Abstract
Our research tests the hypothesis that the inability to sugar-feed reduces the insemination rate in mosquito populations. To test this, we measured the effects of sugar availability on cumulative insemination performance of male Anopheles gambiae Giles s.s. (Diptera: Culicidae) during 10-d periods of continual emergence of equal numbers of both sexes, and we evaluated the implications at the population level with a matrix population model. On each day of each of four replicates, 20 newly emerged mosquitoes of each sex were recruited into the populations within two mesocosms, large walk-in enclosures with simulated natural conditions. Each mesocosm contained a cage to replicate the experiment on a small scale. Scented sucrose was absent or present (control). A human host was available nightly as a bloodmeal source in both mesocosms. Sugar availability and enclosure size significantly influenced female insemination. In the mesocosms, with sugar 49.7% of the females were inseminated, compared with 10.9% of the females without sugar. In the small cages, the insemination rates were 76.0 and 23.5%, respectively. In the mesocosms, cumulative survival of females after 10 d was 51.6% with sugar and 25.6% without sugar. In the cages, female survival was 95 and 73%, respectively. Sensitivity analysis of the population projection matrix shows that both reduced male survival and reduced mating capability due to a lack of sugar contributed to lower insemination rates in females, and in the absence of sugar the insemination rate was lowered to an extent that led to population decline.
Keywords: mosquito, sugar feeding, mating performance, population projection matrix
Anopheles gambiae Giles s.s. (Diptera: Culicidae) is one of the main vectors of malaria in sub-Saharan Africa, yet the behavioral ecology of the males remains insufficiently explored (Ferguson et al. 2005). Current interest in developing sterile male or genetically modified male release programs makes this need for basic knowledge urgent. Here, we examine the mating capability of males in relation to the availability of sugar sources in the environment. Plant sugar is the only exogenous source of energy available to adult An. gambiae males (Foster 1995). In the absence of sugar, survival of males is dependent solely on accumulation of larval reserves, and in this species those reserves are severely limited (Foster and Takken 2004, Walker 2008). For all females of a cohort to become inseminated under these circumstances, either each male must successfully mate at least once within the first few days of his life, before reaching his peak mating capability (Verhoek and Takken 1994), or some proportion of males must inseminate multiple females during this brief period. This demand on males may increase if polyandry in nature is as common as in some laboratory settings (Klowden 2006, Helinski et al. 2008).
A recently growing body of work on the use of plant sugars by An. gambiae indicates sugar feeding in this species is more common than traditionally thought (Muirhead-Thompson 1951, Gillies and De Meillon 1968, McCrae 1989). A wind tunnel test of newly emerged females showed a preference for honey odor over human foot odor (Foster and Takken 2004). Furthermore, studies on plant feeding demonstrated that some plant species promote the survival of both males and females of this species (Gary and Foster 2004, Impoinvil et al. 2004, Manda et al. 2007a). Laboratory studies have revealed that sugar feeding is a common and recurring event in the lives of both males and females, and in females, sugar feeding is strongly influenced by the availability of blood and/or oviposition sites (Gary and Foster 2006). A recent cage study (Manda et al. 2007b) showed An. gambiae to be selective in the plants it feeds upon, which may indicate a preference for particular plant species as sugar sources in the field.
Whether An. gambiae feeds on sugar as a necessity or uses this resource only opportunistically and under limited circumstances (such as in laboratory cages), is a question that remains unanswered. Certainly without sugar, females remain capable of completing gonotrophic cycles (Briegel and Hörler 1993) and seem to have a higher reproductive success despite a diminished expected life span (Straif and Beier 1996). Truly adverse effects of sugar deprivation on An. gambiae mating were unknown until Gary et al. (2009) focused on the behavior of male mosquitoes and found that the presence of sugar, in combination with advanced male age, larger body size, lower temperature, and smaller cage size, significantly enhanced male mating performance. Under the most favorable conditions (small cage, large males, 23°C) males with or without sugar were equally capable of mating on the first two nights. On the third night, males without sugar were less capable of mating, and after the third night had virtually starved to death. Although this clearly indicated that males require sugar to reach their peak mating performance, it did not allow for a direct extrapolation to mating success in nature at the population level, where daily cohorts of both sexes overlap, environmental factors fluctuate, and cumulative sex ratios depend on both male and female survival rates. For example, one could imagine that continuous recruitment of newly emerged males might provide sufficient mating stock for inseminating most or all females. In our study, therefore, we simulated a more natural situation by releasing cohorts of newly emerged males and females daily into mesocosms in the presence or absence of sugar, to determine how sugar-source availability may affect reproductive potential in the field. Concurrently we ran the same experiment in cages located within the mesocosms, to compare the effect of sugar under the less natural conditions of close confinement on mating behavior in this species. To explore the implications of reduced male performance in the absence of sugar, we developed a population-dynamics theory using a two-sex population projection matrix (Caswell 1989, Fujiwara and Caswell 2002).
Materials and Methods
Mosquitoes
The mosquitoes used for these experiments came from a colony established in 2001 by staff at the International Centre of Insect Physiology and Ecology from the local population of An. gambiae s.s. in Mbita Point, Suba District, Nyanza, Kenya, and identified by polymerase chain reaction (PCR). This Mbita strain has been maintained in acrylic cages at 26.6 ± 1°C and 80 ± 5% RH since 2006. Water and 10% sucrose solution were available to colony adults ad libitum. Experimental mosquitoes were reared by transferring 100 newly hatched first instar larvae into 22.8- by 33-cm pans filled with 450 ml of aged tap water and feeding them 0.2 mg of finely ground Tetramin fish flakes per larva during the first 3 d of larval development, 0.4 mg for the next 3 d, and 0.8 mg for subsequent days until pupation. The day before adult emergence, each pupa was placed in a glass test tube three-quarters filled with aged tap water and corked. Adults were released the following day into two types of enclosures: walk-in mesocosms and laboratory cages located within these mesocosms.
Mesocosms and Cages
Two contiguous rooms 2.4 by 1.8 by 2.1 m (=9.1 m3) in The Ohio State University Biological Sciences Greenhouse, Columbus, OH, allowed for a more natural situation than a typical cage can provide, particularly for the energetic demands associated with swarming, mating, and locating water, sugar, blood, and dark resting sites within a large space. Prototype mesocosms previously had been used successfully by Gary et al. (2009) and a fully developed version is described in more detail by Stone et al. (2009). In brief, humidity was maintained by a low flow of water through a soaker hose on the floor, whereas temperature was maintained within a preset range (20–26°C) by a thermostatically controlled greenhouse climate-control system, automatically heated by steam wall radiators and cooled by overhead vents and floor-level evaporative coolers. Temperature and humidity were recorded with Hobo data loggers (Onset Computer Corp., Bourne, MA).
Each mesocosm contained a cement block “hut” (80 by 62 by 139 cm), which provided daytime resting sites for the mosquitoes. The front of the hut was a dark wooden board with 30 circular holes into which were inserted 10-cm-diameter cardboard mailing tubes cut to 20-cm lengths and painted black on the inside. Experiments were performed between September and November, when natural photoperiods range from 13:11 (L:D) to 10:14 (L:D) h. A combination of a string of incandescent light bulbs and fluorescent shop lights maintained a minimum 12-h photophase, timed so that after the artificial light switched off, the natural evening crepuscular light could facilitate swarming and coupling. Both were observed during twilight, although they were not quantified. Males engaged in the dance-like, zig-zagging flight typical of swarming male mosquitoes.
Eight glass vials containing either water or sugar solution were suspended upside-down from wires along the length of the 121-cm lighting fixture. The opening of each vial was stopped with a cotton-wool dental wick. A plastic tray (26 by 32 cm), containing a thin layer of water, was placed on the floor to serve as an oviposition site to avoid potential consequences of delayed oviposition.
To compare the performance of mosquitoes in the large mesocosm environment to their performance in cages of a size often used in colony maintenance, a clear acrylic plastic cage (30 by 30 by 45 cm) was situated on the floor of each mesocosm, in which the same experiment was performed simultaneously. Black plastic cups, oriented sideways and glued against the cage wall, served as resting sites. A small plastic cup (10 cm in diameter, 4 cm in depth) one-third filled with aged water served as an oviposition site.
Experimental Procedures
Each day, 20 male and 20 female mosquitoes were released into each of the mesocosms the afternoon after their emergence to simulate the natural daily emergence of new cohorts. At the same time, five males and five females were released into each of the plastic cages. Field observations by Marchand (1984) showed swarms to typically consist of 50–100 mosquitoes, although with no lower limit. The numbers we released, taking survival into account, allowed for roughly comparable numbers. Typical resting densities in and around households also fall within this range (Minakawa et al. 2002).
In the water-only (experimental) mesocosm and cage, all glass vials were filled with water: eight in the mesocosm, four in the cage. In the sugar-access (control) mesocosm, four were filled with water and four with a 10% sucrose solution that contained 0.05 ml of verbenone per liter to provide an attractive scent (Gary and Foster 2006). In the cage, two were filled with water and two with scented sucrose. The wicks that delivered the fluid were replaced every 2 d to ensure an ad libitum supply of water or fresh sucrose solution.
Each evening, between 2000 and 2300 hours, the females in one of the mesocosms and the cage within it were allowed to blood-feed on the lower legs and feet of a human volunteer (C.M.S.) for 20 min, after which the females in the other mesocosm and its cage were allowed to blood-feed (in accordance with Human Subjects Research protocol 2004 H0193 and Bio-hazard Research protocol 2005N0020). The order in which the treatments were given access to blood was alternated each day. Using a headlamp with a red filter to avoid disturbing the mosquitoes, the volunteer observed and recorded the total number of bites that occurred during the exposure period and the time (minutes) between the beginning of the exposure period and the initiation of each bite, i.e., settling on the skin. Settling was invariably followed by blood engorgement. The time of the median bite after the start of exposure was used to assess female responsiveness to a potential bloodmeal on each of the 10 nights in each treatment. Mean blood-feeding frequency per female in each treatment during each of the 10 nights was calculated from estimates of female survivorship (as described below).
After 10 d, all surviving mosquitoes were collected by aspirator, frozen, and later counted. Every female was dissected in saline, the spermatheca removed and inspected visually for the presence of sperm at 100 and 450× magnification. Because body size is known to affect behavior and survival of mosquitoes, 20 males and females from each treatment were selected randomly to determine wing length, measured from the axillary incision to the tip, excluding the fringe (Packer and Corbet 1989).
Daily survival rates of all released females and males were based on the number of mosquitoes that were still alive at the end of the experiment (see Fig. 2). Daily survival could be derived from this value, because the number alive after 1–10 d in an enclosure should equal the sum of the survivors of all 10 released cohorts. The survivors from day one, for example, would be based on n = 20*daily survival (to the power of 10), and so on, for each of the cohorts (assuming no significant variation in cohort survival). In a similar manner the daily male insemination rate could be deduced. For example, for the cohort released on the first day the number of surviving, inseminated females at the end of the experiment would be as follows: 10 nights*sex ratio*male insemination rate *20 females*daily female survival10. The sum over all 10 cohorts should then be equal to the total number of inseminated females at the end of the experiment. The average sex ratio over all 10 nights was used to deduce daily male insemination rates (see further elaboration below). The experiment was performed four times, each time switching treatments between mesocosms to eliminate any effects of mesocosm differences unrelated to dietary treatment.
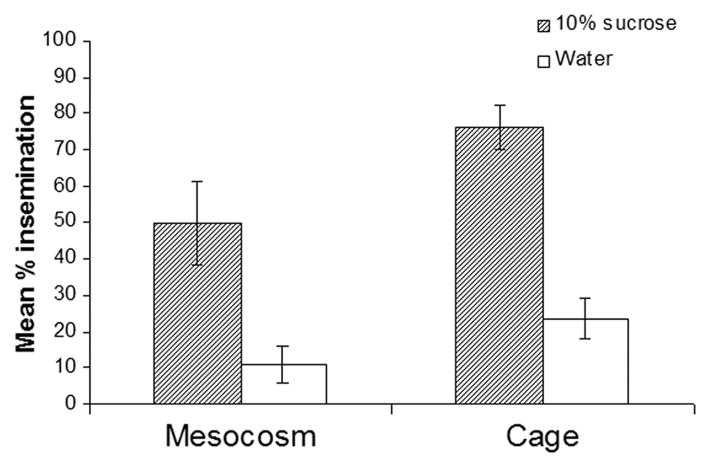
Fig. 2.
Mean percentage of four replicates of females inseminated after 10 d with or without sugar, both with blood nightly, in large or small enclosures (mesocosm versus cage, respectively). Newly emerged males and females were introduced daily.
Temperature and Humidity
Temperature and relative humidity differed slightly between different areas within the experimental enclosure. Mean ± SD values for temperature and humidity were as follows: within the plastic cage set on the floor 23.0 ± 2.67°C and 63.3 ± 16.0%, within a resting tube in the mesocosm 24.5 ± 2.05°C and 60.6 ± 19.8%, and on top of the hut in the mesocosm, 25.4 ± 3.21°C and 56.6 ± 20.5%. The narrower range of temperature and humidity within the resting sites, and the slightly lower temperature and higher humidity on the floor, compared with the top of the hut, illustrate that the mosquitoes would be capable of evading the more inhospitable conditions. This was confirmed observationally; typically all mosquitoes were resting either close to the floor outside of the resting hut, on the soaker hose, or (the majority) inside the resting tubes, with a preference for the lower tubes. Based on all 74 tallies throughout the experimental periods, an average of 81 ± 15% of mosquitoes were found resting in the tubes during the daytime, as opposed to elsewhere in the mesocosm.
Population Projection Matrix
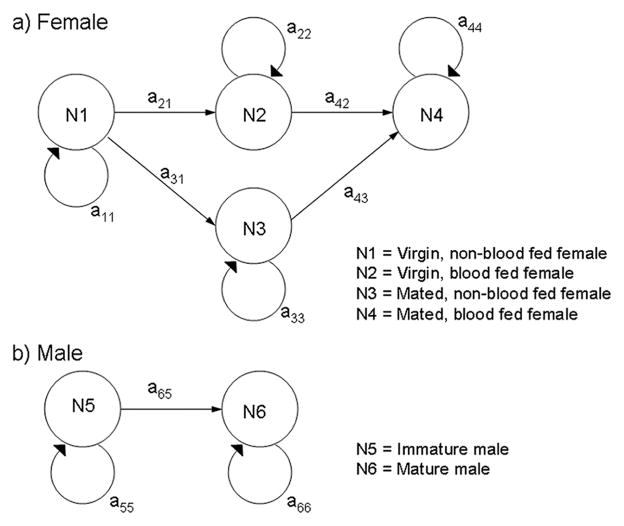
The goal of developing a population model was to evaluate the consequences of reduced male insemination performance on population growth over many generations. We chose to use a population projection matrix, because mosquito populations are structured according to mating status (virgin and mated females, whereas males were not competent until rotation of the terminalia was complete) and, in the case of females, blood-feeding status (i.e., those that have obtained a blood-meal, and those that have not). When reproduction and survivorship are status-dependent, models that explicitly consider population structure are appropriate (Caswell 1989). Our model consisted of a stage-distribution vector and a transition matrix. The stage distribution vector, At, was composed of the elements N1–N6, which represent the numbers of males or females in any given stage at any point in time (Fig. 1). Equations for the transition values, a, between any two elements, that is, aij, are provided below. An overview of the parameters used in these equations is given in Table 1.
Fig. 1.
Stage structures for male and female An. gambiae, schematically depicting the stage distribution vector (N1–6) and the transition matrix elements (aij).
Table 1.
Parameters used in equations for stage transitions
| Parameter | Description | Value (lit.) | Value (exp.) | Source |
|---|---|---|---|---|
| ξ | Mating term | Varies | ||
| τ | Sex ratio (proportion m:f) | Varies | ||
| φ | Male insemination rate (♀ ♀/d) | 0.5; 0.3 | 0.245; 0.09 | A. C. Rodriguez, R. L. Aldridge, and W. A. F. (unpublished data) |
| β | Blood feeding probability/female/d | 0.2 | ||
| μ | Death rate in environments with sugar or sugar-free (s/sf) of: | Gary and Foster 2001; Midega et al. 2007 | ||
| Males | ||||
| Females, blood fed | 0.034; 0.34 | 0.192; 0.693 | ||
| Females, unfed | 0.028; 0.034 | 0.128; 0.16 | ||
| 0.23 | 0.46 | |||
| η | Adult male maturation rate | 0.7 | Gary et al. 2009 | |
| R | Daily production of offspring that survive to emerge as adults of a mated, blood fed female | 0.099 | 1.03 | Fitted to give pop growth rates of 50x/yr; or 10× in 100 d |
lit., values estimated from literature or other sources; exp., experimental results from this study.
The transition matrix, Tt, corresponding to the stage structure in Fig. 1, had three regions and can be written as follows:
The upper-left sector contains transition values for females (aij) as well as recruitment of females via reproduction from mated, blood-fed females (F4α), where α is the primary sex ratio that we set at 0.5. The lower left sector provides recruitment of males via reproduction from mated, blood-fed females. The lower right sector harbors transition values for males. Furthermore, there are several nontransition elements (e.g., a11), where individuals remain within their current stage, as explained below.
Female transitions are determined by the following three parameters: 1) a mating term, ξ(0,1), which is a function of sex ratio; in the model described here, ξ = τφ, where φ is the sex ratio (i.e., ratio of competent males to virgin females) and φs and φsf are male insemination rates in sugar-present and sugar-free environments, respectively; 2) a blood-feeding probability β; and 3) survivorship terms e−μfs and e−μfsf in sugar-present and sugar-free environments, respectively. Finally, as we assume no natal control of sex (i.e., α = 0.5), recruitment of females into the population is simply N4 R α, where R is the daily fecundity of a mated, blood-fed female. For males, recruitment is identical to females, and immature males (i.e., adult males that are not yet competent) mature at rate e−η, and survivorship terms are e−μms and e−μmsf in sugar and sugar-free environments, respectively. This yields the following transitions (daily probabilities):
a11 = (1 − ξ) (1 − β) e−(μf) (a virgin, unfed female will survive but remain in the same state)
a21 = (1 − ξ) β e−(μf) (a virgin, unfed female will survive, blood feed, but fail to mate)
a31 = ξ (1 − β) e−(μf) (a virgin, unfed female will survive, mate, but fail to blood feed)
a22 = (1 − ξ) e−(μf) (a virgin, blood-fed female will survive but fail to mate)
a33 = (1 − β) e−(μf) (a mated, unfed female will survive but fail to blood feed)
a42 = ξe−(μf) (a virgin, blood-fed female will survive and mate)
a43 = βe−(μf) (a mated, unfed female will survive and blood feed)
a44 = e−(μf) (a mated, blood-fed female will survive)
a55 = (e−(η+μm)) (an immature male will survive but not mature)
a65 = (1 − e−(η)) e−(μm) (an immature male will survive and mature)
a66 = e−(μm) (a mature male will survive)
There are several key assumptions:
Males are capable of fewer inseminations per capita, per night, as sugar availability declines.
Females and males die at faster rates as sugar availability declines, i.e., μfsf > μfs and μmsf > μms, respectively.
Probability of insemination is a linear positive function of the sex ratio.
Daily fecundity, R, reflects recruitment of adults into the population and therefore combines fecundity in terms of eggs laid per female and larval survival which is assumed to be density-independent.
We used the model to evaluate the importance of sugar availability (e.g., nectar) on the growth of a theoretical anopheline population. We started with an initial population with a quasi-stable age distribution and under environmental conditions allowing exponential growth. Because there are functions in the transition matrix, we were not able to solve for the stable distribution directly. Instead, we ran the model for 50 generations, obtained a nearly constant R, and used that distribution. The model was iterated over time (1 d at a time) with given values for the different parameters, and we observed population size and sex ratios. This was conducted independently for environments with and without sugar sources.
To confirm that the results of the simulations were not only valid for our experimentally derived values, we ran the simulations separately using values for survivorship and male insemination rate estimated from the literature (Gary and Foster 2001; Midega et al. 2007; Rodriguez, Aldridge, and W.A.F., unpublished), and at different levels of fecundity. For the literature-based simulations, R was determined numerically to generate population growth that would yield an arbitrary 50-fold population increase in one year when sugar was readily available. Although this R (0.099) indicates a fairly low fecundity per blood-meal taken, this figure for production of adult mosquitoes incorporates considerable egg and larval mortality. Further note that we do not incorporate the time investment and probability of finding a suitable oviposition site and that females once blood-fed and mated do not revert to a previous stage, so that time spent on obtaining subsequent blood meals is also corrected for in our value of R. For the mesocosm-experiment-based simulations we set R at 1.03, giving a tenfold population-level increase over 100 d, which is comparable with the reported increase in mosquito density in houses over the course of the long rainy season in western Kenya (Minakawa et al. 2001, 2002). The rate of maturation, η, was set at 0.7 to yield 50% maturation within the first day of emergence. Finally, note that field populations normally do not continue to grow unimpeded for such a large number of generations.
In model runs, we arbitrarily implemented a sugar shortage starting at day 183. Here, we considered two scenarios: 1) Only males are impacted in terms of insemination rate (60% of normal, or 27% based on our experiment) and survivorship (10% of normal, or 22% based on our experiment). 2) Males and females are both impacted by sugar elimination. Here, we assumed that blood-feeding females without sugar survive at 80% longevity relative to females with access to both sugar and blood, whereas females that do not blood-feed with probability (1 − β) survive at the same reduced rate as males.
Statistics
Effects of sugar availability on male mating performance, quantified as the proportion of females inseminated at the end of 10 d, were analyzed with chi-square tests. Survivorship data were analyzed with Mann–Whitney tests. Wing lengths were normally distributed and compared with t-tests. Biting data were analyzed with Friedman tests. All facets of the analysis employed SPSS, version 16 software (SPSS Inc., Chicago, IL).
Results
Experiments
Presence of sugar had a significant and profound effect on the insemination of females (Fig. 2), both in the mesocosms (χ2 = 88.7, P < 0.05; n = 598) and plastic cages (χ2 = 89.3, P < 0.05; n = 335). In the mesocosms, over four replicates the mean percentage of females that had mated after 10 d was 49.7% when sugar was present and 10.9% when it was absent. This effect also was observed in the cages, yet here the insemination rates were higher, with sugar 76.1% and without sugar 23.5%. Calculations based on survivorship and sex-ratio estimates gave average daily male insemination rates of 0.25 and 0.09 in mesocosms with sugar present and absent, and 0.28 and 0.20 in cages with sugar present and absent, respectively.
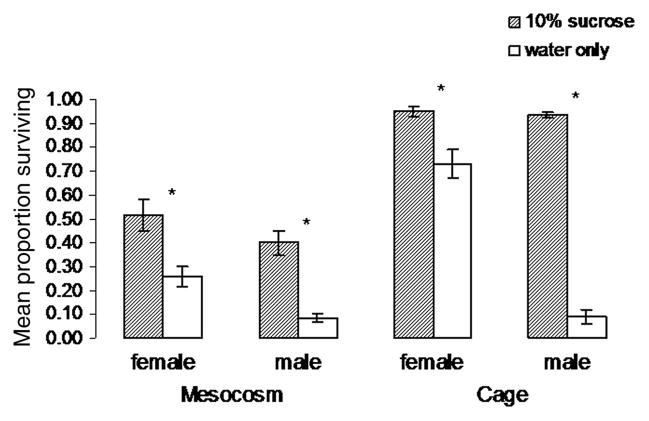
Survival of mosquitoes, measured as the number of survivors present after 10 d, depended strongly on whether a sugar source was present or absent in the environment (Fig. 3). Mann–Whitney tests indicated that this difference was significant for both males and females in both mesocosms and cages (U = 0, P = 0.029 for all four comparisons; n = 8). Average daily survival of females was 0.88 and 0.72 in mesocosms with sugar present and absent and 0.99 and 0.94 in cages with sugar present and absent, respectively. Corresponding values for males were 0.83 and 0.50 in mesocosms with sugar present and absent and 0.99 and 0.50 in cages with sugar present and absent, respectively.
Fig. 3.
Survival of males and females after 10 d with or without sugar, both with blood nightly, in large or small enclosures (mesocosm versus cage, respectively), based on four replicates and daily releases of newly emerged males and females.
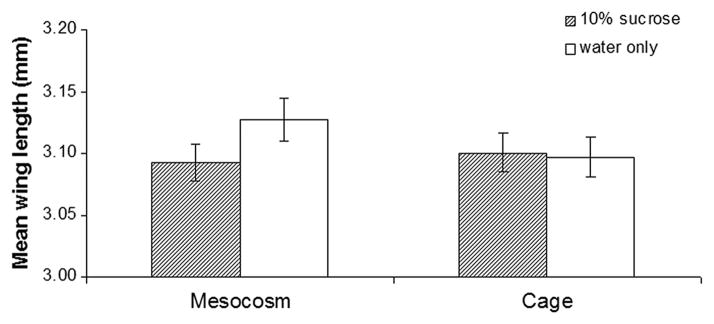
The body size of females collected after 10 d from the mesocosm with only water was slightly, but not significantly, greater than those collected from the mesocosm where sugar was present, (t-test, df = 158, P = 0.11; 46.5% power) (Fig. 4). Surviving females in the plastic cages, and males in the mesocosms, showed no treatment differences in body size after 10 nights. Survival of males in cages without sugar was too low to allow a comparison.
Fig. 4.
Mean size of females (wing length, n = 80) surviving after 10 d, with or without sugar in large or small enclosures (mesocosm versus cage, respectively).
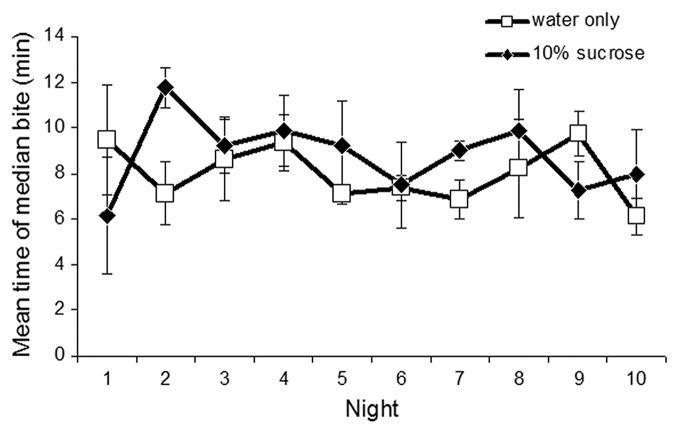
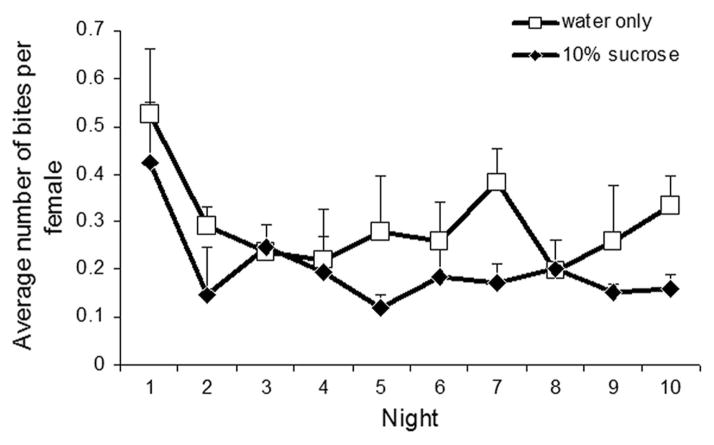
Across the replicates, median time to initiate biting (Fig. 5), averaged among the four replicates, was 8.2 min for females without sugar and 9.9 min for females with sugar, a difference that is not significant (Friedman test, χ2 = 3.6, df = 1, P > 0.05). It seemed that only on the second night, females with sugar present took substantially longer to land on their host (median bite, 16 min) than females without sugar (median bite, 7 min). Figure 6 depicts the average number of bites per female per night for mesocosms with and without sugar. Estimates of the number of females present in the mesocosms on a given night were derived from survivorship values as described above. On eight of the 10 nights, females without sugar had a higher biting rate than females with sugar. Over all 10 nights females took an average of 0.30 and 0.20 bloodmeals per female per day, in mesocosms without and with sugar, respectively. Nonetheless, this difference was not significant (Friedman test, χ2 = 3.6, df = 1, P = 0.058).
Fig. 5.
Mean time (±SE) of median bite recorded within 20 min host-exposure period each night, for females in mesocosms with 10% sucrose versus only water available.
Fig. 6.
Average number of bites per female (±SE) per night, for females in mesocosms with 10% sucrose versus only water available.
Models
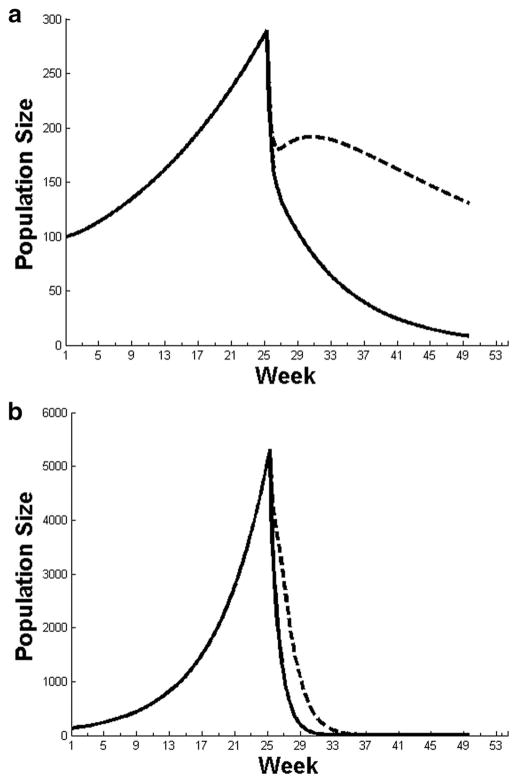
We explored the results of eliminating access to sugar on population size after 183 d of growth using a two-sex population projection matrix by using values derived from the literature (Fig. 7a) and values based on mesocosm experiments (Fig. 7b). Both graphs show male-only (dashed lines) and male-plus-female effects (solid lines).
Fig. 7.
Population decline after the removal of carbohydrate sources in the environment assuming only males are affected (dashed line) or both sexes are affected (solid line) by a lack of sugar, for simulations based on values from the literature (a) or from the mesocosm experiments (b).
For both scenarios in Fig. 7a, there is an exponential increase in the mosquito population until day 183, after which the populations rapidly decline, most dramatically within the scenario with effects on both males and females. In Fig. 7a, the male-only effect scenario shows a rapid decrease in mosquito numbers, mostly due to shifts in survivorship of males (μms = 0.034 versus μmsf = 0. 34), i.e., median survivorship drops from 20 to 2 d. From there, both male and female numbers declined due to reduced insemination effects and altered sex ratios, although more weeks were required to drive the population to near-zero than in the male-plus-female effect scenario. The importance of insemination rate in this decline was emphasized by the result that before reducing sugar, virgin females made up 4% of the population versus 19% afterward. The same pattern of exponential growth, then a plummeting of numbers, can be seen in Fig. 7b. Here, the difference between scenarios was less pronounced, and the population decline sharper, due to the lower insemination rates and more severe mortality estimates obtained from our mesocosm experiments.
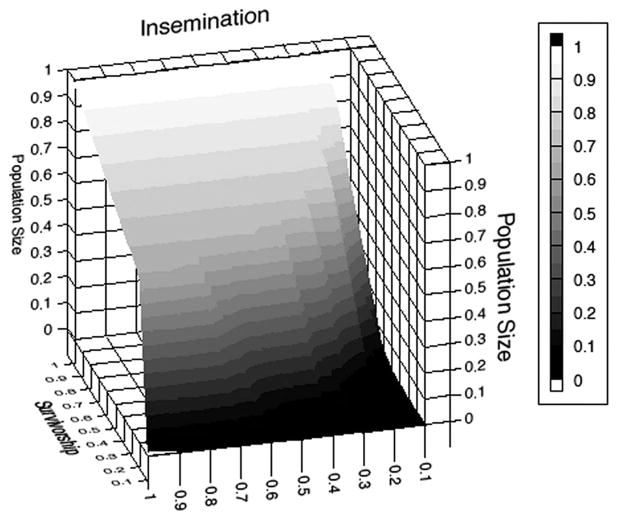
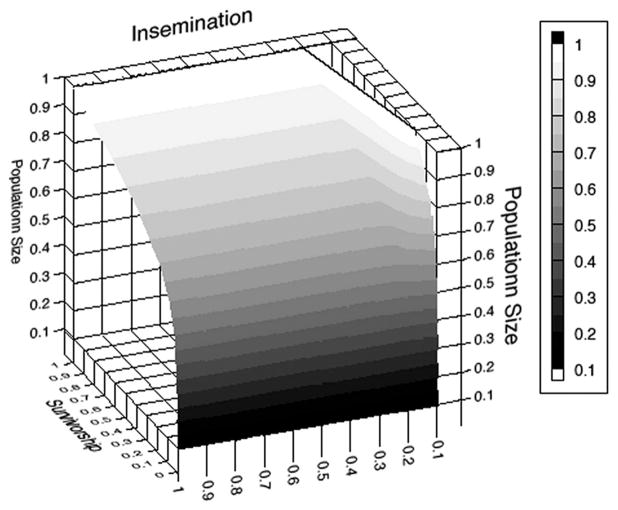
To explore the impact of reduced insemination rates and survivorship on population growth, we ran a sensitivity analysis with stepwise reductions of 10% for each of these parameters in each of the two scenarios. Figures 8 and 9 show final population size after 183 d with sugar elimination, normalized against the maximal population size in the presence of sugar. As expected, each of the two parameters contributed to population reduction, but it was the survivorship parameter that had effects throughout the surface. This was because reduced insemination rates due to behavioral factors could be mitigated by large numbers of males when they were able to survive for extended periods (we assumed no senescence or any loss of fertility across nights). However, when survivorship was low, there was a strong effect of insemination loss as well. This interaction was less obvious in the plots with two-sex effects, because female death shifts the sex ratio back toward a 1:1 ratio, thereby reducing the impact of limited per-capita insemination.
Fig. 8.
Population size after 183 d at different levels of reduced male insemination rate and survival, expressed as a proportion of population size after 183 d when sugar is readily available, under the assumption that males are the only sex affected by a lack of carbohydrates in the environment.
Fig. 9.
Population size after 183 d at different levels of reduced male insemination rate and survival, expressed as a proportion of population size after 183 d when sugar is readily available, under the assumption that both males and females are affected by a lack of carbohydrates in the environment.
Discussion
Insemination of female An. gambiae was shown to depend heavily upon the presence of sugar in the environment. Female An. gambiae, like female Aedes aegypti (L.) (Harrington et al. 2001), are generally thought to survive primarily by blood feeding and only rarely take sugar meals in nature (Beier 1996). Therefore, the sex that affects insemination rates due to the absence of sugar in the environment can reasonably be assumed to be the males, which do (as adults) rely completely on sugar as a food source (Yuval 1992, Foster 1995).
Without crop sugar and glycogen, both of which play an important role as fuel for flight in mosquitoes (Nayar and Van Handel 1971, Yuval et al. 1994), males are completely dependent on the reserves carried over from the larval stage to provide them with enough energy to sustain the flight activity necessary for swarming and mating behavior; here they were severely disadvantaged by an absence of sugar.
Besides affecting male An. gambiae by limiting flight energy, a lack of sugar also affected their survival. Holliday-Hanson et al. (1997) postulated that lipids converted from sugars may be used for resting metabolism, because starved males of An. freeborni were found to have drastically reduced lipid content. The lack of a chance to build up and replenish these reserves induced such mortality that these males were unlikely to reach an age of >2–3 d (Gary and Foster 2004). An. gambiae males show a peak in insemination capability around 7 d of age (Verhoek and Takken 1994), suggesting that sugar-deprived males not only have less energy to sustain patrolling and capturing flight within a swarm, but also may never reach the age at which their mating capabilities are at their full potential. The combination of these factors likely explains the reduced insemination rate of females in the environment where sugar was unavailable, with the decreased survival of males most strongly reducing insemination rates (Fig. 8).
Although the presence of sucrose increased insemination rates in both the plastic cages and the mesocosms, reduced enclosure volume also increased insemination rates, such that rates overall were higher in the cages than in the mesocosms. This may be because chance encounters between males and females are simply more frequent in a confined space; or females may be disturbed more often in these smaller confines, taking flight more frequently, consequently increasing their chance of being heard and seized by a male; or it may reflect the adaptation of the mosquito strain to mating in colony cages. It is noteworthy that daily male insemination rate, φ, in cages (following the formula ξ = τφ) without sugar was 71% of φ (0.2/0.28) when sugar was present, whereas in a sugar-deprived mesocosm environment, φ is only 37% (0.09/0.245) of that when sugar is available, clearly indicating that the effect of sugar availability on male insemination rate is itself influenced by enclosure size.
In a similar mesocosm study, within a single cohort of sugar-deprived mosquitoes, Gary et al. (2009) found lower insemination than we did, whereas in cages the level of insemination was comparable to that found here. Although Gary et al. (2009) found mesocosm males to be practically incapable of mating with females if sugar was not available, we found that on average 10.9% of females became inseminated even in the absence of sugar. A direct comparison was not possible, however, due to the differences in experimental design: 10 consecutive cohorts of mosquitoes released in this experiment versus a single cohort in theirs. In addition, Gary et al. (2009) used the Suakoko strain, established in 1987 by M. Coluzzi from mosquitoes originating in Suakoko, Bong, Liberia. This strain has been used in laboratories for a significantly longer time than the 2001 Mbita strain used here. Although little is known about how fast An. gambiae adapts to mating in small cages and whether it simultaneously loses the ability to mate under more natural circumstances, it may help explain why the Suakoko strain performed worse under mesocosm conditions than the Mbita strain.
Our observation that sugar-deprived males with a high mortality could still inseminate 10% of the females poses the question whether some males sexually matured quickly and mated with females early in life, or whether a minority of males managed to survive longer and achieved most of the mating. Although Verhoek and Takken (1994) found that on the second day after emergence male An. gambiae were capable of inseminating females to some extent, how that holds up in the absence of sugar and in a much larger environment remains unclear. A relevant question that was not addressed in the current study is to what extent, when sugar is in short supply but not absent, the few males that gain access to sugar can make up for the insemination deficit. Survival of females in mesocosms with sugar was lower than survival in cages, but it did reflect mortality estimates of An. gambiae in the field. In our experiments, 52% of females were present at the end of the 10-d experiment, which equals 12% mortality per day. This mortality is close to field estimates obtained from mark-release-recapture experiments, which range from 16% (Gillies 1961) and 12–20% (Costantini et al. 1996) to 22% per day (Takken et al. 1998). Daily mortality at the Kenyan coast has been estimated to be 5% (Midega et al. 2007).
Although the differences in energy expenditure between mesocosms and cages may have contributed to the difference in survival, females might be expected to survive well in both types of enclosures in both the presence and absence of sugar, because they could compensate for the absence of sugar by producing fewer eggs or taking more blood. Figure 6 indicates that the latter indeed occurred (though not significantly), but that this higher rate of blood feeding did not allow females to compensate for the lack of sugar in terms of survival. This observation is consistent with the results of Straif and Beier (1996) and Gary and Foster (2001). Blood feeding also may have been much easier, and therefore more frequent in both treatments, in the cages. Another difference may have been the absence of predators in cages. Occasionally other arthropods, such as ants, small spiders (Salticidae), and centipedes, were found in the mesocosms and likely disturbed and preyed upon mosquitoes, perhaps contributing to a field-like level of mortality.
Insemination levels for An. gambiae found in the sugar treatment of this experiment were comparable to rates in the field (88–98% for An. gambiae in Tanzania [Gillies and Chir 1956]; 78% for An. arabiensis in Ethiopia [Ameneshewa and Service 1996]), which suggests that sugar feeding must be a part of the behavioral repertoire of male An. gambiae in nature. If only 10% of females can become inseminated in the absence of sugar sources, does that mean this species is confined to areas where plants with nectar, or other sugar sources, are present? Our matrix population model certainly suggests that this is the case. Several of the underlying assumptions do require more information on the biology of this species. To obtain a more realistic R, for example, more information on egg fertility and survivorship of immatures would be useful. How swarm size in nature affects mating (locating a small swarm of males might be considerably easier for females in a mesocosm than under true field conditions, where an Allee effect may occur [Courchamp et al. 1999]) is relevant to the assumption that mating probability is a linear positive function of sex ratio. Even so, nectar availability in nature must certainly have a considerable impact on the dynamics and size of An. gambiae populations, which suggests a possibility of employing environmental management as a means of population control. The degree of population suppression by eliminating sugar availability, as exhibited in this study, is significant and certainly in line with that of other methods of population control. For example, Fillinger and Lindsay (2006) reported a 90% population reduction from using various Bacillus larvicidal formulations. Similarly, Perez-Pacheco (2005) reported a 30–88% reduction of Anopheles larvae from using mermithid nematodes. Finally, Ansari and Razdan (2004) reported ≈90% suppression of Anopheles culicifacies Giles from deltamethrin indoor spraying. Our work is similar to that of Gu et al. (2006) insofar as we also considered nontraditional control by manipulation of a life-history component on mosquito population growth rates: in our case, insemination rates, and in Gu et al. (2006) oviposition site arrival rates. In both cases, simple changes in rates of one component can have profound effects at the population level.
Acknowledgments
We thank James Cannon for constructing the framework of the mesocosms and are grateful to Joan Leonard, George Keeney, and Cindy Schroeder for expert advice and assistance in the greenhouse. This project was supported in part by National Institutes of Health grant R21-AI062957.
References Cited
- Ameneshewa B, Service MW. The relationship between female body size and survival rate of the malaria vector Anopheles arabiensis in Ethiopia. Med Vet Entomol. 1996;10:170–172. doi: 10.1111/j.1365-2915.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Razdan RK. Follow-up studies after withdrawal of deltamethrin spraying against Anopheles culicifacies and malaria incidence. J Am Mosq Control Assoc. 2004;20:424–428. [PubMed] [Google Scholar]
- Beier JC. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and An. funestus (Diptera: Culicidae) in western Kenya. J Med Entomol. 1996;33:613–618. doi: 10.1093/jmedent/33.4.613. [DOI] [PubMed] [Google Scholar]
- Briegel H, Hörler E. Multiple bloodmeals as a reproductive strategy in Anopheles (Diptera: Culicidae) J Med Entomol. 1993;30:975–985. doi: 10.1093/jmedent/30.6.975. [DOI] [PubMed] [Google Scholar]
- Caswell H. Matrix population models: construction, analysis, and interpretation. Sinauer; Sunderland, MA: 1989. [Google Scholar]
- Costantini C, Li S, Della Torre A, Sagnon N, Coluzzi M, Taylor CE. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a West African Sudan savanna village. Med Vet Entomol. 1996;10:203–219. doi: 10.1111/j.1365-2915.1996.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Courchamp F, Clutton-Brock T, Grenfell B. Inverse density dependence and the Allee effect. Trends Ecol Evol. 1999;14:405–410. doi: 10.1016/s0169-5347(99)01683-3. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Bernadette J, Ng’habi K, Knols BJG. Redressing the sex imbalance in knowledge of vector biology. Trends Ecol Evol. 2005;20:202–209. doi: 10.1016/j.tree.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Fillinger U, Lindsay S. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya Trop. Med Int Health. 2006;11:1629–1642. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Foster WA, Takken W. Nectar-related vs. human-related volatiles: behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding. Bull Entomol Res. 2004;94:145–157. doi: 10.1079/ber2003288. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Caswell H. Estimating population projection matrices from multi-stage mark-recapture. Ecology. 2002;83:3257–3265. [Google Scholar]
- Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Med Vet Entomol. 2004;18:102–107. doi: 10.1111/j.0269-283X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Diel timing and frequency of sugar feeding in the mosquito Anopheles gambiae, depending on sex, gonotrophic state and resource availability. Med Vet Entomol. 2006;20:308–316. doi: 10.1111/j.1365-2915.2006.00638.x. [DOI] [PubMed] [Google Scholar]
- Gary RE, Cannon JW, III, Foster WA. Effect of sugar on male Anopheles gambiae Giles (Diptera: Culicidae) mating performance, as modified by temperature, space, and body size. Parasit Vectors. 2009;2:19. doi: 10.1186/1756-3305-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT. Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and release experiments. Bull Entomol Res. 1961;52:99–127. [Google Scholar]
- Gillies MT, Chir B. A new character for the recognition of nulliparous females of Anopheles gambiae. Bull WHO. 1956;15:451–459. [PMC free article] [PubMed] [Google Scholar]
- Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region) South African Institute for Medical Research; Johannesburg, South Africa: 1968. [Google Scholar]
- Gu W, Regens JL, Beier JC, Novak RJ. Source reduction of mosquito larval habitats has unexpected consequences on malaria transmission. Proc Natl Acad Sci USA. 2006;103:17560–17563. doi: 10.1073/pnas.0608452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- Helinski MEH, Hood RC, Knols BJG. A stable isotope dual-labelling approach to detect multiple insemination in un-irradiated and irradiated Anopheles arabiensis mosquitoes. Parasit Vectors. 2008;1:9. doi: 10.1186/1756-3305-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday-Hanson ML, Yuval B, Washino R. Energetics and sugar-feeding of field collected anopheline females. J Vector Ecol. 1997;22:83–89. [PubMed] [Google Scholar]
- Impoinvil DE, Kongere JO, Foster WA, Njiru BN, Killeen GF, Githure JI, Beier JC, Hassanali A, Knols BJG. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Med Vet Entomol. 2004;18:108–115. doi: 10.1111/j.0269-283X.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Switchover to the mated state by spermathecal activation in female Anopheles gambiae mosquitoes. J Insect Physiol. 2006;52:679–684. doi: 10.1016/j.jinsphys.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Foster WA, Jackson RR, Beier JC, Githure JI, Hassanali A. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar J. 2007a;6:113. doi: 10.1186/1475-2875-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Nyandat E, Kabiru W, Jackson RR, Foster WA, Githure JI, Beier JC, Hassanali A. Discriminative feeding behaviour of Anopheles gambiae s.s on endemic plants in Western Kenya. Med Vet Entomol. 2007b;21:103–111. doi: 10.1111/j.1365-2915.2007.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand RP. Field observations on swarming and mating in Anopheles gambiae mosquitoes in Tanzania. Neth J Zool. 1984;34:367–387. [Google Scholar]
- McCrae AWR. Differences in sugar-feeding activity between tropical and temperate mosquitoes: field observations and their implications. Vector Ecol Newsl. 1989;20:16. [Google Scholar]
- Midega JT, Mbogo CM, Mwambi H, Wilson MD, Ojwang G, Mwangangi JM, Nzovu JG, Githure JI, Yan G, Beier JC. Estimating dispersal and survival of Anopheles gambiae and Anopheles funestus along the Kenyan Coast by using mark–release–recapture methods. J Med Entomol. 2007;44:923–929. doi: 10.1603/0022-2585(2007)44[923:edasoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakawa N, Githure JI, Beier JC, Yan G. Anopheline mosquito survival strategies during the dry period in western Kenya. J Med Entomol. 2001;38:388–392. doi: 10.1603/0022-2585-38.3.388. [DOI] [PubMed] [Google Scholar]
- Minakawa N, Seda P, Yan G. Influence of host and larval habitat distribution on the abundance of African malaria vectors in western Kenya. Am J Trop Med Hyg. 2002;67:32–38. doi: 10.4269/ajtmh.2002.67.32. [DOI] [PubMed] [Google Scholar]
- Muirhead-Thompson RC. Mosquito behavior in relation to malaria transmission in the tropics. E. Arnold; London, United Kingdom: 1951. [Google Scholar]
- Nayar JK, Van Handel E. The fuel for sustained mosquito flight. J Insect Physiol. 1971;17:471–481. doi: 10.1016/0022-1910(71)90095-3. [DOI] [PubMed] [Google Scholar]
- Packer MJ, Corbet PS. Size variation and reproductive success of female Aedes punctor (Diptera: Culicidae) Ecol Entomol. 1989;14:297–309. [Google Scholar]
- Perez-Pacheco R. Control of the mosquito Anopheles pseudopunctipennis (Diptera: Culicidae) with Romanomermis iyengari (Nematoda: Mermithidae) in Oaxaca, Mexico. Biol Control. 2005;32:137–142. [Google Scholar]
- Stone CM, Taylor RM, Foster WA. An efficient sampling structure within an indoor mesocosm for studying populations of Anopheles gambiae. J Am Mosq Cont Asso. 2009 doi: 10.2987/08-5885.1. (in press) [DOI] [PubMed] [Google Scholar]
- Straif SC, Beier JC. Effects of sugar availability on the blood feeding behaviour of Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 1996;33:608–612. doi: 10.1093/jmedent/33.4.608. [DOI] [PubMed] [Google Scholar]
- Takken W, Charlwood J, Billingsley P, Gort G. Dispersal and survival of Anopheles funestus and A. gambiae s.l. (Diptera: Culicidae) during the rainy season in southeast Tanzania. Bull Entomol Res. 1998;88:561–566. [Google Scholar]
- Verhoek BA, Takken W. Age effects on the insemination rate of Anopheles gambiae s.l. in the laboratory. Entomol Exp Appl. 1994;72:167–172. [Google Scholar]
- Walker K. M.S. Thesis. Simon Fraser University; Burnaby, BC, Canada: 2008. Nutritional ecology of the malaria vector Anopheles gambiae. [Google Scholar]
- Yuval B. The other habit: sugar feeding by mosquitoes. Bull Soc Vector Ecol. 1992;17:150–156. [Google Scholar]
- Yuval B, Holliday-Hanson ML, Washino R. Energy budget of swarming male mosquitoes. Ecol Entomol. 1994;19:74–78. [Google Scholar]