Abstract
AIMS
Angiotensin-converting enzyme inhibitors (ACEi) are frequently prescribed for various cardiovascular and renal diseases. A common side-effect of these drugs is a persistent dry cough. Physicians who fail to recognize a dry cough to be ACEi-related may attempt to treat it with antitussive agents instead of recommended ACEi substitution. Prescription behaviour in the general population considering treatment of the side-effect with antitussive agents has not been studied before.
METHODS
Drug dispensing data between 2000 and 2007 were retrieved from the IADB.nl database. A prescription sequence symmetry analysis was used to determine whether antitussive agents were prescribed more often following ACEi initiation than the other way around. A logistic regression model was fitted to determine predictors.
RESULTS
We identified 27 446 incident users of ACEi therapy. One thousand and fifty-four patients were incident users of both ACEi and antitussives within a half-year time span. There was an excess of patients being prescribed antitussive agents after ACEi initiation (703 vs. 351), adjusted sequence ratio 2.2 [confidence interval (CI) 1.9, 2.4]. Female patients were more likely to be prescribed antitussive agents following ACEi therapy initiation, odds ratio 1.4 (CI 1.1, 1.9), age and co-medications were not significant predictors.
CONCLUSIONS
There was a significant and clinically relevant excess of patients receiving antitussives after ACEi initiation. The results suggest that cough as a side-effect of ACEi is not recognized as being ACEi-related or is symptomatically treated with antitussive agents instead of ACEi substitution. The estimated frequency of antitussive treatment of ACEi-induced dry cough is 15%.
Keywords: ACE inhibitor, cough, side-effect
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Dry cough is a common and well-documented side-effect of angiotensin-converting enzyme inhibitor (ACEi) treatment.
The recommended course of action in case of ACE-induced cough is substitution of the ACEi with other antihypertensive agents.
Misdiagnosis and mistreatment of ACEi-induced cough has not been studied before.
WHAT THIS STUDY ADDS
In the general population, a significant and clinically relevant proportion of patients with ACE-induced cough are treated with antitussive agents.
These results suggest misdiagnosis and mistreatment of a well-known side-effect.
The estimated frequency of antitussive treatment of ACEi-induced cough is 15%.
Introduction
Angiotensin-converting enzyme inhibitors (ACEi) can be prescribed for various cardiovascular and renal diseases. Cardiovascular indications for ACEi therapy include hypertension and prevention of myocardial infarction, stroke and heart failure [1]. In chronic kidney disease, ACEi are renoprotective in both diabetic [2] and nondiabetic patients [3, 4]. Unfortunately, a well-documented side-effect of ACEi is a persistent dry cough, the frequency of which ranges from 5–20% [5, 6]. The side-effect usually develops within a few weeks after ACEi initiation; is not dose-dependent and is more common in women [5, 7, 8]. The persistent and troublesome nature of the cough often warrants discontinuation of the ACEi, after which the side-effect will usually abate within a few days [9, 10]. Substitution of the ACEi with alternative agents, preferably angiotensin II antagonists, is recommended [9, 11].
Despite the fact that ACEi-associated cough is well documented, some studies have noted a delay in the correct diagnosis of the side-effect [12], possibly related to poor knowledge of the side-effect and the recommended course of action [13]. In patients with congestive heart failure the side-effect might be overlooked because it may be ascribed to pulmonary congestion [5]. Patients in whom a dry cough is not recognized to be ACEi-related, which can often easily be determined by means of a dechallange test [14], might be subjected to extensive and unnecessary evaluations, diagnostic tests, and consultations. Physicians may attempt to treat the cough with antitussive agents [5, 12], such as noscapine or codeine. Prescribing antitussive agents for ACEi-induced dry cough instead of ACEi treatment substitution constitutes irrational pharmacotherapy [5, 12, 15].
In the present study we analysed whether there is an excess of antitussive treatments following ACEi initiation. Such prescription behaviour would indicate that ACEi-induced cough is either not recognized or, arguably irrationally, treated with antitussive agents by physicians and pharmacists. The influence of patient characteristics on this irrational prescription behaviour was determined.
Methods
Drug dispensing data at the individual level were retrieved from the IADB.nl database, which holds prescription records of approximately 500 000 individuals. In the IADB.nl database, each prescription record contains basic patient characteristics (anonymous identifier, gender and date of birth) and information on drug name, anatomical therapeutic chemical (ATC) code, dosage, and dispensing date (http://www.IADB.nl) [16, 17]. The use of over-the-counter drugs and in-hospital prescriptions are not included. Due to high patient commitment to their pharmacy in the Netherlands [18], complete medication histories of individuals could be retrieved. Data between January 2000 and December 2006 were used for the analyses.
All incident users of both ACEi (ATC codes ‘C09A’; ‘C09B’) and antitussive agents (ATC code ‘R05D’) were identified. Incidence was defined as not having been prescribed the drug in question for at least 1 year while being known in the database for that period. A prescription sequence symmetry analysis was used to determine whether antitussive agents were prescribed more often following ACEi initiation than the other way around [19]. To this end, incident users of both treatments with a half-year time span of each other were selected for analysis. The number of individuals starting ACEi first and antitussive agents second, divided by the number of individuals starting antitussive agents first and ACEi second, is called the sequence rate. This sequence rate is an estimate of the incidence rate ratio of antitussives prescribing in ACEi-exposed vs. non-exposed person time [19, 20]. The calculated sequence rate should be adjusted for time trends in use of the study drugs, because if a drug is prescribed with increasing incidence there will be a nonspecific excess of that drug being prescribed last. The rationale, advantages and limitations of the prescription sequence analysis and the adjustment for time trends in drug use are discussed in detail elsewhere [19, 20].
A multivariate logistic regression model was fitted on the data to determine predictors of being prescribed antitussive agents following ACEi therapy initiation. Specifically, we focused on age; sex; comorbidity obstructive airway disease (by proxy of ATC codes ‘R03’); comorbidity diabetes mellitus (by proxy of ATC codes ‘A10’); and co-medication of angiotensin II antagonists (ATC codes ‘C09C’ and ‘C09D’); β-blockers (ATC codes ‘C07’); calcium channel antagonists (ATC codes ‘C08’); and diuretics (ATC codes ‘C03’). Co-medication was defined as having received a minimum of three prescriptions for the drug(s) in question within a time interval of 1 year. The model was also adjusted for the date of ACEi prescription. Statistical analyses were performed using SPSS, version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
We identified 27 446 incident users of ACEi therapy. Of these, 1082 patients were selected who were incident users of antitussive agents before or after a half-year time span of ACEi initiation. Twenty-eight patients (2.6%) started both therapies on the same day and were excluded from the analysis. In the remaining group of 1054 patients, the mean age at ACEi initiation was 65.3 years (SD 13.9); 61.3% were female, 11.6% had recorded use of medication for obstructive airway diseases and 18.8% for diabetes mellitus.
Of the 1054 patients, 703 started ACEi therapy first and antitussive agents second against 351 patients who started antitussive therapy first, yielding a sequence ratio of 2.0 [95% confidence interval (CI) 1.8, 2.3]. Adjusted for incidence trends in drug use, the sequence ratio was 2.2 (95% CI 1.9, 2.4).
Multivariate logistic regression analysis (Table 1) showed that female patients were more likely to be prescribed antitussive agents following ACEi therapy initiation, while age and co-medications were not significant predictors.
Table 1.
Predicting factors for receiving antitussive agents following angiotensin-converting enzyme inhibitor (ACEi) initiation over the opposite prescription order, total study population of 1054
| Variable | n (%) | Odds ratio | 95% confidence interval |
|---|---|---|---|
| Date of ACEi prescription (per month) | 1.00 | (0.99, 1.00) | |
| Age (per year) | 1.00 | (0.99, 1.01) | |
| Female gender | 646 (61.3) | 1.45 | (1.11, 1.90) |
| Recorded use of co-medication | |||
| Obstructive airway disease | 122 (11.6) | 1.18 | (0.78, 1.80) |
| Diabetes mellitus | 198 (18.8) | 1.40 | (0.99, 1.98) |
| AT-2 antagonists | 23 (2.2) | 0.93 | (0.39, 2.26) |
| β-Blockers | 358 (34.0) | 1.01 | (0.76, 1.33) |
| Calcium channel antagonists | 110 (10.4) | 1.52 | (0.96, 2.42) |
| Diuretics | 298 (28.3) | 0.84 | (0.63, 1.14) |
Discussion
Although ACEi-induced dry cough is a well-documented side-effect, prescription behaviour in the general population considering treatment of the side-effect with antitussive agents has not been studied before. Prescribing antitussive agents for ACEi-induced cough instead of ACEi treatment substitution constitutes irrational pharmacotherapy, because of avoidable polypharmacy [15], low evidence of effectiveness of the antitussives [5, 12] and exposure to side-effects of the antitussive agents, which include drowsiness and nausea.
Our data identified 27 446 incident users of ACEi therapy; 2745 of those can be expected to have developed an ACEi-induced dry cough, assuming a frequency of 10% [6]. The prescription symmetry analysis revealed a crude excess of 703–351 = 352 patients with the prescription order ACEi–antitussive. Adjusting for incidence trends in use of the study drugs resulted in an excess of 376 patients, which can be considered an estimate of the number of antitussive treatments attributable to ACEi-induced cough [19]. Therefore, the estimated frequency of antitussive treatment of the side-effect within half a year of ACEi initiation is 376/2745 = 15.2%. It should be noted that this estimate is inversely related to the assumed prevalence of ACEi-induced cough.
Multivariate logistic regression analysis confirmed that women are more likely to develop an ACEi-induced cough [5, 7, 8]. Age and comorbidities were not found to influence the prescription order, confirming earlier studies in which no other predictive factors for ACEi-induced dry cough other than gender were identified [5]. Albeit nonsignificantly, recorded use of co-medication for diabetes mellitus and calcium channel antagonists showed an increased odds ratio for the prescription order ACEi–antitussive agent. Possibly, physicians are unwilling or hesitant to substitute ACEi therapy in these frail patient groups because of the cardiovascular- and renoprotective properties of ACEi.
Possibly, the physicians in our study did recognize the dry cough as an ACEi-induced side-effect and discontinued or substituted ACEi therapy, but also prescribed an antitussive agent for symptomatic treatment. ACEi-induced cough is often not susceptible to antitussive treatment [5, 12]. However, we performed a second analysis in which we excluded patients who, after the first antitussive prescription, did not receive new ACEi prescriptions; results were similar, adjusted sequence ratio 1.7 (95% CI 1.4, 1.9). Therefore, antitussive agents are prescribed for ACEi-induced cough while the ACEi treatment is continued.
In our analysis incident users of ACEi and antitussive agents within a half-year time span were included. To test the validity of this time span, an exploratory analysis with a 1-year time span was performed. The prescription asymmetry of first antitussive prescription before and after ACEi initiation is shown in Figure 1. Most of the excess of antitussives are prescribed within half a year of ACEi initiation, validating the half-year time-span chosen in our analysis.
Figure 1.
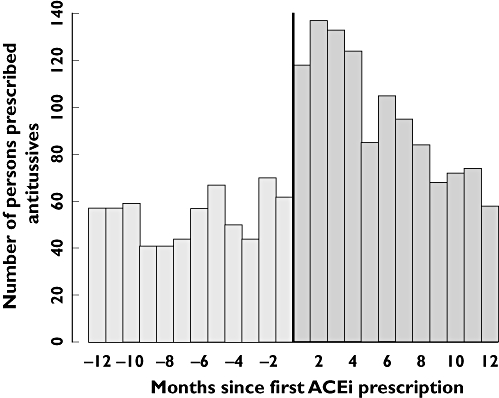
Prescription asymmetry of first antitussive prescription within 1 year before or after angiotensin-converting enzyme inhibitor (ACEi) initiation (n = 1802)
Conclusion
We found a significant and clinically relevant excess of patients receiving antitussive agents following the first half-year after ACEi initiation. This prescription sequence asymmetry suggest that the dry cough is either not recognized as being ACEi-related or symptomatically treated with antitussive agents instead of the pharmacotherapeutically more rational ACEi substitution with other agents such as angiotensin II antagonists. The estimated frequency of antitussive treatment of the ACEi-induced dry cough is 15%.
Competing interests
None to declare.
REFERENCES
- 1.Brugts JJ, Ninomiya T, Boersma E, Remme WJ, Bertrand M, Ferrari R, Fox K, MacMahon S, Chalmers J, Simoons ML. The consistency of the treatment effect of an ACE-inhibitor based treatment regimen in patients with vascular disease or high risk of vascular disease: a combined analysis of individual data of ADVANCE, EUROPA, and PROGRESS trials. Eur Heart J. 2009;30:1385–94. doi: 10.1093/eurheartj/ehp103. [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Lordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–51. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 3.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349(9069):1857–63. [PubMed] [Google Scholar]
- 4.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354(9176):359–64. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 5.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–42. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 6.Overlack A. ACE inhibitor-induced cough and bronchospasm. Incidence, mechanisms and management. Drug Saf. 1996;15:72–8. doi: 10.2165/00002018-199615010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Gibson GR. Enalapril-induced cough. Arch Intern Med. 1989;149:2701–3. [PubMed] [Google Scholar]
- 8.Os I, Bratland B, Dahlof B, Gisholt K, Syvertsen JO, Tretli S. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens. 1994;7:1012–5. doi: 10.1093/ajh/7.11.1012. [DOI] [PubMed] [Google Scholar]
- 9.Morice AH, Kastelik JA. Cough. 1: chronic cough in adults. Thorax. 2003;58:901–7. doi: 10.1136/thorax.58.10.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavord ID, Chung KF. Management of chronic cough. Lancet. 2008;371(9621):1375–84. doi: 10.1016/S0140-6736(08)60596-6. [DOI] [PubMed] [Google Scholar]
- 11.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1) Suppl.:169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 12.Olsen CG. Delay of diagnosis and empiric treatment of angiotensin-converting enzyme inhibitor-induced cough in office practice. Arch Fam Med. 1995;4:525–8. doi: 10.1001/archfami.4.6.525. [DOI] [PubMed] [Google Scholar]
- 13.Lombardi C, Crivellaro M, Dama A, Senna G, Gargioni S, Passalacqua G. Are physicians aware of the side effects of angiotensin-converting enzyme inhibitors?: a questionnaire survey in different medical categories. Chest. 2005;128:976–9. doi: 10.1378/chest.128.2.976. [DOI] [PubMed] [Google Scholar]
- 14.Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795–801. doi: 10.7326/0003-4819-140-10-200405180-00009. [DOI] [PubMed] [Google Scholar]
- 15.Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315(7115):1096–9. doi: 10.1136/bmj.315.7115.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schirm E, Monster TB, de Vries R, van den Berg PB, de Jong-van den Berg LT, Tobi H. How to estimate the population that is covered by community pharmacies? An evaluation of two methods using drug utilisation information. Pharmacoepidemiol Drug Saf. 2004;13:173–9. doi: 10.1002/pds.882. [DOI] [PubMed] [Google Scholar]
- 17.Tobi H, van den Berg PB, de Jong-van den Berg LT. The interaction database: synergy of science and practice in pharmacy. In: Brause RW, Hanisch E, editors. Medical Data Analysis. Berlin: Springer-Verlag; 2000. pp. 206–11. [Google Scholar]
- 18.Leufkens HGM, Urquhart J. Automated pharmacy record linkage in the Netherlands. In: Strom BL, editor. Pharmacoepidemiology. Chichester: John Wiley & Sons Ltd.; 2008. pp. 347–60. [Google Scholar]
- 19.Hallas J. Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiology. 1996;7:478–84. [PubMed] [Google Scholar]
- 20.Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiol Drug Saf. 2009;18:483–91. doi: 10.1002/pds.1736. [DOI] [PubMed] [Google Scholar]


