Abstract
We have reported previously the existence of an Mr 70,000 form of the α6 integrin called α6p in a variety of human epithelial cell lines. Four different experimental conditions were used to examine the regulation of α6 and α6p integrin. The production of the α6 integrin was decreased by 45% using a protein translation inhibitor (2.25 μM puromycin), whereas production of the α6p variant was unaffected. The α6p variant was decreased 60% by actin depolymerization (10 μM cytochalasin D) corresponding to a decrease in its surface expression, whereas α6 integrin production was unaffected. The α6p variant was resistant to endoglycosidase H treatment, whereas the α6 integrin was both sensitive and resistant to endoglycosidase H treatment, indicating retention in the endoplasmic reticulum and processing through the Golgi apparatus. Additionally, digestion by endoglycosidase F demonstrated both α6p and α6 integrin contained NH2-linked glycosylations and both shifted Mr ~10,000 on enzymatic digestion. Finally, inhibition of serine/threonine phosphatases by either calyculin A (15 nM) or okadaic acid (62 μM) did not affect α6p, whereas the production of α6 integrin was decreased by 50%. These data suggest that the production of the α6p variant is distinct from α6 integrin and may involve a post-translational processing event at the cell surface.
Introduction
Integrins are signaling receptors that link the intracellular cytoskeleton to the extracellular matrix and play important roles in adhesion, migration, proliferation, signaling, differentiation, and cell survival (1–8). The α6 integrin is a laminin receptor in epithelial cells (9–14). Previously, studies demonstrated a loss of theα6β4 heterodimer during prostate tumor progression (15–17) and a persistent expression of the α6β1 integrin (18). Additionally, expression of α6β1 integrin is maintained in micrometastases (15, 16, 19–21).
Our previous studies identified a novel Mr 70,000 variant of the α6 integrin, called α6p, for the Latin word parvus, in prostate carcinoma cell lines (22). The variant paired with both β1 and β4 integrin subunits and was present in a number of epithelial carcinoma cell lines, as well as in a normal immortalized human keratinocyte cell line. Two-dimensional gel analysis and Western blotting data indicated the cytoplasmic light chain of the variant was identical to that of the full-length α6 integrin and that the primary alteration was a shortened extracellular heavy chain. The shortened extracellular domain was missing the putative ligand-binding domain contained within the β-propeller (23–25).
Adhesion to extracellular matrix proteins has been shown to play a role in cytoskeletal organization (26). The α6β1 integrin localizes to the focal adhesion, functioning to link the extracellular matrix to the actin cytoskeleton via the β1 cytoplasmic domain for both signal transduction and mechanical stability of the cell during migration (8, 15, 27–30). This interaction has been shown to be important for integrin signaling and recruitment of scaffolding molecules, such as paxillin and filamentous-actin (31, 32).
The production of a variant form of the integrin, missing the ligand-binding region of the molecule, may influence these events. It is of particular interest to understand the circumstances surrounding the production of α6p and whether it is subject to similar regulatory controls as the production of the α6 integrin. We have extended our studies to examine the effect of known experimental perturbations of integrin function on the production of α6 and α6p integrin. The following experiments demonstrated that the α6 and α6p integrins responded differently to the inhibition of translation, the alteration of actin filaments, endoglycosidase digestion, and the action of serine/threonine phosphatase inhibitors. These data indicate that the mechanism of α6 and α6p production differs significantly. Furthermore, these data are consistent with the hypothesis that the α6p integrin is produced by a processing event after the molecule reaches the cell surface.
Results
Production of α6 Integrin, but not α6p, was Translation Dependent
Recently, Alais et al. (33) demonstrated that the expression of α1 integrins could be regulated through translation-dependent mechanisms. Our previous studies indicated that the α6p variant was generated independent of a transcription event, such as an alternative mRNA splicing (22). We used the translation inhibitor, puromycin, to determine the importance of translation on the production of both α6 and α6p integrins. The human prostate carcinoma DU145H cells were exposed to 2.25 μM puromycin for 18 h or DMSO vehicle. The α6 and α6p integrin proteins were identified at Mr 160,000 and 70,000 respectively, from a whole cell lysate (Fig. 1A). Puromycin treatment resulted in a 45% reduction of the α6 integrin as compared with the vehicle control (Fig. 1B). No effect on α6p integrin protein levels was observed. Although the production of the α6 integrin was dependent on translation, the level of the α6p variant was not affected by the inhibition of translation.
Fig. 1.
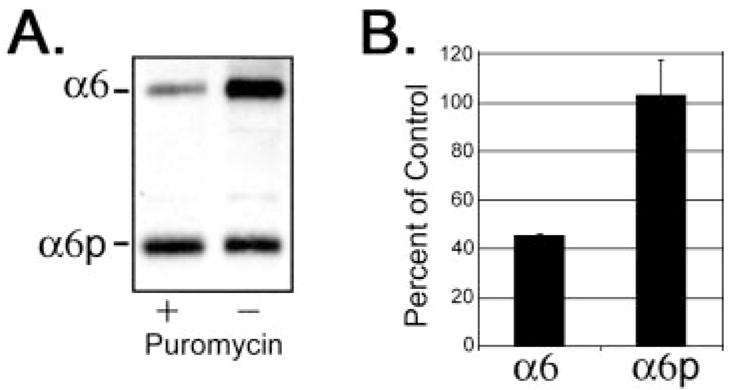
Expression of the α6 integrin, but not α6p, was dependent on translation. Human prostate carcinoma DU145H cells were treated with either 2.25 μM puromycin or DMSO vehicle for 18 h. Whole cell lysate (10 –15 μg) was loaded and electrophoresed on a 7.5% polyacrylamide gel under nonreducing conditions. Proteins were transferred to PVDF membrane followed by Western blot analysis with anti-α6 integrin antibody, AA6A (A). Protein bands in A were quantified and graphed in Excel (B). Data shown were representative of three independent experiments.
Production of α6p, but not α6 Was Dependent on the Actin Cytoskeleton
The actin cytoskeleton influences integrin behavior on the cell surface, such as integrin clustering, dispersal from focal adhesions, and integrin-mediated adhesion to extracellular matrix proteins (34). Disruption of the actin cytoskeleton, but not the tubulin network, has been previously shown to inhibit α6β1-mediated cell adhesion to laminin (35). If the α6p variant was produced on the cell surface, one would expect the production of the variant to be dependent on the actin cytoskeleton. The human prostate carcinoma DU145H cell line was used for these studies because of the abundance of the α6β1 and α6pβ1 integrins (22).
The actin staining in the DU145H cells revealed primarily a cortical staining pattern surrounding the periphery of the cells with few stress fibers, and treatment with cytochalasin D resulted in a loss of cortical actin replaced with perinuclear distribution of disorganized actin (data not shown). Microtubule networks were observed to radiate throughout the cytoplasm, originating from the microtubule organization centers near the nuclei of the DU145H cells, and treatment with nocodazole resulted in a loss of the tubulin network (data not shown). The total production of α6 and α6p integrins was examined after the addition of cytochalasin D. A time-dependent decrease in totalα6p protein levels to ~60% of the control level at 18 h was observed, whereas the total α6 integrin protein levels were relatively unaltered (Fig. 2, A and B). The differential change in the α6 and α6p integrin proteins was apparent by 12 h postaddition of cytochalasin D, and by 18 h, the α6p integrin form had decreased to 60% of the vehicle control (DMSO; Fig. 2, A and B). The microtubule network was disrupted using 8 μM nocodazole, and the total amount of α6 and α6p integrins was examined to determine whether nonspecific effects on the cytoskeleton were responsible for the altered production of α6p variant (Fig. 2, C and D). No significant difference was observed in the total amount of the α6 and α6p integrin forms on depolymerization of the microtubules, suggesting that the tubulin network was not important for production of either α6 or α6p integrins.
Fig. 2.
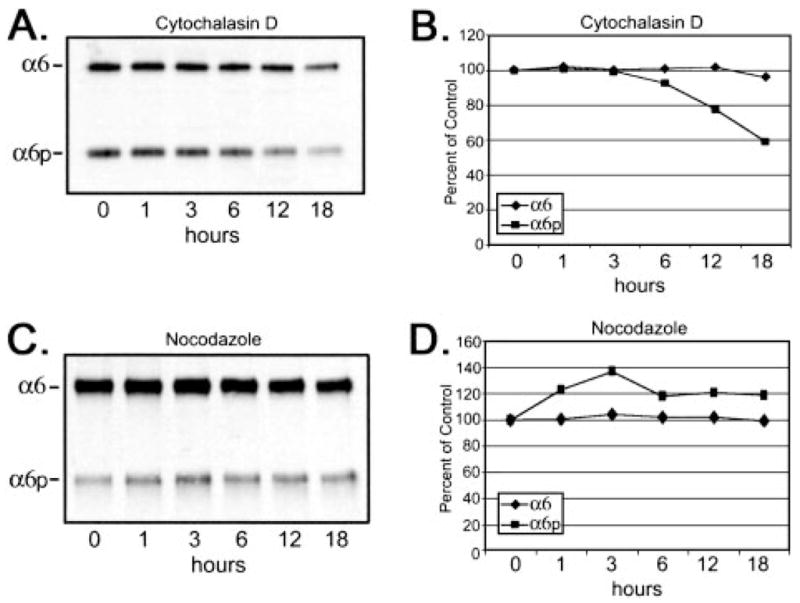
Disruption of the actin cytoskeleton reduced total protein expression of α6p but not α6 integrin. Human prostate carcinoma DU145H cells were treated with either 10 μM cytochalasin D (A and B) or 8 μM nocodazole (C and D) over a 24-h period of time. Identical amounts of whole cell lysates (10 μg) were loaded and electrophoresed on a 7.5% polyacrylamide gel under nonreducing conditions. Proteins were transferred to a PVDF membrane followed by Western analysis for α6 integrin (A and C). The α6 and α6p protein bands were scanned and quantitated using Scion Image Analysis software and graphed in Excel (B and D). Data shown were representative of three experiments.
Cytochalasin D Reduced Cell Surface Expression of α6, α6p, and β1 Integrins
Because the data suggested that the α6p was produced on the cell surface, we next determined if the loss of the α6p production by cytochalasin D could be accounted for by the loss of α6p cell surface expression. To distinguish between surface and cytoplasmic integrin subunits, cell surface proteins were labeled using biotin before adding either cytochalasin D (10 μM) or nocodazole (8 μM) to the cells. Depolymerization of actin by cytochalasin D resulted in a significant loss of α6 and β1 integrins from the cell surface to 36 and 30% of vehicle controls, respectively (Fig. 3, A and B), whereas the total production of α6 integrin was not affected (Fig. 2). In contrast, the surface protein levels of the α6p decreased to 67% of the control value (Fig. 3, A and B), and the total production of the α6p integrin was reduced to ~65% of the control value (Fig. 2). No change in cell surface α6, β1, or α6p integrins was observed in cells treated with nocodazole (Fig. 3, A and B). These data indicated that cytochalasin D decreased the cell surface expression of α6, whereas the total level of α6 integrin was unaffected (Figs. 2 and 3). In contrast, both the α6p cell surface expression and the total α6p production was significantly decreased (Fig. 3). These data taken together suggested again that the α6p variant was produced at the cell surface, dependent on the actin cytoskeleton.
Fig. 3.
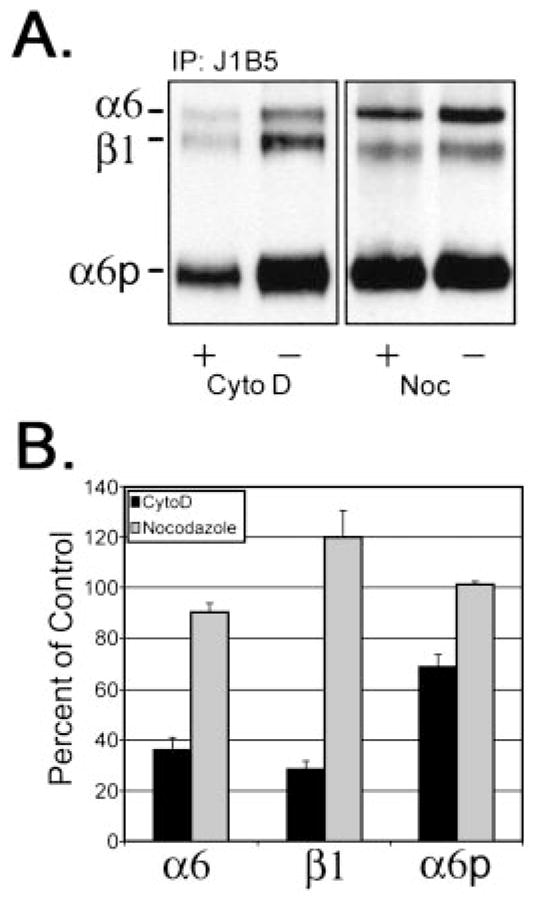
Disruption of the actin cytoskeleton significantly reduced cell surface expression of α6, α6p, and β1 integrins. Surface changes of α6, β1, and α6p were determined by surface of DU145H cells with biotin before treatment with either 10 μM cytochalasin D or 8 μM nocodazole for 18 h. Labeled cells were lysed, and 200 μg of total protein were used for immunoprecipitations with anti-α6 integrin antibody, J1B5. Samples were separated on a 7.5% polyacrylamide gel under nonreducing conditions. Proteins were transferred to PVDF membrane followed by incubation with HRP conjugated to streptavidin (A). Resulting α6, β1, and α6p integrin protein bands were quantified and graphed (B).
Differential Intracellular Processing of the α6 and α6p Integrin
It is known that the integrins can be modified after translation by glycosylation (2). There are nine potential NH2-linked glycosylation sites contained in the α6 integrin (36, 37); five are contained within exons 13–25, the region present within the α6p integrin (22). The enzyme endoH3 is frequently used in combination with endoF to distinguish between complex and high-mannose oligosaccharides. Proteins sensitive to cleavage by endoH are not fully processed, i.e., retained in the Golgi apparatus, whereas proteins sensitive to endoF cleavage are fully processed by the Golgi (38). We determined whether or not the α6p integrin variant was differentially glycosylated compared with the full-length α6 integrin. Human prostate carcinoma DU145H cells were lysed, immunoprecipitated with anti-α6 integrin antibody J1B5, and subjected to digestion with either endoH or endoF as detailed in “Materials and Methods.” EndoH digestion resulted in the appearance of at least three α6 integrin intermediates, indicating the retention of these forms within the ER (Fig. 4). The majority of the α6 integrin was both endoH and endoF resistant, indicating successful passage through the ER and entrance into the medial Golgi compartment. In contrast, the α6p variant was not sensitive to endoH digestion but was sensitive to endoF (Fig. 4). No ER-retained forms of the α6p integrin were detected, although the α6p does contain high mannose type oligosaccharides. The data suggested that the variant may be produced after the molecule arrives at the cell surface, because the α6p was not processed through the ER and was not dependent on active protein translation.
Fig. 4.
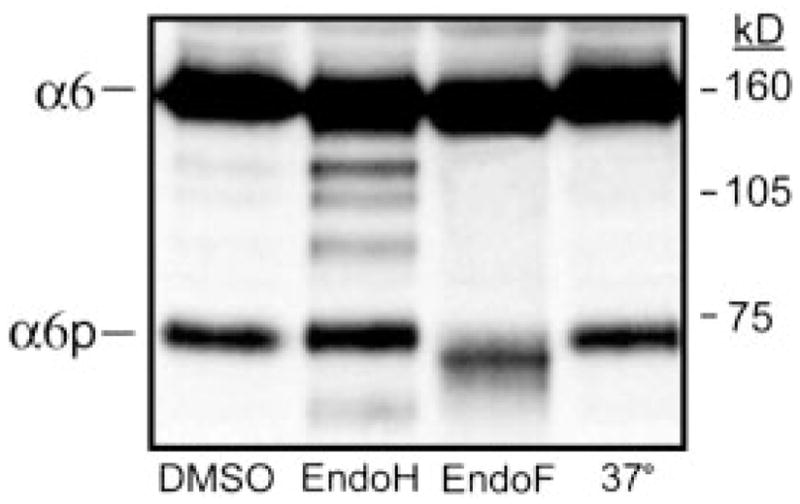
The α6 integrin sensitivity to endoglycosidase treatment. Human prostate carcinoma DU145H cells were immunoprecipitated for α6 integrin using J1B5 antibody and then subjected to digestion with either endoH or endoF overnight at 37°C. Resulting protein samples were analyzed by 7.5% SDS-PAGE under nonreducing conditions followed by Western blot analysis using anti-α6 integrin antibody, AA6A. The migration of the molecular weight standards and integrins are indicated.
The α6, but not α6p Integrin, Was Altered by Serine/Threonine Phosphatase Inhibitors
Inhibition of serine/threonine phosphatases using pharmacological inhibitors has been shown previously to regulate integrin phosphorylation (39, 40) and integrin function (41–43). Calyculin A is a potent inhibitor of protein phosphatase type 1 and 2A, whereas okadaic acid inhibits both, but it preferentially inhibits type 2A (44, 45).
To examine the role for protein phosphatase inhibitors on α6 and α6pintegrins, calyculin A and okadaic acid were tested. Using 15 nM calyculin A to inhibit serine/threonine phosphatases, the total amount of α6 and α6p integrins was examined. After treatment for 6 h with 15 nM calyculin A, we observed a 50% decrease in total protein production of α6 integrin but only a 10% decrease in the variant α6p form (Fig. 5, A and B). Cells also were treated with 62 μM okadaic acid for 18 h. Two α6 integrin forms were observed after treatment (Fig. 5C). The molecular weight shift observed in the lower form was consistent with a dephosphorylated α6 integrin protein similar to that observed for α4 integrin (39). There was a 2-fold increase of the faster migrating form of α6 integrin, with a corresponding 50% decrease in the slower migrating form (Fig. 5D). No alteration in electrophoretic mobility of α6p integrin was observed under the same experimental conditions. The pharmacological inhibitors used in this study were not toxic to the cells (data not shown).
Fig. 5.
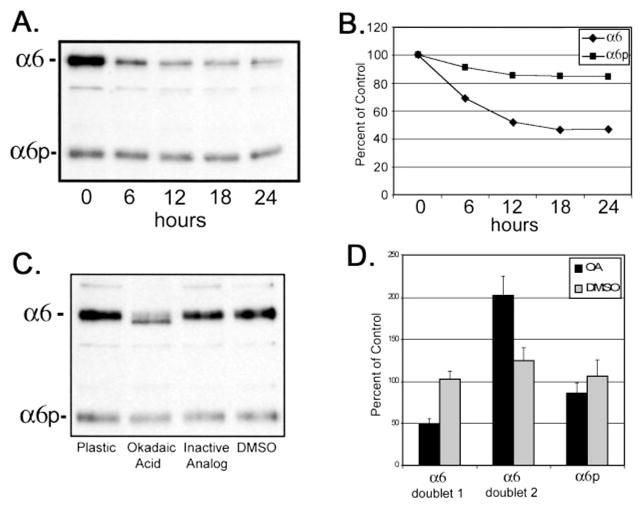
Calyculin A and okadaic acid treatment of DU145H cells decreased α6 integrin protein levels but not α6p. Human prostate carcinoma DU145H cells were treated with 15 nM calyculin A over a 24-h period of time. Identical amounts of whole cell lysate (10) were loaded and electrophoresed on a 7.5% polyacrylamide gel under nonreducing conditions. Proteins were transferred to PVDF membrane followed by Western analysis for α6 integrin (A). Protein bands in A were scanned and quantified using Scion Image Analysis software and graphed in Excel (B). Data shown were representative of three independent experiments. DU145H cells were treated with 50 μM okadaic acid, the inactive analogue 1-nor-okadaone, or vehicle (DMSO) for 18 h. Whole cell lysates were examined for α6 integrin protein expression as above (C). Resulting α6 and α6p bands from three independent experiments were quantified and graphed (D).
Discussion
Previous studies have indicated that the α6 integrin-containing heterodimer is altered in prostate carcinoma progression, shifting from the α6β4 to α6β1 integrin. Previously, we identified a novel variant of the α6 integrin, called α6p, which paired with both β1 and β4 subunits (22). The variant was missing a large portion of the extracellular domain, including the postulated ligand-binding region, but retained an identical cytoplasmic light chain. Four different experimental strategies were used here to determine whether α6p and α6 were regulated in a similar or distinct manner. It was found that the response of the α6p and the α6 integrin to the experimental conditions was distinct, indicating a disassociation between the appearance of these two integrin forms.
The most striking difference in the forms was the susceptibility of the α6 integrin and the resistance of the α6p integrin to the inhibition of protein translation using puromycin. Our previous studies identified only one mRNA transcript for the α6 integrin in the DU145H cells (22). One formal possibility to explain the production of a smaller version of α6 (α6p) was that an altered translation of the α6 mRNA occurred. Previous work has shown that isoforms of cell surface receptors can be generated by the selective use of internal ribosome entry sites or alternative translational start sites (46). Recently, expression of β1 integrin was altered by a translation-dependent mechanism (33). Our studies indicated that production of the α6 integrin could be suppressed using an inhibitor of translation but that expression of the α6p variant was unaltered by translation inhibition. These data suggested that the α6p variant was generated through a post-translational mechanism. These data also may indicate that a larger “pool” of the wild-type α6 integrin exists relative to the α6p form. Inhibition of α6 production by puromycin may trigger processing of the α6 to the α6p form.
The integrin α6β1 is processed after translation in the ER. The intracellular processing of the integrin can be monitored by determining the susceptibility of the protein to cleavage by endoH. Our results are similar to the findings of others that the α6 integrin contains both endoH-sensitive and -resistant forms, consistent with passage of the molecule through the ER and the Golgi compartment (47). In contrast, the α6p integrin was resistant to endoH cleavage, indicating that it was not resident within the ER. Because the α6p is on the cell surface and does not traffic through the ER, it suggests that the protein was produced by a post-translational event occurring at the cell surface.
Integrins on the cell surface are known to be regulated by the cytoskeleton (26, 32, 48 –50), e.g., the actin cytoskeletal attachment to integrins is important for modulation of integrin clustering and dispersal from focal contacts and “inside-out” signaling. In this study, we observed that the cell surface abundance of the α6 and β1 integrins was dependent on the actin cytoskeleton, whereas the total cellular production of the α6 integrin was unaltered. In contrast, both the production and the cell surface expression of the α6p form of the integrin were uniquely susceptible to actin depolymerization. These data combined with the resistance of the α6p to endoH suggests that the α6p variant formed after the integrin arrived on the surface of the cell.
The processing of cell surface receptors has been described previously as ectodomain shedding and plays an essential role in mammalian development (51). We note with interest that collagen XVII/BP180, an epidermal adhesion molecule, exists as a full-length transmembrane protein and is processed into a Mr 120,000 ectodomain that is shed from the keratinocyte surface (52). In addition, CD44, a specific adhesion receptor for hyaluronan, can be shed in a process that can be reduced by disruption of actin assembly with cytochalasin D (53). Current work is under way to determine whether the α6p form of the integrin is generated in a manner similar to the process of ectodomain shedding. The data presented are consistent with a proteolytic processing of the α6 integrin on the cell surface to the α6p form. Experiments are under way to determine the nature of the protease activity involved. At the present, we know that broad-based metalloproteinase inhibitors are ineffective in blocking the α6p production (data not shown). This is in contrast to recent findings that the integrin αV can be processed by MT1-MMP (54).
A final experimental approach to examine α6 and α6p function was the use of phosphatase inhibitors. Inhibition of serine/threonine phosphatases has been shown previously to decrease cell-cell adhesion (55, 56) and integrin-dependent adhesion and motility (40 –43). Inhibitors of serine/threonine phosphatases, such as okadaic acid and calyculin A, resulted in dephosphorylation of α4 integrin, resulting in high-avidity binding of VCAM-1 (39). In our experiments, treatment with calyculin A and okadaic acid resulted in a differential alteration of the electrophoretic properties of the α6 integrin (Fig. 5). The α6 integrin in treated cells existed as a protein doublet. The α6p variant was not altered by treatment with serine/threonine phosphatase inhibitors. Although the significance of phosphorylation of the α6 integrin cytoplasmic domain is understood incompletely, it has been shown to induce tyrosine phosphorylation of paxillin and other unknown proteins on ligand binding (57, 58). Our results were suggestive that the cytoplasmic domain of the α6 integrin was responsive to a signaling event, whereas the α6p variant was not, despite having identical cytoplasmic domains (22). In this instance, the α6p variant may play a dominant negative role. However, we note that ectopic expression of the α6 cytoplasmic domain alone in myoblasts is active in suppressing proliferation, induction of differentiation, and suppression of focal adhesion signaling (59, 60). These data would support the notion that an integrin lacking the extra-cellular ligand-binding domain may still retain a role in altering the cellular response to growth. Experiments are underway currently to determine the role of the α6p variant in the alteration of cellular adhesion and proliferation.
Materials and Methods
Cell Culture
Human prostate carcinoma cell line, DU145H, was isolated by us as described previously (19). Cells were grown in IMDM (Life Technologies, Inc., Gaithersburg, MD) plus 10% fetal bovine serum and incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Antibodies and Reagents
Anti-α6 integrin antibodies were obtained as follows: GoH3, rat IgG2a (Accurate Chemicals, Westbury, NY; Ref. 61), J1B5, rat monoclonal was a generous gift from Dr. Caroline Damsky (University of California, San Francisco, CA; Ref. 62), and AA6A rabbit polyclonal, which was raised and purified using Bethyl Laboratories, Inc. (Montgomery, TX) specific for 16 amino acids (CIHAQPSDKERLTSDA) at the COOH terminus of the human α6A sequence (9) as done previously (11). Cytoskeletal inhibitors cytochalasin D and nocodazole were obtained from Sigma Chemical Co. (St. Louis, MO). Serine/threonine phosphatase inhibitors were obtained as follows: calyculin A, Okadaic acid (Alexis Biochemicals, San Diego, CA), and inactive analogue 1-nor-okadaone (LC Laboratories, Woburn, MA). For inhibition of translation, puromycin was obtained (Sigma Chemical Co.).
Immunoprecipitations/Western Blot Analysis
For immunoprecipitations, 200 μg of total protein lysate were used for each reaction and incubated with 35 μl of protein G Sepharose and 1 μg of antibody. The final volume of the lysate was adjusted to 500 μl with RIPA buffer [150 mM NaCl, 50 mM Tris, 5 mM EDTA, 1% (volume for volume) Triton X-100, 1% (w/v) deoxycholate, and 0.1% (w/v) SDS (pH 7.5)]. The mixture was rotated for 18 h at 4°C. After incubation, complexes were washed three times with cold RIPA and eluted in 2 × nonreducing sample buffer. Immunoprecipitation and whole cell lysate samples were boiled for 5 min before loading onto a 7.5% SDS-polyacrylamide gel for analysis. Proteins resolved in the gel were electrotransferred to Millipore Immobilon-P PVDF membrane (Millipore, Bedford, MA), incubated with either peroxidase-conjugated streptavidin or Western blotting antibodies plus secondary antibody conjugated to HRP and visualized by chemiluminescence (ECL Western Blotting Detection System; Amersham, Arlington Heights, IL), and exposed to film. Protein bands were quantitated using Scion Image Analysis software as described previously (63) and graphed using Excel software.
Alteration of α6 and α6p Integrins by Pharmacological Inhibitors
Human prostate carcinoma DU145H cells were treated in serum-free IMDM media containing 0.1% BSA with drug (10 μM cytochalasin D, 8 μM nocodazole, 15 nM calyculin A, 62 μM okadaic acid, 62 μM 1-nor-okadaone, and 2.25 μM puromycin) for 18 h in the dark. For time courses, media were exchanged for serum-free IMDM containing 0.1% BSA at the start of the time course, and drug was added at appropriate time points. Cells were then collected by scraping, centrifuged for 5 min at 800 × g, and washed two times in HEPES buffer. Cell pellets were lysed in RIPA buffer with protease inhibitors and sonicated. Whole cell lysate (10 – 15 μg) was loaded and electrophoresed on a 7.5% SDS-polyacrylamide gel under nonreducing conditions. Proteins were transferred to PVDF membrane followed by Western analysis for α6 integrin with anti-α6 integrin antibody, AA6A. Protein bands for α6 and α6p were scanned and quantified using Scion Image Analysis software as described previously (63) and graphed using Excel software.
Surface changes of α6, β1, and α6p were determined by surface biotinylation of DU145H cells followed by 18 h of drug treatment for cytochalasin D. For nocodazole studies, DU145H cells were labeled after the 18-h drug treatment. Biotinylated DU145H cells were lysed, and 200 μg of total protein were used for immunoprecipitations with anti-α6 integrin antibody, J1B5. Samples were analyzed as above, and PVDF membrane was incubated with HRP-streptavidin. Resulting protein bands for α6, β1, and α6p from treated or vehicle samples were quantitated and graphed.
EndoH and EndoF Digestions
For digestions, 200 μg of whole cell lysate were first immunoprecipitated overnight with anti-α6 integrin antibody, J1B5, in microcentrifuge tubes. The following day, the beads were washed three times with RIPA buffer, and the sample was resuspended in 35 μl of 2 × nonreducing sample buffer containing 4 mM CaCl2 plus 1 mUnit either endoH or endoF (obtained from Sigma Chemical Co.), which had been diluted in 10% glycerol. Tubes containing reactions were placed in a shaking hot water bath at 37°C overnight. The following morning, the samples were analyzed by 7.5% SDS-PAGE under nonreducing conditions followed by Western blot analysis using anti-α6 integrin antibody, AA6A.
Acknowledgments
We thank Dr. Caroline Damsky for the contribution of the J1B5 hybridoma and discussions with Drs. S. Dedhar and J. C. R. Jones.
Footnotes
The abbreviations used are: endoH, endoglygosidase H; endoF, endoglycosidase F; ER, endoplasmic reticulum; IMDM, Iscove’s modified Dulbecco’s medium; RIPA, radioimmunoprecipitation assay; PVDF, polyvinylidene fluoride; HRP, horseradish peroxidase.
Supported by Grants CA56666 and CA75152 from the National Cancer Institute.
References
- 1.Sonnenberg A. Integrins and their ligands. Curr Top Microbiol Immunol. 1993;184:7–35. doi: 10.1007/978-3-642-78253-4_2. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 3.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura Y. Integrins: expression, modulation, and signaling in fertilization, embryogenesis and implantation. Keio J Med. 1997;46:16–24. doi: 10.2302/kjm.46.16. [DOI] [PubMed] [Google Scholar]
- 5.Ruoslahti E. Integrins as signaling molecules and targets for tumor therapy. Kidney Int. 1997;51:1413–1417. doi: 10.1038/ki.1997.193. [DOI] [PubMed] [Google Scholar]
- 6.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 7.Menko S, Philp N, Veneziale B, Walker J. Integrins and development: how might these receptors regulate differentiation of the lens. Ann N Y Acad Sci. 1998;842:36–41. doi: 10.1111/j.1749-6632.1998.tb09629.x. [DOI] [PubMed] [Google Scholar]
- 8.Dedhar S. Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol. 2000;12:250–256. doi: 10.1016/s0955-0674(99)00083-6. [DOI] [PubMed] [Google Scholar]
- 9.Tamura RN, Rozzo C, Starr L, Chambers J, Reichardt LF, Cooper HM, Quaranta V. Epithelial integrin α 6 β 4: complete primary structure of α 6 and variant forms of β 4. J Cell Biol. 1990;111:1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aumailley M, Timpl R, Sonnenberg A. Antibody to integrin α 6 subunit specifically inhibits cell-binding to laminin fragment 8. Exp Cell Res. 1990;188:55–60. doi: 10.1016/0014-4827(90)90277-h. [DOI] [PubMed] [Google Scholar]
- 11.Cooper HM, Tamura RN, Quaranta V. The major laminin receptor of mouse embryonic stem cells is a novel isoform of the α 6 β 1 integrin. J Cell Biol. 1991;115:843–850. doi: 10.1083/jcb.115.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin α 6 β 4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niessen CM, Hogervorst F, Jaspars LH, de Melker AA, Delwel GO, Hulsman EH, Kuikman I, Sonnenberg A. The α 6 β 4 integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 14.Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by α 3 β 1, α 6 β 1 and α 6 β 4 integrins. J Cell Sci. 2000;113:869–876. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- 15.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The α 6 β 1 and α 6 β 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 16.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–1507. [PMC free article] [PubMed] [Google Scholar]
- 17.Davis TL, Cress AE, Dalkin BL, Nagle RB. Unique expression pattern of the α6β4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46:240–248. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knox JD, Cress AE, Clark V, Manriquez L, Affinito KS, Dalkin BL, Nagle RB. Differential expression of extracellular matrix molecules and the α 6-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinovitz I, Nagle RB, Cress AE. Integrin α 6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metastasis. 1995;13:481–491. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonkhoff H, Stein U, Remberger K. Differential expression of α 6 and α 2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol. 1993;24:243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- 21.Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, Zahn R, Riethmuller G, Pantel K. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59:241–248. [PubMed] [Google Scholar]
- 22.Davis T, Rabinovitz I, Futscher B, Schnölzer M, Burger F, Liu Y, Kulesz-Martin M, Cress A. Identification of a novel structural variant of the α 6 integrin. J Biol Chem. 2001;276:26099–26106. doi: 10.1074/jbc.M102811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer TA. Folding of the N-terminal, ligand-binding region of integrin α - subunits into a β -propeller domain. Proc Natl Acad Sci USA. 1997;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mould AP, Askari JA, Humphries MJ. Molecular basis of ligand recognition by integrin α 5β 1. I Specificity of ligand binding is determined by amino acid sequences in the second and third NH2-terminal repeats of the α subunit. J Biol Chem. 2000;275:20324–20336. doi: 10.1074/jbc.M000572200. [DOI] [PubMed] [Google Scholar]
- 25.Oxvig C, Springer TA. Experimental support for a β-propeller domain in integrin α- subunits and a calcium binding site on its lower surface. Proc Natl Acad Sci USA. 1998;95:4870–4875. doi: 10.1073/pnas.95.9.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honore S, Pichard V, Penel C, Rigot V, Prevt C, Marvaldi J, Briand C, Rognoni JB. Outside-in regulation of integrin clustering processes by ECM components per se and their involvement in actin cytoskeleton organization in a colon adenocarcinoma cell line. Histochem Cell Biol. 2000;114:323–335. doi: 10.1007/s004180000189. [DOI] [PubMed] [Google Scholar]
- 27.Imanaka-Yoshida K, Enomoto-Iwamoto M, Yoshida T, Sakakura T. Vinculin, Talin, Integrin α6β1 and laminin can serve as components of attachment complex mediating contraction force transmission from cardiomyocytes to extracellular matrix. Cell Motil Cytoskeleton. 1999;42:1–11. doi: 10.1002/(SICI)1097-0169(1999)42:1<1::AID-CM1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Virtanen I, Korhonen M, Kariniemi AL, Gould VE, Laitinen L, Ylanne J. Integrins in human cells and tumors. Cell Differ Dev. 1990;32:215–227. doi: 10.1016/0922-3371(90)90034-t. [DOI] [PubMed] [Google Scholar]
- 29.Tentori L, Leonetti C, Aquino A. Temozolomide reduces the metastatic potential of Lewis lung carcinoma (3LL) in mice: role of α-6 integrin phosphorylation. Eur J Cancer. 1995;31A:746–754. doi: 10.1016/0959-8049(94)00521-6. [DOI] [PubMed] [Google Scholar]
- 30.Walker JL, Menko AS. α6 integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev Biol. 1999;210:497–511. doi: 10.1006/dbio.1999.9277. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alais S, Allioli N, Pujades C, Duband J, Vainio O, Imhof B, Dunon D. HEMCAM/CD146 downregulates cell surface expression of β1 integrins. J Cell Sci. 2001;114:1847–1859. doi: 10.1242/jcs.114.10.1847. [DOI] [PubMed] [Google Scholar]
- 34.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 35.Haier J, Nasralla M, Nicolson GL. Different adhesion properties of highly and poorly metastatic HT-29 colon carcinoma cells with extracellular matrix components: role of integrin expression and cytoskeletal components. Br J Cancer. 1999;80:1867–1874. doi: 10.1038/sj.bjc.6690614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnenberg A, Linders CJ, Daams JH, Kennel SJ. The α 6 β 1 (VLA-6) and α 6 β 4 protein complexes: tissue distribution and biochemical properties. J Cell Sci. 1990;96:207–217. doi: 10.1242/jcs.96.2.207. [DOI] [PubMed] [Google Scholar]
- 37.Hogervorst F, Kuikman I, van Kessel AG, Sonnenberg A. Molecular cloning of the human α 6 integrin subunit. Alternative splicing of α 6 mRNA and chromosomal localization of the α 6 and β 4 genes. Eur J Biochem. 1991;199:425–433. doi: 10.1111/j.1432-1033.1991.tb16140.x. [DOI] [PubMed] [Google Scholar]
- 38.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J, editors. Molecular Biology of the Cell. 3. New York: Garland Publishing; 1994. pp. 1–1294. [Google Scholar]
- 39.Hedman H, Lundgren E. Regulation of α 4 integrin avidity in human B cells: requirement for dephosphorylation events for high avidity VCAM-1 binding. Scand J Immunol. 1996;44:239–242. doi: 10.1046/j.1365-3083.1996.d01-305.x. [DOI] [PubMed] [Google Scholar]
- 40.Mulrooney J, Foley K, Vineberg S, Barreuther M, Grabel L. Phosphorylation of the β 1 integrin cytoplasmic domain: toward an understanding of function and mechanism. Exp Cell Res. 2000;258:332–341. doi: 10.1006/excr.2000.4964. [DOI] [PubMed] [Google Scholar]
- 41.Seminario MC, Sterbinsky SA, Bochner BS. β1 integrin-dependent binding of Jurkat cells to fibronectin is regulated by a serine-threonine phosphatase. J Leukoc Biol. 1998;64:753–758. doi: 10.1002/jlb.64.6.753. [DOI] [PubMed] [Google Scholar]
- 42.Hangan-Steinman D, Ho WC, Shenoy P, Chan BM, Morris VL. Differences in phosphatase modulation of α4β1 and α5β1 integrin-mediated adhesion and migration of B16F1 cells. Biochem Cell Biol. 1999;77:409–420. [PubMed] [Google Scholar]
- 43.Coppolino MG, Dedhar S. Ligand-specific, transient interaction between integrins and calreticulin during cell adhesion to extracellular matrix proteins is dependent upon phosphorylation/dephosphorylation events. Biochem J. 1999;340:41–50. [PMC free article] [PubMed] [Google Scholar]
- 44.Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa M, Toyoda H, Saito M, Morita K, Tawara I, Deguchi K, Kuno T, Shima H, Nagao M, Shirakawa S. Calyculin A and okadiac acid inhibit human platelet aggregation by blocking protein phosphatases types 1 and 2A. Cell Signal. 1994;6:59–71. doi: 10.1016/0898-6568(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 46.Vivier MA, Sollitti P, Pretorius IS. Functional analysis of multiple AUG codons in the transcripts of the STA2 glucoamylase gene from Saccharomyces cerevisiae. Mol Gen Genet. 1999;261:11–20. doi: 10.1007/s004380050936. [DOI] [PubMed] [Google Scholar]
- 47.Rigot V, Andre F, Lehmann M, Lissitzky JC, Marvaldi J, Luis J. Biogenesis of α6β4 integrin in a human colonic adenocarcinoma cell line involvement of calnexin. Eur J Biochem. 1999;261:659–666. doi: 10.1046/j.1432-1327.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- 48.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 49.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985–996. doi: 10.1016/S0002-9440(10)64966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 51.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 52.Schumann H, Baetge J, Tasanen K, Wojnarowska F, Schacke H, Zillikens D, Bruckner-Tuderman L. The shed ectodomain of collagen XVII/BP180 is targeted by autoantibodies in different blistering skin diseases. Am J Pathol. 2000;156:685–695. doi: 10.1016/S0002-9440(10)64772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi M, Dennis K, Peschon JJ, Chandrasekaran R, Mikecz K. Antibody-induced shedding of cd44 from adherent cells is linked to the assembly of the cytoskeleton. J Immunol. 2001;167:123–131. doi: 10.4049/jimmunol.167.1.123. [DOI] [PubMed] [Google Scholar]
- 54.Ratnikov BI, Rozanov DV, Postnova TI, Baciu PG, Zhang H, DiScipio RG, Chestukhima GG, Smith JW, Deryugina EI, Strongin AY. An alternative processing of integrin αV subunit in tumor cells by membrane type-1 matrix metalloproteinase. J Biol Chem. 2002;277:7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- 55.Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, Vincent C, Schmitt D. The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res. 2000;257:255–264. doi: 10.1006/excr.2000.4895. [DOI] [PubMed] [Google Scholar]
- 56.Serres M, Grangeasse C, Haftek M, Durocher Y, Duclos B, Schmitt D. Hyperphosphorylation of β-catenin on serine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exp Cell Res. 1997;231:163–172. doi: 10.1006/excr.1996.3443. [DOI] [PubMed] [Google Scholar]
- 57.Shaw LM, Turner CE, Mercurio AM. The α 6A β 1 and α 6B β 1 integrin variants signal differences in the tyrosine phosphorylation of paxillin and other proteins. J Biol Chem. 1995;270:23648–23652. doi: 10.1074/jbc.270.40.23648. [DOI] [PubMed] [Google Scholar]
- 58.Jewell K, Kapron-Bras C, Jeevaratnam P, Dedhar S. Stimulation of tyrosine phosphorylation of distinct proteins in response to antibody-mediated ligation and clustering of α 3 and α 6 integrins. J Cell Sci. 1995;108:1165–1174. doi: 10.1242/jcs.108.3.1165. [DOI] [PubMed] [Google Scholar]
- 59.Sastry SK, Lakonishok M, Wu S, Truong TQ, Huttenlocher A, Turner CE, Horwitz AF. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J Cell Biol. 1999;144:1295–1309. doi: 10.1083/jcb.144.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin α subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonnenberg A, Janssen H, Hogervorst F, Calafat J, Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987;262:10376–10383. [PubMed] [Google Scholar]
- 62.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 63.Cress A. Quantitation of phosphotyrosine signals in human prostate cell adhesion sites. Biotechniques. 2000;29:776–781. doi: 10.2144/00294st03. [DOI] [PubMed] [Google Scholar]


