss of PINCH-1, whereas PINCH-1 can fully compensate for the loss of PINCH-2 both in vivo and in vitro (Braun et al, 2003; Fukuda et al, 2003; Li et al, 2005; Stanchi et al, 2005). Deletion of α-parvin leads to an upregulation of β-parvin expression, which acts to stabilize ILK and PINCH-1 levels, resulting in successful recruitment of this particular IPP complex to FAs. This is, however, not sufficient to functionally compensate for the loss of α-parvin (Montanez et al, 2009). Thus, a model of signalling specificities through molecularly distinct IPP complexes is beginning to emerge. This will be discussed in more detail later in this review.
An update of the functions of the IPP complex in vivo and in vitro
The biological functions of the IPP complex proteins have been extensively studied in several organisms and cell types (Table 2). Genetic ablation of ILK or PINCH-1 in mice results in embryonic lethality (Sakai et al, 2003; Li et al, 2005). Mice lacking ILK expression die during peri-implantation because of a failure in epiblast polarization, which is associated with severe defects in F-actin organization at adhesion sites (Sakai et al, 2003). ILK-deficient fibroblasts display defects in cell adhesion, spreading, and migration because of a delay in the formation of FAs that also fail to mature and are poorly linked to a disorganized actin cytoskeleton (Sakai et al, 2003; Stanchi et al, 2009). The defective maturation of ILK-deficient FAs into fibrillar adhesions leads to defects in deposition of the fibronectin matrix (Stanchi et al, 2009). Interestingly, this function requires the interaction of ILK with α-parvin but not with PINCH-1 (Stanchi et al, 2009). The essential role of ILK in linking integrins to the actin cytoskeleton has been further confirmed in several tissue and cell types (McDonald et al, 2008a). Recent studies suggest that ILK does not only regulate the actin cytoskeleton but can also modulate the microtubule network and influence mitotic spindle orientation (Dobreva et al, 2008; Fielding et al, 2008). However, as loss of ILK can lead to both increased or decreased proliferation rates in vivo, depending on the cellular context (Grashoff et al, 2003; Sakai et al, 2003; Lorenz et al, 2007; Gkretsi et al, 2008), the relevance of these functions needs to be established.
Table 2.
In vivo analyses of IPP proteins
| Gene | Organism | Tissue | Phenotype | References |
|---|---|---|---|---|
| ILK | Caenorhabditis elegans | Embryonic lethality; muscle attachment defect | Mackinnon et al (2002) | |
| Drosophila melanogaster | Embryonic lethality; actin detachment from muscle membrane, adult wing blisters | Zervas et al (2001) | ||
| Xenopus laevi | Embryonic lethality; blastopore closure and axis elongation defects | Yasunaga et al (2005) | ||
| Zebrafish | Embryonic lethality; cardiovascular defects | Bendig et al (2006); Friedrich et al (2004); Postel et al (2008) | ||
| Mus musculus | Constitutive | Embryonic lethality between E5.5 and E6.5; abnormal epiblast polarization, impaired cavitation, cell detachment from the ECM | Sakai et al (2003) | |
| Bone (chondrocytes; Col2-Cre) | Perinatal lethality; chondrodysplasia, dwarfism, and respiratory distress | Grashoff et al (2003); Terpstra et al (2003) | ||
| Cardiovascular system (cardiomyocytes; Mck-Cre) | Cardiomyopathy and heart failure | White et al (2006) | ||
| Cardiovascular system (EC; Tie2-Cre) | Embryonic lethality between E10.5 and E12.5; embryonic and extra-embryonic vascular defects | Friedrich et al (2004) | ||
| Cardiovascular system (vSMC; PDGFRβ-Cre) | Embryonic lethality between E13.5 and E18.5; abnormal vessel wall formation | Kogata et al (2009) | ||
| Skin (keratinocytes; K5-Cre and K14-Cre) | Epidermal defects and hair loss | Lorenz et al (2007); Nakrieko et al (2008) | ||
| Skeletal muscle (HSA-Cre) | Mild progressive muscular dystrophy | Gheyara et al (2007); Wang et al (2008a) | ||
| Immune system (T cells; Lck-Cre) | T-cell chemotaxis and survival defects | Liu et al (2005) | ||
| Haematopoietic system (platelets; Mx1-Cre) | Abnormal platelet aggregation, granule secretion, and thrombus formation | Tucker et al (2008) | ||
| Liver (hepatocytes; AFP-Cre) | Hepatocyte differentiation defect | Gkretsi et al (2008) | ||
| Kidney (podocytes; podocin-Cre) | Fibrosis and proteinuria | Dai et al (2006); El-Aouni et al (2006); Kanasaki et al (2008) | ||
| Central nervous system (nestin-Cre) | Granule cell precursor proliferation defects and Bergmann glial cell differentiation defects | Belvindrah et al (2006); Mills et al (2006) | ||
| Central nervous system (neuroepithelium; Emx1-Cre) | Cortical lamination defects | Niewmierzycka et al (2005) | ||
| Peripheral nervous system (Schwann cells; Dhh-Cre) | Abnormal radial sorting and remyelination of axons | Pereira et al (2009) | ||
| Mammary gland (mammary epithelial cells; Blg-Cre and Wap-Cre) | Defects in post-pregnancy mammary gland development and differentiation | Akhtar et al (2009) | ||
| PINCH-1 | Caenorhabditis elegansa | Embryonic lethality; muscle attachment defect | Hobert et al (1999) | |
| Drosophila melanogastera | Embryonic lethality; actin detachment from muscle membrane, adult wing blisters | Clark et al (2003); Kadrmas et al (2004) | ||
| Mus musculus | Constitutive | Embryonic lethality between E6.5 and E7.5; abnormal epiblast polarization, impaired cavitation, cell detachment from the ECM, abnormal cell–cell contacts, impaired endoderm survival | Li et al (2005); Liang et al (2005) | |
| Mus musculus | Cardiac muscle (ventricular cardiomyocytes; MLC2v-Cre) | Viable | Liang et al (2005) | |
| PINCH-2 | Mus musculus | Constitutive | Viable | Stanchi et al (2005) |
| PINCH-1 and PINCH-2 | Mus musculus | Skeletal and cardiac muscle (cTNT-Cre) | Early postnatal lethality; dilated cardiomyopathy | Liang et al (2009) |
| α-Parvin | Caenorhabditis elegansb | Embryonic lethality; muscle attachment defect | Lin et al (2003) | |
| Mus musculus | Constitutive | Embryonic lethality between E10.5 and E14.5; cardiovascular and kidney defects | Lange et al (2009); Montanez et al (2009) | |
| β-Parvin | Mus musculus | Constitutive | Viable | Thievessen and Fässler (unpublished) |
| γ-Parvin | Mus musculus | Constitutive | Viable | Chu et al (2006) |
| Abbreviations: AFP, α-feto protein; Blg, bovine β-lactoglobulin; Col2, collagen-2; cTNT, cardiac troponin T; Dhh, desert hedgehog homolog; E, embryonic day; EC, endothelial cell; ECM, extracellular matrix; Emx1, empty spiracles homeobox 1; HSA, human skeletal α-actin; K5, keratin 5; K14, keratin 14; Lck, lymphocyte protein tyrosine kinase; Mck, muscle creatine kinase; MLC2v, myosin light chain 2v; Mx1, myxovirus resistant protein1; PDGFRβ, platelet-derived growth factor receptor β; vSMC, vascular smooth muscle cell; Wap, whey acidic protein. | ||||
| aPossess only a single PINCH isoform. | ||||
| bPossess only a single parvin isoform. | ||||
PINCH-1 is ubiquitously expressed throughout mammalian development and adult life, whereas PINCH-2 expression is observed during the second half of embryonic development and has a slightly more restricted expression pattern (Braun et al, 2003). Ablation of PINCH-2 does not affect mouse development, but loss of PINCH-1 results in abnormal epiblast polarity, impaired cavitation, and detachment of endoderm and epiblast from basement membranes (Li et al, 2005). However, the functions of PINCH-1 are not restricted to the regulation of cell-matrix adhesions as PINCH-1 has been shown to regulate cell–cell adhesion of the endoderm and epiblast as well as cell survival in the endoderm layer (Li et al, 2005). As ILK has not been shown to have a role in these processes, it seems that several functions of PINCH are independent of the IPP complex. How PINCH regulates cell–cell adhesion is not understood, but the mechanisms by which it regulates cell survival are beginning to unravel. In Drosophila, PINCH antagonizes the activation of c-Jun N-terminal kinase (JNK) during dorsal closure and seems to fine tune JNK signalling and mediates its cross talk with integrin signalling to allow epithelial morphogenesis. This occurs through binding of PINCH to Ras suppressor 1 (RSU-1), a negative regulator of JNK signalling (Kadrmas et al, 2004). Interestingly, PINCH-2 cannot bind RSU-1, which might partly explain why it is unable to compensate for the loss of PINCH-1 in mammals (Kadrmas et al, 2004; Dougherty et al, 2005). PINCH-1 is found frequently to be upregulated in human tumours (Eke et al, manuscript submitted), and depletion of PINCH-1 results in reduced phosphorylation of Akt on both Ser473 and Thr308 accompanied by decreased cell survival (Eke et al, manuscript submitted; Fukuda et al, 2003). In addition, PINCH-1 can protect cancer cells from apoptosis through regulation of the Erk-Bim pathway (Chen et al, 2008). As Akt activation has been shown to suppress JNK, which in turn downregulates Erk (Junttila et al, 2008; Kim et al, 2009), it is possible that PINCH-1 regulates all of these pathways by modulating Akt phosphorylation. In line with this possibility is a recent report showing that PINCH-1 binds and inhibits the protein phosphatase 1α (PP1α) (Eke et al, manuscript submitted), which can directly dephosphorylate Akt. The inhibition of PP1α activity leads to increased levels of Akt phosphorylation and increased cell survival upon radiation. Consequently, deletion of PINCH-1 revokes PP1α inhibition, decreased Akt phosphorylation, and compromised cell survival (Eke et al, manuscript submitted).
Like ILK and PINCH, parvins have a role in modulating cell spreading and actin organization downstream of integrins. However, the role of parvins in these processes is more complex and the precise functions of the different isoforms in vivo are not clear. Mice lacking β- or γ-parvin show no obvious phenotypes, whereas α-parvin-null mice die between E11.5 and E14.5, suggesting that the parvin isoforms can functionally substitute for each other during development (Chu et al, 2006; Montanez et al, 2009; Thievessen and Fässler, in preparation). All parvins contain two CH domains and bind F-actin in vitro, but the functional significance of this interaction is unknown (Olski et al, 2001; Yamaji et al, 2001, 2004). The primary sequences of both CH domains of α-parvin are highly diverged from the typical CH domains found in actin-binding domains (Gimona et al, 2002), and it has been shown that α-parvin uses these domains to interact with paxillin (Nikolopoulos and Turner, 2000; Lorenz et al, 2008; Wang et al, 2008b). As the C-terminal region containing the CH domains is highly conserved throughout the parvin family, it has been suggested that all parvin paralogues may be able to bind paxillin and its homologue Hic-5 (Lorenz et al, 2008). Interestingly, however, γ-parvin can interact with paxillin (Yoshimi et al, 2006), to which β-parvin seems not to bind (Yamaji et al, 2001), despite being more homologous to α-parvin than γ-parvin is. In contrast, β-parvin binds the actin-modulatory protein α-actinin (Yamaji et al, 2004). The differential interactions might allow distinct functions for the individual isoforms. Indeed, knockdown of β-parvin in HeLa cells results in reduced cell spreading, whereas depletion of α-parvin increases cell spreading (Fukuda et al, 2003; Yamaji et al, 2004). However, most of the cellular studies have been carried out with a single parvin isoform instead of directly comparing the properties of the three proteins. Therefore, additional studies are required to understand the precise functions and signalling specificities of the parvin isoforms.
The use of lower eukaryotes, such as C. elegans and D. melanogaster as model organisms, has allowed functional analysis of the IPP complex in a simplified system, as they express only a few integrin subunits and single orthologues for PINCH and parvin. Studies in C. elegans have shown that ILK (PAT-4), PINCH (UNC-97), and parvin (PAT-6) co-localize with β integrin (PAT-3) at muscle attachment sites termed dense bodies and M-lines, which attach actin filaments to the basal sarcolemma. Deletion of β integrin or any member of the IPP complex leads to a paralysed, arrested elongation at twofold (Pat) phenotype, characterized by embryonic lethality and detachment of muscles from the body wall because of defects in dense body and M-line assembly (Mackinnon et al, 2002; Lin et al, 2003; Norman et al, 2007). In Drosophila, PINCH and ILK co-localize with βPS integrins at muscle attachment sites and the basal junctions of the wing epithelium. Similar to the deletion of βPS, both ILK- and PINCH-null flies show detachment of muscles from the body wall (Zervas et al, 2001; Clark et al, 2003). A parvin mutant has not been described so far. Although in the integrin mutant the detachment occurs between the ECM and the plasma membrane, the ILK- and PINCH-null phenotypes are characterized by detachment of the actin filaments from the muscle ends, establishing an essential function for these proteins in reinforcing the link between integrins and the actin cytoskeleton (Zervas et al, 2001; Clark et al, 2003). However, loss of integrin function causes additional defects in midgut morphogenesis and dorsal closure (Newman and Wright, 1981; Roote and Zusman, 1995), which are present in the PINCH-null flies (Kadrmas et al, 2004), but not in the ILK mutants (Zervas et al, 2001). This suggests that ILK is indispensable for only a subset of integrin functions and that ILK and PINCH have independent functions in mediating integrin signalling in the fly. In addition, IPP proteins can localize to adhesion sites independently of each other both in Drosophila and C. elegans (Zhang et al, 2002; Clark et al, 2003; Lin et al, 2003). Thus, despite extensive similarities in its role as an essential link between integrins and the actin cytoskeleton, the molecular regulation of the IPP complex seems not to be conserved among species, which might further reflect differences in its function between invertebrates and vertebrates.
ILK/α-parvin complex: a negative regulator of cell contractility
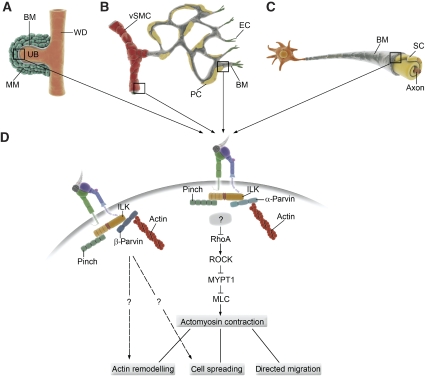
Genetic studies have firmly established a role for the IPP complex in adhesion strengthening and organization of the actin cytoskeleton downstream of integrins. However, as several of the functions in mice seem to be tissue specific, the key question as to how this specificity is achieved remains unanswered. The analysis of α-parvin-null mice has revealed a specific function for this protein in regulating cell contractility in a subset of cell types. Unlike ablation of ILK or PINCH-1, disruption of the α-parvin gene in mice does not lead to peri-implantation lethality. Despite the ubiquitous expression pattern of α-parvin, these mice survive to E14.5, because of the ability of β-parvin to compensate during early development (Montanez and Fässler, unpublished data), and die as a result of severe cardiovascular defects (Montanez et al, 2009). The absence of α-parvin causes impaired investment of vSMCs to developing vessel walls resulting in defective stabilization of the vasculature and subsequent dilation of vessels, formation of microaneurysms, and vessel rupture. These defects are caused by increased RhoA activity that leads to elevated MLC phosphorylation and aberrant actomyosin contractility (Montanez et al, 2009). RhoA is a small GTPase that regulates cell contractility through activation of its downstream target ROCK, which in turn can either indirectly activate MLC through phosphorylation and inactivation of MLC phosphatase, or by directly phosphorylating MLC (Amano et al, 1996; Kimura et al, 1996). Spatiotemporal regulation of RhoA activity is critical for directed cell motility, as activation of RhoA is necessary for the retraction of the trailing edge (Burridge and Wennerberg, 2004), whereas suppression of RhoA activity is required to promote lamellipodial protrusion at the leading edge (Arthur et al, 2000; Arthur and Burridge, 2001). On the other hand, RhoA/ROCK signalling has been shown to drive fast, random motility, whereas activation of the GTPase Rac promotes persistent cell migration (Sahai and Marshall, 2003; Danen et al, 2005; Sanz-Moreno et al, 2008). Consistently with this, the α-parvin-null vSMCs are hypercontractile and fail to establish a persistent leading edge, resulting in an increase of random motility but a loss of directed migration towards the vessel wall (Montanez et al, 2009). Interestingly, α-parvin-null fibroblasts or endothelial cells do not display a hypercontractile phenotype, suggesting that the function of α-parvin as a negative regulator of RhoA is cell-type specific (Montanez et al, 2009). Tissue-specific ablation of ILK in vSMCs leads to a similar phenotype with impaired coverage of the vasculature by mural cells (Kogata et al, 2009). These mice die around E18.5 because of haemorrhages and oedema caused by failure of the vSMCs to form a unitary, stabilizing cell layer around the endothelial tubes, leading to local vessel constriction and rupture. Like in the α-parvin-null mice, these defects are due to an aberrant upregulation of RhoA/ROCK signalling, resulting in hypercontractility of the vSMCs (Kogata et al, 2009). A similar role for ILK in the regulation of Rho activity has been observed in Schwann cells of the nervous system, where ablation of ILK leads to upregulation of Rho/ROCK signalling, resulting in the inability of the Schwann cells to extend cytoplasmic processes to envelope the nerves (Pereira et al, 2009). Interestingly, point mutations in the potential ATP-binding site of ILK, which selectively disrupt its interaction with α-parvin, induce contractile cell behaviour as well as enhanced random motility and loss of directional cell migration in collecting duct epithelial cells (Lange et al, 2009), suggesting involvement of a similar RhoA/ROCK-dependent mechanism. These studies collectively identify the ILK/α-parvin complex as a negative regulator of cell contractility in certain cell types, and point to α-parvin as the critical modulator of this function. They further suggest that a single parvin isoform seems to be sufficient to fulfil the functions of the IPP complex during early embryonic development of mammals or in less developed organisms. However, later on when differentiation of tissues requires more specialized signalling, such as tight regulation of contractility in the vSMCs or precise spatiotemporal regulation of cell migration in the ureteric bud epithelium, different parvin isoforms construct functionally distinct IPP complexes (Figure 2).
Figure 2.
Model of how the ILK/α-parvin complex regulates tissue morphogenesis by suppressing cell contractility. (A–C) Examples of morphogenetic events that require tight regulation of cell contractility for promoting directional migration and cellular organization. (A) Kidney development is initiated by the outgrowth of the ureteric bud (UB) from the Wolffian duct (WD) to invade the surrounding metanephric mesenchyme (MM) resulting in nephron formation and formation of the collecting duct system. BM, basement membrane. (B) Vascular smooth muscle cells (vSMC) and pericytes (PC) are recruited by the endothelial cells (EC) to surround large arteries and capillaries, respectively. The recruited vSMCs and PCs subsequently spread around the vessels to stabilize the endothelial tubes, to guide vascular remodelling, and to regulate vessel tone. (C) Schwann cells extend cellular processes and wrap axons of neurons to generate a myelin sheet that promotes neuronal survival and enhances the conduction velocity of nerve impulses. (D) Two molecularly distinct IPP complexes associate with integrins at cell-matrix adhesions. The ILK/PINCH/α-parvin complex functions as a mechanosensor to downregulate RhoA signalling downstream of cell adhesion, possibly by recruiting an unidentified negative regulator of RhoA (illustrated with a boxed question mark). Suppression of RhoA activity leads to decreased activation of the downstream target ROCK. ROCK regulates contractility by inactivating the myosin light chain phosphatase (MYPT), which dephosphorylates and inactivates myosin light chain (MLC). ROCK has also been shown to directly phosphorylate and activate MLC. Activated MLC promotes actomyosin contractility. Spatiotemporal regulation of RhoA activity and actomyosin contractility is essential to promote actin remodelling, cell spreading, and directed cell migration. Enhanced RhoA activity, which occurs in the absence of ILK/α-parvin, leads to actomyosin hypercontractility resulting in enhanced actin stress fibre formation, decreased cell spreading, and loss of directional migration. The specific functions of the ILK/PINCH/β-parvin complex are not clear, but are likely to involve remodelling of the actin cytoskeleton downstream of integrin adhesion.
The precise molecular mechanism, by which the ILK/α-parvin complex regulates RhoA/ROCK signalling, remains open for future research. Interestingly, deleting the β1 integrin gene in vSMCs also leads to decreased stability of the vasculature (Abraham et al, 2008), but the mechanism underlying this defect seems to differ from that of the ILK- and α-parvin-null mice. Loss of β1 integrin leads to defective differentiation and aberrant proliferation of vSMC (Abraham et al, 2008), but these cells do not display a hypercontractile phenotype or increased levels of MLC phosphorylation indicative of enhanced RhoA activity (Kogata et al, 2009). This is in agreement with in vitro studies in which the fibronectin receptor α5β1 has been shown to promote RhoA/ROCK signalling, resulting in upregulation of random motility, whereas the fibronectin receptor αvβ3 has been shown to promote directional motility through activation of Rac (Danen et al, 2002, 2005). The downstream signals induced by these two heterodimers are regulated by an elegant mechanism of cross talk, where ligation of αvβ3 integrin inhibits the recycling of α5β1 back to the cell surface, resulting in decreased ability of this integrin to promote RhoA/ROCK-mediated random motility (White et al, 2007). Conversely, inhibition or downregulation of αvβ3 leads to increased recycling of α5β1 to restore ROCK signalling (White et al, 2007). Hence, there are at least two potential scenarios whereby the ILK/α-parvin complex could operate. In the first scenario, the cell-type specificity of the complex is determined by the integrin heterodimer to which the complex is bound. In vSMCs, the ILK/α-parvin complex could specifically regulate signalling downstream of β3 integrin, and ablation of the complex would lead to compromised β3 signalling and subsequent predominance of β1 integrin-mediated RhoA/ROCK activation resulting in hypercontractility and loss of persistent cell migration. In the alternative model, the ILK/α-parvin complex could function as a negative feedback loop for β1 integrin through recruitment of negative regulator(s) of the RhoA/ROCK/MLC2 signalling pathway to this complex. The tissue specificity of the signalling would then be achieved by the relative expression levels of α-/β-parvin and/or by the cell-type-specific expression pattern of the yet unidentified negative regulator(s) downstream of α-parvin (Figure 2).
Concluding remarks
Recent studies have lead to important and exciting advances in the overall as well as specific understanding of IPP complex functions. It is now clear that the putative kinase activity of ILK is non-existent and thus cannot be required for its function in vivo, and that the kinase homology domain is a critical mediator of several protein–protein interactions. Therefore, ILK is an essential scaffold protein, whose central function is to target the IPP complex to integrin adhesion sites. Whether this involves a direct interaction of ILK with β integrins and how kindlin and paxillin cooperate to modulate the recruitment of the complex to FAs require more analyses. Comparative studies using different cell types might further reveal specific modes of recruitment that could enable tissue-specific functions of the IPP complex as well as its individual components.
The assembly of molecularly distinct IPP complexes has turned out to be another critical determinant of tissue specificity, but the mechanisms that regulate the relative abundance of the various complexes, as well as the distinct functional properties of the individual components that ultimately generate this specificity remain unknown. Identification of accessory proteins that cooperate with the IPP complex to regulate tissue-specific functions as well as biochemical and structural studies comparing the different PINCH and parvin isoforms are needed to understand the molecular determinants for the specific functions of these complexes. Finally, as most of the in vivo studies on the IPP complex proteins have been carried out using constitutive or tissue-specific deletions of the components, which leads to a significant reduction in the levels of the other members, further in vivo analyses using point mutations that specifically disrupt the various interactions of these proteins will be an important approach to overcome this limitation and to provide more insights into the functions of these proteins in development and disease.
Note added in proof
After submission of our manuscript Qin and co-workers (Fukuda et al, 2009) reported the high-resolution crystal structure of the ILK kinase domain, which revealed a kinase fold with a distinct pseudosubstrate active site conformation, further corroborating ILK as a pseudokinase.
Acknowledgments
We thank Roy Zent, Mercedes Costell, and members of the Fässler lab for discussion and careful reading of the paper, and Max Iglesias for artwork. SAW is supported by the Sigrid Juselius Foundation, the Academy of Finland and the Finnish Cultural Foundation. The ILK/PINCH/parvin work is supported by the Austrian Science Funds (SFB021) and the Max Planck Society.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abraham S, Kogata N, Fässler R, Adams RH (2008) Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res 102: 562–570 [DOI] [PubMed] [Google Scholar]
- Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R (2007) Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci USA 104: 6782–6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Marlow R, Lambert E, Schatzmann F, Lowe ET, Cheung J, Katz E, Li W, Wu C, Dedhar S, Naylor MJ, Streuli CH (2009) Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development 136: 1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249 [DOI] [PubMed] [Google Scholar]
- Anthis NJ, Wegener KL, Ye F, Kim C, Goult BT, Lowe ED, Vakonakis I, Bate N, Critchley DR, Ginsberg MH, Campbell ID (2009) The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J 28: 3623–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Burridge K (2001) RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell 12: 2711–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Petch LA, Burridge K (2000) Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol 10: 719–722 [DOI] [PubMed] [Google Scholar]
- Attwell S, Mills J, Troussard A, Wu C, Dedhar S (2003) Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell 14: 4813–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Nalbant P, Ding S, Wu C, Bokoch GM, Müller U (2006) Integrin-linked kinase regulates Bergmann glial differentiation during cerebellar development. Mol Cell Neurosci 33: 109–125 [DOI] [PubMed] [Google Scholar]
- Bendig G, Grimmler M, Huttner IG, Wessels G, Dahme T, Just S, Trano N, Katus HA, Fishman MC, Rottbauer W (2006) Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev 20: 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D (2004) Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432: 466–472 [DOI] [PubMed] [Google Scholar]
- Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR (2006) Emerging roles of pseudokinases. Trends Cell Biol 16: 443–452 [DOI] [PubMed] [Google Scholar]
- Braun A, Bordoy R, Stanchi F, Moser M, Kostka GG, Ehler E, Brandau O, Fässler R (2003) PINCH2 is a new five LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp Cell Res 284: 239–250 [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116: 167–179 [DOI] [PubMed] [Google Scholar]
- Chen K, Tu Y, Zhang Y, Blair HC, Zhang L, Wu C (2008) PINCH-1 regulates the ERK-Bim pathway and contributes to apoptosis resistance in cancer cells. J Biol Chem 283: 2508–2517 [DOI] [PubMed] [Google Scholar]
- Chiswell BP, Zhang R, Murphy JW, Boggon TJ, Calderwood DA (2008) The structural basis of integrin-linked kinase-PINCH interactions. Proc Natl Acad Sci USA 105: 20677–20682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Thievessen I, Sixt M, Lämmermann T, Waisman A, Braun A, Noegel AA, Fässler R (2006) Gamma-parvin is dispensable for hematopoiesis, leukocyte trafficking, and T-cell-dependent antibody response. Mol Cell Biol 26: 1817–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, McGrail M, Beckerle MC (2003) Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130: 2611–2621 [DOI] [PubMed] [Google Scholar]
- Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y (2006) Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17: 2164–2175 [DOI] [PubMed] [Google Scholar]
- Danen EH, Sonneveld P, Brakebusch C, Fässler R, Sonnenberg A (2002) The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 159: 1071–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, Sonnenberg A (2005) Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol 169: 515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 95: 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobreva I, Fielding A, Foster LJ, Dedhar S (2008) Mapping the integrin-linked kinase interactome using SILAC. J Proteome Res 7: 1740–1749 [DOI] [PubMed] [Google Scholar]
- Dougherty GW, Chopp T, Qi SM, Cutler ML (2005) The Ras suppressor Rsu-1 binds to the LIM 5 domain of the adaptor protein PINCH1 and participates in adhesion-related functions. Exp Cell Res 306: 168–179 [DOI] [PubMed] [Google Scholar]
- El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, Miner JH, Moeller MJ, St-Arnaud R, Dedhar S, Holzman LB, Wanke R, Kretzler M (2006) Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol 17: 1334–1344 [DOI] [PubMed] [Google Scholar]
- Fan Y, Gong Y, Ghosh PK, Graham LM, Fox PL (2009) Spatial coordination of actin polymerization and ILK-Akt2 activity during endothelial cell migration. Dev Cell 16: 661–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S (2008) Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J Cell Biol 180: 681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko NR, Attwell S, Roskelley C, Dedhar S (2005) Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene 24: 5837–5849 [DOI] [PubMed] [Google Scholar]
- Friedrich EB, Liu E, Sinha S, Cook S, Milstone DS, MacRae CA, Mariotti M, Kuhlencordt PJ, Force T, Rosenzweig A, St-Arnaud R, Dedhar S, Gerszten RE (2004) Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol 24: 8134–8144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Gupta S, Chen K, Wu C, Qin J (2009) The pseudoactive site of ILK is essential for Its binding to a-parvin and localization to focal adhesions. Mol Cell 36: 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Chen K, Shi X, Wu C (2003) PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem 278: 51324–51333 [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez B, de Pereda JM, Calderwood DA, Ulmer TS, Critchley D, Campbell ID, Ginsberg MH, Liddington RC (2003) Structural determinants of integrin recognition by talin. Mol Cell 11: 49–58 [DOI] [PubMed] [Google Scholar]
- Gheyara AL, Vallejo-Illarramendi A, Zang K, Mei L, St-Arnaud R, Dedhar S, Reichardt LF (2007) Deletion of integrin-linked kinase from skeletal muscles of mice resembles muscular dystrophy due to alpha 7 beta 1-integrin deficiency. Am J Pathol 171: 1966–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Djinovic-Carugo K, Kranewitter WJ, Winder SJ (2002) Functional plasticity of CH domains. FEBS Lett 513: 98–106 [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Partridge A, Shattil SJ (2005) Integrin regulation. Curr Opin Cell Biol 17: 509–516 [DOI] [PubMed] [Google Scholar]
- Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St-Arnaud R, Dedhar S, Kaestner KH, Wu C, Michalopoulos GK (2008) Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 48: 1932–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C, Aszodi A, Sakai T, Hunziker EB, Fässler R (2003) Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep 4: 432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241: 42–52 [DOI] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S (1996) Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379: 91–96 [DOI] [PubMed] [Google Scholar]
- Ho E, Irvine T, Vilk GJ, Lajoie G, Ravichandran KS, D'Souza SJ, Dagnino L (2009) Integrin-linked kinase interactions with ELMO2 modulate cell polarity. Mol Biol Cell 20: 3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Moerman DG, Clark KA, Beckerle MC, Ruvkun G (1999) A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J Cell Biol 144: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ (2006) Integrin ligands at a glance. J Cell Sci 119: 3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687 [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J (2008) Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 22: 954–965 [DOI] [PubMed] [Google Scholar]
- Kadrmas JL, Smith MA, Clark KA, Pronovost SM, Muster N, Yates JR III, Beckerle MC (2004) The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J Cell Biol 167: 1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH Jr, Kalluri R (2008) Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N, Taylor SS (2008) Rethinking pseudokinases. Cell 133: 204–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskanokwong T, Shandro HJ, Johnson DE, Kittanakom S, Vilas GL, Thorner P, Reithmeier RA, Akkarapatumwong V, Yenchitsomanus PT, Casey JR (2007) Interaction of integrin-linked kinase with the kidney chloride/bicarbonate exchanger, kAE1. J Biol Chem 282: 23205–23218 [DOI] [PubMed] [Google Scholar]
- Kim MA, Kim HJ, Jee HJ, Kim AJ, Bae YS, Bae SS, Yun J (2009) Akt2, but not Akt1, is required for cell survival by inhibiting activation of JNK and p38 after UV irradiation. Oncogene 28: 1241–1247 [DOI] [PubMed] [Google Scholar]
- Kim YB, Choi S, Choi MC, Oh MA, Lee SA, Cho M, Mizuno K, Kim SH, Lee JW (2008) Cell adhesion-dependent cofilin serine 3 phosphorylation by the integrin-linked kinase.c-Src complex. J Biol Chem 283: 10089–10096 [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248 [DOI] [PubMed] [Google Scholar]
- Kogata N, Tribe RM, Fässler R, Way M, Adams RH (2009) Integrin-linked kinase controls vascular wall formation by negatively regulating Rho/ROCK-mediated vascular smooth muscle cell contraction. Genes Dev 23: 2278–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornev AP, Taylor SS (2009) Pseudokinases: functional insights gleaned from structure. Structure 17: 5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A, Wickström SA, Jakobson M, Zent R, Sainio K, Fässler R (2009) Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature 461: 1002–1006 [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF (2007) Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol 8: 633–644 [DOI] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fässler R (2006) ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7: 20–31 [DOI] [PubMed] [Google Scholar]
- Legate KR, Wickström SA, Fässler R (2009) Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 23: 397–418 [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Mahendra A, Naruszewicz I, Hannigan GE (2001) Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J 20: 2160–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fässler R (2005) PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci 118: 2913–2921 [DOI] [PubMed] [Google Scholar]
- Liang X, Sun Y, Ye M, Scimia MC, Cheng H, Martin J, Wang G, Rearden A, Wu C, Peterson KL, Powell HC, Evans SM, Chen J (2009) Targeted ablation of PINCH1 and PINCH2 from murine myocardium results in dilated cardiomyopathy and early postnatal lethality. Circulation 120: 568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J Jr, Chen J (2005) PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol 25: 3056–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Qadota H, Moerman DG, Williams BD (2003) C. elegans PAT-6/actopaxin plays a critical role in the assembly of integrin adhesion complexes in vivo. Curr Biol 13: 922–932 [DOI] [PubMed] [Google Scholar]
- Liu E, Sinha S, Williams C, Cyrille M, Heller E, Snapper SB, Georgopoulos K, St-Arnaud R, Force T, Dedhar S, Gerszten RE (2005) Targeted deletion of integrin-linked kinase reveals a role in T-cell chemotaxis and survival. Mol Cell Biol 25: 11145–11155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumailley M, Fässler R (2007) Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol 177: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz S, Vakonakis I, Lowe ED, Campbell ID, Noble ME, Hoellerer MK (2008) Structural analysis of the interactions between paxillin LD motifs and alpha-parvin. Structure 16: 1521–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA (2007) Structural basis of integrin regulation and signaling. Annu Rev Immunol 25: 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DK, Ellis CA, Edwards PA, Hiles ID (1999) Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene 18: 8024–8032 [DOI] [PubMed] [Google Scholar]
- Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD (2002) C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol 12: 787–797 [DOI] [PubMed] [Google Scholar]
- McDonald PC, Fielding AB, Dedhar S (2008a) Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci 121: 3121–3132 [DOI] [PubMed] [Google Scholar]
- McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S (2008b) Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res 68: 1618–1624 [DOI] [PubMed] [Google Scholar]
- Mills J, Niewmierzycka A, Oloumi A, Rico B, St-Arnaud R, Mackenzie IR, Mawji NM, Wilson J, Reichardt LF, Dedhar S (2006) Critical role of integrin-linked kinase in granule cell precursor proliferation and cerebellar development. J Neurosci 26: 830–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fässler R (2008) Kindlin-2 controls bidirectional signaling of integrins. Genes Dev 22: 1325–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez E, Wickström SA, Altstätter J, Chu H, Fässler R (2009) alpha-parvin controls vascular mural cell recruitment to vessel wall by regulating RhoA/ROCK signalling. EMBO J 28: 3132–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, Wang HV, Sperandio M, Fässler R (2009a) Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med 15: 300–305 [DOI] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fässler R (2009b) The tail of integrins, talin, and kindlins. Science 324: 895–899 [DOI] [PubMed] [Google Scholar]
- Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R (2008) Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 14: 325–330 [DOI] [PubMed] [Google Scholar]
- Nakrieko KA, Welch I, Dupuis H, Bryce D, Pajak A, St Arnaud R, Dedhar S, D'Souza SJ, Dagnino L (2008) Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation of integrin-linked kinase in the epidermis. Mol Biol Cell 19: 1462–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SM Jr, Wright TR (1981) A histological and ultrastructural analysis of developmental defects produced by the mutation, lethal(1)myospheroid, in Drosophila melanogaster. Dev Biol 86: 393–402 [DOI] [PubMed] [Google Scholar]
- Niewmierzycka A, Mills J, St-Arnaud R, Dedhar S, Reichardt LF (2005) Integrin-linked kinase deletion from mouse cortex results in cortical lamination defects resembling cobblestone lissencephaly. J Neurosci 25: 7022–7031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE (2000) Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol 151: 1435–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE (2001) Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J Biol Chem 276: 23499–23505 [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE (2002) Molecular dissection of actopaxin-integrin-linked kinase-Paxillin interactions and their role in subcellular localization. J Biol Chem 277: 1568–1575 [DOI] [PubMed] [Google Scholar]
- Norman KR, Cordes S, Qadota H, Rahmani P, Moerman DG (2007) UNC-97/PINCH is involved in the assembly of integrin cell adhesion complexes in Caenorhabditis elegans body wall muscle. Dev Biol 309: 45–55 [DOI] [PubMed] [Google Scholar]
- Olski TM, Noegel AA, Korenbaum E (2001) Parvin, a 42 kDa focal adhesion protein, related to the alpha-actinin superfamily. J Cell Sci 114: 525–538 [DOI] [PubMed] [Google Scholar]
- Pasquali C, Bertschy-Meier D, Chabert C, Curchod ML, Arod C, Booth R, Mechtler K, Vilbois F, Xenarios I, Ferguson CG, Prestwich GD, Camps M, Rommel C (2007) A chemical proteomics approach to phosphatidylinositol 3-kinase signaling in macrophages. Mol Cell Proteomics 6: 1829–1841 [DOI] [PubMed] [Google Scholar]
- Pasquet JM, Noury M, Nurden AT (2002) Evidence that the platelet integrin alphaIIb beta3 is regulated by the integrin-linked kinase, ILK, in a PI3-kinase dependent pathway. Thromb Haemost 88: 115–122 [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Goncalves AF, Ozcelik M, Thurnherr T, Tricaud N, Meijer D, Fässler R, Suter U, Relvas JB (2009) Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol 185: 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S (2001) Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276: 27462–27469 [DOI] [PubMed] [Google Scholar]
- Postel R, Vakeel P, Topczewski J, Knoll R, Bakkers J (2008) Zebrafish integrin-linked kinase is required in skeletal muscles for strengthening the integrin-ECM adhesion complex. Dev Biol 318: 92–101 [DOI] [PubMed] [Google Scholar]
- Roote CE, Zusman S (1995) Functions for PS integrins in tissue adhesion, migration, and shape changes during early embryonic development in Drosophila. Dev Biol 169: 322–336 [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ (2003) Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5: 711–719 [DOI] [PubMed] [Google Scholar]
- Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fässler R (2003) Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev 17: 926–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135: 510–523 [DOI] [PubMed] [Google Scholar]
- Stanchi F, Bordoy R, Kudlacek O, Braun A, Pfeifer A, Moser M, Fässler R (2005) Consequences of loss of PINCH2 expression in mice. J Cell Sci 118: 5899–5910 [DOI] [PubMed] [Google Scholar]
- Stanchi F, Grashoff C, Nguemeni Yonga CF, Grall D, Fässler R, Van Obberghen-Schilling E (2009) Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J Cell Sci 122: 1800–1811 [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA (2003) Talin binding to integrin beta tails: a final common step in integrin activation. Science 302: 103–106 [DOI] [PubMed] [Google Scholar]
- Terpstra L, Prud'homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, St-Arnaud R (2003) Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol 162: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troussard AA, Mawji NM, Ong C, Mui A, St-Arnaud R, Dedhar S (2003) Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem 278: 22374–22378 [DOI] [PubMed] [Google Scholar]
- Tu Y, Huang Y, Zhang Y, Hua Y, Wu C (2001) A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 153: 585–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Li F, Goicoechea S, Wu C (1999) The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 19: 2425–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Sage T, Stevens JM, Jordan PA, Jones S, Barrett NE, St-Arnaud R, Frampton J, Dedhar S, Gibbins JM (2008) A dual role for integrin-linked kinase in platelets: regulating integrin function and alpha-granule secretion. Blood 112: 4523–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HV, Chang LW, Brixius K, Wickström SA, Montanez E, Thievessen I, Schwander M, Müller U, Bloch W, Mayer U, Fässler R (2008a) Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J Cell Biol 180: 1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fukuda K, Byeon IJ, Velyvis A, Wu C, Gronenborn A, Qin J (2008b) The structure of alpha-parvin CH2-paxillin LD1 complex reveals a novel modular recognition for focal adhesion assembly. J Biol Chem 283: 21113–21119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ (2006) Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev 20: 2355–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DP, Caswell PT, Norman JC (2007) alphavbeta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol 177: 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji S, Suzuki A, Kanamori H, Mishima W, Yoshimi R, Takasaki H, Takabayashi M, Fujimaki K, Fujisawa S, Ohno S, Ishigatsubo Y (2004) Affixin interacts with alpha-actinin and mediates integrin signaling for reorganization of F-actin induced by initial cell-substrate interaction. J Cell Biol 165: 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H, Mohri H, Ohno S, Ishigatsubo Y (2001) A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol 153: 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Masuda J, Omori T, Usui R, Akiyama H, Maru Y (2009) EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J Cell Sci 122: 243–255 [DOI] [PubMed] [Google Scholar]
- Yasunaga T, Kusakabe M, Yamanaka H, Hanafusa H, Masuyama N, Nishida E (2005) Xenopus ILK (integrin-linked kinase) is required for morphogenetic movements during gastrulation. Genes Cells 10: 369–379 [DOI] [PubMed] [Google Scholar]
- Yoshimi R, Yamaji S, Suzuki A, Mishima W, Okamura M, Obana T, Matsuda C, Miwa Y, Ohno S, Ishigatsubo Y (2006) The gamma-parvin-integrin-linked kinase complex is critically involved in leukocyte-substrate interaction. J Immunol 176: 3611–3624 [DOI] [PubMed] [Google Scholar]
- Zervas CG, Gregory SL, Brown NH (2001) Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol 152: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C (2002) Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci 115: 4777–4786 [DOI] [PubMed] [Google Scholar]