Although anthracyclines are recognized as efficacious chemotherapeutic agents with broad-spectrum anticancer activity, their clinical use is limited by their chronic accumulative dose-dependent cardiac toxicity. Three forms of anthracycline-induced cardiotoxicity have been described: acute or subacute cardiac injury, chronic cardiotoxicity resulting in cardiomyopathy, and late-onset ventricular dysfunction with arrhythmias. Acute and subacute anthracycline toxicities are most often manifested as arrhythmias, pericarditis-myocarditis, subclinical cardiomyopathy, left ventricular dysfunction, and sudden death [1, 2]. However, acute cardiac toxicity is uncommon and is usually subclinical in nature or is associated with mild clinical symptoms lacking significant consequences. This report describes a case of severe subacute congestive heart failure (CHF) and ventricular tachycardia (VT) developing in response to the first exposure to idarubicin.
A pale-faced 34-year-old woman presented to our haematology outpatient clinic with the complaint of general malaise of 1 month's duration. No systemic disease had occurred prior to this illness and she had no history of coronary artery disease, smoking, or illegal substance abuse. Bone marrow examination confirmed the presence of acute myeloid leukaemia (AML), M6 subtype. Induction chemotherapy with idarubicin and cytarabine was therefore initiated. Idarubicin was administered at 12 mg m−2 day−1 for 3 days by a 30-min intravenous infusion, and cytarabine was administered at 100 mg m−2 day−1 for 7 days by continuous (24 h) intravenous infusion. A neutropenic fever developed on day 10, and empirical antibiotic therapy with piperacillin/tazobactam and amikacin was prescribed. Because the fever persisted, antibiotic therapy was changed to meropenem on day 15 and fluconazole was added on day 18. When blood laboratory analyses conducted on day 17 revealed the presence of hypokalaemia, potassium (KCl solution) was given intravenously. Even after aggressive potassium chloride supply, serum levels of potassium on day 27 were 2.53 mmol l−1. On day 20, the patient displayed tachypnoea and shortness of breath. Chest x-ray revealed cardiomegaly and right pleural effusion. Echocardiography performed on day 26 revealed chamber dilation of the left atrium and left ventricle, generalized hypokinetic wall motion, and a left ventricular ejection fraction (LVEF) of 23% (Figure 1). Prior to the development of CHF, the patient exhibited no symptoms or signs of arrhythmia or pericarditis-myocarditis. Cardiac marker measurements of creatine kinase isoenzyme (CK-MB) and troponin-I on day 24 were all within normal ranges.
Figure 1.
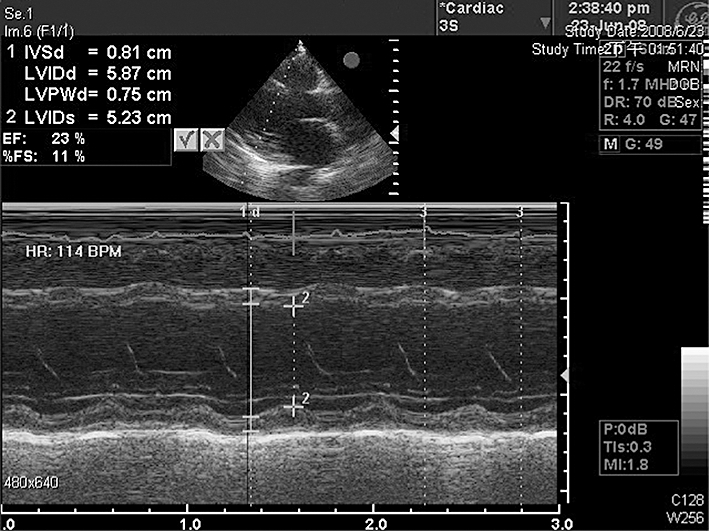
The echocardiogram on day 26 shows chamber dilation of left atrium and left ventricle and left ventricle generalized hypokinetic wall motion with left ventricular ejection fraction of 23%
Frequent ectopic premature ventricular beats were detected on day 27, with the arrhythmia progressing to VT and ventricular fibrillation on day 28. Because of a sudden loss of consciousness, cardiopulmonary resuscitation with defibrillation was performed immediately. Intubation with ventilatory support and an intra-arterial balloon pump were placed to manage cardiogenic shock and pulmonary oedema.
On day 38 the intra-arterial balloon pump and ventilator were no longer required, and the patient was transferred to a general ward. Echocardiography performed 4 months after the induction chemotherapy showed no chamber dilation and adequate global left ventricular performance with a LVEF of 64.2%.
Idarubicin (4-demethoxydaunorubicin) is a newer congener with lesser degrees of cardiotoxicity compared with other anthracyclines. In a retrospective investigation of idarubicin-related cardiac toxicities [3], only one of 115 idarubicin-treated patients developed definite CHF with four patients developing possible CHF. No patient with asymptomatic decreased LVEF progressed to CHF. The patient who developed definite CHF received a total idarubicin dose of 92 mg m−2. The investigators therefore concluded that idarubicin-related cardiomyopathy is uncommon with cumulative idarubicin doses of up to 290 mg m−2.
Our patient, a 34-year-old woman, had no risk factors for heart disease. Nonetheless, she developed severe CHF after her first induction chemotherapy involving a total cumulative idarubicin dose of 36 mg m−2. To our knowledge, no previous reports of subacute CHF developing after first exposure to idarubicin have been published. However, the present report underscores the possibility of severe CHF developing even after an initial exposure to this drug. The causality assessment of this adverse drug reaction (ADR) according to the Naranjo Algorithm is 6, placing the reaction in the probable ADR category [4].
The clinical course of the severe subacute CHF exhibited by our patient was complicated by VT. We postulate that this arrhythmia was precipitated by the hypokalaemia in the situation of idarubicin-associated cardiomyopathy. Another possible precipitating factor was fluconazole-induced prolongation of the cardiac QTc interval; however, the electrocardiogram measured 2 h before the attack of VT revealed sinus rhythm and frequent premature ventricular contractions with QTc interval of 400 ms.
Chronic anthracycline-induced cardiomyopathy is irreversible and the prognosis for cancer patients with this complication is poor. The prognosis for patients with subacute symptomatic anthracycline-induced CHF is unknown because of the low incidence of this disorder. Our patient developed clinically significant CHF after her first treatment with idarubicin. Four months later, however, her LVEF had increased to 64.2%. This observation supports the hypothesis that the subacute CHF developing in response to short-term anthracycline therapy can be reversed totally or partially.
The present case illustrates that severe subacute anthracycline-induced CHF, although rare, can develop after the first exposure to idarubicin. To our knowledge, this report is the first to describe a patient with AML who developed severe subacute CHF with VT after the first cycle of induction chemotherapy with idarubicin and cytarabine. For such patients, early detection and aggressive treatment of these cardiac disturbances are anticipated to restore cardiac function partially or fully.
Competing interests
None declared.
REFERENCES
- 1.Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med. 1978;65:823–32. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- 2.Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–58. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 3.Anderlini P, Benjamin RS, Wong FC, Kantarjian HM, Andreeff M, Kornblau SM, O'Brien S, Mackay B, Ewer MS, Pierce SA. Idarubicin cardiotoxicity: a retrospective study in acute myeloid leukemia and myelodysplasia. J Clin Oncol. 1995;13:2827–34. doi: 10.1200/JCO.1995.13.11.2827. [DOI] [PubMed] [Google Scholar]
- 4.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


