Abstract
In a prospective passive diarrhea surveillance cohort study of 1034 infants of low-socioeconomic communities in Lima, Peru we determined the prevalence and antimicrobial susceptibility of the diarrheagenic Escherichia coli. The prevalence of diarrheagenic E. coli was 29% (161/557) in children with gastroenteritis and 30% (58/195) in the control group without diarrhea. The most common E. coli pathogens in diarrhea were enteroaggregative-EAEC (14%), enteropathogenic-EPEC (7%), diffusely adherent-DAEC (4%) and enterotoxigenic E.coli-ETEC (4%). Diarrheagenic E. coli as a group exhibited high levels of antimicrobial resistance in diarrheal cases to ampicillin-85%, cotrimoxazole-79%, tetracycline-65% and nalidixic acid-28%. Among the individual E. coli groups in diarrhea, DAEC and EAEC exhibited significant higher frequency of resistance to ampicillin, cotrimoxazole, tetracycline and nalidixic acid, than EPEC and ETEC. Antimicrobial resistance to ampicillin and cotrimoxazole were more frequent in E. coli isolated from diarrheal samples than controls, reflecting the greater antibiotic exposure in patients with gastroenteritis.
INTRODUCTION
Diarrheal illness remains one of the leading causes of morbidity and mortality worldwide, despite ongoing progress in our basic understanding of its epidemiology, pathogenesis, and treatment. In developing areas, infectious diarrhea is a major cause of childhood mortality; there are an estimated 2 million deaths/yr among children globally1. Beyond this, however, are its under-recognized long term effects, which include permanent shortfalls in physical and cognitive development attributable to early repeated childhood diarrhea episodes and to enteric parasitic infections2–6. The longitudinal prevalence of diarrhea seems to be a stronger predictor of diarrhea’s long term health effects, than the incidence rates7; therefore, the duration of the diarrheal episodes in small children may be critical.
Diarrheagenic Escherichia coli are major causes of gastroenteritis in children in the developing world and are associated with high antibiotic resistance levels8–11. E. coli associated with diarrhea have been classified into 6 groups based on clinical, epidemiological and molecular criteria12: (1) Enteropathogenic E. coli (EPEC); (2) Enterotoxigenic E. coli (ETEC); (3) Shigatoxin producing E. coli (STEC), also known as Enterohemorrhagic E. coli (EHEC) or Verotoxin producing E. coli (VTEC); (4) Enteroinvasive E. coli (EIEC); (5) Enteroaggregative E. coli (EAEC or EAggEC); and (6) Diffusely Adherent E. coli (DAEC). The course of illness with many of these pathogens might be ameliorated by specific antimicrobial therapy. However, specific etiologic diagnosis, as well as the knowledge of the local antibiotic susceptibility patterns, is necessary if treatment interventions are to be considered. Our hypothesis was that due to frequent inappropriate empiric antibiotic use in Lima, there should be a high prevalence of antimicrobial resistance in strains of diarrheagenic E. coli isolated from children whether the strains were isolated from infants with diarrhea or controls without diarrhea. The objective of this study was to determine the prevalence, antimicrobial susceptibility patterns, and association of these patterns with specific classes of diarrheagenic E. coli. Such information might be useful both in discouraging inappropriate use and guiding physicians to more appropriate choices when therapy is necessary. We focused on infants in the first year of life to determine whether resistant organisms were acquired very early.
PATIENTS AND METHODS
This study was a prospective passive diarrhea surveillance cohort study of 1034 Peruvian infants followed from 2 to 12 months of age in low-socioeconomic communities in the southern districts of Lima, Peru. A complete description of the patients and methods of the cohort diarrhea study is presented elsewhere13. Children were enrolled in the community; written informed consent was obtained from the infants’ parents. The study was approved by the Ethical Review Board of the Instituto de Investigacíon Nutricional and Universidad Peruana Cayetano Heredia, Lima, Perú. Diarrheal episodes that required medical attention at our study clinic were evaluated. Treatment advice was provided to parents, including use of oral rehydration solution (ORS) and antibiotics if needed, as well as advice regarding dietary management. Additionally, control stool samples were obtained monthly from 20–30 randomly selected healthy study children; these children had no diarrhea for at least 7 days before and 7 days after the stool sample collection. Children were followed at home one week later to confirm the absence of diarrhea after the control stool samples was taken. Stool samples were analyzed for the presence of common enteric viruses, bacteria and parasites using conventional methods. Five lactose fermenting colonies were selected from each MacConkey plate for analysis for the presence of the diarrheagenic E. coli. Detection was by a real time multiplex PCR system14 with primers described in table 1, using a PTC-200 thermal cycler with Chromo 4 optical detector (MJ Research/Biorad, Hercules, CA). E. coli strains positive for any diarrheagenic E. coli gene were analyzed for their antimicrobial susceptibility by disk diffusion according to the Clinical Laboratory Standards Institute (CLSI) guidelines15. The antibiotics analyzed were: ampicillin (AMP, 10 μg disk), amoxicillin-clavulanic acid (AMC, 30 μg disk), azythromycin (AZD, 15 μg disk), cefotaxime (CTX, 30 μg disk), ceftazidime (CAZ, 30 μg disk), chloramphenicol (CAF, 30 μg disk), ciprofloxacin (CIP, 5 μg disk), cotrimoxazole (SXT, 23.75/1.25 μg disk), gentamicin (GTM, 10 μg disk), nalidixic acid (NAL, 30 μg disk), nitrofurantoin (NIT, 300 μg disk), and tetracycline (TET, 30 μg disk).
Table 1.
Primers for multiplex real time PCR for diarrheagenic E. coli genes.
| E. coli | Gene | Primer sequence 5-3′* | Size** (bp) | Tm† (C°) | |
|---|---|---|---|---|---|
| EAEC | aggR | F | CGAAAAAGAGATTATAAAAATTAAC | 100 | 77 |
| R | GCTTCCTTCTTTTGTGTAT | ||||
| ETEC | stIa | F | TTTCCCCTCTTTTAGTCAGTCAA | 159 | 81 |
| stIb | F | TGCTAAACCAGTAGAGTCTTCAAAA | 138 | ||
| st | R | GCAGGATTACAACACAATTCACAGCAG | |||
| lt | F | TCTCTATGTGCATACGGAGC | 322 | 86 | |
| R | CCATACTGATTGCCGCAAT | ||||
| EPEC | eaeA | F | ATGCTTAGTGCTGGTTTAGG | 248 | 83 |
| R | GCCTTCATCATTTCGCTTTC | ||||
| STEC‡ | stx1 | F | CTGGATTTAATGTCGCATAGTG | 150 | 87 |
| R | AGAACGCCCACTGAGATCATC | ||||
| stx2 | F | GGCACTGTCTGAAACTGCTCC | 255 | 89 | |
| R | TCGCCAGTTATCTGACATTCTG | ||||
| EIEC | ipaH | F | GTTCCTTGACCGCCTTTCCGATACCGTC | 619 | 91 |
| R | GCCGGTCAGCCACCCTCTGAGAGTAC | ||||
| DAEC | daaD | F | TGAACGGGAGTATAAGGAAGATG | 444 | 93 |
| R | GTCCGCCATCACATCAAAA | ||||
Primers previously describe by Guion et al13;
Amplicon size in base pars;
Tm, melting temperature for each amplicon;
STEC strains could be eaeA positive and should always have stx1 or stx2 or both stx1+ stx2.
The overall prevalence of each type of diarrheagenic E. coli in gastroenteritis and control stool samples and the prevalence by age groups as well as antibiotic resistance rates were compared. Statistical differences were evaluated through Chi-square or Fisher’s exact tests.
RESULTS
The diarrhea surveillance study for antimicrobial susceptibility was conducted from September 2006 to May 2007. During this 8-month period we have studied 557 stool samples from children with diarrhea and 195 control samples from children without diarrhea. Some children had more than one episode of diarrhea requiring medical attention during the study period and some had none. The mean age of the children studied was 5.2 ± 1.8 months; the oldest child in this group had 10 months of age. The prevalence of the diarrheagenic E. coli was 29% (161/557) in the diarrhea group and 30% (58/195) in the control group. The prevalence of each diarrheagenic E. coli group in diarrhea and control samples was EAEC 14% (78/557) vs. 18% (35/195), EPEC 7% (37/557) vs. 7% (13/195), DAEC 4% (21/557) vs. 3% (6/195), ETEC 4% (20/557) vs. 2% (3/195) and STEC 1% (5/557) vs. 0.5% (1/195). No EIEC strains were isolated. The prevalence of other enteric pathogens in diarrhea and control samples was Campylobacter spp. 14% (78/557) vs. 8% (16/195), Salmonella spp. 0% vs. 0.5% (1/195). Rotavirus was found in 7% (37/548) of diarrhea samples; control samples were not tested for rotavirus. There were no Shigella spp. or Vibrio cholerae isolated.
The inappropriate use of antibiotics during illnesses was very common in this population. Children from the diarrhea group received more courses of antibiotics (for any illness) in the previous 3 months prior to the stool sample collection than children in the control group (1.0 ± 1.3 courses of antibiotics/child vs. 0.6 ± 0.8, respectively, p=0.023). The most common antibiotics used were amoxicillin (31% vs. 42%, p=0.050), macrolides (32% vs. 19%, p=0.032) and cotrimoxazole (15% vs. 23%, p=0.076) in the diarrhea and control group respectively (288 course of antibiotics in the diarrhea group and 78 in the control group). Empiric antibiotic (selected by the parents without medical advice or prescribed by a physician) was used in 46% of diarrhea episodes in which a diarrheagenic E. coli was isolated. The most commonly used antibiotics for diarrhea episodes were macrolides 26% (33/129) (erythromycin 20%, azythromycin 6%), furazolidone 8% (10/129), amoxicillin 5% (7/129) and cotrimoxazole 2% (3/129).
Eighty percent of the diarrheagenic E. coli from diarrhea cases (129/161) and 98% of the control samples (57/58) were available for the antibiotic susceptibility studies. The diarrheagenic E. coli as a group were frequently resistant to ampicillin, cotrimoxazole, tetracycline, nalidixic acid, and chloramphenicol (Table 2). All strains were fully susceptible to cefotaxime and ceftazidime. There were no strains with high-level resistance to amoxicillin/clavulanic, however 12% had intermediate resistance among diarrheal samples and 9% among controls. Similarly, 13% of strains showed intermediate resistance to nitrofurantoin among diarrheal samples and 2% in controls. Although azythromycin is commonly used as therapy for some enteric pathogens, there are no approved resistance criteria for disc diffusion analysis of E. coli. The distribution of the growth inhibitory zones by disc diffusion in 167 diarrheagenic E. coli from diarrhea and control samples were: 10% (17 strains) had azythromycin inhibitory diameter zones less than 10 mm, 21% (35 strains) had 11–15 mm, 33% (55 strains) had 16–20 mm, and 36% (60 strains) had more than 21 mm.
Table 2.
Antibiotic resistance rates of Diarrheagenic E. coli from diarrhea (n=129) and control (n=55) samples*
| EAEC, n (%) |
EPEC, n (%) |
ETEC, n (%) |
DAEC, n (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Diarrhea (n=67) | Control (n=34) | Diarrhea (n=32) | Control (n=12) | Diarrhea (n=18) | Control (n=3) | Diarrhea (n=12) | Control (n=6) | |
| Ampicillin | 61 (91) | 26 (76) | 23 (72) | 5 (42) | 14 (78) | 2 (67) | 12 (100) | 6 (100) |
| Cotrimoxazole | 57 (85) | 22 (65) | 23 (72) | 3 (25) | 11 (61) | 3 (100) | 11 (92) | 6 (100) |
| Tetracycline | 49 (73) | 22 (65) | 16 (50) | 2 (17) | 8 (44) | 2 (67) | 11 (92) | 6 (100) |
| Nalidixic Acid | 22 (33) | 5 (15) | 7 (22) | 1 (8) | 1 (6) | 0 (0) | 6 (50) | 2 (33) |
| Chloramphenicol | 26 (39) | 13 (38) | 2 (6) | 0 (0) | 1 (6) | 0 (0) | 5 (42) | 3 (50) |
| Gentamicin | 4 (6) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0 (0) |
| Nitrofurantoin | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 3 (17) | 0 (0) | 0 (0) | 0 (0) |
| Ciprofloxacin | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 1 (17) |
| Multi-resistance** | 47 (70) | 19 (57) | 15 (47) | 2 (17) | 7 (39) | 2 (67) | 12 (100) | 6 (100) |
Data on STEC is not presented, do to small number of samples.
Resistance to 3 or more antibiotics.
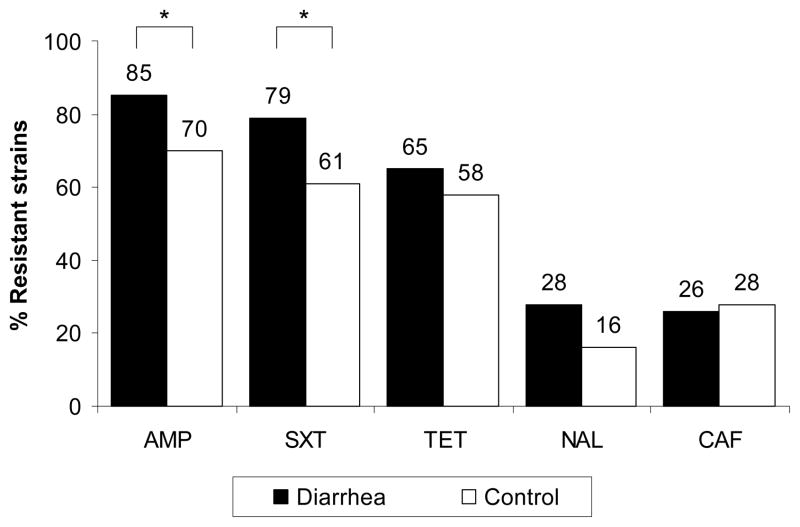
There was a higher frequency of resistance to all antibiotics in diarrheal samples than in controls; this difference was significant for ampicillin (85% vs. 70%, p=0.039) cotrimoxazole (79% vs. 61%, p=0.024) (Figure 1). Multidrug-resistance, defined as resistance to 3 or more antibiotics, was common in diarrheal (63%, 81/129) and control samples (53%, 29/57). There were 14 different antibiotic resistance patterns among the diarrheagenic E. coli (diarrhea and control strains grouped together) (Table 3). The most common resistance patterns were AMP-SXT-TET, present in 24% of all strains (41/168), and AMP-SXT, present in 19% of strains (25/168).
Figure 1.
Antibiotic resistance of diarrheagenic E. coli as a group in infants with diarrhea (■) (n=129) and without diarrhea (□) (n=57). Percentage of strains with high level resistance by disk diffusion method. AMP=ampicillin, SXT= cotrimoxazole, TET=tetracycline, NAL=nalidixic acid, CAF=chloramphenicol. * p< 0.05 for the comparison between diarrhea and control.
Table 3.
Antibiotic resistance patterns for the diarrheagenic E. coli (n=168) from diarrhea and control samples.
| Pattern number | Antibiotic resistance patters | EAEC (n=94) | EPEC (n=39) | DAEC (n=18) | ETEC (n=17) |
|---|---|---|---|---|---|
| I | AMP-SXT-TET-NAL-GTM-CIP | 1 | 0 | 0 | 0 |
| II | AMP-SXT-TET-NAL-CAF | 19 | 1 | 3 | 0 |
| II | AMP-SXT-TET-CAF-GTM | 3 | 0 | 2 | 0 |
| IV | AMP-SXT-TET-NAL-CIP | 0 | 0 | 2 | 0 |
| V | AMP-SXT-TET-CAF | 13 | 1 | 3 | 0 |
| VI | AMP-SXT-TET-NAL | 4 | 3 | 0 | 0 |
| VII | AMP-SXT-TET-NIT | 0 | 0 | 0 | 3 |
| VIII | AMP-SXT-CAF-GTM | 2 | 0 | 0 | 0 |
| IX | AMP-TET-NAL-CAF | 0 | 0 | 1 | 0 |
| X | SXT-TET-NAL-CIP | 1 | 0 | 0 | 0 |
| XI | AMP-SXT-TET | 18 | 12 | 6 | 5 |
| XII | AMP-SXT-NAL | 3 | 0 | 0 | 0 |
| XIII | AMP-SXT-CAF | 1 | 0 | 1 | 0 |
| XIV | AMP-TET-NAL | 1 | 0 | 0 | 0 |
| Resistance to two antibiotics* | 17 | 10 | 0 | 5 | |
| Resistance to only one antibiotic | 6 | 6 | 0 | 3 | |
| Pan-susceptible | 5 | 6 | 0 | 1 |
Resistance patterns: AMP-SXT (25 strains), AMP-TET (3 strains), SXT-TET(2 strains), SXT-NAL (1 strain), TET-NAL (1 strain)
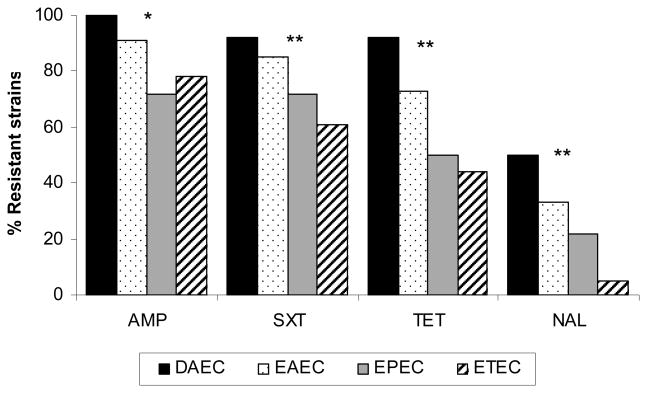
Among the individual E. coli groups there was a striking difference in susceptibility between them. DAEC consistently exhibited more resistance to ampicillin, cotrimoxazole, tetracycline and nalidix acid, than EAEC; which was more resistant than EPEC; which was more resistant than ETEC. This trend was significant for all 4 antibiotics (Figure 2). Multiple resistance also differed strikingly with DAEC and EAEC much more resistant (table 3) than the other categories of E. coli. Resistant to three or more agents: DAEC (100%), EAEC (70%), ETEC (47%), EPEC (44%) (p=0.0001).
Figure 2.
Antibiotic resistance among the different diarrheagenic E. coli groups in infants with diarrhea. DAEC (n=12), EAEC (n=67), EPEC (n=32) and ETEC (n=18). AMP=ampicillin, SXT= cotrimoxazole, TET=tetracycline and NAL=nalidixic acid. * p< 0.05, ** p<0.01 (chi squared for trend), for the comparison of the resistance percentage among the different E. coli.
DISCUSSION
For many episodes of infectious diarrhea only fluid and electrolyte management is appropriate. However, antimicrobial therapy is indicated for children and adults with acute infectious gastroenteritis in a variety of circumstances16. These indications may include dysentery, severe or prolonged disease, eradication of fecal shedding and transmission, and prevention of sequellae and death16. However, antimicrobial agents do not benefit most children with acute diarrhea because viral enteropathogens are so common. Thus, in children with acute gastroenteritis, use of specific antimicrobials should be limited to well-defined bacterial and protozoal agents. Among the diarrheagenic E. coli, there is an established benefit for the use of antibiotics on ETEC, EIEC and EAEC infections; these conclusions are based primarily on data in traveler’s diarrhea17. However, the role of antibiotic therapy in children with acute diarrhea due to a diarrheagenic E. coli is not fully defined18. This is due primarily to the lack of rapid diagnostic tests available at the outpatient or hospital settings19. The time frame in which treatment choices must be made is short. Prior to the development of the mulitplex real time PCR there was no rapid, sensitive and inexpensive diagnostic technique for determining presence of a diarrheagenic E. coli in the community setting. Now that such rapid assays are available in research laboratories, local epidemiological studies on etiology and antimicrobial susceptibility are relevant.
Diarrheagenic E. coli were the most commonly isolated pathogens in Peruvian infants with diarrhea. This is similar to studies from other developing countries, where the diarrheagenic E. coli, as a group, are responsible for 30% to 40 % of acute diarrhea episodes in children19. However, in this study the diarrheagenic E. coli were found with similar frequency in children with and without diarrhea, demonstrating that infants in this setting where breastfeeding is universal are frequently exposed to these bacteria. This study clearly demonstrates that multiply resistant organisms are acquired very early in life.
These organisms are exposed to antibiotic pressure when both enteric and non enteric infections occur. In this study antimicrobial resistance in E. coli was associated both with a high frequency of antibiotic use for diarrhea (46%) and with a higher frequency of previous antibiotic exposure for non enteric infections. Recent antibiotic use, particularly one month before exposure, is a risk factor for developing infection or colonization with resistant bacterial pathogens20,21. Excessive and inappropriate use of antimicrobials for proven or presumed infections in many organ systems, including the gastrointestinal tract, is a common problem. This use occurs in part because of inappropriate prescribing practices and in part because of misguided beliefs and expectations of parents who often lack an awareness of the dangers of antimicrobial use20. In Peru, as in many other developing countries, antimicrobials are sold over the counter without a prescription. Thus, unrestricted accesses to antimicrobial drugs coupled with lack of understanding regarding their use are major factors driving multiresistance22–24.
The problem of antimicrobial resistance is not unique to Peru. It is particularly critical in many developing countries where frequent illnesses coupled with ready access to unregulated antibiotics diminishes the value of these agents for those patients who actually need them. We found a high frequency of antimicrobial resistance of diarrheagenic E. coli to commonly used antibiotics such as ampicillin (85%) and cotrimoxazole (79%). This finding is similar to what has been recently described in children from Vietnam (86% and 88%, respectively)10, Tanzania (85% and 87%)8, México (73% and 65%)11, Argentina (75% and 64%)25 and Mozambique (72% and 58%)26. Of interest, neither ampicillin nor cotrimoxazole is appropriate empiric therapy for common enteric bacteria such as Shigella and Salmonella, because of the high frequency of resistance in many areas of the world16. The growing problem of multidrug-resistant enteric pathogens is especially common in Africa and Asia27–29.
Resistance patterns were strikingly different among the individual E. coli groups. DAEC and EAEC had higher resistance levels than EPEC and ETEC. This is likely to result from several factors. It is possible that DAEC and EAEC are more resistant because they are exposed to antimicrobials more often, which may be because they cause persistent diarrhea and/or are often carried asymptomatically. Thus the long time within human hosts increases the chance that they will be exposed to antimicrobials and/or acquire resistant genes from the resident flora. These data could also reflect an association of resistance genes with plasmid-associated virulence genes, such as adherence factors present in DAEC (i.e. Dr adhesins)30 and EAEC (i.e. AAF or aggregative adherence fimbria)31. It is known that resistance to multiple antibiotics can be due to a variety of mobile genetic elements such as plasmids, transposons, and gene cassettes in integrons32, or to alteration in the E. coli multiple antibiotic resistance operon (mar)33. The Mar phenotype includes resistance to structurally unrelated antibiotics such as tetracycline, chloramphenicol, β-lactams, fluoroquinolones, puromycin, nalidixic acid, rifampicin, and others34. Integron-associated antibiotic resistance has been described for EIEC, EAEC and cell-detaching E. coli35,.36; and a conjugative multi-resistance plasmid for EPEC37.
In summary, dirrheagenic E. coli are the most common isolated pathogens in Peruvian infants with diarrhea as well as in asymptomatic controls, reflecting the high and early exposure to pathogens in this setting. Dirrheagenic E. coli were associated with a high frequency of antibiotic resistance, with different resistance patterns among the individual E. coli groups. Overall, resistance to ampicillin and cotrimoxazole were so common, that both drugs should not be use empirical for the treatment of invasive or persistent bacterial gastroenteritis in children from Lima. Antimicrobial resistance was more frequent in E. coli isolated from diarrheal samples than controls, associated with more antibiotic exposure in patients with gastroenteritis. The impact of unsupervised antibiotic use is clear. Inappropriate antibiotics are expensive, potentially toxic, used with dubious indication that is unlikely to result in benefit to the recipient, and select organisms that are multiply resistant so that the usefulness of the agents is limited when legitimate medical indications exist. It is imperative to implement strategies to prevent and control the emergence and spread of resistant organisms by improving diagnosis and reducing the selective pressure caused by overuse and misuse of antibiotics in children.
Acknowledgments
TO is supported by PHS - FIC 1K01TW007405 (National Institutes of Health, USA); JR is supported by FIS - CP05/0130 (Fondo de Investigaciones Sanitarias, Spain);TC is supported by the PHS - NICHD R01-HD051716 (National Institutes of Health, USA). This work has been partially funded by the AECI (Ageñcia Espanola de Cooperacíon Internacional) grant number A/4892/06; ACCD (Agència Catalana de Cooperació al Desenvolupament, Generalitat de Catalunya) grant U2006; and CCD-UPC (Centre de Cooperació per al Desenvolupament, Universitat Politècnica de Catalunya) to LJdV.
Footnotes
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the U.S. Government, nor the National Institutes of Health and other funding institutions.
Contributor Information
Theresa J. Ochoa, Email: Theresa.J.Ochoa@uth.tmc.edu, Instituto de Medicina Tropical “Alexander von Humboldt”, Universidad Peruana Cayetano Heredia, Av. Honorio Delgado 430, San Martin de Porras, Lima 31, Perú, Phone 51-1-482-3910, Fax: 51-1-482-3404.
Joaquím Ruiz, Email: joruiz@clinic.ub.es, Centre de Recerca en Salut Internacional de Barcelona (CRESIB), C/Rosselló 132, 4. 08036, Barcelona, Spain, Phone: +34932275400 ext 3388, Fax: +34932279853.
Margarita Molina, Email: micro@iin.sld.pe, Instituto de Investigación Nutricional, Av. La Molina 1885, La Molina, Lima 12, Perú, Phone: (+51-1) 349-6023, Fax: +51-1-349-6025.
Luis J. del Valle, Email: Luis.Javier.Del.Valle@upc.edu, Departament d’Enginyeria Agroalimentària i Biotecnologia (DEAB), Escola Superior d’Agricultura de Barcelona (ESAB), Universitat Politècnica de Catalunya (UPC), Av. Canal Olímpic 15, 08860 Castelldefels (Barcelona), Spain, Phone:. +0034-935521229, Fax +0034-935521001.
Martha Vargas O, Email: mvargas@clinic.ub.es, Hospital Clinic de Barcelona Villarroel 179, 08036 Barcelona, Spain, Phone: +0034-93 227 54 00- Ext2424, Fax: +0034-93 227 57 83.
Ana I Gil, Email: agil@iin.sld.pe, Instituto de Investigacion Nutricional Av. La Molina 1885, La Molina, Lima12, Perú, Phone: (+51-1) 349-6023 or 349-6024 Fax: +51-1-349-6025.
Lucie Ecker, Email: lecker@iin.sld.pe, Instituto de Investigacion Nutricional Av. La Molina 1885, La Molina, Lima 12, Perú, Phone: (+51-1) 349-6023 or 349-6024 Fax: +51-1-349-6025.
Francesca Barletta, Email: francescabarletta@yahoo.es, Instituto de Medicina Tropical “Alexander von Humboldt”, Universidad Peruana Cayetano Heredia, Av. Honorio Delgado 430, San Martin de Porras, Lima 31, Perú, Phone 51-1-482-3910, Fax: 51-1-482-3404.
Eric R. Hall, Email: eric.hall@med.navy.mil, Director for Administration, Public Affairs Officer, Naval Medical Research Center, 503 Robert Grant Avenue, Silver Spring, MD, 20910-7500, Phone: 301-319-7581, Fax: 240-478-8769.
Thomas G. Cleary, Email: Thomas.G.Cleary@uth.tmc.edu, Center for Infectious Diseases, University of Texas School of Public Health, P.O. Box 20186, Houston, TX 77225, Phone: 713 702 2860, Fax: 713-500-9359.
Claudio F. Lanata, Email: clanata@iin.sld.pe, Instituto de Investigación Nutricional, Av. La Molina 1885, La Molina, Lima12, Perú, Telf: (+51-1) 349-6023, Fax: (+51-1) 349-6025.
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, Guerrant RL. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 3.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 4.Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, Guyatt H, Lima AM, Guerrant RL. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25:513–520. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 5.Steiner TS, Samie A, Guerrant RL. Infectious diarrhea: new pathogens and new challenges in developed and developing areas. Clin Infect Dis. 2006;43:408–410. doi: 10.1086/505874. [DOI] [PubMed] [Google Scholar]
- 6.Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118:1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris SS, Cousens SN, Kirkwood BR, Arthur P, Ross DA. Is prevalence of diarrhea a better predictor of subsequent mortality and weight gain than diarrhea incidence? Am J Epidemiol. 1996;144:582–588. doi: 10.1093/oxfordjournals.aje.a008968. [DOI] [PubMed] [Google Scholar]
- 8.Vila J, Vargas M, Casals C, Urassa H, Mshinda H, Schellemberg D, Gascon J. Antimicrobial resistance of diarrheagenic Escherichia coli isolated from children under the age of 5 years from Ifakara, Tanzania. Antimicrob Agents Chemother. 1999;43:3022–3024. doi: 10.1128/aac.43.12.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franzolin MR, Alves RC, Keller R, Gomes TA, Beutin L, Barreto ML, Milroy C, Strina A, Ribeiro H, Trabulsi LR. Prevalence of diarrheagenic Escherichia coli in children with diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz. 2005;100:359–363. doi: 10.1590/s0074-02762005000400004. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TV, Le PV, Le CH, Weintraub A. Antibiotic resistance in diarrheagenic Escherichia coli and Shigella strains isolated from children in Hanoi, Vietnam. Antimicrob Agents Chemother. 2005;49:816–819. doi: 10.1128/AAC.49.2.816-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrada-García T, Cerna JF, Pacheco-Gil L, Velázquez RF, Ochoa TJ, Torres J, DuPont HL. Drug-resistant Diarrheogenic Escherichia coli, Mexico. Emerg Infect Dis. 2005;11:1306–1308. doi: 10.3201/eid1108.050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochoa TJ, Ecker L, Barletta F, Mispireta M, Gil AI, Contreras C, Molina M, Amemiya I, Verastegui H, Hall ER, Cleary TG, Lanata LF. Age-related susceptibility to infection with diarrheagenic E. coli in infants from peri-urban areas of Lima, Peru. 2009 doi: 10.1086/648069. Manuscript submitted to Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guion CE, Ochoa TJ, Walker CM, Barletta F, Cleary TG. Diagnosis of the diarrheagenic Escherichia coli using melting curve analysis of a real-time multiplex polymerase chain reaction. J Clin Microbiol. 2008;46:1752–1757. doi: 10.1128/JCM.02341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement. 2006;26(3) M100-S16. [Google Scholar]
- 16.Pickering LK. Antimicrobial resistance among enteric pathogens. Semin Pediatr Infect Dis. 2004;15:71–77. doi: 10.1053/j.spid.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Cabada MM, White AC., Jr Travelers’ diarrhea: an update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473–479. doi: 10.1007/s11894-008-0087-7. [DOI] [PubMed] [Google Scholar]
- 18.Leibovitz E, Janco J, Piglansky L, Press J, Yagupsky P, Reinhart H, Yaniv I, Dagan R. Oral ciprofloxacin vs. intramuscular ceftriaxone as empiric treatment of acute invasive diarrhea in children. Pediatr Infect Dis J. 2000;19:1060–1067. doi: 10.1097/00006454-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 19.O’Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16:125–136. doi: 10.1053/j.spid.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Vanden Eng J, Marcus R, Hadler JL, Imhoff B, Vugia DJ, Cieslak PR, Zell E, Deneen V, McCombs KG, Zansky SM, Hawkins MA, Besser RE. Consumer attitudes and use of antibiotics. Emerg Infect Dis. 2003;9:1128–1135. doi: 10.3201/eid0909.020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magee JT, Pritchard EL, Fitzgerald KA, Dunstan FD, Howard AJ. Antibiotic prescribing and antibiotic resistance in community practice: retrospective study, 1996–8. BMJ. 1999;319:1239–1240. doi: 10.1136/bmj.319.7219.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bojalil R, Calva JJ. Antibiotic misuse in diarrhea. A household survey in a Mexican community. J Clin Epidemiol. 1994;47:147–156. doi: 10.1016/0895-4356(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 23.Bartoloni A, Cutts F, Leoni S, Austin CC, Mantella A, Guglielmetti P, Roselli M, Salazar E, Paradisi F. Patterns of antimicrobial use and antimicrobial resistance among healthy children in Bolivia. Trop Med Int Health. 1998;3:116–123. doi: 10.1046/j.1365-3156.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- 24.Howteerakul N, Higginbotham N, Dibley MJ. Antimicrobial use in children under five years with diarrhea in a central region province, Thailand. Southeast Asian J Trop Med Public Health. 2004;35:181–187. [PubMed] [Google Scholar]
- 25.Binsztein N, Picandet AM, Notario R, Patrito E, De Lesa ME, De Petris A, Maurel D, Nader O, Rivas M, Szefner M, Vergara M. Antimicrobial resistance among species of Salmonella, Shigella, Escherichia, and aeromonas isolated from children with diarrhea in 7 Argentinian centers. Rev Latinoam Microbiol. 1999;41:121–126. [PubMed] [Google Scholar]
- 26.Mandomando IM, Macete EV, Ruiz J, Sanz S, Abacassamo F, Vallès X, Sacarlal J, Navia MM, Vila J, Alonso PL, Gascon J. Etiology of diarrhea in children younger than 5 years of age admitted in a rural hospital of southern Mozambique. Am J Trop Med Hyg. 2007;76:522–527. [PubMed] [Google Scholar]
- 27.Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, Pablos-Mendez A, Klugman KP. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 28.Okeke IN, Aboderin OA, Byarugaba DK, Ojo KK, Opintan JA. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg Infect Dis. 2007;13:1640–1646. doi: 10.3201/eid1311.070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hien BT, Scheutz F, Cam PD, Serichantalergs O, Huong TT, Thu TM, Dalsgaard A. Diarrheagenic Escherichia coli and Shigella strains isolated from children in a hospital case-control study in Hanoi, Vietnam. J Clin Microbiol. 2008;46:996–1004. doi: 10.1128/JCM.01219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes LM, Fabbricotti SH, Ferreira AJ, Kato MA, Michalski J, Scaletsky IC. Heterogeneity among strains of diffusely adherent Escherichia coli isolated in Brazil. J Clin Microbiol. 2005;43:1968–1972. doi: 10.1128/JCM.43.4.1968-1972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington SM, Dudley EG, Nataro JP. Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol Lett. 2006;254:12–18. doi: 10.1111/j.1574-6968.2005.00005.x. [DOI] [PubMed] [Google Scholar]
- 32.Brueggemann AB. Antibiotic resistance mechanisms among pediatric respiratory and enteric pathogens. Pediatr Infect Dis J. 2006;25:969–973. doi: 10.1097/01.inf.0000239365.60595.d5. [DOI] [PubMed] [Google Scholar]
- 33.Barbosa TM, Levy SB. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J Bacteriol. 2000;182:3467–3474. doi: 10.1128/jb.182.12.3467-3474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maira-Litrán T, Allison DG, Gilbert P. Expression of the multiple antibiotic resistance operon (mar) during growth of Escherichia coli as a biofilm. J Appl Microbiol. 2000;88:243–247. doi: 10.1046/j.1365-2672.2000.00963.x. [DOI] [PubMed] [Google Scholar]
- 35.Gassama A, Aïdara-Kane A, Chainier D, Denis F, Ploy MC. Integron-associated antibiotic resistance in enteroaggregative and enteroinvasive Escherichia coli. Microb Drug Resist. 2004;10:27–30. doi: 10.1089/107662904323047763. [DOI] [PubMed] [Google Scholar]
- 36.Okeke IN, Steinrück H, Kanack KJ, Elliott SJ, Sundström L, Kaper JB, Lamikanra A. Antibiotic-resistant cell-detaching Escherichia coli strains from Nigerian children. J Clin Microbiol. 2002;40:301–305. doi: 10.1128/JCM.40.1.301-305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nwaneshiudu AI, Mucci T, Pickard DJ, Okeke IN. A second large plasmid encodes conjugative transfer and antimicrobial resistance in O119:H2 and some typical O111 enteropathogenic Escherichia coli strains. J Bacteriol. 2007;189:6074–6079. doi: 10.1128/JB.00349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]