Abstract
Redox dysregulation originating from metabolic alterations and dependence on mitogenic and survival signaling through reactive oxygen species represents a specific vulnerability of malignant cells that can be selectively targeted by redox chemotherapeutics. This review will present an update on drug discovery, target identification, and mechanisms of action of experimental redox chemotherapeutics with a focus on pro- and antioxidant redox modulators now in advanced phases of preclinal and clinical development. Recent research indicates that numerous oncogenes and tumor suppressor genes exert their functions in part through redox mechanisms amenable to pharmacological intervention by redox chemotherapeutics. The pleiotropic action of many redox chemotherapeutics that involves simultaneous modulation of multiple redox sensitive targets can overcome cancer cell drug resistance originating from redundancy of oncogenic signaling and rapid mutation. Moreover, some redox chemotherapeutics may function according to the concept of synthetic lethality (i.e., drug cytotoxicity is confined to cancer cells that display loss of function mutations in tumor suppressor genes or upregulation of oncogene expression). The impressive number of ongoing clinical trials that examine therapeutic performance of novel redox drugs in cancer patients demonstrates that redox chemotherapy has made the crucial transition from bench to bedside. Antioxid. Redox Signal. 11, 3013–3069.
-
Molecular Targets for Anticancer Redox Chemotherapy
I. Introduction
A. The (redox) war on cancer
Almost 40 years after the war on cancer was declared in 1971, when the National Cancer Act was signed into United States federal law by then U.S. President Richard Nixon, the fighting continues with ever increasing intensity worldwide. Even though many heroic battles have been won changing the face of this dreadful disease for many patients, curative chemotherapy of cancer still represents an ultimate pharmacological frontier to be conquered only by future drug discovery. The biological revolution of the late 20th century has fundamentally changed the way in which this disease is being understood, diagnosed, treated, and prevented; yet, it is now evident that the challenge to eliminate the suffering and death from cancer by 2015 issued in 2003 by Andrew von Eschenbach, then director of the National Cancer Institute, will not be met soon (359). Due to increased life expectancy resulting in a demographic shift towards older populations worldwide, combined with the age-related increase in cancer incidence, this disease represents a major medical challenge of our time. In the United States, cancer and cardiovascular disease are now competing as the leading cause of death (390).
Therapeutic breakthroughs have been achieved in various areas including pediatric oncology targeting childhood leukemia and urogenital oncology targeting testicular cancer where spectacular survival rates can now be achieved by chemotherapeutic intervention (182). In contrast, marginal progress has been made in the chemotherapeutic treament of other important malignancies including metastatic melanoma, pancreatic carcinoma, and glioblastoma where no efficacious chemotherapeutic options are currently available and patient survival after diagnosis is often measured in months (249).
Reactive oxygen species (ROS), the key mediators of cellular oxidative stress and redox dysregulation involved in cancer initiation and progression, have recently emerged as promising targets for anticancer drug discovery; extensive research has documented a causative involvement of redox alterations in tumor progression, particularly for oncological indications with little treatment options, including metastatic melanoma and pancreas carcinoma (48, 117, 118, 210, 249, 349). It is now widely accepted that constitutively elevated levels of cellular oxidative stress and dependence on mitogenic and anti-apoptotic ROS-signaling in cancer cells represent a specific vulnerability that can be selectively targeted by direct- or indirect-acting pro- and antioxidants and redox modulators that will be jointly referred to as redox chemotherapeutics, representing a novel class of promising anticancer agents (Fig. 1). This review will present an update on drug discovery, target identification, and mechanisms of action of experimental redox chemotherapeutics with a focus on agents that have shown efficacy in advanced preclinical animal models and have now moved into human clinical trials.
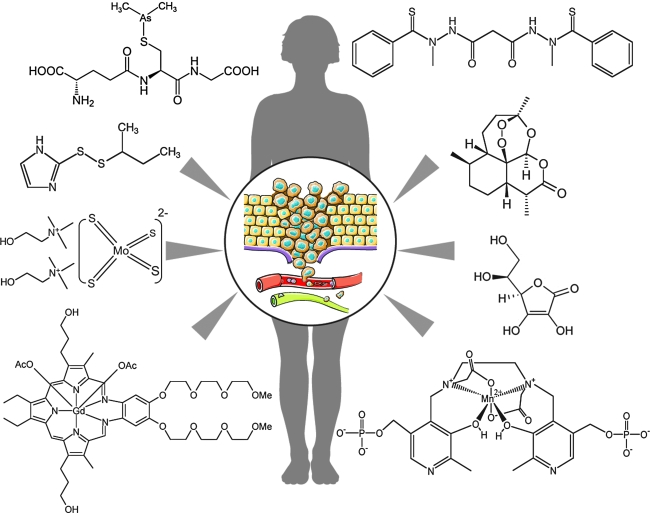
FIG. 1.
Selected redox chemotherapeutics in clinical development for oncological indications. The therapeutic performance of redox chemotherapeutics in advanced phases of clinical testing suggests that they represent novel pharmacological agents that target cancer depicted here as an invasive tumor in the process of metastatic cell dissemination (center insert). Selected investigational redox chemotherapeutics are depicted (clockwise from upper right): the orally active prooxidants elesclomol and artemisinin, the infusional redox drug ascorbate, the infusional SOD mimetic mangafodipir, the infusional redox cycler motexafin gadolinium, the orally active SOD1 inhibitor ATN-224, the infusional thioredoxin inhibitor PX-12, and the infusional arsenical darinaparsin. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
B. Developing anticancer redox chemotherapeutics
Discovery and development of anticancer chemotherapeutics is a challenging endeavor, and redox chemotherapeutics are no exception. As with other novel experimental chemotherapeutics that enter clinical testing, promising redox-based investigational agents with good efficacy and selectivity documented in animal studies have failed in early human trials due to lack of activity or unacceptable adverse drug reactions (232). Unfavorable pharmacokinetics, unexpected off-target activity, and systemic toxicity not predicted from simple cell culture- and short-term murine xenograft models may pose serious obstacles during later stages of development.
The following specific considerations illustrate both the formidable challenges and the exciting opportunities associated with discovery and development of redox chemotherapeutics as a promising class of novel anticancer agents:
1. Redox chemotherapeutics: More than neocytotoxics?
Remarkably, many of the novel molecularly targeted chemotherapeutic agents that have entered advanced clinical trials or received FDA-approval, including the proteasome inhibitor bortezomib, cyclin-dependent-kinase inhibitors, MAP-kinase antagonists, and mTOR inhibitors, target molecules essential to normal cells (181). Therefore, these agents have been referred to as ‘neocytotoxics’, agents that exert anticancer activity based on the preferential sensitivity of rapidly dividing cells to cytotoxic agents, a mechanism of action that they share with classic cytotoxic anticancer chemotherapeutics including alkylating agents, spindle poisons, and topoisomerase inhibitors. Indeed, during early stages of anticancer drug discovery, general cytotoxicity and cancer cell-selective induction of cell death are often dealt with synonymously, and in many studies that address anticancer effects of experimental redox chemotherapeutics, little experimentation is performed on nontransformed primary cells. However, as discussed in much detail below, increasing experimental evidence suggests that redox dysregulation represents a specific vulnerability associated with many tumors that provides a therapeutic window of sufficient width for redox intervention in the absence of unacceptable off-target effects and systemic toxicity. It is particularly encouraging that experimental redox chemotherapeutics may function according to the concept of synthetic lethality (i.e., drug cytotoxicity is confined to cancer cells that display loss of function mutations in tumor suppressor genes or upregulation of oncogene expression) (85, 176, 350, 388, 393).
2. Redox chemotherapeutics: Pleiotropic ‘dirty’ drugs?
Pharmacological agents that modulate cellular redox homeostasis through direct or indirect alteration of ROS generation, signaling, and turnover are generally considered ‘dirty’ drugs, a term that refers to pharmacological agents that modulate multiple molecular targets through pleiotropic interactions (111). In the age of molecularly targeted therapy, development of dirty drugs is generally pursued with reduced enthusiasm due to the tendency of these promiscuous agents to produce toxic off-target effects. Moreover, pharmacodynamic effects of redox modulators often depend on the presence of chemically reactive pharmacophores that display an obvious potential for uncontrolled reactivity and untargeted cytotoxicity. Covalent adduction and generation of reactive intermediates (282) is associated with numerous reactive pharmacophore contained in redox chemotherapeutics (e.g., bisfunctional Michael acceptors, discussed in Section II.G). However, recent research suggests that it is exactly this pleiotropic mode of action associated with numerous redox chemotherapeutics (i.e., the simultaneous modulation of multiple redox sensitive targets) that seems to be uniquely tailored to overcome cancer cell drug resistance originating from redundancy of oncogenic signaling and rapid mutation, as discussed extensively throughout this review (50, 111, 199). Moreover, lead optimization of prototype redox chemotherapeutics has led to the development of advanced preclinical candidates with pharmacophores that display attenuated and more targeted redox reactivity such as quinones that target Cdc25 phosphatases with attenuated or absent redox cycler activity (discussed in Section III.F) (124). Structure-based approaches are now used to identify redox-silent pharmacophores that modulate cellular redox status and signaling through ligand-based interactions such as redox-inactive vitamin E analogues (discussed in Section IV.B.1) (257). In addition, drug discovery strategies that combine the superior target specificity provided by ligand approaches with potent target modulation by reactive electrophilic pharmacophores have now led to the generation of a novel class of anticancer receptor protein kinase inhibitors including neratinib (discussed in Section II.H) (376).
3. Redox chemotherapeutics: Combinatorial or stand-alone drugs?
Many developmental redox therapeutics have shown a potentiating effect on pharmacodynamic activity of other anticancer agents and radiation, but offer only modest or no chemotherapeutic benefit if used as single agents (9, 29, 193). Therefore, many ongoing clinical studies test activity of these agents as combinatorial drugs, consistent with the mechanism of action that involves induction of deviations from redox homeostasis that preferentially sensitize cancer cells to the cytotoxic effects of chemotherapeutic agents (9, 29, 232, 246, 288). However, other ongoing studies examine feasibility of single agent redox chemotherapy as detailed throughout this review.
4. Redox chemotherapeutics and personalized medicine
Accumulating evidence gained from preclinical and clinical studies indicates that the therapeutic benefit provided by redox chemotherapeutics depends on careful patient selection based on genotypic and phenotypic profiling that matches the individual patient with a specific redox intervention.
In the emerging age of personalized medicine, complexity and variability of redox dysregulation in tumors depends on tumor type and progressional stage, localization, and prior chemotherapeutic exposure, diminishing the general efficacy of redox intervention. Indeed, in some tumors, alterations in redox signaling and metabolic profile may represent mere epiphenomena downstream of crucial mechanisms that drive tumor progression, and therapeutic benefit of redox intervention may therefore be compromised in these cases. In contrast, careful patient selection based on detailed tumor redox pheno- and genotyping should guide the selection of specific drugs that efficiently target the redox Achilles heel of the individual tumor, a form of personalized redox medicine that depends on discovery and validation of predictive redox biomarkers (19, 44, 239, 287, 320).
Recent experimental evidence suggests that total antioxidant capacity measurements from clinical tumor samples predict paclitaxel chemosensitivity (287). Other advances in the identification of predictive redox biomarkers have been achieved recently. For example, circulating thioredoxin is secreted by cancer cells and elevated in the plasma of patients with pancreatic and hepatocellular cancers, and efficacy of redox chemotherapy using a small molecule thioredoxin inhibitor has indeed been monitored by following drug-induced suppression of plasma thioredoxin and VEGF levels, suggesting that thioredoxin may serve as a redox biomarker amenable to rapid standard detection (19). In addition, tumor genotyping for Keap1 mutational status is emerging as a potential predictor of therapeutic success associated with redox intervention targeting Nrf-2 downstream effectors such as heme oxygenase 1 (as discussed briefly in Section III.D) (320).
It is now established that prooxidant intervention using metabolic inhibitors and hypoxia-activated agents should be based on phenotypic profiling measuring tumor glucose uptake and hypoxia, respectively (as discussed in Sections IV.A and IV.C.1). Indeed positron emission tomography (PET) imaging with [18F]-fluoromisonidazole is clinically used for selection of hypoxic tumors to be targeted by hypoxia-activated drugs (239), and tumor glucose uptake can be assessed by [18F]2-fluoro-2-deoxy-D-glucose PET imaging (44). Rapid progress in this area suggests that oncological redox biomarkers provide a unique opportunity for optimization of personalized redox chemotherapeutic intervention.
C. Redox dysregulation as anticancer drug target
Molecular mechanisms by which redox alterations contribute to cancer cell proliferative control, survival, invasion, and metastasis are of equal interest to researchers focusing on fundamental cancer biology or translational anticancer drug discovery, as expertly reviewed recently (48, 115, 117, 127, 129, 210, 296, 309, 310, 349). The involvement of ROS in cancer initiation and progression is now firmly established. Apart from its role as a causative factor in carcinogenesis through ROS-induced mutagenesis, redox dysregulation contributes to malignant transformation and progression through ROS-mediated mitogenic signaling and redox modulation of apoptotic and survival pathways (127, 210, 296). Following early studies that described increased production of ROS including superoxide radical anions and hydrogen peroxide (H2O2) by human tumor cells (335, 348), recent research supports a causative role of altered redox regulation in tumorigenesis and has identified numerous cellular sources of ROS production in cancer cells, including overexpression of ROS-generating NOX family members and enhanced electron leakage from the mitochondrial respiratory chain (115, 133, 134, 252, 356, 389). Indeed, NOX-dependent ROS generation driving angiogenesis has recently emerged as a promising target for pharmacological anticancer redox intervention as suggested by prototype studies performed in murine hemangioma (276).
Early studies observed a correlation between expression of oncogenes and cellular ROS levels (e.g., increased ROS production in response to ras oncogenic activity has been described in H-RASv12-transformed NIH3T3 fibroblasts) (161). It is now established that constitutive upregulation of ras protein signaling through overexpression or mutational activation, one of the most common genetic events observed in carcinogenesis, is associated with increased ROS production, cellular oxidative stress, and mutagenesis observed in many tumors (198, 315, 349). It is therefore not surprising that RAS-transformed cells are more sensitive to pharmacological depletion of glutathione, suggesting that an elevated rate of constitutive ROS production in Ras-transformed cells may represent a functional target for pharmacological intervention that undermines the cellular antioxidant capacity (349, 350).
Another oncogene, the chimeric BCR-ABL tyrosine kinase responsible for chronic myelogenous leukemia (CML), increases intracellular oxidative stress and causes inactivation of protein phosphatases and genomic instability in an ROS-dependent manner, providing another example of oncogene-controlled redox dysregulation in cancer cells (296, 309, 349). Interestingly, BCR/ABL activates ROS-producing signaling pathways leading to oxidative DNA damage and transitional mutations that encode clinically relevant amino acid substitutions in the BCR/ABL kinase domain causing imatinib resistance (199).
In the same manner, inactivation of tumor suppressor genes may cause deviations from redox homeostasis that increase mutagenesis and tumorigenesis. For example, recent mouse studies suggest that p53 mutational inactivation impairs p53 antioxidant function through transcriptional downregulation of key mediators including TP53INP1 (tumor protein 53-induced nuclear protein 1) resulting in increased oxidative stress, accelerated mutational rate, and increased tumor growth, all of which can be suppressed by antioxidant supplementation (303). Taken together, these exemplary studies suggest that numerous oncogenes and tumor suppressor genes exert their functions in part through redox mechanisms that may be amenable to pharmacological intervention by redox chemotherapeutics.
Redox dysregulation observed in cancer cells is a complex phenomenon that integrates many aspects of the cancerous phenotype, including alterations in metabolism, proliferative control, and anti-apoptotic survival signaling, as reviewed extensively elsewhere (48, 117, 118, 296, 349). In many human cancer cell lines and tumors, alterations of proliferative and apoptotic control have been shown to depend partly on constitutive activation of multiple redox sensitive targets through autocrine production of ROS, including components of signaling cascades (e.g., Akt/protein kinase B and MAP kinases) as well as transcription factors [e.g., nuclear factor κB (NFκB) and activator protein 1 (AP-1)] (141, 296, 330, 349). The role of ROS-dependent redox dysregulation in tumor progression has been studied recently in much detail in human melanoma where overexpression of Akt converts radial (noninvasive) to vertical (invasive) growth phase tumors with increased generation of superoxide originating from NOX4 upregulation, preferential glycolytic energy metabolism, and VEGF-dependent angiogenesis (115, 134). In this context, it should also be mentioned that the antagonist of phosphoinositide-dependent Akt activation and tumor suppressor PTEN and other members of the protein tyrosine phosphatase superfamily are established molecular targets of ROS signaling, chemically inactivated by ROS-dependent oxidation of essential cysteine residues facilitating tumorigenic tyrosine kinase receptor signaling (36, 214, 215, 247, 387).
Paradoxically, apart from being involved in proliferative, anti-apoptotic, metastatic, and angiogenic signaling, ROS may also exert cytotoxic and proapoptotic functions that would limit tumorigenicity and malignant progression (48, 117). For example, changes in cellular redox homeostasis and ROS levels will affect viability through redox modulation of the mitochondrial permeability transition pore opening leading to cytochrome C release, apoptosome assembly, and activation of executioner caspases, if cellular ROS levels reach a certain threshold incompatible with cellular survival (117, 349). Consequently, redox homeostasis in cancer cells that produce ROS at elevated levels due to glycolytic metabolic adaptations, mitochondrial insufficiencies, and ROS-dependent survival signaling depends on a concerted upregulation of antioxidant defense mechanisms, most notably the glutathione- and thioredoxin-dependent redox systems (255, 279), but also involves upregulation of fundamental stress response signaling including the heat shock response and the electrophilic stress response mediated by the Nrf2/Keap1-ARE pathway (discussed in Section III.D) (208).
Accumulating evidence suggests feasibility of chemotherapeutic redox intervention by modulation of constitutively elevated levels of cellular oxidative stress using novel pro- and antioxidant redox chemotherapeutics that target mitogenic and anti-apoptotic ROS-signaling (48, 117, 118, 210, 349). It has been suggested that differential redox set points in cancer cells versus nontransformed normal cells represent a therapeutic window of sufficient width permitting redox intervention that selectively targets cancer cells with constitutively upregulated levels of ROS. Much attention has therefore focused on the identification and development of experimental chemotherapeutics that induce positive deviations from redox homeostasis through prooxidant action, either by direct production of oxidizing species or by modulation of specific cellular targets involved in redox homeostasis. Theoretically, prooxidant deviation induces a redox shift that redlines the cancer cell proliferative engine leading to cell cycle arrest and cell death without compromising viability of untransformed cells based on the redox differential between normal and tumor cells (Fig. 2). Remarkably, the requirements for prooxidant proliferative and survival signaling encountered in rapidly dividing cancer cells also suggest feasibility of antioxidant intervention by pharmacological induction of negative deviations from redox homeostasis expected to attenuate the cancer cell proliferative engine.
FIG. 2.
Targeting cancer cell redox homeostasis by prooxidant therapeutic intervention. Constitutive upregulation of oxidative stress contributes to genotypic and phenotypic changes characteristic of cancer cells and represents a redox vulnerabilty that can be targeted by prooxidant therapeutic intervention (POX). Differential redox set points in normal and malignant cells (as represented in a virtual ‘redox tachometer’) suggest that prooxidant-induced upregulation of cellular ROS specifically targets cancer cells. This process can be referred to as ‘redlining’ in analogy to the red bar (redline) displayed on car tachometers denoting the maximum speed at which an internal combustion engine is designed to operate without causing damage. In malignant cells already at a high setpot of constitutive oxidative stress, prooxidant deviation induces a redox shift that ‘redlines’ the cancer cell proliferative engine, leading to functional impairment, cell cycle arrest, and cell death. In contrast, the same prooxidant deviation from redox homeostasis is tolerated by nonmalignant cells. The width of the therapeutic window will be determined by the redox differential between normal and tumor cells and may limit efficacy of redox intervention. Exemplary redox chemotherapeutics for POX are depicted in the lower box including ( from left to right) the semisynthetic endoperoxide artemether, the metabolic modulator 2-deoxy-D-glucose, and the synthetic glutathione depleting agent imexon. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
D. ROS in cancer chemotherapy: From toxicological liability to therapeutic asset
It is well established that dose-limiting off-target toxicity of anthracycline tumor antibiotics can result in potentially fatal cardiomyopathy, attributed to the generation of free radical-mediated damage originating from site-specific oxidative metabolism of these anthraquinone-derived redox active drugs in cardiac sarcoplasmic reticulum and mitochondria (65). Indeed, considerable effort has pursued the identification of cytoprotective metal chelators (e.g., dexrazoxane hydrochloride) and antioxidant cytoprotective adjuvants (e.g., amifostine) that can serve as combinatorial agents for prevention of chemotherapy-associated organ toxicity without compromising chemotherapeutic efficacy of these agents (65, 240). Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimetic mangafodipir has been demonstrated recently (7), and mangafodipir protective activity against oxaliplatin neurotoxicity is currently evaluated in a Phase II clinical trial (ClinicalTrials.gov Identifier: NCT00727922).
Apart from being invoved in mediating undesirable collateral damage in cancer chemotherapy, it has long been known that formation of genotoxic free radical species is a crucial mechanism of action associated with potent anticancer antibiotics including calicheamicin, leinamycin (discussed in Section II.F), and bleomycin. For example, it is well established that the glycopeptide bleomycin, an established component of standard combination chemotherapy, destroys malignant cells based on a metal-dependent prooxidant free radical mechanism leading to DNA fragmentation (61, 69). Bleomycin binds ferrous iron and oxygen and after reduction in vivo produces an activated intermediate, a Fe3+ hydroperoxide [BLM-Fe(III)-OOH, ferric peroxide complex] that cleaves DNA by hydrogen abstraction.
More recently, accumulative evidence suggests that induction of ROS formation and redox dysregulation is causatively involved in the chemotherapeutic efficacy of many established anticancer drugs that have traditionally not been associated with a free radical mechamism of action (76). Numerous studies have demonstrated the causative involvement of ROS formation in the mediation of cancer cell apoptosis induced by various standard chemotherapeutic agents including paclitaxel (5, 6), cisplatin (30), bortezomib (114), and etoposide (266). Remarkably, it has been demonstrated that cisplatin apoptogenicity depends on formation of ROS and occurs independent of nuclear DNA damage, suggesting that apoptogenic oxidative stress is the crucial mechanism of cisplatin-induced cancer cell death (30).
Based on this largely unappreciated prooxidant aspect of anticancer activity associated with established chemotherapeutic agents, combination therapy employing a chemotherapeutic drug with an experimental prooxidant provides a promising therapeutic strategy for potentiation of cancer cell cytotoxicity currently examined in clinical trials as discussed in detail below.
II. Reactive Pharmacophores for Anticancer Redox Chemotherapy
Small molecule anticancer redox drugs now in various phases of preclinical and clinical development are a heterogenous group of therapeutics distinguished by their ability to alter cellular redox status. Importantly, target modulation by redox chemotherapeutics can occur by structure-based mechanisms (i.e., the activity of molecular targets is altered through binding of a ligand). However, more often target modulation by redox chemotherapeutics involves a reactivity-based mechanism, that is, the activity of a molecular target is altered through a chemical reaction. Indeed, reactants (reactive molecules) represent important anticancer redox chemotherapeutics with documented preclinical and clinical efficacy. These agents produce or inactivate ROS, antagonize or mimic activity of enzymes involved in redox metabolism, or chemically alter redox sensitive protein targets involved in proliferative and survival signaling.
The following section discusses mechanism of action, structure activity relationship (SAR), and preclinical and clinical efficacy of selected anticancer redox drugs that are defined by their exemplary reactive pharmacophores. It should be mentioned that photodynamic pharmacophores that produce ROS including singlet oxygen by photon-driven energy and electron transfer reactions, an important class of FDA-approved prooxidant anticancer chemotherapeutics for photodynamic therapy (PDT), will not be included here since they have been covered extensively elsewhere (264, 381).
A. Organic endoperoxides: Artemisinins
Artemisinin (Fig. 3A-1), a sesquiterpene endoperoxide natural product isolated from Artemisia annua (qinhao, sweet wormwood), targets malaria protozoa through iron-dependent induction of oxidative stress (95, 96, 177, 321). Lead optimization by medicinal chemistry has led to the development of a whole range of 1,2,4-trioxane-based, semisynthetic artemisinin-derivatives with retained endoperoxide pharmacophore and improved pharmacokinetic profile and stability, including dihydroartemisinin (DHA, Fig. 3A-3), artemether (Fig. 3A-4), arteether (Fig. 3A-5), and artesunate (Fig. 3A-6), which constitute the most potent and rapidly acting group of antimalarial drugs available today. Indeed, artemisinin combination therapies (ACT) combining artemisinin derivatives with longer half-life drugs (e.g., artemether‱lumefantrine), are now used as internationally approved antimalaria chemotherapeutics of major global significance. In plasmodia, the cytotoxicity inducing cleavage of the endoperoxide moiety is triggered by heme iron released during hemoglobin digestion in host erythrocytes. In addition, artemisinins are potent inhibitors of the malaria parasite PfATP6 enzyme, an orthologue of the thapsigargin-sensitive sarcoendoplasmic reticulum Ca2+ (SERCA) ATPase (96).
FIG. 3.
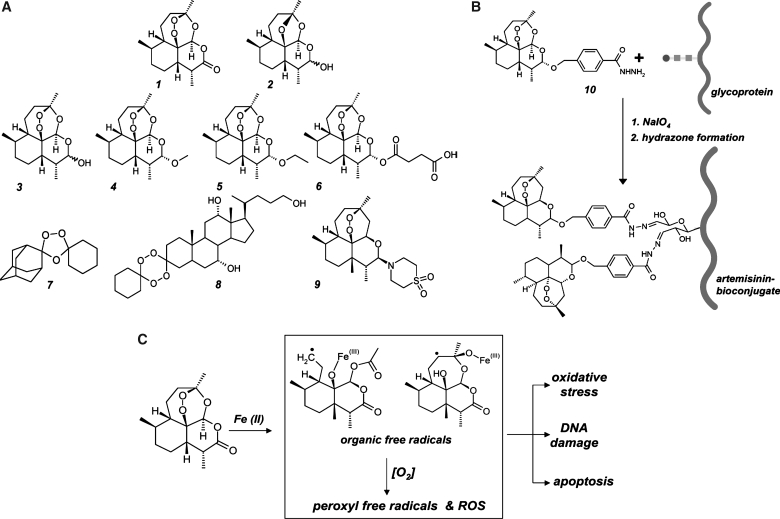
Artemisinins: A novel class of endoperoxide-based anticancer redox chemotherapeutics. (A) The sesquiterpene endoperoxide artemisinin (1) and 1,2,4-trioxane-based, semisynthetic artemisinin-derivatives including dihydroartemisinin (3), artemether (4), arteether (5), artesunate (6), and artemisone (9) are established prooxidant antimalarial drugs and investigational chemotherapeutics for oncological indications. Deoxyartemisinin (2) is a redox-inactive artemisinin derivative. OZ277 (7) and the steroidal tetraoxane 5β-cholan-7α,12α,24-triol-3-spiro-6′-(1′,2′,4′,5′-tetraoxacyclohexane)-3′-spiro-cyclo-hexane (8), are fully synthetic drug-like 1,2,4-trioxolane-derivatives. (B) Artemisinin–glycoprotein bioconjugates for targeted prooxidant intervention. Artemisinin bioconjugation of transferrin and other glycoproteins (curved line: protein; squares and circle: oligosaccharide chain) occurs via hydrazone formation using artelinic acid hydrazide (10) after periodate-induced oxidation of vicinal sugar diol-groups. (C) The free radical mechanism of artemisinin drug action. The pharmacophoric moiety of artemisinins is an endoperoxide bridge that can be fragmented by intracellular iron [Fe(II)] species, leading to the formation of carbon-centered electrophilic radical species including a sterically unhindered primary radical (middle panel, upper left) and a sterically more congested secondary radical (middle panel, upper right). These organic free racicals together with ROS formed through Fenton chemistry are the suggested key mediators of artemisinin-induced cancer cell inactivation.
Recent research has demonstrated that artemisinin and its semisynthetic derivatives target human cancer cells based on intracellular prodrug activation with formation of prooxidant reactive species triggered by redox-active iron ions, the common pharmacodynamic basis underlying both antimalarial and anticancer activity of artemisinin-type stable endoperoxides (95, 96, 321). Importantly, concentrations required for antagonizing cancer cells, usually in the upper nanomolar to low micromolar range, are about one to two orders of magnitude higher than those required for killing plasmodia. The active moiety of artemisinins is an endoperoxide bridge that can be activated and fragmented by intracellular iron [Fe(II)] leading to the formation of carbon-centered electrophilic radical species and ROS with involvement of Fenton chemistry (Fig. 3C). Importantly, earlier studies have shown that artemisinin cytotoxicity against various cancer cell lines is completely abolished upon chemical replacement of the endoperoxide functional group by an ether bridge producing the redox-inactive deoxyartemisinin (Fig. 3A-2), a versatile molecular probe and negative control for the interrogation of redox mechanisms in artemisinin drug action (177).
The endoperoxide-pharmacophore contained in artemisinin and its derivatives imparts a unique chemical reactivity (96, 337). Unlike other organic peroxide compounds, artemisinin displays drug-like stability, and the endoperoxide core does not react with regular reducing agents such as NaBH4, providing large-scale semisynthetic access to artemisinins with retained Fe(II)-dependent reactivity via reduction of the artemisinin lactone group to dihydroartemisinin. Subsequent esterification and ether formation generate numerous derivatives with improved stability and pharmacokinetic profile. Recently, a series of experimental antimalarials and anticancer agents containing synthetically accessible tetraoxane or ozonide pharmacophores has been generated (337). These fully synthetic organic endoperoxide-derivatives either retain the unique pharmacodynamic characteristics of artemisinin or display significantly dissociated antimalarial and antiproliferative potencies such as OZ277 (Fig. 3A-7), a synthetic ozonide (1,2,4-trioxolane-derivative) now in clinical development for antimalaria intervention (337), and the steroidal tetraoxane 5β-cholan-7α, 12α,24-triol-3-spiro-6′-(1′,2′,4′,5′-tetraoxacyclohexane)-3′-spiro-cyclo-hexane (Fig. 3A-8), a compound that displays potent antiproliferative effects in the nanomolar concentration range as determined in the NIH 60 cancer cell line screen without displaying significant antimalarial effects (268, 337).
Recent studies indicate that induction of cellular oxidative stress leading to mitochondrial dysfunction and rapid induction of mitochondrially-triggered apoptosis plays a major role in the anticancer activity of artemisinins (94–96, 321). Moreover, induction of DNA damage by artesunate based on rapid formation of DNA comets and γ-H2AX phosphorylation has been demonstrated (217). Remarkably, cell lines over-expressing genes that confer resistance to established antitumor drugs (e.g., MDR1, MRP1, dihydrofolate reductase, ribonucleotide reductase) are not crossresistant to artesunate, indicating that this drug has a different target and is not subject to multidrug resistance. Expression of antioxidant genes, including thioredoxin reductase and catalase, is an important determinant of artesunate activity against tumor cells (97). For example, WEHI7.2 cells selected for resistance to hydrogen peroxide or transfected with thioredoxin, manganese superoxide dismutase, catalase, or Bcl-2 displayed resistance to artesunate compared to the parental cell line (97). Other research has demonstrated potent antiangiogenic activity of artemisinin linked to artemisinin-mediated downregulation of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) expression, which control endothelial cell growth (79, 96). Other research has demonstrated that dihydroartemisinin increases generation of ROS and blocks HIF-1α activation and expression of its downstream target VEGF in C6 glioma cells with little cytotoxicity observed in rat primary astrocytes (157).
Recent findings indicate that artemisinin blocks prostate cancer growth and cell cycle progression by structure-based interference with Sp1-cyclin-dependent kinase-4 promoter interactions leading to inhibition of CDK4 gene expression, and it was suggested that this activity occurs independent of a redox mechanism of action (374). However, promotor binding studies were performed at very high supratherapeutic concentrations (300 μM) of artemisinin, and no control experiments employing metal chelators or the redox inactive analogue deoxyartemisinin (introduced above) were employed to exclude involvement of redox mechanisms.
Most cancer cells display increased rates of iron uptake that supports rapid proliferation (204), and recent data strongly suggest that altered iron metabolism occurs in oncogenic Ras-transformed cells, providing a valid therapeutic window for prooxidant intervention using artemisinins (393). It is now well established that the susceptibility of tumor cells to artemisinin and its derivative, artesunate, can be enhanced by co-administration of ferrous iron, and oral co-administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma tumor growth in rats (253). Recent research demonstrates that the transferrin receptor (TfR) is overexpressed in tumors, and the effect of ferrous iron on artesunate can be reversed by a monoclonal antibody that competes with transferrin for TfR-binding (256). Interestingly, TfR-dependent cytotoxicity of artemisinin-transferrin conjugates provides a promising strategy for targeted artemisinin-dependent prooxidant intervention. Design of artemisinin-bioconjugates for targeted delivery is facilitated by availability of the artemisinin-derivative artelinic acid hydrazide (Fig. 3B-10) that can be covalently attached to any glycoprotein via hydrazone adduction after periodate-induced protein carbonylation (256, 265). Artemisinin–transferrin conjugates induce TfR-dependent cytotoxicity in DU145 prostate cancer cells with induction of oxidative stress that triggers the mitochondrial pathway of apoptosis (256). In a different study, small artemisinin–heptapeptide conjugates targeting the transferrin receptor diplayed selective cytotoxicity in molt-4 leukemia cells but not normal human leukocytes (265).
The anticancer activity of artemisinin-drugs in vivo has been demonstrated employing various murine xenograft tumors models as recently reviewed (96). Growth of fibrosarcoma in Fisher 344 rats was significantly delayed by the daily administration of dihydroartemisinin plus ferrous sulfate. In a rat 7,12-dimethylbenzo[a]anthracene (DMBA) breast carcinogenesis model, oral administration of artemisinin delayed tumor development and reduced tumor multiplicity and size (205). Growth of subcutaneously injected KS-IMM Kaposi sarcoma cells in nude mice was strongly suppressed in artesunate-treated animals (79). Importantly, anticancer activity of artemisinins also covers human papilloma virus (HPV)-induced tumors as demonstrated in a canine model of oral mucosal papillomavirus-induced tumors that were inhibited by topical application of dihydroartemisinin. Earlier research demonstrated that dihydroartemisinin is cytotoxic to HPV-infected epithelial cells in vitro and in vivo, and clinical usefulness of artemisinins as prooxidant chemotherapeutics targeting HPV-infection and cervical dysplasia has been proposed (82). Stability and hydrophobicity of certain artemsinine derivatives may also enable topical application, which could simplify the treatment of early cervical lesions, including those in immunocompromised patients. A case report on the therapeutic benefit of artesunate chemotherapy in patients with metastatic uveal melanoma has been published (28). In an ongoing phase I study of artesunate in metastatic and locally advanced breast cancer, tolerability of an add-on therapy using artesunate is evaluated (ClinicalTrials.gov Identifier: NCT00764036).
In this context it will be fascinating to examine the anticancer activity of novel endoperoxide drugs currently in advanced clinical testing as antimalaria agents including artemisone (BAY 44-9585; Fig. 3A-9) and the fully synthetic 1,2,4 trioxane OZ277 (Fig. 3A-7). These investigational drugs, optimized for improved oral availability, pharmacokinetics, and drug safety, retain the redox active endoperoxide pharmacophore and may therefore display potent anticancer activity, a hypothesis to be tested by future experimentation (337).
B. Arsenicals: As2O3 and darinaparsin
1. As2O3
Trivalent arsen [As(III)]-containing inorganic and organic compounds are an important class of thiol-reactive prooxidant redox chemotherapeutics targeting acute promyelocytic leukemia (APL) (363). Importantly, the prototypical inorganic arsenical arsenic trioxide (As2O3) received FDA-approval in 2000 for treatment of APL in patients who have relapsed or are refractory to first-line intervention using retinoid and anthracycline chemotherapy. Clinical use of As2O3 in APL has recently advanced to first-line therapy. Remarkably, in newly diagnosed patients, high rates of complete remission (up to 95%) and 2-year leukemia-free survival rates (80%) are usually achieved using As2O3 as single agent. However, beyond APL, the therapeutic benefit of As2O3 in other hematologic malignancies is very limited.
Based on the known reactivity of trivalent arsenicals towards thiol-containing molecules leading to covalent crosslinking of vicinal thiols (396), As2O3 exerts antiproliferative and apoptogenic effects on cancer cells by prooxidant mechanisms that include covalent adduction and oxidation of redox-sensitive cellular cysteine residues in GSH and proteins (117, 229). Most APL cases are characterized by t(15;17)(q22;q21) chromosomal translocation and the resulating PML–RARalpha chimeric gene product, the fusion protein promyelocytic leukemia/retinoic acid receptor, blocks granulocytic differentiation and induces tumorigenesis (363). As2O3 is a potent inducer of the mitochondrial pathway of apoptosis causing enhanced electron leakage from the mitochondrial respiratory chain (274), but it is not clear how these activities facilitate rapid degradation of PML–RARalpha observed in As2O3-treated APL (170). Experimental evidence obtained in IM-9 mutiple myeloma cells has been presented demonstrating that the voltage-dependent anion channel (VDAC), an outer mitochondrial membrane protein and critical component of the permeability transition pore complex, represents a crucial molecular target of As2O3 upstream of cytochrome c release and caspase activation, and As2O3-induced cross-linking of cysteine residues within the transmembrane domain of VDAC may be the crucial molecular mechanism underlying induction of permeability transition pore opening (402). Moreover, recent evidence suggests that irreversible inhibition of thioredoxin reductase 1 by covalent adduction of critical selenocysteine- and cysteine residues is the key mechanism of prooxidant redox disturbance underlying As2O3-induced breast cancer cell apoptosis (229).
Importantly, the magnitude of As2O3-induced cytotoxicity is inversely correlated with the intracellular GSH pool of cancer cells, and molecular interventions that deplete cellular glutathione levels sensitize cells towards As2O3-induced apoptosis as observed upon pharmacological downregulation of cellular glutathione levels using L-buthionine-S,R-sulfoximine or L-ascorbate as reviewed recently (117, 118). Indeed, potentiation of As2O3 chemotherapeutic efficacy by combination with BSO was demonstrated in various mouse xenograft tumor models including an orthotopic model of prostate cancer metastasis, suggesting that prooxidant intervention using As2O3 in combination with an inhibitor of the antioxidant GSH system is effective in terms of both efficacy and selectivity (235). Clinical development of As2O3 for oncological applications beyond APL is ongoing. In recently completed Phase II trials, As2O3 did not offer therapeutic benefit in patients with adenocarcinoma of the pancreas or patients with metastatic melanoma (17), but promising results were obtained in a Phase I/II study of As2O3/bortezomib/L-ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma (27).
Based on the clinical success of As2O3, anticancer arsenicals are an expanding class of experimental redox chemotherapeutics. Interestingly, the cubic isoform As4O6 {2,4,6,8,9,10-hexaoxa-1,3,5,7-tetraarsa-tricyclo[3.3.1.13,7]decane}, a more potent inducer of apoptosis and inhibitor of tumor growth than As2O3 in SiHa HPV 16-immortalized human cervical carcinoma cell xenografted nude mice, has received attention as an alternative inorganic arsenical for anticancer intervention (55).
2. Darinaparsin
The investigational drug darinaparsin (S-dimethylarsino-glutathione; ZIO-101; Fig. 4-11) is a synthetic organic arsenical with improved toxicity profile that displays antitumor activity towards As2O3-resistant and MRP1/ABCC1-overexpressing cell lines (283). Darinaparsin treatment results in higher intracellular arsenic accumulation when compared to As2O3 treatment since As2O3, but not darinaparsin, is efficiently exported by ABCC1, suggesting increased therapeutic efficacy of darinaparsin in ABCC1-overexpressing tumors (81). Darinaparsin induces high levels of cellular oxidative stress and apoptosis with more potency than As2O3, but its detailed molecular mechanism of action remains to be elucidated. Numerous clinical trials in Phases I and II examine the use of darinaparsin in patients with various oncological conditions such as hematological cancers (ClinicalTrials.gov Identifiers: NCT00592046; NCT00421213) and advanced hepatocellular carcinoma (ClinicalTrials.gov Identifier: NCT00423306).
FIG. 4.
A selection of experimental and developmental redox chemotherapeutics and related derivatives. Agents discussed throughout the text and not shown in other specialized figures: Darinaparsin (11), motexafin gadolinium (12), menadione (13), toluidine blue O (14), geldanamycin (15), 17-allylamino-17-demethoxygeldanamycin (16), radicicol (17), 2-(phenyltelluryl)-3-methyl-[1,4]naphthoquinone (18), ebselen (19), 4,4′-dihydroxydiphenyl telluride (20), disulfiram (21), Dp44mT (22), triapine (23), lissoclinotoxin A (24), leinamycin (25), L-ascorbic acid (26), ATN-224 (27), 2-methoxyestradiol (28), M40403 (29), mangafodipir (30), TEMPO (31), imexon (32), PX-916 (33), auranofin (34), chaetocin (35), ES936 (36), DIBA (37), and elesclomol (38).
C. Redox cyclers: Motexafin gadolinium
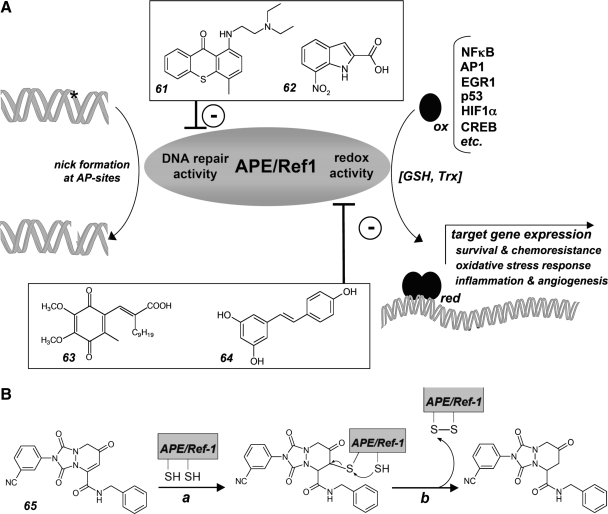
Redox cycling is an important chemical mechanism underlying formation of ROS by numerous clinical and experimental anticancer agents containing quinone pharmacophores (including anthracyclines, geldanamycin, and menadione) as well as other redox active pharmacophores (e.g., texaphyrin macrocycles and polysulfide agents), and drug-induced ROS formation has important toxicological and pharmacodynamic consequences. Experimental therapeutics that can undergo spontaneous or enzyme-driven redoxcycling with production of cytotoxic organic free radicals and ROS can target cancer cells through selective induction of oxidative stress as reviewed extensively elsewhere (120, 237, 379). As prooxidant catalysts, redox cyclers mediate electron transfer from a cellular reducing agent [e.g., glutathione or NAD(P)H] onto oxygen with production of ROS (120, 128, 237, 358, 379). After electron transfer to oxygen, the redox catalyst is regenerated by spontaneous or enzyme-driven reduction. Importantly, cancer cell-selectivity of these agents may be based on (a) the established prooxidant redox vulnerability of certain cancers (as detailed in Section I.C), (b) the increased availability of bioreductive equivalents in glycolytic and hypoxic tumor tissue, and (c) the potential cancer cell selective expression of bioreductive enzymes such as NQO1 involved in driving redoxcycling by reductive regeneration of the reduced form of the catalyst. Generally, redox cyclers act in low concentrations due to their catalytic mechanism of action that is based on reversible electron transfer reactivity as discussed elsewhere (48, 120).
1. Motexafin gadolinium
Motexafin gadolinium (MGd; Fig. 4-12) is a prototype anticancer redox catalyst far advanced in clinical studies, as extensively reviewed in (237). It is interesting to note that MGd is derived from paramagnetic probes used for magnetic resonance bioimaging. MGd comprises a central paramagnetic gadolinium(III) ion coordinated by five pyrrole- and imine-derived nitrogens contained in the planar polyaromatic texaphyrin macrocycle. The amphiphilic drug-like molecule easily penetrates cellular membranes, and water solubility depends on hydroxypropyl and triethyleneglycol monomethyl ether substituents. It is now established that redox cycling of MGd depletes cellular antioxidant factors such as protein-bound and free thiols and ascorbate, and releases thiolate-bound Zn ions from the antioxidant protein factor metallothionein. In addition to pharmacodynamic activity as redox cycler, MGd is also a potent inhibitor of the key antioxidant enzyme thioredoxin reductase 1 that is also inhibited by free Zn ions that are intracellularly released as a consequence of MGd-metallothionein interaction, a mechanism of action that may underlie MGd activity as a potent radiosensitizer (144, 236, 237). Moreover, recent experimental evidence suggests that MGd targets ribonucleotide reductase by directly binding and inactivating the redox sensitive R1 subunit of this key enzyme involved in deoxyribonucleotide biosynthesis (395).
The SAR of MGd-induced prooxidant effects as determined by texaphyrin-gadolinium (III) cation interactions is well defined (237). Presence of the gadolinium (III) cation is essential for drug action and is lost upon replacement by other paramagnetic cations such as lutetium and europium. MGd displays spontaneous redox reactivity and reversibly accepts electrons from cellular redox factors with sufficiently negative reduction potential (e.g., NAD(P)H, ascorbate, glutathione, and small molecule and protein-bound vicinal thiols) followed by electron transfer to molecular oxygen. Gadolinium (III) does not participate in redox catalysis, which depends on high electron affinity and oxidizing properties of the extended cationic π-electron system of the texaphyrin ligand. However, the paramagnetic properties of the Gd(III) cation ensure the harmless return of the photoexcited molecule to the ground state and thereby minimize photodynamic activity of the large texaphyrin chromophore that would interfere with drug action. NAD(P)H-driven MGd redox cycling is potentiated by the presence of intracellular reductases such as P450 reductase, cytochrome c reductase, and thioredoxin reductase, leading to extensive ROS formation and cellular oxidative stress.
Importantly, MGd cotreatment exerts potent radio- and chemosensitization of cancer cells and may induce the mitochondrial pathway of apoptosis by prooxidant mechanisms when used as single agent against cancer cells with constitutively elevated levels of ROS, including human B cell lymphoma cells (98, 237). The drug has completed promising Phases I and II clinical trials (9, 288). In an international randomized Phase III trial patients with brain metastases from non-small cell lung cancer (NSCLC) given MGd in addition to whole brain radiation therapy had a median time to neurological progression of 15.4 months, compared to 10.0 months for patients who received only radiation, a trend in favor of the MGd-treated arm (246). In addition, MGd is now undergoing evaluation as a monotherapy and in various combinations with chemotherapy and monoclonal antibodies for several tumor types including lymphomas and leukemias (ClinicalTrials.gov Identifier: NCT00100711; NCT00076401), lung cancer (ClinicalTrials.gov Identifier: NCT00365183), renal cell cancer (ClinicalTrials.gov Identifier: NCT00134186) and glioblastoma (ClinicalTrials.gov Identifier: NCT00305864).
2. Menadione
Redox cycling of quinone pharmacophores contained in experimental and clinical chemotherapeutics represents an important mechanism underlying cancer cell directed cytotoxicity and may also induce unwanted off-target toxicity associated with established chemotherapeutics such as anthracyclin tumor antibiotics (65, 358). The experimental redox chemotherapeutic menadione (2-methylnaphthalene-1,4-dione, vitamin K3; Fig. 4-13) contains a naphthoquinone pharmacophore that undergoes intracellular single electron reduction to the cytotoxic semiquinone free radical. The semiquinone radical is rapidly reoxidized to its quinone form by electron transfer reaction with molecular oxygen leading to superoxide formation driven by the reducing activity of cellular NAD(P)H-dependent reductases or redox factors including ascorbate. Feasibility of harnessing menadione redox cycling for experimental redox chemotherapy with or without ascorbate potentiation has been substantiated in various murine xenograft models (358). Remarkably, ascorbate/menadione-induced cancer cell death occurs without caspase-3 activation in an ROS-dependent mode that involves oxidative DNA damage associated with massive PARP activation and mitochondrial release of AIF, a mechanism of cytotoxicity that may overcome chemoresistance in cancer cells with redundant anti-apoptotic survival signaling. It is important to note that apart from disturbing cancer cell redox homeostasis by redox cycling, menadione exerts anticancer activity through covalent electrophilic adduction of the catalytic domain of the redox sensitive Cdc25A phosphatase leading to Cdk1 hyperphosphorylation and cell cycle arrest, an important mechanism of anticancer redox intervention (discussed in Section III.F) (382).
Anticancer activity of prooxidant quinone-derivatives has been documented in many studies but the potential role of redox cycling in ROS production is not always established. For example, anticancer activity of the naphthoquinone derivative plumbagin seems to result from ROS formation upstream of cell death-associated pathways, including inhibition of topoisomerase II followed by DNA strand breaks and induction of apoptosis (187). Similarly, shikonin, another naphthoquinone natural product, induces apoptogenic oxidative stress in human hepatoma cells; ROS-dependent activation of the stress-related JNK pathway and subsequent mitochondrial dysfunction, cytochrome c release, caspase activation, and preferential apoptosis were observed in CML cells (238).
3. Acetaminophen and O-acetylsalicylic acid
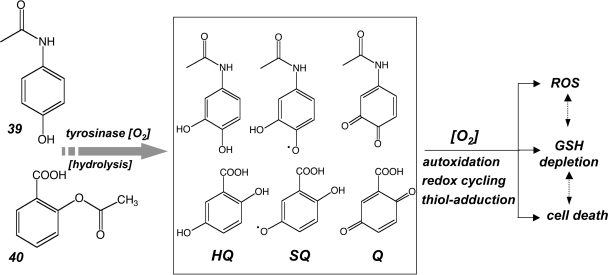
A recent report has attributed the experimental antimelanoma activity of the nonsteroidal anti-inflammatory drug (NSAID) N-acetyl-para-aminophenol (acetaminophen; Fig. 5-39) to prooxidant glutathione depletion by the 3-hydroxy-1,4-quinone-imine-metabolite, a redox active metabolite that forms specifically by tyrosinase-catalyzed transformation, suggesting the melanocyte-specific manifestation of this cytotoxic effect (352). A Phase I trial of high dose acetaminophen and carmustine in patients with metastatic melanoma has been conducted (378), and further testing is ongoing in a Phase I/II clinical study that assesses the effectiveness of acetaminophen combination chemotherapy in treating patients with stage III or stage IV melanoma. (ClinicalTrials.gov Identifier: NCT00003346). However, a potential for hepatotoxicity known to be associated with excessive acetaminophen quinone-metabolism may compromise the width of the therapeutic window of acetaminophen-based redox chemotherapy and may present an obstacle for future clinical development. In a similar manner, tyrosinase-dependent metabolism of the phenolic drug aspirin (O-acetylsalicylic acid; Fig. 5-40) induces formation of quinone metabolites that display pronounced redox cycling and oxidative stress-induced cytotoxicity in human melanoma cells (353), providing an example of experimental redox chemotherapy that may be based on cancer cell-selective formation of prooxidant quinone metabolites of approved drugs with established safety profile. However, prooxidant activity of NSAIDs targeting cancer cells may depend on tyrosinase-independent mechanisms (1). Moreover, no epidemiological evidence in support of a chemopreventive effect of NSAID use on melanoma incidence has been obtained in a recent large cohort study, suggesting that NSAID use for potential melanoma chemotherapeutic intervention remains speculative and must await further preclinical and clinical validation (13). In this context it should be mentioned that the design of nitric oxide (NO) releasing NSAID-prodrugs has recently emerged as a promising strategy for the targeted induction of oxidative stress in cancer cells harnessing the cytotoxic activity of reactive nitrogen species (RNS) (294, 332). Indeed, NO-aspirin has shown anticancer efficacy in murine xenograft models and has now progressed into early stages of clinical testing for colorectal cancer chemopreventive activity (ClinicalTrials.gov Identifier: NCT00331786). However, recent SAR studies have revealed that the anticancer activity of NO-aspirin does not depend on the presence of the NO-releasing moiety, but rather originates from spacer group-dependent formation of quinone-methide reactive intermediates with the NO-releasing group functioning as an effective leaving group (185).
FIG. 5.
N-Acetyl-para-aminophenol and O-acetylsalicylic acid as tyrosinase-activated prooxidants. In melanoma cells, phenolic metabolites of the nonsteroidal anti-inflammatory agents N-acetyl-para-aminophenol (39) and O-acetylsalicylic acid (40) form hydroquinone (HQ), semiquinone free radical (SQ), and quinone (Q) metabolites through tyrosinase-dependent hydroxylation. These prooxidant metabolites are thought to induce therapeutically relevant glutathione depletion and other cytotoxic effects directed against tyrosinase expressing cells. Acetaminophen is now an investigational drug in clinical trials targeting metastatic melanoma.
4. Geldanamycin
The molecular chaperone heat shock protein 90 (Hsp90) is an important cancer drug target due to its crucial involvement in folding and activation of signaling proteins that promote cancer cell proliferation and survival (121, 324, 329). The benzoquinone antibiotic geldanamycin (Fig. 4-15) along with its analogues 17-allylamino-17-demethoxygeldanamycin (17-AAG; Fig. 4-16) and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) targets Hsp90 as a specific inhibitory ligand masking its ATP binding site. Geldanamycin-induced target inactivation results in degradation of oncogenic client proteins (such as ErbB2, Braf, Akt/PKB, CDK4, cyclin D1, Aurora B, Polo-1 kinase, and hTERT) offering the therapeutic benefit of simultaneously antagonizing multiple targets in tumors (329). Anticancer activity of geldanamycin-derivatives is now examined in numerous clinical trials (324). It has been noted that the Hsp90 inhibitors geldanamycin, 17-AAG, and DMAG contain a redox-active benzoquinone pharmacophore, and strong experimental evidence has been presented that supports an involvement of ROS production in the antitumor effects of 17-AAG and DMAG (121). In HT29 colon cancer cells, 17-DMAG/DMAG treatment inactivated the kinase activity of nondegraded BRAF(V600E), a constitutively active oncogenic kinase, through a mechanism at least partially dependent on ROS production, suggesting that Hsp90 client proteins may be exposed to geldanamycin-induced oxidative stress. The close proximity of both the ROS generating geldanamycin benzoquinone pharmacophore and BRAF, while simultaneously bound to HSP90, would facilitate ROS-induced damage to the client protein enhancing both substrate degradation and loss of enzymatic activity. Consistent with this hypothesis, other HSP90 inhibitors including radicicol (Fig. 4-17) and novobiocin devoid of the benzoquinone redox pharmacophore, do not induce cellular oxidative stress and also fail to inhibit MAPkinase signaling through BRAF (V600E) inactivation (121). In this context it is important to note that bioreduction of the geldanamycin quinone moiety by NAD(P)H:quinone oxidoreductase 1 increases inhibition of HSP90 through enhanced binding of the hydoroquinone form of the drug, a bioreductive potentiation of geldanamycin activity independent of potential redox cycling or ROS formation (140).
5. 3,7-Diaminophenothiazinium redox dyes
Recently, it has been demonstrated that experimental agents containing a redox active 3,7-diaminophenothiazinium pharmacophore including methylene blue and the more potent toluidine blue (Fig. 4-14) selectively target melanoma and other cancer cells with induction of mitochondrial apoptosis based on bioreductive activation and redox cycling (194, 379). Remarkably, the phenothiazinium redox cycler (PRC) methylene blue is in clinical use worldwide as infusional redox antidote against cyanide poisoning and methemoglobinemia, a therapeutic application that suggests feasibility of systemic administration for investigational evaluation of potential oncological applications (48, 219). PRC compounds are two-electron redox systems with standard reduction potentials compatible with nonenzymatic and enzyme-dependent cycling between the oxidized dye-form and the colorless reduced leuco-form under cellular redox conditions (188, 293). Spontaneous electron transfer from the PRC leuco-form to molecular oxygen may induce the nonenzymatic formation of ROS, including H2O2 (379). Using PRC lead compounds against human metastatic melanoma cell lines, apoptosis occurred with phosphatidylserine externalization, loss of mitochondrial transmembrane potential, cytochrome c release, caspase-3 activation, and massive ROS production. Expression of NAD(P)H:quinone oxidoreductase (NQO1) enzymatic activity known to drive PRC bioreductive redox cycling is an important determinant of PRC cytotoxicity observed in numerous cancer cell lines (379). Based on the known overexpression and increased specific enzymatic activity of NQO1 in various human tumors (discussed in Section III.D.2) (74), PRC compounds may represent a novel class of bioreductive experimental anticancer agents that eliminate cancer cells by NQO1-driven redox cycling with induction of apoptosis in the absence of genotoxic alkylating stress observed with conventional bioreductive chemotherapeutic agents (48, 364).
6. 2-(Phenyltelluryl)-3-methyl-[1,4]naphthoquinone
Recently, drug development of multifunctional redox catalysts that selectively enhance oxidative stress in cancer cells through glutathione depletion, ROS formation, and thiol-oxidation of crucial redox target proteins including transcription factors has been initiated (120, 128). These redox catalysts, active in the nanomolar range, display both redox features of quinone-based cyclers and small molecule chalcogen-based glutathione peroxidase mimetics. On one hand, redox catalysts such as the naphthoquinone-derivative 2-(phenyltelluryl)-3-methyl-[1,4]naphthoquinone (Fig. 4-18) induce cancer cell-selective therapeutic enhancement of oxidative stress based on enzymatically-driven bioreductive redox cycling leading to ROS formation. Moreover, as catalytic antioxidants that mimic the action of glutathione peroxidase based on a pharmacophore that incorporates a selenium or tellurium atom in an organic scaffold reminiscent of the antioxidant ebselen (Fig. 4-19) (77), these agents can undergo the same reaction cycle as glutathione peroxidase, but accept any cellular thiol due to lack of specificity for glutathione. Consequently, pro-oxidant and antioxidant redox effects are achieved as a function of cellular redox status and glutathione availability, providing a therapeutic window based on cancer cell redox dysregulation with elevated cellular peroxide and decreased glutathione levels. The promiscuous peroxidase activity of the organochalcogen moiety may result in depletion of cellular thiols and can also lead to oxidative disruption of redox-sensitive zinc finger transcription factors including Sp1 observed with prototype agents including 4,4′-dihydroxydiphenyl telluride (Fig. 4-20) as discussed in Section III.G (128). The potential therapeutic usefulness of these promising experimental redox catalysts with transparent SAR active in cell culture models awaits further validation in stringent preclinical in vivo models.
D. Metal chelators: Disulfiram and triapine
A number of anticancer redox chemotherapeutics now in advanced clinical trials are defined by their metal-binding pharmacophores that display pharmacodynamic effects as a function of metal binding. The clinically active copper ion chelator and SOD1 antagonist ATN-224 will be discussed in the context of anticancer intervention targeting the SOD system (Section III.A.I).
1. Disulfiram
Disulfiram [Bis(N,N-(diethylthiocarbamoyl) disulfide, DSF; Fig. 4-21)] is an FDA-approved drug used as alcohol-abuse deterrant based on inhibitory activity on aldehyde dehydrogenase, as discussed elsewhere (53). Disulfiram, the disulfide form of N,N-diethyldithiocarbamate, undergoes thiol–disulfide exchange targeting specific protein sulfhydryl groups (e.g., a critical Cys residue in aldehyde dehydrogenase). In addition, S-thiolation and regulatory modulation of PKC isozymes by thiuram disulfide exposure has been associated with DSF anticancer activity (70). Recently it has been demonstrated that the dithiocarbamate-derivative DSF is a potent sulfur-based copper chelator, and the redox active copper(II)-bis-N,N-diethyl-dithiocarbamate complex, in which the metal ion is bound to four sulfur atoms from two dithiocarbamate anions, was shown to be the ultimate causative agent underlying DSF-induced cancer cell apoptosis (53). It is known that dithiocarbamate cytotoxicity involves copper-catalyzed conversion to thiuram disulfides, which then oxidize cellular glutathione in a redox cycle without the release of ROS (45), and DSF was found earlier to induce glutathione oxidation, DNA fragmentation, and cell death. DSF has recently been shown to preferentially target cultured human melanoma cells by oxidative stress-induced apoptosis without exerting cytotoxicity in primary melanocytes (53). Apoptosis occurs with intracellular copper ion accumulation, and both intracellular copper uptake and DSF-apoptogenicity were blocked by co-incubation with bathocuproine disulfonic acid, a nonmembrane-permeable Cu chelator. Recent studies support an array of relevant molecular targets that mediate redox dependent disulfiram anticancer effects that include inhibition of activating transcription factor/cyclic AMP-responsive element binding protein. In the same study, activity in a mouse xenograft model of human melanoma was demonstrated (39). Moreover, activity of DSF-copper in a breast cancer mouse xenograft model was recently demonstrated, but attributed to inhibition of proteasomal activity (60).
The potential role of DSF as a developmental redox chemotherapeutic targeting metastatic melanoma with clinically relevant selectivity has recently been reviewed (117, 118). Differential redox regulation between untransformed primary melanocytes and melanoma cells known to display constitutively elevated levels of ROS seems to originate from formation of redox-reactive pathological melanins, potential melanosomal leakage of prooxidant melanin precursors, and elevated levels of enzymatic ROS production, providing a rational for preferential apoptogenicity of DSF against melanoma cells (248, 249). Safety profile and prior clinical experience with this approved drug have encouraged ongoing clinical PhaseI/II studies that aim at establishing clinical efficacy of DSF in human metastatic melanoma (ClinicalTrials.gov Identifier: NCT00256230). A potential prooxidant potentiation that results in improved therapeutic benefit may exist between DSF and arsenic trioxide, another FDA approved anticancer prooxidant agent (discussed in Section II.B), a combination currently evaluated in patients with metastatic melanoma who underwent at least one prior systemic therapy (ClinicalTrials.gov Identifier: NCT00571116). Initial assessment of the effect of the addition of disulfiram to standard chemotherapy in NSCLC is the subject of an ongoing Phase I trial (ClinicalTrials.gov Identifier: NCT00312819). Moreover, another Phase I study examines disulfiram and copper gluconate for the treatment of refractory solid tumors involving the liver (ClinicalTrials.gov Identifier: NCT00742911).
2. Triapine and others
Potent prooxidant effects on cancer cells can be achieved using small molecule agents that target cellular iron ions leading to the intracellular formation of redox active chelates (179). However, it is important to note that iron chelation can exert anticancer activity without involvement of prooxidant mechanisms, presumably due to iron depletion of rapidly proliferating tumor cells. For example, inhibiton of MDA-MB231 breast tumor growth in nude mice by the clinically approved iron chelator desferal has been demonstrated to occur through iron depletion (149). In contrast to its effect on tumor cells, desferal did not inhibit growth of normal breast epithelial cells, suggesting that desferal may find future application as an adjunctive chemotherapeutic agent.
The design of potent iron chelators that achieve clinically useful anti-tumor activity by iron depletion and stimulation of iron-dependent free radical damage is a rapidly developing area of anticancer drug discovery (31, 179). Novel iron chelators derived from aroylhydrazones and thiosemicarbazones demonstrate selective antiproliferative activity against tumor cells (372). Selective anti-tumor activity of iron chelators derived from di-2-pyridylketone isonicotinoyl hydrazone has been attributed to induction of cellular oxidative stress by Fenton-type free radical generation, and the SAR of redox active dipyridyl-thiosemicarbazone chelators has been elucidated. The higher antiproliferative efficacy of dipyridyl-thiosemicarbazone chelators relative to the related isonicotinoyl hydrazone derivatives correlates with the redox potentials of their Fe complexes determining their ability to form ROS (179). The most effective anticancer iron chelators possess considerable lipophilicity and are charge neutral at physiological pH, allowing access to intracellular Fe pools. The iron chelator di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT; Fig. 4-22) displayed potent anticancer activity in a number of xenograft tumor models without induction of systemic iron depletion (372). Importantly, anticancer effects of intracellular iron chelation, in addition to its role in the targeted induction of cellular oxidative stress, may also originate from the inhibition of crucial iron-dependent cellular target proteins including ribonucleotide reductase and topoisomerase IIα (290). Recently, the iron chelator and inhibitor of ribonucleotide reductase, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (triapine; Fig. 4-23), has entered clinical trials as an anticancer agent. A multicenter Phase II trial of triapine and gemcitabine in advanced NSCLC with pharmacokinetic evaluation using peripheral blood mononuclear cells was finished recently (232). It was concluded that triapine did not enhance clinical response to gemcitabine in patients with prior exposure to gemcitabine for advanced NSCLC. Moreover, further development of triapine in lung cancer is compromised by adverse drug reactions including methemoglobinemia and hypoxia that seem particularly problematic in patients with reduced pulmonary reserves. In another Phase II trial of intravenous triapine for advanced pancreatic adenocarcinoma in both chemotherapy-naive and gemcitabine-refractory patients, no therapeutic benefit could be detected (233).
E. Di- and polysulfides: Varacin and diallyltrisulfide
The well-established versatility of sulfur-based redox chemistry observed under physiological conditions combined with an increasing body of evidence that demonstrates significant chemotherapeutic activity of compounds containing redox active di-and polysulfide-pharmacophores has stimulated considerable research activity in this area of anticancer redox drug discovery (166, 281).
Organic disulfides can undergo reductive clevage to two thiol-group containing moieties leading to the oxidation of the reducing reaction partner (e.g., two adjacent thiol residues in a protein forming a disulfide bridge). Moreover, disulfide exchange reactions can occur upon reaction of a disulfide with a nucleophilic thiol, where the attacking thiol forms a new disulfide bond and a thiol-moiety is released from the initial disulfide. These simple reactions can dramatically modulate the biological function of a target peptide or protein as exemplified by the redox chemotherapeutic PX-12, a disulfide-pharmacophore containing inhibitor of the redox factor thioredoxin (discussed in detail in Section III.C.1). Other important examples of redox active chemotherapeutic agents with an essential disulfide-pharmacophore are represented by the developmental drug NOV-002 (discussed in Section III.B) and the anticancer epipolythiodioxopiperazines chaetocin and gliotoxin (discussed in Section III.C.4).
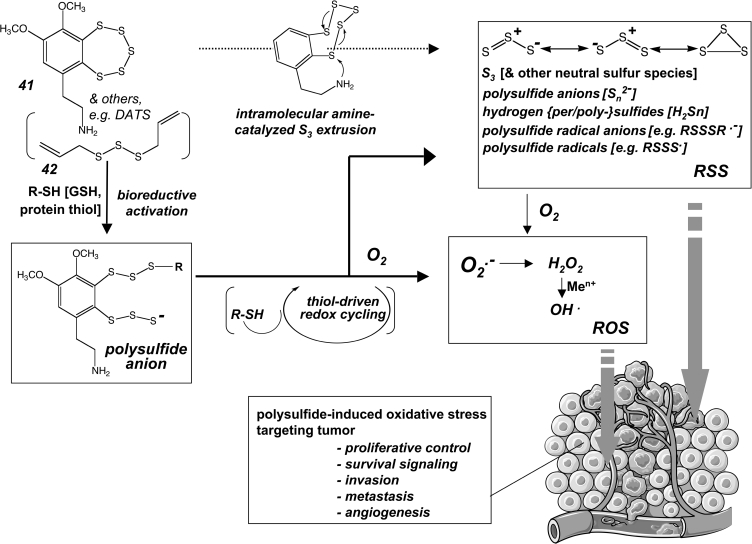
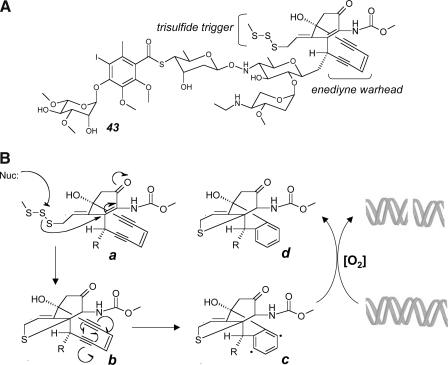
Polysulfide-based redox chemistry displays more complexity than disulfide-related reactivity, and accumulating experimental evidence suggets that polysulfide-based redox reactivity may serve as the crucial cytotoxic determinant of experimental anticancer agents, including varacin (Fig. 6-41) and diallyltrisulfide (Fig. 6-42) (57, 58, 166, 281). Moreover, polysulfide-related reactions may merely provide an upstream redox active switch that initiates a molecular rearrangement activating a cytotoxic pharmacophore as observed with the enediyne-tumor antibiotic calicheamicin γ1I (Fig. 7-43) (260).
FIG. 6.
Polysulfide drugs as prooxidant redox chemotherapeutics. Polysulfide-based redox reactivity is thought to underly the prooxidant cytotoxic activity of experimental anticancer agents including varacin (41) and diallyltrisulfide (DATS) (42). Bioreductive activation of polysulfide pharmacophores, through reaction with cellular thiols, leads to formation of strongly reducing polysulfide anion species upstream of ROS and reactive sulfur species (RSS) formation. RSS comprise a wide range of redox reactive molecules that also include neutral sulfur species such as S3. Polysulfide-dependent formation of ROS and RSS occurs in a quasi-catalytic redox cycle driven by cellular reducing thiols.
FIG. 7.
Free radical mechanism of DNA cleavage by the polysulfide derivative calicheamicin γ1I. (A) Calicheamicin γ1I (43) with enediyne warhead and trisulfide bioreductive trigger. (B) After intracellular bioreductive activation of the trisulfide trigger by formation of a thiolate intermediate (a), an intramolecular Michael adduction enables subsequent Bergman cycloaromatization of the enediyne warhead (b), leading to the generation of a highly reactive diradical intermediate (c) in close proximity to target DNA. Oxygen-dependent DNA cleavage is initiated by hydrogen abstraction, leading to the formation of an unreactive reaction product (d).
Polysulfide redox chemistry displays a wide range of chemical reactivity that can lead to bioreductive formation of superoxide radical anions after reaction of the polysulfide with a cellular thiol such as glutathione (Fig. 6). Remarkably, polysulfide-dependent formation of oxidizing species can occur in a quasi-catalytic redox cycle that will generate large amounts of ROS until reducing thiols that drive redox cycling are depleted (57, 58, 166). The reaction of polysulfides with glutathione or protein-bound thiols will generate mixed di- or polysulfides and additional reactive sulfur species (RSS) including hydropersulfide or hydropolysulfide species (R-SnH, n ≥ 2) that display enhanced acidity, nucleophilicity, and redox reactivity compared to the original thiol compound (R-SH). In particular, due to their increased acidity a large fraction of hydroper- and polysulfides occurs in the strongly reducing, anionic form (R-Sn-S−, n ≥ 1) under conditions of physiological pH. Moreover, formation of protein-bound mixed polysulfide species formed upon nucleophilic attack of protein-cysteine residues on polysulfide agents induces functional alterations of target proteins and could initiate the next cycle of ROS formation in close proximity to target proteins.
1. Calicheamicin γ1I
An early focus on polysulfide-based anticancer drug discovery was initiated by identification and subsequent structural and mechanistic studies of the polysulfide calicheamycin γ1I isolated from micromonospora echinospora (Fig. 7-43) (260). Calicheamicin γ1I is a potent genotoxic agent that induces oxidative DNA strand scission at pyrimidine-rich recognition sites. The trisulfide substituent serves as a bioreductive trigger activated through trisulfide-exchange reaction with reducing intracellular thiols after the compound has reached the intracellular compartment. Intramolecular Michael addition then induces cycloaromatization of the enediyne warhead, forming a highly reactive diradical species (1,4-dehydrobenzene) that abstracts hydrogen atoms from the sugar backbone of DNA, followed by oxidative strand cleavage. A calicheamycin antibody conjugate, gemtuzumab ozogamicin, contains a recombinant humanized anti-CD33 monoclonal antibody linked to calicheamicin (326). CD33 antigens are expressed on acute myelogenous leukemia (AML) cells and hematopoietic progenitor cells, but not on nonhematopoietic cells or pluripotent hematopoietic stem cells. Consequently, gemtuzumab selectively targets CD33-presenting cells and spares nonhematopoietic cells and stem cells from toxicity. In the United States, the drug is FDA-approved for use in patients with relapsed AML who are not considered candidates for standard chemotherapy.
2. Varacin and other polysulfides
Hydropolysulfide-dependent redox chemistry associated with formation of electrophilic ROS has been implicated in the cytotoxic mechanism of action of the anticancer agent varacin A (Fig. 6-41) (57, 58, 166). The marine natural product varacin is a benzopentathiepin-type pentasulfide, representative of a number of polysulfides isolated from natural sources including (I) the linear compounds allylmethyltrisulfide, 2-hydroxyethyltrisulfide, diallyltrisulfide (Fig. 6-42), diallytetrasulfide, and (II) the cyclic polythianes leptosins A, B, E, and F, sirodesmins B and C, lissoclinotoxin A (Fig. 4-24), and NN,dimethyl-5-(methylthio)varacin. This class of polysulfides capable of releasing ROS and RSS after bioreductive activation is associated with potent cytotoxic properties targeting bacteria, fungi, and cancer cells. Using the synthetically accessible prototype pharmacophore 7-methylbenzopentathiepin, it has been demonstrated that polysulfides, in the presence of reducing thiols and transition metal ions, are thiol-dependent DNA-cleaving agents, a reactivity dependent on formation of hydropolysulfides as crucial reactive intermediates and blocked in the presence of metal chelators and antioxidants (Fig. 6). It was also suggested that the positively charged aminoethyl substituent present in the natural benzopentathiepin derivatives may facilitate DNA-binding through electrostatic interactions and may also be involved in catalyzing the initial thiol attack that induces reductive opening of the polysulfur ring. Additional experimental evidence supports the formation of strongly electrophilic neutral sulfur species (Sn, 1 < n < 8) including S3, an isoelectronic analogue of the ROS ozone, extruded from polysulfide anion species, supporting the involvement of another class of RSS in polysulfide-induced cellular oxidative stress (136).
3. Leinamycin
The 1,2-dithiolan-3-one-1-oxide pharmacophore contained in the Streptomyces-derived experimental tumor antibiotic leinamycin (Fig. 4-25) is another thiol-activated pharmacophore capable of releasing a disulfide anion thought to induce the reductive formation of ROS involved in DNA cleavage by this highly cytotoxic redox chemotherapeutic. In addition to ROS formation, further rearrangement of bioreductively activated leinamycin generates an electrophilic episulfonium ion that can alkylate guanine residues in duplex DNA leading to the formation of abasic sites after spontaneous depurination of 7-alkylguanine bases (59, 166).
Even though the sulfoxide leinamycin displays impressive cytotoxicity in the low nanomolar concentration range directed against cancer cell lines, its potential for further development as anticancer chemotherapeutic beyond preclinical experimentation seems to be limited by the high general cytotoxicity associated with nonselective bioreductive activation of the alkylating pharmacophore. Research aiming at the develoment of leinamycin-derived molecules that display attenuated alkylating properties with retention of persulfide-dependent prooxidant reactivity has recently demonstrated that the nonsulfoxide leinamycin-derivative S-deoxyleinamycin containing the modified 1,2-dithiolan-3-one pharmacophore is markedly less cytotoxic than leinamycin but retains thiol-activated persulfide-dependent prooxidant activity inducing oxidative cleavage of target DNA (322). It has been suggested that the 1,2-dithiolan-3-one heterocycle may provide a new redox pharmacophore for targeted delivery of therapeutically active polysulfides, hydrogen sulfide, and ROS to the interior of cancer cells.
4. Diallyldisulfide and diallyltrisulfide
Diallyldisulfide (DADS) and diallyltrisulfide (DATS; Fig. 6-42), formed among other polysulfides as secondary products in mechanically processed garlic after allinase-catalyzed conversion of alliin (S-Allyl-L-cysteine sulfoxide) to the alkyl alkanethiosulfinate allicin (2-propene-1-sulfinothioic acid S-2-propenyl ester), have been studied extensively as dietary factors displaying significant chemopreventive and chemotherapeutic anticancer activity. The involvement of oxidative stress in anticancer action of these and other organosulfur compounds has recently been reviewed (11, 281). Rapid induction of cellular ROS formation and disruption of calcium homeostasis have been implicated in DADS- and DATS-induced cancer cell apoptosis. In human glioblastoma T98G and U87MG cells, diallylsulfide (DAS), DADS, and DATS induced ROS formation associated with increases in intracellular free calcium ions, expression of calreticulin, activation of caspase-4 indicative of endoplasmic reticulum stress, signaling through stress kinase pathways (p38 and JNK1), and activation of cysteine proteases including calpain, caspase-9, and caspase-3 (75). DADS-induced apoptosis in SH-SY5Y neuroblastoma cells, accompanied by ROS formation and oxidation of cellular lipids and proteins, was efficiently reversed by overexpression of CuZn-superoxide dismutase or pretreatment with the spin trap 5,5-dimethyl-1-pyrroline N-oxide (105). DATS has also been shown to induce superoxide-dependent G2-M arrest targeting cultured human DU145 and PC-3 prostate cancer cells associated with proteasomal ferritin degradation and relase of labile, Fenton-active iron ions (10). In this study, DATS-induced inhibition of G2-M cell cycle progression in human PC-3 and DU145 prostate cancer cells was shown to result from ROS-induced Ser216 hyperphosphorylation of the dual specificity phosphatase Cdc25C (discussed as an anticancer redox drug target in Section III.F). Ser216-phosphorylated Cdc25 undergoes 14-3-3 mediated cytoplasmic sequestration, resulting in blocked entry into mitosis due to maintained inactivating phosphorylation of the Cdc25 substrate complex Cdk1/cyclin B1.
Detailed target identification studies suggest a crucial involvement of covalent adduction and functional alteration of the microtubule component β-tubulin in the anticancer activity of DATS with specific oxidative modification of cysteine residues Cys-12β and Cys-354β with formation of a protein-bound S-allylmercaptocysteine resulting from a thiol–disulfide exchange reaction (153). These data suggest that pharmacological suppression of spindle dynamics blocking cell mitosis at the transition from metaphase to anaphase, an important mechanism of action of standard cancer chemotherapeutics including vinca alkaloids and paclitaxel, can also be achieved by the dietary prooxidant DATS. In a subsequent detailed SAR study, it was demonstrated that the alkenyl group is a necessary component of the pharmacophore responsible for disrupting the microtubule network in human HT29 colon cancer cells (154).
Anticancer activity of the experimental redox chemotherapeutics DADS and DATS In murine xenograft models has been widely documented (11, 281). DADS and DATS administered intraperitoneally or intragastrically suppressed growth of human HCT-15 colon tumor cell xenografts in athymic nude mice (153). DATS was active against PC-3 human prostate cancer xenografts associated with Bax and Bak induction, increased the effectiveness of TRAIL inhibiting prostate cancer growth in an orthotopic model, and prevented development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP (transgenic adenocarcinoma of mouse prostate) mice (313). Anti-tumor effects of hepatic targeted polybutylcyanoacrylate nanoparticles of DATS, prepared by an emulsion polymerization method with polybutylcyanoacrylate as carrier material, were demonstrated in a murine orthotopic transplantation tumor model of human hepatocellular carcinoma (400).
F. Isothiocyanate organosulfur agents: β-Phenylethylisothiocyanate
It is now widely accepted that preferential induction of apoptosis in aberrant cells contained in precancerous lesions is an important mechanism of chemopreventive activity during early stages of carcinogenesis, as reviewed extensively elsewhere (11, 143). Prooxidant biofactors that induce deviations from bioenergetic and redox homeostasis incompatible with rapid proliferation and survival signaling of malignant cells are therefore promising experimental chemopreventive agents; in addition, their apoptogenic activity also imparts direct chemotherapeutic efficacy as discussed below.
Isothiocyanates are electrophilic organosulfur compounds containing a reactive isothiocyanate (R-N = C = S) pharmacophore underlying the prooxidant and thiol-adducting reactivity of these molecules. Dietary isothiocyanates including sulforaphane (R-1-isothiocyanato-4-methylsulfinylbutane), β-phenyethyl-isothiocyanate (PEITC), benzyl-isothiocyanate, and 6-methylsulfinylhexyl-isothiocyanate are important chemopreventive factors that display preferential apoptogenicity towards premalignant and cancer cells associated with their pleiotropic prooxidant effects that involve ROS formation (189), mitochondrial functional impairment (384), adduction, export and depletion of cellular glutathione, oxidation of mitochondrial peroxiredoxin 3 (43), and adduction of cysteine-thiols in crucial target proteins, including β-tubulin and Keap1 (151, 250). In this context it is also important to note that anticancer activity of benzyl–isothiocyanate has recently been attributed to complex III inhibition of the mitochondrial respiratory chain upstream of ROS formation and apoptotic elimination of breast cancer cells (384), a molecular mechanism of action that links respiratory and functional mitochondrial impairment with ROS production (as discussed in Section IV.B.1). Recently, proteomic analysis has provided experimental evidence that relative tubulin binding affinity of isothiocyanate-electrophiles correlates with their potency of cell growth inhibition and apoptosis induction in A549 NSCLC cells (250).
It is well established that chemopreventive isothiocyanates activate the cellular electrophilic/oxidative stress response mediated by the redox-sensitive Nrf2/Keap1-ARE pathway that controls expression of many antioxidant and phase II-detoxification target genes thought to interfere with carcinogenesis (discussed as a redox drug target in Section III.D) (208). For example, it has been shown that sulforaphane acts through thionoacyl-adduction of Cys151 in Keap1 (Kelch-like ECH-associated protein 1), the negative regulator of Nrf2 (nuclear factor-E2-related factor 2), with formation of a thionoacyl-alkylation product that compromises Keap1 function (151). Interference with Keap1-dependent Nrf2-degradation by ubiquitination induces Nrf2 nuclear translocation followed by Nrf2-dependent transcriptional activation of target genes containing an antioxidant response element (ARE)-promotor sequence (208, 319, 362).
An increasing number of studies conducted in cell culture and animal models demonstrates that prooxidant chemopreventive factors containing electrophilic isothiocyanate and other pharmacophores including Michael acceptors may be useful as interventional redox chemotherapeutics, particularly at apoptogenic concentrations that exceed those needed to activate chemopreventive signaling through the Nrf2/Keap1-ARE pathway (143).
1. Sulforaphane
Sulforaphane-induced apoptosis associated with ROS formation has been observed in many cancer cell lines. Moreover, systemic administration of sulforaphane significantly inhibits growth of numerous murine xenograft models and is also the subject of early clinical trials for cancer chemoprevention, as recently reviewed (11, 71). Recent experimental evidence suggests that sulforaphane targets highly therapy-resistant pancreatic tumor-initiating cells by interference with NFκB-induced survival signaling (180). In a pancreas carcinoma xenograft model, sulforaphane blocked tumor growth and angiogenesis, and in combination therapy with TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) sulforaphane had an additive effect without cytotoxic effects on normal cells. Identification and synthesis of a chemical library of improved glucosinolate sulforaphane prodrugs for potential oral anticancer intervention has been described (245).
2. β-Phenylethylisothiocyanate
A recent landmark study suggests that isothiocyanate-induced cellular oxidative stress selectively kills oncogenically transformed cells, providing a striking example of a molecular Achilles heel presented only by oncogene-expressing cancer cells that display increased constitutive levels of oxidative stress and preferential vulnerability to prooxidant redox intervention according to the concept of synthetic lethality (176, 349, 350, 393). Oncogenic transformation of ovarian epithelial cells with H-Ras (V12) or expression of Bcr-Abl in hematopoietic cells caused elevated ROS generation and rendered the malignant cells highly sensitive to redox intervention using β-phenylethylisothiocyanate (PEITC). Excessive ROS formation and GSH depletion observed in oncogene-expressing cells exposed to PEITC was associated with oxidative mitochondrial damage and cardiolipin oxidation, inactivation of glutathione peroxidase, inhibition of basal and TNFα-stimulated NF-κB (p65/50) DNA binding activity, and massive nonapoptotic cell death. Importantly, PEITC exhibited chemotherapeutic activity in a human ovarian clear cell carcinoma xenograft model. In a related study, PEITC displayed strong cytotoxicity even in imatinib-resistant CML cells with T315I mutation associated with induction of oxidative stress and redox-mediated degradation of the BCR-ABL protein, leading to massive death of wild-type or mutant BCR-ABL-expressing leukemia cells (397). More detailed studies elucidating pharmacokinetics and potential off-target toxicity associated with isothiocyanate pharmacophore-containing biofactors are needed before these promising findings can be translated into trials that assess the potential use of organosulfur electrophiles for redox chemotherapeutic intervention in human cancer patients (11).
G. Electrophilic Michael acceptors: Parthenolide and neratinib
Selective prooxidant redox intervention that substantially increases cellular ROS and modulates specific redox sensitive targets can be achieved using small molecule electrophiles containing a Michael acceptor (α, β-unsaturated carbonyl) pharmacophore contained in numerous experimental anticancer agents such as parthenolide (Fig. 8A-45) (123), curcumin (Fig. 8B-47) (323), dimethylfumarate (225), inuviscolide (299), and cinnamic aldehyde (Fig. 8C-50) (47). Generally, Michael acceptors target thiol-group containing reaction partners through covalent adduction (thioalkylation), and Michael acceptor-induced redox alterations in target cells can therefore originate from glutathione adduction and ROS formation downstream of glutathione depletion. Moreover, Michael acceptor-dependent redox modulation can occur by covalent adduction of critical thiol residues in redox-sensitive target proteins that regulate celluar redox status such as components of the NFκB signaling or thioredoxin redox system. For example, the novel thioredoxin antagonist PMX464 can act as a dual-Michael acceptor that alkylates cysteine residues contained in the thioredoxin active site thereby inducing dramatic redox alterations in cancer cells (as discussed in Section III.C).
FIG. 8.
Michael acceptors as experimental redox chemotherapeutics. Selective prooxidant redox intervention through ROS upregulation and covalent modification of redox sensitive target proteins can be achieved using Michael acceptor pharmacophores contained in small molecule natural products and synthetic derivatives. (A) The sesquiterpene lactone parthenolide (45) represents a lead compound containing a Michael acceptor pharmacophore that imparts anticancer activity through covalent modulation of redox targets. Helenalin (44) represents a structural analogue with unfavorable toxicity profile associated with its dual Michael acceptor pharmacophore. The Michael-inactive prodrug dimethylamino-parthenolide (46) represents a clinical candidate with improved solubility, oral bioavailability, and pharmacokinetic profile. (B) The pleiotropic anticancer Michael acceptor curcumin (47) has served as a lead compound for the design of improved drug-like derivatives, including the bisbenzylidenecyclopentanone-derivative (48) and the fluorinated drug-like analogue EF24 (49). (C) Michael acceptor-induced upregulation of oxidative stress response gene expression in A375 melanoma cells. The dietary factor trans-cinnamic aldehyde (CA, 50) contains a Michael pharmacophore. CA, but not closely related derivatives devoid of Michael acceptor activity including DHCA (51), COH (52), and CAC (53), displays antimelanoma activity in vivo accompanied by induction of a complex oxidative stress response, as revealed by gene array analysis performed on cultured A375 melanoma cells (25 μM CA, 24 h; RT2 Human Oxidative Stress ProfilerTM PCR Expression Array technology) with upregulation of heme oxygenase-1 (HMOX1; 129-fold), glutathione peroxidase 2 (GPX2; sevenfold), sulfiredoxin 1 homolog (SRXN1; sevenfold), thioredoxin reductase 1 (TXNRD1; fourfold); early growth response gene 1 (EGR1; fivefold), DNA-damage-inducible transcript 3 (DDIT3; 13-fold), and cyclin-dependent kinase inhibitor 1A (CDKN1A; 11-fold) (modified with permission from (47)).
Much research has focused on Michael acceptor containing anticancer agents, sequiterpenelactones in particular, as reflected by abundant coverage in the research literature. It has to be mentioned that the SAR underlying cytotoxicity and potential anticancer activity associated with experimental agents that contain an electrophilic Michael acceptor functional group is often obscure and poorly understood. For example, isoobtusilactone A, a novel natural product butanolide-type α-methylene-lactone Michael acceptor, displays impressive cancer cell apoptogenicity and chemotherapeutic efficacy in a MDA-MB-231 breast cancer xenograft model, attributed to the rapid induction of oxidative stress and activation of the apoptosis signal-regulating kinase 1 (ASK1) upstream of c-Jun NH(2)-terminal kinase and p38 MAPkinase (201). Antioxidants decreased apoptosis by inhibiting ASK1 dephosphorylation at Ser-967 and by promoting interaction of ASK1 with thioredoxin or 14-3-3 proteins, and siRNA target modulation of ASK1 completely suppressed isoobtusilactone A-induced apoptosis. However, SAR and molecular target of isoobtusilactone A are unknown, and the mechanistic role of the Michael acceptor pharmacophore contained in this prooxidant chemotherapeutic is unresolved, a situation that applies to many oxidative stress inducing anticancer agents with Michael acceptor functional groups.
The following section will focus on a representative selection of experimental therapeutics that contain a Michael acceptor pharmacophore with established functional significance and are currently in preclinical and clinical phases of development for oncological indications.
1. Parthenolide
The sesquiterpene lactone parthenolide (Fig. 8A-45), a natural product isolated from feverfew (Tanacetum parthenium), contains an electrophilic α-methylene-γ-lactone pharmacophore thought to be the crucial determinant of anticancer activity associated with this Michael acceptor electrophile. Interestingly, the structurally related sesquiterpene lactone helenalin (Fig. 8A-44), a bisfunctional Michael acceptor, displays unacceptable systemic toxicity associated with a potential for bifunctional target alkylation and crosslinking. It is now established that parthenolide is a potent inhibitor of the NFκB signaling pathway in cancer cells where ROS-dependent constitutive activation of NFκB transcriptional activity is involved in tumor progression through upregulation of anti-apoptotic genes (including Bcl-2, Bcl-xL, survivin, XIAP) and the chemoresistance-conferring transporter P-glycoprotein (mdr1) (349). Parthenolide targets the DNA binding domain of p65 by covalent cysteine adduction (Cys 38) (123). Moreover, parthenolide shows inhibitory activity on IκB degradation through inhibition of IKKβ, inactivated through Michael addition reaction between parthenolide and Cys179 of IKKβ (203). Parthenolide administered as single or combinatorial agent displays potent anticancer activity in numerous xenograft models (267, 334).
Parthenolide lead optimization has led to the identification of an orally available derivative with improved pharmacokinetic profile and 1,000-fold increased water solubility, dimethylamino-parthenolide (DMAPT, LC-1; Fig. 8A-46), that selectively eradicates acute myelogenous leukemia stem and progenitor cells (142). Remarkably, DMAPT most likely represents a Michael inactive prodrug that regenerates the electrophilic Michael pharmacophore after systemic uptake and spontaneous dimethylamine elimination. DMAPT induced death of primary human AML, ALL, and CML cells, but not normal hematopoietic cells, and DMAPT-based chemotherapy, associated in vivo with induction of an oxidative stress response, inhibition of NFκB, and activation of p53, displayed activity in mouse xenograft models and spontaneous acute canine leukemias (142). Using global gene expression analysis of CD34+ primary AML specimens, strong upregulation of Nrf2 and its transcriptional target genes heme oxygenase 1 (HO-1) were detected in response to parthenolide treatment, suggesting that expression analysis of these genes may serve as a potential biomarker for parthenolide-based drug responses in tumors.
2. Curcumin
Another natural product containing an electrophilic Michael acceptor-pharmacophore is the dietary factor curcumin (diferuloylmethane; Fig. 8B-47). This multi-pharmacophore bearing natural product, containing β-dicarbonyl, α,β-unsaturated carbonyl, and phenolic substructures, is a classic pleiotropic redox modulator displaying reactivity as pro- and antioxidant, metal chelator, and electrophilic Michael acceptor, as recently reviewed (227). Many studies have documented curcumin chemopreventive and chemotherapeutic activity observed in numerous cell culture and animal systems as a function of these reactive pharmacophores modulating various molecular targets, including thioredoxin reductase, NFkB, AP1, and topoisomerase II. Moreover, curcumin can also modulate molecular targets through ligand activity (227). Multiple p53–dependent and -independent pathways of curcumin-induced cancer cell death have been elucidated, and mitochondrial release of proapoptogenic factors including cytochrome c and AIF as well as the activation of the extrinsic Fas receptor/caspase-8 death pathway have been observed in response to curcumin treatment (46, 184, 314). Recently, Michael acceptor-based covalent adduction and inactivation of thioredoxin reductase 1 at cysteine 496 and selenocysteine 497 by curcumin have been demonstrated (99), and based on detailed SAR studies more potent simplified Michael acceptor inhibitors of thioredoxin reductase 1 derived from the curcumin structural scaffold including a bisbenzylidenecyclopentanone derivative (Fig. 8B-48) have been designed (223). EF24 {3,5-bis(2-fluorobenzylidene)piperidin-4-one; Fig. 8B-49}, a drug-like fluorinated Michael active analogue of curcumin, displays potent apoptogenicity associated with induction of cellular oxidative stress in vitro and in vivo (3).
A recent phase II clinical trial has examined curcumin chemotherapeutic efficacay in pancreatic cancer (80). Out of 21 evaluable patients, an anti-tumor response was observed in only two patients, a response rate that may originate from low bioavailability. However, given the lack of efficacious chemotherapeutic agents that provide therapeutic benefit in patients with advanced pancreatic cancer, even this moderate response rate led to the suggestion that curcumin derivatives with more favorable bioavailability may be attractive drugs targeting pancreatic cancer. Anticancer chemotherapeutic efficacy of curcumin as single or combinatorial agent is currently evaluated in numerous clinical trials, including multiple myeloma (ClinicalTrials.gov Identifier: NCT00113841), rectal cancer (ClinicalTrials.gov Identifier: NCT00745134), metastatic colon cancer (ClinicalTrials.gov Identifier: NCT00295035), advanced osteosarcoma (ClinicalTrials.gov Identifier: NCT00689195), and pancreatic cancer (ClinicalTrials.gov Identifier: NCT00192842).
3. Cinnamaldehyde
Prototype Michael acceptors of the α,β-unsaturated aldehyde-class including acrolein, crotonaldehyde, and trans-4-hydroxy-2-nonenal display very high electrophilicity and chemical reactivity that are associated with cytotoxicity, carcinogenic potential, and an acute toxicity profile unacceptable for controlled systemic delivery and therapeutic testing in vivo (54). Interestingly, it has been shown that acrolein targets and inactivates the p50 subunit of NFκB by covalent modification of Cys-61 and Arg-307, two amino acids residing in the DNA binding domain, suggesting a therapeutically useful activity of related Michael acceptors with attenuated reactivity and improved safety profile (206).
In search for a drug-like Michael acceptor with potential anticancer activity and established animal pharmacokinetics devoid of dose-limiting systemic toxicity, recent studies have focused on the acrolein-derivative trans-cinnamic aldehyde (cinnamaldehyde, trans-3-phenylpropenal, CA; Fig. 8C-50) (47, 152, 169, 380). CA contains a Michael acceptor pharmacophore that displays attenuated reactivity towards thiol adduction due to aromatic conjugation with the adjacent benzene ring. CA spontaneously forms covalent adducts with model thiols and activates Nrf2-regulated antioxidant response element (ARE)-mediated gene expression (380). Nonaldehyde cinnamoyl derivatives with further attenuated Michael acceptor reactivity have been designed serving as noncytotoxic experimental Nrf2 activators for skin cell protection against photooxidative stress (380). Remarkably, CA, but not closely related CA-derivatives devoid of Michael acceptor activity, impairs melanoma cell proliferation, viability, invasiveness, and NFκB transcriptional activity, and high oral doses of CA display significant antimelanoma activity in vivo with induction of a complex oxidative stress gene expression response (Fig. 8C) (47).
The molecular targets of the CA derivative 2′-hydroxycinnamaldehyde (HCA), a compound that displays anticancer activity in murine xenograft models, were elucidated recently by proteomic analysis of a biotinylated HCA derivative (biotin-HCA) revealing five different proteasomal subunits as predominant protein targets modified by covalent adduction (152). Therefore, it was proposed that HCA- and CA-associated proteasomal alterations interfere with IκB degradation, thereby antagonizing NFκB transcriptional activity. Optimization of the lead compound HCA has led to the identification of novel experimental chemotherapeutics with potent prooxidant apoptogenic activity as demonstrated in a human colon tumor model in nude mice (169).
4. Neratinib
Lead optimization of Michael acceptor pharmacophore-containing drug candidates aims at attenuating electrophilic reactivity associated with unselective adduction of protein targets and general cytotoxicity. Additionally, dramatic improved target selectivity can be achieved by attaching an attenuated Michael acceptor pharmacophore to a ligand moiety that guides the electrophilic pharmacophore to a specific binding site and a particular target cysteine residue as illustrated by the development of the investigational cancer chemotherapeutic neratinib (376, 398). Even though this ligand-guided electrophilic Michael acceptor pharmacophore exerts anticancer activity independent of modulation of cancer cell redox regulation, the molecular design of neratinib provides a prototype approach for the future development of redox chemotherapeutics with reactive pharmacophores that display high target selectivity through ligand-based interactions.
Based on the rapidly emerging complexity and causative involvement of dysregulated kinase signaling in tumorigenesis, small molecule inhibitors of oncogenic kinases are now an important class of novel cancer chemotherapeutics (398). As mentioned above, Inhibition of the tyrosine kinase activity of the oncogenic BCR-ABL1 fusion protein by imatinib, the first clinically available member of this novel class of therapeutic agents, represented a breakthrough in the treatment of CML and has set an early precendent for the structure-based design and development of molecularly-targeted cancer chemotherapeutics. Guided by the prospect of generating drugs that target cancer cells with superior potency and selectivity and driven by insufficient efficacy or rapid emergence of resistance associated with available kinase inhibitors, this class of cancer chemotherapeutics has undergone rapid expansion with over 10 different agents available in the clinic by early 2009 and numerous molecules in advanced clinical trials (376, 398).
The clinically available anilinoquinazoline drugs gefitinib (Fig. 9A-54) and erlotinib (Fig. 9A-55) are ATP-competitive inhibitors of EGFR kinases, overexpressed or mutated in many cancer types (376, 398). These receptor tyrosine kinases are composed of an extracellular ligand-binding domain, a single membrane-spanning region, and intracellular tyrosine kinase and regulatory domains. Recently, the structure-based design of small molecule kinase inhibitors containing an electrophilic Michael acceptor pharmacophore has been described, and lead compounds that act as suicide ligands of the ErbB family of receptor tyrosine kinases are now undergoing phase II clinical evaluation targeting NSCLC and breast cancers (351, 376, 377).
FIG. 9.
Neratinib and peltinib: ligand-guided Michael acceptors targeting a specific cysteine residue in oncogenic tyrosine receptor kinases. (A) ATP-competitive inhibitors of EGFR kinases such as the established anilinoquinazoline drugs gefitinib (54) and erlotinib (55) are ligands that inactivate receptor tyrosine kinases through reversible binding. (B) The 4-anilino-3-cyano quinoline derivatives neratinib (56) and peltinib (57) are investigational receptor tyrosine kinase inhibitors that act as irreversible suicide ligands of ErbB-1 and ErbB-2, combining ligand-based selectivity (ATP-competitive ligand moiety) with irreversible target inactivation through covalent adduction by an electrophilic pharmacophore (Michael acceptor moiety). (C) A conserved cysteine residue (Cys-805 in HER-2) contained in the ATP active site attacks the 4-(dimethylamino)crotonamide Michael acceptor pharmacophore (blue) of the suicide ligand neratinib facilitated by intramolecular amine catalysis. (D) Model of neratinib (Michael acceptor moiety in blue, covalently bound to Cys-805 (thiol in yellow) in the ATP active site of the HER-2 kinase catalytic domain (ATP-competitive ligand moiety in red). (Panel D adapted from (376) with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In an attempt to develop improved inhibitors of the epidermal growth-factor receptor [EGFR (ErbB-1)] and human epidermal growth factor receptor-2 (HER2 (ErbB-2)], 4-anilino-3-cyano quinoline derivatives were recently designed to irreversibly inhibit the kinase domain, a valid strategy for overcoming drug resistance and binding competition due to high intracellular concentrations of ATP associated with reversibly binding ligands. Structure- and reactivity-based strategies led to the development of two investigational drugs, HKI-272 (neratinib; Fig. 9B-56) and EKB-569 (peltinib; Fig. 9B-57), that inhibit the function of the kinase domains of these proteins by forming a covalent bond with a conserved cysteine residue, Cys-773 (or Cys-797) and Cys-805 in EGFR and HER-2, respectively (351, 376, 377). The suicide ligands neratinib and peltinib contain a Michael-acceptor pharmacophore with attenuated electrophilic reactivity [4-(dimethylamino)crotonamide] that assures target-specific activation by intramolecular amine catalysis of a Michael reaction facilitated by target binding (Fig. 9C and D). Thus, EGFR/HER-2-target inactivation by these 4-anilino-3-cyanoquinoline derivatives occurs as a result of structure-based target binding and reactivity-based target-adduction. This novel class of electrophilic kinase inhibitors that target the chemical reactivity of a specific cysteine residue (Cys-773) in the ATP binding region of the active site illustrates a specific anticancer drug design strategy that combines ligand-based selectivity with the attenuated reactivity of an electrophilic warhead pharmacophore. This approach avoids toxicity due to untargeted reactivity, a limitation associated with many reactivity-based pharmacophores contained in redox chemotherapeutics.
Promising clinical interim results have been obtained with HKI-272 and EKB-569 in human colon, lung, and breast cancers; antitumor activity of these inhibitors in NSCLC patients is enhanced by their potential to overcome drug resistance observed with gefitinib and erlotinib, as recently reviewed (376).
H. Sacrificial antioxidants: L-ascorbate
Consistent with recently performed genetic target validation studies that demonstrate the significant tumor suppressive effects of overexpression of cellular antioxidant enzymes including catalase and SOD (108, 371), sacrificial and catalytic antioxidants may display potent anticancer activity in cellular systems and xenograft models. Pro- and antioxidant pharmacodynamic effects observed with small molecule SOD mimetics will be discussed in the context of anticancer intervention targeting the SOD system (Section III.A.2). Sacrificial antioxidant agents, including amifostine [2-(3-aminopropylamino)ethylsulfanyl phosphonic acid] and MESNA (sodium 2-sulfanylethanesulfonate), are important palliative drugs, FDA approved for the suppression of free radical-induced chemotherapy- and radiation-associated collateral tissue damage from oto-, cardio, and nephrotoxicity (65). However, modest or negligible chemotherapeutic efficacy has been observed with sacrificial antioxidants, including NAC, tiron (4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt), and sodium thiosulfate (Na2S2O3) tested in murine xenograft models, as discussed in detail elsewhere (48).
Anticancer activity associated with oral administration of the prototype antioxidant vitamin and reducing agent ascorbic acid (Fig. 4-26) is generally disappointing. However, recent preclinical animal studies suggest a significant anticancer activity of infusional ascorbate delivered at very high doses that raise plasma concentrations to millimolar levels not achievable by oral administration (62–64). It was demonstrated that pharmacological doses of ascorbate exert prooxidant activity generating H2O2-dependent cytotoxicity in a variety of cancer cell lines in vitro, without adversely affecting normal cells including lymphocytes, monocytes, and fibroblasts. The semidehydroascorbyl free radical and H2O2 formed selectively within tumor interstitial fluid as assessed by real-time parenchymal microdialysis performed upon acute parenteral administration of ascorbate in mice bearing glioblastoma xenografts (64). Upon chronic pharmacological treatment, tumor growth rates were significantly reduced in ovarian, pancreatic, and glioblastoma murine xenografts. In a number of ongoing exploratory Phase I clinical trials in human cancer patients (ClinicalTrials.gov Identifier: NCT00284427, NCT00441207, and NCT00626444) infusional ascorbate is administered at doses that achieve millimolar peak plasma concentrations (327). Paradoxically, anticancer activity of very high doses of intravenous ascorbate is most likely based on prooxidant effects via H2O2 formation through autoxidation that occurs in the extracellular fluid but not in whole blood, indicating that ascorbate at chemotherapeutic concentrations in blood serves as a prodrug for H2O2 delivery to tissue (62–64). It is also possible that, after spontaneous oxidation of ascorbate to the semidehydroascorbyl free radical, chemical dismutation generates ascorbic acid and dehydroascorbic acid, the oxidized form of ascorbate actively transported into cells and transformed to ascorbic acid in a reductive enzymatic process that is glutathione-dependent and may therefore induce cellular glutathione depletion (367). Indeed, ascorbic acid-induced glutathione depletion enhances As2O3 induced apoptosis in animal models of lymphoma and leukemia, and the minimal toxicity of infusional ascorbic acid compares favorably with other glutathione-depleting agents discussed below (18, 135). On the basis of these promising preclinical data, various Phase I/II clinical trials examine ascorbic acid potentiation of As2O3 chemotherapeutic effects (56), for example, in the treatment of adult non-APL acute myelogenous leukemia (AML) (ClinicalTrials.gov Identifier: NCT00184054). In addition to prooxidant potentiation based on glutathione depletion, anticancer activity of ascorbic acid observed in animal tumor models may also result from its ability to reductively drive redox cycling of cytotoxic quinone species, including the experimental anticancer quinone menadione (358), providing an example of antioxidant-dependent reductive induction of oxidative stress, a paradoxical prooxidant effect observed with many reducing antioxidants.
III. Molecular Targets for Anticancer Redox Chemotherapy
Rapid progress in the areas of fundamental cancer and redox biology, combined with the increasing availability of powerful drug discovery tools that allow rapid genetic and pharmacological target validation and lead compound identification, has generated an ever increasing number of valid molecular cancer targets amenable to redox intervention, as highlighted in the following section (48).
A. Targeting the SOD system
Modulation of SOD enzymatic activity for anticancer intervention is a multifaceted therapeutic approach that can be based on both pharmacological down- or upregulation of SOD activity, and many studies have provided evidence that pharmacological agents that either inhibit or mimic SOD activity are promising redox chemotherapeutics.
1. SOD inhibitors: ATN-224
Consistent with the established role of SOD1 and 2 in cellular antioxidant defense, pharmacological inhibition of cellular SOD activity may enhance oxidative stress and induce cytotoxic effects preferentially in cells already exposed to high levels of ROS from endogenous sources such as mitochondrial leakage and NAD(P)H oxidases (34, 117). Consistent with this hypothesis, it has been observed that SOD1 knockdown using RNAi induces senescence in untransformed fibroblasts, whereas the same intervention causes cell death in cancerous cells (35). Moreover, targeting Mn-SOD (SOD2) using antisense oligodeoxynucleotides sensitizes melanoma cells to tumor necrosis factor-alpha-induced mitochondrial apoptosis in a murine B16 metastasis model (25). Paradoxically, pharmacological inhibition of the antioxidant enzyme SOD1 can also result in suppression of prooxidant proliferative and survival signaling since formation of the SOD reaction product H2O2, an essential prooxidant signaling molecule in cancer cells, will be impaired by SOD1 inhibition. Indeed, in A431 and other cancer cells SOD1-derived H2O2 oxidizes and thereby inactivates redox-sensitive thiol residues in the active sites of tumor-suppressive tyrosine phosphatases that anatagonize signaling in response to tumor-associated growth factors such as EGF, IGF-1, PDGF, and VGEF, providing another rational for anticancer intervention based on pharmacological inhibition of SOD1 (174).
a. TETA
Recent research suggests therapeutic efficacy of targeting SOD1 for anticancer intervention based on copper chelation. For example, cisplatin sensitization of human ovarian cancer cells by triethylenetetramine (TETA) has been attributed to inhibition of SOD1 enzymatic activity (41), but anticancer effects of this copper (II) ion chelator may originate from off-target activities associated with this pleiotropic agent, including general depetion of cellular copper and telomerase inhibition by G-quadruplex binding (224).
b. ATN-224
The bis-choline salt of tetrathiomolybdate (ATN-224; Fig. 4-27), a copper chelator with antiangiogenic activity, specifically targets and inactivates the CuZn-enzyme SOD1 in tumor and endothelial cells leading to elevation of intracellular superoxide levels and induction of tumor cell apoptosis, as demonstrated in various cellular and animal models (173). In cellular studies it has been shown that SOD1 inhibition by ATN-224 is accompanied by attenuation of growth factor signaling thought to originate from protection of protein phosphatases such as the EGFR antagonist PTP1B against SOD1-controlled oxidative inactivation by H2O2 (174). Interestingly, A549 cells expressing constitutively activated Ras associated with highly upregulated SOD-independent ROS production were resistant to ATN-224. Thus, ATN-224 anticancer effects seem to originate from a complex pattern of redox alterations including prooxidant (increased levels of superoxide radical anion) and antioxidant (reduced levels of H2O2) effects.
Suppression of angiogenesis and tumor growth observed with ATN-224 can be causatively attributed to SOD1 inhibition, since effects of ATN-224 on endothelial and tumor cells are substantially reversed using the SOD mimetic MnTBAP (173). Moreover, ATN-224 selectively induces apoptosis in tumor cells but targets endothelial cells only by inhibition of proliferation, thus affecting tumor cell survival and angiogenesis in different but potentially synergistic ways. However, anticancer activity of this SOD1 inhibitor may at least in part originate from copper depletion known to exert antiangiogenic and antiprolferative effects, and systemic copper depletion has indeed been observed in a recent Phase I study that examined pharmacokinetic profile and dose-limiting toxicity in patients with advanced solid tumors (228). However, in a recent biomarker study in mice and macaques aiming at the identification of clinical routine parameters that indicate antiangiogenic and antitumor activity of ATN-224, it was found that inhibition of angiogenesis occurred before a measurable decrease in systemic copper as followed by tracking ceruloplasmin, a biomarker for systemic copper (87). In the same study, a rapid drop in blood cell SOD1 activity was observed upon ATN-224 exposure, supporting the importance of SOD1 inhibition as an early pharmacodynamic effect of this investigational cancer drug.
After successful completion of a Phase I trial (228), ATN-224 is currently completing various advanced clinical trials, including a multicenter Phase II study to evaluate the safety and efficacy of oral ATN-224 plus temozolomide in patients with advanced melanoma (ClinicalTrials.gov Identifier: NCT00383851), a multicenter Phase II study of the safety and efficacy of two dose levels of oral ATN-224 in patients with prostate cancer (ClinicalTrials.gov Identifier: NCT00405574), and a Phase II trial using exemestane with or without ATN-224 in treating postmenopausal women with recurrent or advanced breast cancer (ClinicalTrials.gov Identifier: NCT00674557).
c. 2-Methoxyestradiol
Interestingly, the cancer cell-selective apoptogenicity of estrogen derivatives including 2-methoxyestradiol (2-ME; Fig. 4-28) against human leukemia and ovarian carcinoma cells has been attributed to their ability to serve as specific inhibitors of SOD1, leading to free radical-mediated damage to mitochondrial membranes followed by release of cytochrome c (156). Among structurally related estrogen derivatives, 2-ME, 2-hydroxyoestradiol, and 2-methoxyoestrone substantially inhibited SOD activity, whereas 17-β-estradiol and estrone showed minimal activity against SOD, indicating a structural requirement for a 2-OH or 2-OCH3 substituent. However, a subsequent study has questioned the specific inhibitory activity of 2-ME on SOD1, and anticancer activity of the improved derivative 2-methoxyestradiol-3,17-O,O-bis-sulfamate (STX140) was attributed to microtubular disruption (175, 336). Anticancer efficacy of a 2-methoxyestradiol nanocrystal colloidal dispersion is currently evaluated in a series of Phase II clinical trials targeting metastatic renal cell carcinoma (ClinicalTrials.gov Identifier: NCT00444314) and relapsed or plateau phase myeloma (ClinicalTrials.gov Identifier: NCT00592579).
2. SOD mimetics: Mangafodipir
Recent experiments using various cellular transfection systems have demonstrated that SOD, both the mitochondrially located Mn-SOD and the cytosolic CuZn-SOD, inhibits cancer cell growth in mouse xenograft tumor models (371). Importantly, adenovirus vector-mediated CuZnSOD- or MnSOD-gene transfer via intratumoral injection of the adenoviral vector in nude mice bearing human pancreatic tumor xenografts resulted in tumor growth suppression not observed in the control group treated with empty adenovirus vector only, providing a prototype example of redox directed anticancer gene therapy (340). Based on this stringent genetic target validation, small molecule SOD mimetics, antioxidant catalysts that display SOD activity and combine catalytic efficacy of the enzyme with favorable drug-like properties of small molecules, are promising anticancer agents (48). Indeed, antioxidant antiproliferative intervention using small molecule SOD mimetics may be based on downregulation of cellular levels of mitogenic superoxide levels. Moreover, it also possible that pharmacological agents that act as SOD mimetics exert prooxidant cytotoxic effects through increased generation of H2O2, which may occur in cells with insufficient peroxide metabolism, providing a rational for prooxidant cytotoxic intervention using small molecule SOD-mimetics.
Due to the established involvement of superoxide in various disease states associated with redox dysregulation and increased oxidative stress, small molecules with SOD mimetic activity are attractive therapeutics, and discovery and development of small molecule SOD mimetics is an area of intense investigation. Small molecule mimetics of SOD, including nonmetal based nitroxide free radicals and metal-based agents such as manganese(III) salens, manganese(III) meso-porphyrins, and copper(II) diisopropylsalicylate, are more potent than regular chemical antioxidants and may circumvent the pharmacokinetic difficulties associated with a SOD-protein drug, including deliverability, inaccessibility of intracellular targets, chemical stability, and immunogenicity. SAR and developmental aspects of drug-like SOD mimetics have been discussed in detail elsewhere (48). It should be mentioned that small molecule catalase mimetics are another important class of catalytic antioxidants with emerging therapeutic applications in the nononcological areas of anti-inflammatory, neuro-, and cardioprotective intervention, as reviewed recently (77). Remarkably, most small molecule catalase mimetics such as the salen EUK-134 display dual SOD- and catalase-activity and will therefore not be covered separately (83).
a. M40403
In humans, the macrocyclic manganese-based SOD mimetic M40403 (Fig. 4-29) has shown efficacy in the palliative management of radiation therapy-induced side effects, and in 2008 the FDA has granted orphan drug designation for M40403 for prevention of radiation- or chemotherapy-induced oral mucositis in cancer patients. Moreover, M40403 has shown promise as a cotreatment for prevention of side effects associated with interleukin-2 (IL-2), an immune-stimulating cytokine drug that is approved for use in metastatic melanoma and renal cell carcinoma (306).
In addition to using SOD mimetics as combinational antioxidants for the management of oxidative stress-related side effects of chemotherapy, recent evidence suggests that coadministration of SOD mimetics with cytotoxic anticancer agents enhances their prooxidant activity, since SOD-derived H2O2 may preferentially target rapidly dividing cancer cells with compromised peroxide metabolism and high levels of endogenous oxidative stress (7, 210).
b. Mangafodipir
Mangafodipir [N,N′-bis(pyridoxal-5-phosphate)ethylenediamine-N,N′-diacetic acid: Fig. 4-30], a chelate of manganese (II) and the vitamin B6-derived dipyridoxyl diphosphate ligand fodipir, is an approved clinical contrast agent for magnetic resonance imaging (MRI) of the liver. As a potent antioxidant, mangafodipir limits oxidative tissue damage and protects normal liver cells against ROS-induced apoptosis. Experimental combination therapy using this SOD mimetic improves the therapeutic index of anticancer drugs by both protecting normal cells and increasing antitumoral activity, leading to decreased hematotoxicity and enhanced cytotoxicity of anticancer agents as demonstrated recently in a tumor xenograft model (7). Mangafodipir has catalase, SOD-, and glutathione reductase-like properties, suggesting a multifunctional interference with cancer cell redox homeostasis.
c. cis-FeMPy2P2P
The SOD mimetic cis-FeMPy2P2P {([5,10-bis(N-methyl-4-pyridyl)-15,20-diphenyl]porphinato-iron(III)} displays strong prooxidant apoptogenicity against various cancer cell lines that inversely correlates with cellular total SOD enzymatic activity. Moreover, genetic antagonism of SOD1 expression using antisense technology sensitized cells towards cis-FeMPy2P2P cytotoxicity (12). Further studies suggest that rather than behaving as an SOD mimetic, cis-FeMPy2P2P generates superoxide through cytochrome P450-reductase driven redox cycling in cancer cells, a prooxidant reactivity documented earlier for cationic manganoporphyrins.
d. MnTBAP and others
SOD mimetics, including copper(II) diisopropylsalicylate and manganese(III) tetrakis-(5,10,15,20)-benzoic acid porphyrin (MnTBAP), are able to potentiate the cytotoxicity of anticancer drugs by increasing intracellular levels of H2O2 (210). The SOD mimetic Mn(III)tetrakis(N-ethylpyridinium-2-yl)porphyrin [Mn(III)TE-2-Py(P5+)] has been shown to protect normal tissue from radiation injury and also enhances tumor radio-responsiveness. Indeed, various rodent tumor and endothelial cell lines exposed to MnTE-2-PyP(5+) displayed enhanced radiosensitivity in vitro, and combined treatment with radiation and MnTE-2-PyP(5+) achieved synergistic tumor devascularization in vivo and also resulted in synergistic tumor growth delay.
e. TEMPO and others
Small molecule nitroxides are stable free radicals able to scavenge radicals and act as radical chain terminators. The SAR of nonmetal nitroxide-based catalytic antioxidants is well understood and many prototype agents amenable to lead optimization have been synthesized (200). Based on one-electron redox cycles, which allow the reversible formation of the oxoammonium cation or the hydroxylamine, nitroxides can act catalytically as SOD and catalase mimetics. Antioxidant activity can also be based on the hydroxylamine form of this triple state redox sytem that acts as potent reducing agent. Selective cytotoxicity of the nitroxide TEMPO (2, 2, 6, 6-tetramethyl-piperidine-1-oxyl; Fig. 4-31) against various cancer cell lines is well documented and the chemotherapeutic efficacy of TEMPO and the TEMPO-derivative 4-ferrocenecarboxyl-2,2,6,6-tetramethyl piperidine-1-oxyl (FC-TEMPO) has been demonstrated in various xenograft models including human hormone-dependent and -independent prostate carcinoma (333). Delayed onset of thymic lymphoma was observed in Atm(−/−) mice using the nitroxide antioxidant 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO) (138).
B. Targeting the glutathione redox system: Imexon and NOV002
The cellular glutathione-based antioxidant redox system is a major determinant of the cellular redox status (40, 76, 218, 310). Millimolar intracellular concentrations of reduced glutathione are maintained by pentosephosphate shunt-dependent NADPH generation linking biosynthesis of nucleic acid pentoses, glucose energy metabolism, and cellular thiol redox status. This central redox system requires glutathione reductase-dependent regeneration of glutathione by NADPH that allows metabolism of electrophilic species and ROS by enzymes such as glutathione peroxidases, glutaredoxins, and glutathione transferases. Moreover, glutathione-dependent redox signaling can also involve covalent target modification (e.g., by S-glutathionylation), affecting signaling through key pathways including PKA, PKC, ASK1, and NFκB involved in cancer cell proliferation and survival (40, 76, 218).
Pharmacological or genetic disruption of crucial molecular components of the glutathione redox system induces oxidative stress through impairment of intracellular metabolism of H2O2 and lipid peroxides and may also weaken the cellular defense to NO and associated RNS. Importantly, this intervention weakens the cellular defense against other electrophilic species including chemotherapeutic agents, a promising therapeutic strategy for overcoming tumor chemoresistance often associated with upregulation of the glutathione redox system that occurs as a consequence of an adaptive or genetic upregulation of the Nrf2/Keap1-ARE signaling pathway (208).
1. NOV-002
Experimental evidence obtained in preclinical and clinical studies demonstrates that pharmacological administration of the oxidized form of glutathione, glutathione disulfide (GSSG), exerts significant anticancer activity based on modulation of cellular redox homeostasis, as reviewed recently (346, 347). A complex of glutathione disulfide with cis-platinum at an approximate ratio of 1000:1 (NOV-002) has shown clinical efficacy in combination with chemotherapy in the treatment of NSCLC, a significant redox chemotherapeutic intervention currently being evaluated in an ongoing pivotal Phase III trial that builds on earlier studies that indicated therapeutic benefit of NOV-002 codaministration in patients with advanced NSCLC receiving cisplatin and paclitaxel standard chemotherapy (ClinicalTrials.gov Identifier: NCT00347412). In HL-60 human leukemia, NOV-002 or GSSG administered in the absence of cisplatin induced a significant prooxidant deviation from redox homeostasis based on increased intracellular levels of H2O2, a decreased GSH:GSSG ratio, and extensive S-glutathionylation of intracellular proteins, including actin associated with a changed cytoskeletal morphology (346). Moreover, GSSG-induced protein S-glutathionylation was accompanied by activating phosphorylation of the MAPK pathway kinases JNK, ERK, and p38, consistent with earlier work on MAPK activation by GSSG administration. In promonocytic U937 cells, GSSG administration resulted in pro-oxidant activation of the p38 MAPK apoptotic pathway (106), and superoxide-induced JNK-dependent apoptosis was triggered by GSSG in human SH-SY5Y neuroblastoma cells previously depleted of GSH (104). Extensive oxidation of cell surface protein thiols resulted from NOV-002 and GSSG treatment, suggesting that cell surface proteins are primary targets of the cell-impermeable disulfide GSSG in cancer cells, specifically the enzymes γ-glutamyl transpeptidase, involved in enzymatic production of H2O2 and S-thiolation (86), and protein disulfide isomerase (PDI), an important redox modulator of cancer cell adhesion and invasiveness located in cytoplasmic and endoplasmatic membranes (132).
2. Imexon
The electrophilic cyanoaziridine-derivative imexon (4-imino-1,3-diazabicyclo-[3.1.0]hexan-2-one; Fig. 4-32) is another prooxidant agent holding significant promise as a potent redox chemotherapeutic that has been studied intensely as an anticancer agent in vitro and in vivo (92, 278). In leukemia, myeloma, and pancreatic cancer cell lines in vitro, this very small thiol-reactive organic electrophile (molecular weight = 111.1 Da) induces apoptotic cell death by causing oxidative stress and mitochondrial changes involving spontaneous formation of thiol-adducts with cellular glutathione and protein-bound cysteine residues depleting glutathione in the absence of nuclear genotoxic stress (92, 93). The SAR of thiol reactivity of imexon and related electrophilic cyanoaziridines has been elucidated in detail (165), and accumulative data suggest that intracellular thiol conjugation and subsequent depletion may be a crucial factor determining Imexon pharmacodynamic effects on cancer cells. Interestingly, thiol depletion from erythrocytes may serve as a potential clinical biomarker of imexon drug action.
Recently, using cDNA expression array analysis, it has been demonstrated that imexon activates a broad antioxidant gene expression response and that a change in redox state can be detected in surrogate normal tissues, for example, in peripheral blood mononuclear cells (PBMC), including increased DNA binding of redox-sensitive transcription factors (AP-1, Nrf2) and increased antioxidant gene expression (thioredoxin reductase-1, glutaredoxin-2, and peroxiredoxin-3) (21).
A Phase I trial of imexon in patients with advanced malignancy has recently been completed, defining the maximum tolerated dose and demonstrating that imexon exposure is accompanied by a decrease in plasma thiols (89). Imexon is currently in Phase I/II clinical trials in patients with advanced solid tumors. Combination chemotherapy using gemcitabine and imexon is being evaluated in previously untreated pancreatic adenocarcinoma [ClinicalTrials.gov Identifier: NCT00327327], and safety of chemotherapy using imexon plus docetaxel is examined in lung, breast, and prostate cancer patients [ClinicalTrials.gov Identifier: NCT00327288].
3. L-Buthionine-S,R-sulfoximine
Pharmacological inhibition of glutathione biosynthesis by L-buthionine-S,R-sulfoximine (BSO) has long been known to induce cellular oxidative stress by depletion of cellular glutathione levels. Its use in experimental combination chemotherapy in order to overcome cancer cell resistance to cytotoxic anticancer agents including arsenic trioxide, cisplatin, doxorubicin, and melphalan has been covered elsewhere (117, 119, 394). Efficacy of BSO-mediated chemosensitization has been demonstrated in various experimental tumor models (119). BSO drug action is based on specific inhibition of γ-glutamylcysteine synthetase catalyzing the reaction of L-glutamate with MgATP with formation of γ-glutamylphosphate as an enzyme-bound intermediate followed by reaction of γ-glutamylphosphate with the α-amino group of L-cysteine (137). BSO is a structural mimic of the γ-glutamylphosphate cysteine adduct and serves as a suicide substrate that forms an irreversible buthionine sulfoximine-phosphate adduct with the enzyme. Based on promising results achieved with the prototype agent BSO and the availability of a γ-glutamylcysteine synthetase crystal structures, rational design of more potent human γ-glutamylcysteine synthetase inhibitors structurally unrelated to BSO has been achieved (147).
4. PABA/NO
Glutathione-S-transferase π (GSTπ), a protein that possesses both catalytic and ligand properties, is expressed abundantly in many solid tumors and its overexpression is associated with cancer cell drug resistance (345). A structure-based prodrug, O2-[2,4-dinitro-5-(N-methyl-N-4-carboxyphenylamino) phenyl] 1-N,N-dimethylamino)diazen-1-ium-1,2-diolate (PABA/NO), has recently been designed that induces JNK- and p38-dependent cell death after metabolism to nitric oxide by GSTπ catalysis (302). Moreover, PABA/NO displayed antitumor effects in a human ovarian cancer xenograft in SCID mice (109). A peptidomimetic GSTπ inhibitor, γ-glutamyl-S-(benzyl)cysteinyl-R-phenylglycine diethylester (TLK199) stimulated both lymphocyte production and bone marrow progenitor proliferation associated with elevated activities of JNK1 and ERK1/ERK2, consistent with TLK199 disassociating GSTπ from its binding partner JNK followed by kinase phosphorylation and signaling through this kinase cascade (301). In contrast, 7-nitro-2,1,3-benzoxadiazole derivatives, representing a novel class of mechanism-based non-GSH peptidomimetic suicide inhibitors that eliminate GST enzymatic activity through covalent binding, trigger apoptosis in several P-glycoprotein expressing human cancer cell lines, and specifically overcome multidrug resistance in MDR1-expressing NSCLC and leukemia cells (107). These data provide prototype evidence that supports feasibility of using GSTπ inhibitors with low systemic toxicity as novel redox chemotherapeutics for the selective treatment of MDR1 P glycoprotein-positive tumors.
C. Targeting the thioredoxin system: PX-12 and PMX464
The cellular thioredoxin redox system contributes to alterations of redox balance in cancer cells associated with enhanced mitogenic signaling and suppression of apoptosis (191, 229, 255, 259, 370). As crucial factors involved in cancer cell redox control, all components of the thioredoxin redox system including (a) the thioredoxin (Trx) family of small redox proteins, (b) thioredoxin reductases, the family of corresponding seleno-enzymes involved in NADPH-dependent Trx regeneration, (c) the endogenous thioredoxin inhibitor thioredoxin-interacting protein, and (d) peroxiredoxins (thioredoxin peroxidases) are important cancer drug targets for redox chemotherapeutic intervention validated by numerous mechanistic studies (20, 122, 259, 262, 279). Cancer cell survival is regulated by Trx-1-dependent inhibition of proapoptotic factors including ASK1 (305), and pharmacological inhibition of Trx-1 may induce apoptosis (191, 192). Moreover, Trx-1 overexpression has been shown to increase VEGF production and promote tumor angiogenesis (369). Importantly, Trx-1 is overexpressed in many human tumors where it is associated with decreased patient survival (178, 255).
1. PX-12
Much research has aimed at the rational design and development of specific inhibitors that interfere with cancer cell redox regulation by either targeting Trx-1 or thioredoxin reductase (191, 255, 370). Small molecule prototype agents with promising efficacy in various animal tumor models are currently evaluated in clinical trials. Among various prototype agents that would antagonize these novel redox targets, alkyl-2-imidazolyl disulfides that inactivate thioredoxin by thioalkylation of critical cysteine residues (Cys73) or oxidative protein dimerization have been developed (Fig. 10) (191, 192). In an early landmark study that detailed the rational process of anticancer redox drug discovery, development of PX12 (1-methylpropyl-2-imidazolyldisulfide; Fig. 10-58) started with parallel combinatorial synthesis of disulfides that were first screened for biological activity assessing inhibition of thioredoxin-dependent reduction of insulin by thioredoxin reductase and NADPH (191). Hit compounds that displayed activity in this rapid biochemical in vitro screen were then assessed for apoptogenicity against HT-29 colon cancer cells. In a confirmatory screen, acquisition of drug resistance after thioredoxin transfection of compound-sensitive wild-type MCF-7 cells was examined in order to confirm that compound apoptogenicity occurs as a direct mechanistic consequence of thioredoxin-antagonism. Further assessment of anticancer activity was then performed in human MCF7 breast cancer and HL-60 xenografts growing in SCID mice. PX-12 has advanced successfully through Phase I clinical assessment (370). An ongoing Phase Ib trial examines PX-12 administered as a 72-h infusion every 21 days in patients with a histologically or cytologically confirmed diagnosis of advanced or metastatic cancer that is refractory to standard treatment (ClinicalTrials.gov Identifier: NCT00736372). PX-12 is currently evaluated in a randomized Phase II open-label study at two different dose levels in patients with advanced carcinoma of the pancreas whose tumors have progressed on gemcitabine or on a gemcitabine-containing combination. (ClinicalTrials.gov Identifier: NCT00417287).
FIG. 10.
Thioredoxin: A redox target for anticancer intervention. The small redox protein thioredoxin (Trx-1) is an important cancer drug target for redox chemotherapeutic intervention. Akyl-2-imidazolyl disulfides including the investigational drug PX-12 (58) inactivate thioredoxin by disulfide exchange leading to thioalkylation of a critical cysteine residue (Cys73) or oxidative formation of a disulfide bridge between Cys32 and Cys35. PMX464 (59) is an electrophilic double-Michael pharmacophore containing 4-hydroxycyclohexa-2,5-dien-1-one that targets cysteine residues 32 and 35 of the thioredoxin active site, potentially through dual Michael adduction.
2. PMX464
Recent medicinal chemistry has identified the quinol PMX464 {4-benzothiazol-2-yl)-4-hydroxy-2,5-cyclohexadien-1-one; Fig. 10-59}, an electrophilic dual-Michael acceptor that targets cysteine residues 32 and 35 of the thioredoxin active site as revealed by mass spectrometry of the covalently adducted target protein (Fig. 10) (38). SAR of the reactive 4-hydroxycyclohexa-2,5-dien-1-one pharmacophore contained in this heteroaromatic quinol has been elucidated in much detail, and further lead optimization has generated numerous potent anticancer quinols (255). PMX464 displays potent cytotoxicity directed against many human cancer cell lines and in vivo antitumor activity against subcutaneously growing human RXF 944XL renal cancer cells (254). Anticancer activity in tumor xenografts displayed by various heteroaromatic-substituted 4-hydroxycyclohexa-2,5-dienone-derivatives has also been attributed to covalent adduction and inactivation of thioredoxin reductase through direct quinol attack on the penultimate C-terminal selenocysteine residue (32, 67). Moreover, as typically observed with reactivity-based redox chemotherapeutics that execute target modulation through chemical reactions, PMX464 displays a certain target promiscuity, for example, PMX464 modulation of proinflammatory signaling through NFκB as observed in alveolar epithelial cells seems to occur without mechanistic involvement of thioredoxin (49).
3. PX-916
PX-916 (Fig. 4-33) is a prodrug optimized for water solubility by glycyl derivatization of the thioredoxin reductase lead inhibitor palmarumycin, a napthoquinone-spiroketal natural product. PX-916 is a potent inhibitor of thioredoxin reductase-1 activity in tumor cells, antagonizing the downstream targets of Trx-1 signaling including HIF-1α and VEGF in tumors (280). Moreover, PX-916 showed pronounced activity in several animal tumor models.
Interestingly, other redox agents with considerable anticancer activity have been shown to modulate thioredoxin reductase-1 including the texaphyrin MGd discussed above (237), the promiscuous anticancer polyphenol curcumin (99), redox active gold thiosugars including auranofin (Fig. 4-34) (241), and the prooxidant anticancer drug As2O3, as detailed above (229). Remarkably, auranofin, a drug approved for anti-inflammatory intervention targeting rheumatoid arthritis, has recently been shown to inhibit IKK by modifying Cys-179 of the IKKβ subunit (168), consistent with a pleiotropic mechanism of action characteristic of many redox chemotherapeutics. Auranofin also exerts potent antimalarial activity based on inhibition of plasmodial thioredoxin reductase (307), providing another example of a prooxidant redox drug with combined antiprotozoal and anticancer activity, a situation reminiscent of the artemisinin class of chemotherapeutic endoperoxides.
4. Chaetocin and gliotoxin
Chaetocin (Fig. 4-35) and gliotoxin (Fig. 11-60) are prooxidant antineoplastic members of the epipolythiodioxopiperazine class of fungal secondary metabolites that have recently been associated with therapeutic interference with the thioredoxin redox system. The toxicity of these epipolythiodioxopiperazine mycotoxins has been attributed to the presence of a disulfide bridge, which can inactivate proteins via oxidation or covalent thiol adduction, generate ROS by redox cycling, and cause glutathione depletion (365). Recently, it has been demonstrated that the antineoplastic epipolythiodioxopiperazine chaetocin is a competitive and selective substrate of thioredoxin reductase 1 (TrxR1) (343). Chaetocin displays lower Km than the TrxR1 native substrate thioredoxin, thereby attenuating reductive regeneration of thioredoxin resulting in the induction of cellular oxidative stress. Transient overexpression of the downstream TrxR1 substrate Trx rescues cancer cells from chaetocin-induced, but not from doxorubicin-induced cell death, providing strong evidence for chaetocin target validity. Importantly, chaetocin does not act as a pure inhibitor of TrxR1, but rather as a competitive TrxR1 substrate. Chaetocin has been tested as a promising new antimyeloma agent with in vitro and in vivo activity mediated via imposition of oxidative stress (163).
FIG. 11.
Gliotoxin: A prooxidant peroxiredoxin mimetic and experimental redox chemotherapeutic. The disulfide-based mycotoxin gliotoxin (60) causes catalytic oxidation of thioredoxin [reaction cycle (1)] and glutathione depletion with ROS formation [redox cycle (2)]. In addition, gliotoxin inactivates target proteins by either covalent modification or free radical damage mediated by redox cycling.
Gliotoxin, a virulence factor associated with invasive aspergillosis in immunocompromised patients, causes apoptotic and necrotic cancer cell death. Gliotoxin inactivates alcohol dehydrogenase and NFκB by either covalent modification or free radical damage mediated by redox cycling (365). Necrosis of thymocytes caused by gliotoxin has been attributed to increased cellular calcium levels thought to result from interaction of gliotoxin with a redox-sensitive thiol residue in the plasma membrane calcium channel (158). Calcium dysregulation may then cause subsequent oxidative damage. Gliotoxin has recently been shown to display potent peroxiredoxin mimetic activity at nanomolar concentrations, catalyzing thioredoxin oxidation with concomitant H2O2 reduction to water, a redox activity that results in decreased intracellular levels of H2O2 in HeLa cells and antiangiogenic effects in cultured human endothelial cells (Fig. 11) (68). Moreover, as observed with other epipolythiodioxopiperazines, gliotoxin can be involved in one-electron redox cycling, leading to formation of superoxide and other ROS. Interestingly, glutathione promotes gliotoxin-induced cytotoxicity, probably by facilitating gliotoxin redox cycling through reduction of the disulfide bridge followed by superoxide formation upon reaction of the dithiol with molecular oxygen, and glutathione intensifies gliotoxin-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. Preclinical efficacy of gliotoxin as redox chemotherapeutic has been observed against human MCF7 breast and C1-2 colon carcinoma xenografts in SCID-mice (271). Fluorinated analogs of gliotoxin have been prepared by fluorophenylalanine-based microbial biosynthesis in order to improve pharmacokinetic profile and to enhance antimicrobial and tumoricidal activity (160).
D. Targeting the Nrf2/Keap1-ARE pathway
Cancer cell resistance to chemotherapeutic agents poses a major obstacle to effective anticancer intervention. Recent research suggests that molecular inhibition targeting the redox sensitive Nrf2/Keap1-ARE signaling pathway may be a promising strategy to achieve chemo- and probably radiosensitization of human tumors (208, 319, 362). As mentioned above (Section II.G), Nrf2, a redox-sensitive cap ‘n’ collar basic leucine zipper transcription factor, mediates the expression of key enzymes involved in antioxidant protection and detoxification of xenobiotics. Nrf2-target genes encode antioxidant proteins and enzymes including γ-glutamyl cysteine synthetase catalytic subunit, glutathione reductase, glutathione peroxidase, thioredoxin-1, thioredoxin reductase, and peroxiredoxins (341). With particular relevance to cancer cell chemoresistance, Nrf2 controls xenobiotic metabolism and efflux through upregulation of enzymes such as NQO1, UDP-glucuronosyltransferase, and glutathione-S-transferase family members, and also controls expression of ATP-dependent multidrug-resistant drug efflux pumps, including ABCC1 and ABCC2 (208, 319).
It was recently demonstrated that stable overexpression of Nrf2 resulted in enhanced resistance of cancer cells to chemotherapeutic agents, including cisplatin, doxorubicin, and etoposide (362). Nrf2 was validated as a potential drug target for chemosensitization of cancer cells by demonstrating that downregulation of the Nrf2-dependent response by overexpression of the Nrf2 inhibitor Keap1 or Nrf2-siRNA intervention sensitized cancer cells to chemotherapeutic drugs (319, 362). Importantly, it has been shown that loss of function mutations in Keap1 result in constitutive activation of Nrf2 function in NSCLC that promote tumorigenicity and confer chemoresistance by upregulation of glutathione, thioredoxin, and drug efflux pathways (320). Inhibiting Nrf2 expression by intratumoral delivery of naked siRNA duplexes in combination with carboplatin significantly inhibited tumor growth in a subcutaneous murine model of lung cancer, an example of experimental anticancer redox therapy based on genetic intervention using siRNA technology in vivo (319).
Small molecule inhibitors of the Nrf2/Keap-1 pathway may therefore represent a promising class of adjuvant agents for tumor chemosensitization. However, apart from the recent finding that ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in imatinib-resistant cancer cells, no prototype agents that qualify as specific inhibitors of Nrf2-induced transcriptional activation have been identified (338). Interestingly, in striking contrast to the emerging situation in NSCLC, Nrf2 downregulation has been demonstrated in human prostate tumors where the loss of Nrf2 function initiates a pathological cascade of increased levels of constitutive oxidative stress, genetic instability, and tumorigenesis, demonstrating the complexity of Nrf2 involvement in cancer cell redox dysregulation (116).
In the absence of specific small molecule inhibitors that would undermine Nrf2-controlled tumor antioxidant defense for chemosensitization, agents that target crucial downstream effectors of Nrf2 including HO-1 and NQO1 may offer therapeutic benefit for prooxidant intervention targeting tumors with constitutive Nrf2 upregulation.
1. Targeting HO-1: Zinc protoporphyrin IX
Another class of experimental agents that target specific components of the antioxidant defense system of cancer cells is represented by prototype inhibitors of heme oxygenase-1 (HO-1) that have shown preclinical efficacy in animal models of human cancer (84). HO-1, a stress inducible enzyme under Nrf2 transcriptional control that belongs to the heat shock protein (Hsp32) family, represents an important cellular defense enzyme that protects mammalian cells from oxidative damage, and recent experimental evidence suggests that HO-1 is an essential antioxidant defense enzyme involved in cancer cell resistance to oxidative stress imposed by prooxidant anticancer agents and host leucocytes (84). Importantly, chemosensitization of cancer cells has been achieved using prototype HO-1 inhibitors, including competitive antagonists of the metalloporphyrin type such as zinc protoporphyrin and more soluble pegylated derivatives (PEG-ZnPP) with improved tumor delivery (101). Moreover, HO-1 inhibitors administered i.v. suppressed intratumoral HO-1 activity and displayed cytotoxic activity in a murine hypoxic solid tumor model (100). In another study, zinc protoporphyrin IX administered perorally or intraperitoneally displayed potent antitumor effects against C-26 adenocarcinomas in mice but did not potentiate antitumor effects of standard chemotherapeutics (263).
It should also be noted that HO-1 upregulation can protect cardiomyocytes from anthracycline-induced oxidative stress and cell death, suggesting that the therapeutic index of anthracycline cancer chemotherapeutics can be improved by protecting cardiomyocytes by cotreatment with HO-1 upregulators including specific metalloporphyrins (197). In isolated rat cardiac cells, HO-1 enzyme overexpression prevented doxorubicin-induced mtDNA depletion and apoptosis via activation of Akt1/PKB and guanylate cyclase, while HO-1 gene silencing exacerbated doxorubicin-induced mtDNA depletion and apoptosis (331).
2. Targeting NQO1: Dicoumarol and ES936
The Nrf2-downstream target NQO1 (EC 1.6.99.2, also referred to as DT-diaphorase) is an important antioxidant defense enzyme and modulator of cellular redox homeostasis; in addition, NQO1 functions as a regulator of p53 degradation and p53-mediated apoptosis (130, 297). This flavoprotein catalyzes the obligatory two-electron reduction of quinones and quinone imines using NAD(P)H followed by phase-II conjugation (e.g., glucuronidation) and excretion of redox-inactive metabolites, thereby preventing redox cycling of endogenous and xenobiotic quinones that can form ROS via the intermediate semiquinone anion radical (73, 74, 216, 297).
In addition to antioxidant activity through reductive inhibition of quinone redox cycling, recent experiments have elucidated the NAD(P)H-dependent superoxide scavenging activity associated with purified NQO1, suggesting a new role of this enzyme in cellular antixodant defense (316). Moreover, it has recently been demonstrated that NQO1 regulates the ubiquitin-independent pathway of p53 degradation through the 20S proteasome (130). Upregulated expression of NQO1 is associated with various human malignancies, including NSCLC, pancreas carcinoma, and breast and colon adenocarcinoma (74, 297).
NQO1 upregulation in tumor tissue provides a therapeutic window for the bioreductive activation of cytotoxic redox cyclers and alkylating agents (48, 74, 297). The SAR of reductive activation under physiological conditions is particularly well understood in the area of bioreductively activated quinone-type alkylating agents including the aziridinyl-1,4-benzoquinone mitomycin C, the indolequinone EO9, and the nitroarene radiosensitizer misonidazole as reviewed extensively (74, 364).
a. Dicoumarol
Recent research suggests that pharmacological and genetic strategies that target NQO1 overexpression in tumors may exert potent anticancer effects. Dicoumarol is a potent inhibitor of NQO1 that competes with NAD(P)H for binding to NQO1. In addition, dicoumarol also disrupts the binding of NQO1 to p53, p73, and ornithine decarboxylase and induces ubiquitin-independent proteasomal degradation of these binding partners (14). Using this prototypic NQO1 antagonist, cell proliferation and growth in soft agar were suppressed in cultured pancreatic carcinoma cells that displayed high levels of drug-induced superoxide radical anion formation (73, 90). Pilot studies performed in orthotopic pancreatic tumors implanted into nude mice demonstrated the feasibility of targeting NQO1 for intervention in human pancreatic carcinoma (216). Dicoumarol is a promiscous agent with off-target activity, including uncoupling of mitochondrial electron transport, and experimental evidence has been presented suggesting that dicoumarol-induced anticancer effects may occur independent of NQO1-inhibition (90).
b. ES936
Development of novel NQO1 inhibitors is an active area of current anticancer drug discovery (261). Recent advances in NQO1 structural biology have facilitated progress in the rational design of structure-based and mechanism-based inhibitors. The crystal structure of human NQO1 in complex with dicoumarol at 2.75 Å resolution was reported recently facilitating the rational design of potent inhibitors of NQO1 activity (14). Identification of a mechanism-based indolequinone-type inhibitor of NQO1, 5-methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione (ES936; Fig. 4-36) has been reported recently (375). In a subsequent SAR study on NQO1-inhibitory and noninhibitory indolequinone analogues, it was demonstrated that NQO1 inhibitory activity can be dissociated from growth inhibitory activity in MIAPaCa-2 pancreas carcinoma cells, suggesting additional or alternative targets apart from NQO1 that are responsible for the growth inhibitory activity of this series of indolequinones (292). ES936 has displayed significant chemotherapeutic activity in a MIA PaCa-2 xenograft model of pancreas carcinoma (78).
E. Targeting APE/Ref1: E3330 and PNRI-299
Apurinic/apyrimidinic endonuclease/redox effector factor-1 (APE/Ref-1) is a redox enzyme that facilitates DNA binding of redox-sensitive transcription factors including AP-1, NFkB, and EGR-1 through reductive activation (230, 391, 392). Importantly, APE1/Ref-1 is also the major apurinic/apyrimidinic (AP) endonuclease in human cells and was originally identified as a DNA repair enzyme. After removal of damaged bases by glycosylases, APE/Ref-1 introduces a nick at AP sites that allows further processing for DNA repair. Activation of redox signaling through APE/Ref-1 occurs in response to intra- and extracellular prooxidant alterations of redox homeostasis facilitating transcription of stress response genes and efficient repair of oxidative DNA damage. APE/Ref-1 overexpression in human melanoma cells has been interpreted as an adaptive mechanism maintaining proliferative and survival signaling under conditions of constitutively elevated cellular oxidative stress, and upregulation of APE/Ref-1 is now recognized as a major mechanism of redox-dependent chemo- and radioresistance in melanoma and other cancer cells (391).
APE/Ref-1 is a promising target for anticancer intervention that has been validated by the demonstration that genetic knockdown induces chemosensitization (209). For example, pronounced inhibition of ovarian tumor growth was achieved using siRNA-based genetic intervention targeting APE/Ref-1 (110). Recently, identification of various small molecule inhibitors of specific Ape1 functions has been reported, and feasibilty of pharmacological target modulation for anticancer intervention has been examined (Fig. 12) (392).
FIG. 12.
Apurinic/apyrimidinic endonuclease/redox effector factor-1 (APE/Ref-1): A redox target for anticancer intervention. Reductive activation of DNA binding of redox-sensitive transcription factors that are involved in tumor initiation and progression depends on APE/Ref-1 enzymatic activity. (A) DNA repair activity of APE/Ref-1 is specifically inhibited by lucanthone (61) and CRT0044876 (62), investigational drugs that may confer tumor chemo- and radiosensensitization. E3330 (63) and resveratrol (64) are ligands that suppress redox-dependent transcription factor activation by APE/Ref-1, but do not interfer with DNA repair function of the enzyme. (B) PNRI-299 (65) is a small molecule inhibitor of APE/Ref-1 redox function that contains a redox active enedione pharmacophore that covalently traps (step a) and potentially oxidizes (step b) cysteine nucleophiles contained in the target protein APE/Ref-1, leading to functional inhibition of inflammatory signaling through the APE/Ref-1 client protein AP-1.
1. E3330
The quinone-derivative E3330 {(E)-3-(4,5-dimethoxy-2-methyl-3,6-dioxocyclohexa-1,4-dienyl)-2-nonylpropenoic acid; Fig. 12A-63} was recently identified as a specific inhibitory ligand antagonizing APE1-redox function (230). In the same study, E3330 displayed inhibitory activity in NFκB and AP-1 transactivation reporter assays in ovarian cancer cell lines, caused redox-dependent functional interference in EMSA studies of AP-1 or HIF-1α binding, and suppressed retinal vascular endothelial cell angiogenesis. Importantly, E3330 redox inhibition of APE1 had no effect on DNA repair function.
2. PNRI-299 and resveratrol
Using a structure-based approach involving molecular docking studies resveratrol (Fig. 12A-64) has recently been identified as a potent lead compound that acts as inhibitory ligand of the APE/Ref-1 redox domain, resulting in interference with APE/Ref-1 redox function in human melanoma cells (391). In another study, an unbiased chemogenomics approach that screened for small molecule inhibitors of AP-1 transcriptional activity using a chemical template that incorporates an enedione moiety to trap reactive cysteine nucleophiles in the active sites of redox proteins identified a small molecule AP-1 inhibitor, PNRI-299 (Fig. 12B-65). This molecule showed in vivo activity in an inflammatory asthma mouse model (258). Based on subsequent affinity-labeling studies, APE/Ref-1 was determined as the molecular target of PNRI-299.
3. Lucanthone and CRT0044876
The radiation sensitizer lucanthone {1-{[2-(diethylamino)ethyl]amino}-4-methyl-9H-thioxanthen-9-one; Fig. 12A-61} is a potent inhibitor of APE/Ref-1-mediated APE repair activity and increases chemosensitivity of breast cancer cells to alkylating agents without affecting APE/Ref-1 redox activity or exonuclease function (231). However, no research has addressed the important question if pharmacological inhibition of APE repair activity may increase the incidence of therapy-related cancers as a consequence of therapy-induced genomic instability conferred by loss of DNA repair in healthy tissue (8). Using a high-throughput screen for the rapid identification of test compounds that inhibit AP site-processing enzymes by cleaving the phosphodiester backbone, 7-nitro-1H-indole-2-carboxylic acid (CRT0044876; Fig. 12A-62), a novel chemical inhibitor of APE1 AP-endonuclease, 3′-phosphodiesterase, and 3′-phosphatase functions active at low micromolar concentrations has been identified (234). As suggested by in silico modeling involving docking to two X-ray crystal structures of APE1, CRT0044876 targets the active site in the APE1 DNA repair domain where the indole ring of CRT0044876 is located in the abasic deoxyribose binding pocket. At noncytotoxic concentrations, CRT0044876 potentiates the cytotoxicity of several DNA base-targeting compounds associated with an accumulation of unrepaired AP sites, suggesting feasibility of rational design of APE1-targeting drugs for antitumor therapy.
F. Targeting Cdc25 phosphatases: NSC 67121 and F-NSC 67121
Cell division cycle 25 (Cdc25) phosphatases are redox-sensitive key activators of cyclin-dependent kinases that regulate mammalian cell cycle progression (300). In various human malignancies including head and neck, colon, NSCLC, and breast cancers, expression of the oncogenic Cdc25A and Cdc25B isoforms is strongly upregulated, resulting in hyperproliferative dysregulation and mitotic progression through removal of inhibitory protein phosphotyrosine groups on cyclin-dependent kinases/cyclin complexes, including CDK2/cyclin E and CDK1/cyclin B involved in G2-M and G1-S cell cycle transitions, respectively (124). The catalytic cysteine residue (Cys473 in Cdc25B) occurs as a nucleophilic thiolate anion in the active site of the Cdc25 dual specificity phosphatases and forms a covalent phosphocysteine intermediate during catalytic phosphate removal. The active site thiolate renders Cdc25 phosphatases susceptible to covalent modification by organic electrophiles and oxidation by ROS leading to enzymatic inactivation and interference with cell cycle progression (300). Cdc25 phosphatases may therefore represent promising molecular targets for anticancer redox chemotherapeutics (124, 211).
1. NSC 67121 and F-NSC 67121
Various small molecule agents containing electrophilic pharmacophores that act directly through sulfhydrylarylation or indirectly through ROS formation have been designed as specific inhibitors of Cdc25 phosphatases displaying significant anticancer activity (Fig. 13). Active site binding and cysteine-adduction by electrophilic pharmacophores was optimized based on molecular docking studies using the crystal structure of the Cdc25B catalytic domain (124, 183, 212). It is interesting to note that the napthoquinone menadione (vitamin K3) discussed above in the context of prooxidant redox cycling (Section II.C) represents an early prototype inhibitor of Cdc25 phosphatase enzymatic activity (382). Other naphthoquinones including 2-(2-mercaptoethanol)-3-methyl-1,4-napthoquinone (NSC 67121; Fig.13-66) displayed significant growth inhibition against human and murine carcinoma cells. Redox cycling and ROS formation of napthoquinones were reduced by introduction of four fluorine substituents at the benzene moiety (F-NSC 95397; Fig. 13-67) preventing superoxide formation due to the increased reduction potential of the fluorinated derivative originating from the inductive effect of the electronegative fluorine substutuents. In contrast, active site cysteine-adduction was enhanced with the fluorinated derivatives. Another potent Cdc25 phosphatase inhibitor, the electrophilic quinoline-5,8-dione DA3003-1 [6-chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline-5,8-dione, NSC 663284], inhibited the growth of subcutaneous human colon HT29 xenografts in SCID mice (124, 139). Based on detailed SAR and molecular docking studies, a multiple action mode involving irreversible active site cysteine oxidation with sulfonic acid formation through redox cycling, but also reversible and irreversible target binding, have been suggested as a mechanism of action underlying Cdc25B inactivation by NSC 663284 (139).
FIG. 13.
Cdc25 phosphatases: Redox targets for anticancer intervention. Hyperproliferative dysregulation and cell cycle progression in cancer cells can be controlled by oncogenic cell division cycle 25 (Cdc25) phosphatases that are redox-sensitive activators of cyclin-dependent kinases. The active site cysteine thiolate renders Cdc25 phosphatases susceptible to redox intervention by (a) ROS from redox cycler drugs including 2-(2-mercaptoethanol)-3-methyl-1,4-napthoquinone (NSC 67121, 66) and (b) organic electrophiles including F-NSC 67121 (67), leading to target modulation by covalent adduction. (c) Target inactivation may be achieved by inhibitory ligands including 7-[(2-methylbenzyl)-1H-indol-3-yl]-2,5-dihydroxy-1,4-benzoquinone (68).
In an attempt to further reduce ROS-induced nonspecific cellular toxicity, nonquinoid electrophilic pharmacophores that inactivate Cdc25 phosphatases by alkylation of the active site cysteine residue have been designed, including 4-dimethylamino-2-methoxy-6-(methyl-[2-(4-nitrophenyl)-ethyl]-aminomethyl)-phenol (BN82002) and various maleimide derivatives such as N-(4-biphenyl)3,4-bis-(2-hydroxy-ethylsulfanyl)-maleimide (PM-20) that display activity in various tumor xenograft models (183). Equally, the redox-silent nonquinone sulfone analog of vitamin K3 H32 inhibits hepatoma cell growth by targeting the catalytic cysteine residue of Cdc25A without involvement of ROS production (124).
2. Indolyldihydroxyquinones
In addition, structure-based inhibitors that inactivate Cdc25 through reversible binding have been identified including 7-[(2-methylbenzyl)-1H-indol-3-yl]-2,5-dihydroxy-1,4-benzoquinone (Fig. 13-68), an electron-rich quinone-derivative with strongly attenuated electrophilicity and pronounced intramolecular hydrogen bonding that acts as a specific ligand without covalent binding or redox cycling. Optimized derivatives of a similar lead compound {2,5-dihydroxy-3-(1H-indol-3-yl)[1,4]benzoquinone} have been designed following a lead optimization strategy of redox chemotherapeutics that aims at transition from reactivity-based to ligand-based target inhibition (124, 211).
G. Targeting zinc finger transcription factors: DIBA
Zinc fingers consitute a large superfamily of protein domains that can bind to DNA based on a central zinc ion coordinated by cysteine (and sometimes histidine) residues contained in two antiparallel β strands and an α helix (366). Steroid hormone nuclear receptors and redox-sensitive transcription factors including AP1, Sp1, EGR-1, NFkB, and p53 interact with their DNA target sequences through zinc fingers contained in their DNA binding domain. Importantly, the zinc-coordinated cysteine thiolates of zinc fingers are potentially vulnerable to oxidative inactivation and represent a promising target for pharmacological modulation of gene expression by small molecule redox therapeutics (52).
1. DIBA
Earlier research has demonstrated that virus-encoded zinc fingers can selectively be targeted without affecting host cell zinc finger proteins. Antiretroviral drug development targeting the HIV-1 nucleocapsid zinc finger protein NCp7 containing a Zn(II) ion tetrahedrally coordinated by the thiolate of three Cys residues and the imidazole nitrogen of one histidine residue was based on NCp7-inactivation by chemical induction of zinc extrusion from the zinc finger resulting in suppression of viral replication (311). The redox mechanism of action associated with experimental antiretrovirals including the 2,2′-dithiobisbenzamide-derivative DIBA (NSC 654077; Fig. 4-37) that inactivates HIV-1 by NCp7 zinc finger disruption has been elucidated in much detail (52, 155). Initially, the antiviral agent forms an intermolecular disulfide bond via disulfide exchange through electrophilic attack on one of the sulfur atoms coordinating the Zn(II) ion of the zinc finger. This process is followed by ejection of the metal ion from the zinc finger that occurs as a result of the destabilized Zn(II) ion coordination sphere. A similar pharmacological approach has been taken to target the oncogenic human papilloma virus HPV-16 by disrupting the E6 oncoprotein containing two Cys–[X]2–Cys–[X]29–Cys–[X]2–Cys zinc fingers (23). In a large series of redox-active di- and polysulfides, including 4,4′-dithiodimorpholine-derivatives, potent anti-HPV compounds were identified that produced a release of zinc from E6 with induction of cytotoxicity selective for the HPV-infected cells. Thus, the therapeutic disruption of zinc finger domains by experimental redox drugs represents a validated approach for the design of antiviral agents, and has also been pursued successfully for the design of anticancer redox chemotherapeutics.
About two-thirds of human postmenopausal breast carcinoma is estrogen receptor-positive (ER+), rendering these tumors sensitive to pharmacological intervention by antiestrogens and selective estrogen receptor modulators (72). It is therefore tempting to speculate that estrogen-dependent cancer cells can be targeted through redox disruption of zinc finger-dependent ER transcriptional activity. Indeed, recent experimental evidence obtained in a murine xenograft model of hormone-dependent breast cancer suggests feasibility of chemotherapeutic intervention based on targeting redox sensitive zinc fingers in the ER DNA binding domain (360, 361). Electrophilic derivatives of benzamide-disulfide, including DIBA and the benzisothiazolone BITA, were identified as chemical inhibitors capable of oxidatively disrupting Zinc finger integrity and estrogen-dependent proliferative signaling and breast carcinoma progression (360, 361).
The selectivity of electrophilic agents toward zinc fingers depends on multiple physicochemical parameters including redox potential, reactive proximity, and noncovalent ligand-binding affinity (23, 52, 361). For example, electrochemical analysis of the Sp1 zinc finger by cyclic voltammetry revealed a redox potential that thermodynamically favors preferential oxidative disruption of this particular thiol target by redox cycler prototype organochalcogens in oxidatively stressed cells with lowered glutathione content (128). In analogy, computational analysis of steric and electrostatic screening parameters in zinc finger cores revealed the preferential redox vulnerability of the estrogen receptor C-terminal zinc finger that was disrupted through electrophilic zinc ejection by prototype agents, including DIBA and BITA (361). Zinc finger disruption by these compounds efficiently inhibited estrogen receptor dimerization and complex formation with the estrogen responsive element (ERE) promotor sequence upstream of ER-target genes leading to inhibition of functional transactivation in human breast cancer cells transfected with an ERE-reporter gene construct and exposed to estradiol. Moreover, significant variation in the structural environments of nuclear receptor zinc finger cores of known nuclear receptor DNA binding domains suggests feasibility of targeting estrogen receptor DNA binding with minimal effect on other nuclear receptors that depend on similar binding domains.
Importantly, DIBA-based experimental chemotherapy has recently been shown to block ligand-dependent and-independent cell growth of tamoxifen-resistant breast cancer in vitro and in vivo, demonstrating feasibility of overcoming tamoxifen resistance, a notorious obstacle to successful treatment of ER-positive human breast cancer (360).
The combined evidence provided by multiple studies on (a) antiviral intervention targeting zinc finger domains in viral oncogenic proteins (23), (b) Sp1 zinc finger inactivation in oxidatively stressed cells by prooxidant glutathione peroxidase mimetics (128) and (c) pharmacological disruption of the ER DNA-binding domain by small molecule prooxidant modification of the ER zinc finger (360, 361) strongly suggests feasibility of disrupting oncogenic transcriptional control through redox-based inactivation of specific DNA-binding zinc fingers. Further research will determine if these early reports may herald a new era of nongenotoxic redox chemotherapeutics that selectively target nuclear hormone receptors and other transcription factors.
H. In search of a molecular target: elesclomol
The investigational redox chemotherapeutic elesclomol (Fig. 4-38), a symmetric thiobenzoylhydrazide-derivative [N-malonyl-bis (N′-methyl-N′-thiobenzoylhydrazide, STA-4783), was originally derived from a differential phenotypic screen that assessed potentiation of in vitro cytotoxicity of paclitaxel against human tumor cell lines (29, 118, 193). Early preclinical studies indicated that the growth of human tumor xenografts in CD-1 nude mice was unaffected by treatment with elesclomol administered as single therapeutic agent (7, 25, 26). In contrast, combination therapy with paclitaxel significantly potentiated antitumor activity over paclitaxel administered as single agent without altering host toxicity, and synergistic enhancement of chemotherapeutic efficacy with paclitaxel was then demonstrated in murine tumor xenograft models of human lymphoma, breast, and lung cancer. Earlier experimentation performed on cultured cells revealed a potent apoptogenicity of elesclomol directed against human melanoma and other cancer cell lines, not observed in primary human keratinocytes (193). Remarkably, apoptotic cell death was preceded by induction of massive oxidative stress suggesting a prooxidant mode of anticancer activity reminiscent of other redox chemotherapeutics such as DSF, imexon, and MGd, but no molecular target of this experimental chemotherapeutic has been identified. Transcriptional expression profiling of Hs294T melanoma cells exposed to elesclomol revealed upregulated expression of genes encoding heat shock proteins, metal-activated transcription factor MTF1-dependent metallothionein subtypes, and Nrf2-dependent antioxidant response proteins. Importantly, inhibition of oxidative stress by the antioxidants N-acetylcysteine or tiron (4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt) completely blocked drug-induced apoptosis and stress-associated gene transcription. It has been proposed that various molecular pathways may be involved in oxidative stress-induced apoptogenicity of elesclomol including activation of p53 proapoptotic signaling and ROS-activation of ASK1 kinase function upstream of proapoptotic JNK and p38 signaling (193). It may also be speculated that the symmetric bis-thiobenzoylhydrazide group contained in elesclomol can serve as a polydentate metal ion chelator pharmacophore. This would be consistent with metal-dependent induction of cellular oxidative stress, a situation similar to copper ion-dependent antimelanoma activity of DSF (discussed in Section II.E) and reminiscent of structurally related bis-isatin thiocarbohydrazone copper ion complexes that display anticancer activity against murine Ehrlich Ascites carcinoma (308).
Importantly, elesclomol displayed a benign toxicity profile and pharmacokinetic compatibility when tested in a Phase I clinical trial designed to establish the maximum tolerated dose in combination with paclitaxel in patients with refractory solid tumors (27). In a subsequent double-blinded, randomized, controlled Phase II clinical trial that included 81 patients with stage IV metastatic melanoma, elesclomol, in combination with paclitaxel, doubled median progression-free survival compared with paclitaxel alone (p = 0.035), suggesting a significant therapeutic benefit that may be achieved in this notoriously chemoresistant malignancy characterized by very poor prognosis and insufficient chemotherapeutic options (193, 249). However, a global, pivotal Phase III melanoma trial assessing prolonged progression-free survival and overall survival was discontinued in February of 2009 due to serious safety concerns raised by the Data Monitoring Committee (DMC) after more patients died in the group taking elesclomol in combination with paclitaxel than in the control group taking paclitaxel alone. (SYMMETRY, ClinicalTrials.gov Identifier: NCT00522834). Other trials with elesclomol, including a trial in combination with docetaxel in hormone-refractory metastatic prostate cancer (ClinicalTrials.gov Identifier: NCT00808418), are also suspended pending further analysis of the results of the SYMMETRY trial, a recent development that seriously compromises the potential for future clinical development of this investigational redox drug.
IV. Functional Targets for Anticancer Redox Chemotherapy
Functional targets for anticancer intervention are broadly defined as phenotypic alterations that represent a vulnerability of the specific tumor amenable to pharmacological modulation, including changes in tumor glucose metabolism, mitochondrial energy production, and tissue vascularization. Pharmacological interference with cancer cell energy metabolism has been referred to as metabolic targeting (125, 251, 270, 275, 325). Accumulative evidence strongly suggests that metabolic targeting represents an indirect route of prooxidant chemotherapeutic intervention successfully harnessed for radio- and chemosensitization of tumors. Prooxidant metabolic targeting can occur through administration of alternative energy substrates or small molecule modulators that anatagonize or redirect cancer cell metabolic pathways leading to induction of energy crisis, oxidative stress, and apoptotic sensitization.
In this context it is interesting to note that the tumor suppressor gene p53 simultaneously orchestrates cellular energy metabolism and redox homeostasis (26, 51, 244, 303). For example, p53 upregulates expression of TIGAR (TP53-induced glycolysis and apoptosis regulator) encoding a fructose-bisphosphatase that attenuates glycolytic flux by reducing cellular levels of fructose-2,6-bisphosphate, a potent positive allosteric effector of the glycolytic pacemaker enzyme 6-phosphofructose-1-kinase. Simultaneously, TIGAR antagonizes cellular oxidative stress by upregulation of glucose flux through the pentose phosphate pathway associated with NADPH generation and glutathione reduction (26). Moreover, p53 promotes energy generation through oxidative phosphorylation by upregulating expression of the cytochrome c oxidase assembly gene SCO2 (synthesis of cytochrome c oxidase 2), and a glycolytic phenotype characteristic of p53-deficient tumor cells is generated by genetic disruption of SCO2 in cancer cells that express wild-type p53 (244).
Based on the seminal observation that glucose deprivation induces oxidative stress and cytotoxic effects that seem to target cancer cells with sufficient selectivity, small molecule modulators of cancer cell energy metabolism that induce phenotypic changes consistent with induction of oxidative stress are an important class of indirect acting prooxidants (Fig. 14) (4, 125, 167, 251, 270, 325).
FIG. 14.
Prooxidant anticancer intervention targeting glucose metabolism. Metabolic targeting can occur through administration of alternative energy substrates or small molecule modulators. These anatagonize or redirect glucose flux through the glycolytic and pentose phosphate pathways with induction of energy crisis and oxidative stress in cancer cells. Redox chemotherapeutics for metabolic targeting include 2-deoxy-D-glucose (69) and its improved derivative 2-fluoro-2-deoxy-D-glucose (70), 3-bromopyruvate (71), dichloroacetic acid (72), and oxythiamine (73).
A. Prooxidant intervention targeting glucose metabolism: 2-DG and DCA
1. 2-Deoxyglucose
2-Deoxy-D-glucose (2-DG; Fig. 14-69) is a glucose analogue and antimetabolite thought to mimic the effects of glucose deprivation by inhibition of glycolytic flux resulting from noncompetitive inhibition of hexokinase and competitive inhibition of phosphoglucose isomerase by the 2-DG metabolite 2-DG-6-phosphate, as reviewed in ref. 286. A high rate of glucose uptake by tumor cells, exploited clinically for tumor imaging using [18F]2-fluoro-2-deoxyglucose-PET scanning, favors 2-DG accumulation in tumors (44, 339).
Importantly, 2-DG exposure preferentially induces increased levels of superoxide and H2O2 in cultured cancer cells versus nontransformed cells, a prooxidant metabolic effect that seems to result from 2-DG-induced increased ROS leakage from mitochondrial sources and glutathione depletion, amplifying preexisting oxidative deviations from redox homeostasis found constitutively in tumors cells (15). It has been suggested that under conditions of increased mitochondrial respiratory activity imposed by the metabolic modulator 2-DG, alterations of mitochondrial structure and DNA integrity that result in constitutively increased electron leakage are further enhanced in cancer cells. Recent studies suggest an increasingly complex mechanism underlying 2-DG anticancer action beyond activity as a metabolic blocking agent (286, 328). The crucial 2-DG metabolite 2-DG-6-phosphate has been shown to act as a strong activator of the glucose-sensing MondoA:Mlx heterdimeric transcription factor involved in expression of the negative regulator of glucose uptake and thioredoxin antagonist thioredoxin-interacting protein (TXNIP), suggesting that 2-DG-induced oxidative stress may also result from interference with the thioredoxin-based cellular antioxidant defense, an exciting hypothesis be substantiated by future experiments (328). Moreover, interference with N-linked glycosylation has been identified as an additional causative factor contributing to 2-DG apoptogenicity under normoxic conditions (202).
Numerous preclinical studies performed in cell culture and animal xenograft models (including head and neck cancer and pancreatic carcinoma) indicate that 2-DG is a potent sensitizer of cancer cells towards ionizing radiation and chemotherapeutic agents such as cisplatin, adriamycin, and paclitaxel (167, 242, 318). Based on an acceptable systemic toxicity profile and promising clinical prototype data, safety and efficacy of the investigational radio- and chemosensitizer 2-DG are being studied in a large number of ongoing human Phase I and II clinical trials (ClinicalTrials.gov Identifiers: NCT00247403, NCT00096707, NCT00633087). It is interesting to note that a series of synthetic 2-halogenated 2-DG analogs has been generated aiming at lead optimization based on in silico modeling of molecular interactions between 2-DG and hexokinase I. 2-Fluoro-2-deoxy-D-glucose (Fig. 14-70) was identified as a novel preclinical candidate with significantly increased target affinity and more potent hypoxia-selective apoptogenicity than 2-DG (207).
In this context it should be stressed that human tumors display a significant metabolic heterogeneity. For example, in prostate cancer, energy production through fatty acid oxidation is a dominant bioenergetic pathway that can render tumors undetectable by 2-18F-fluoro-2-deoxyglucose-PET and their sensitivity to pharmacological intervention using 2-DG may therefore be limited (221).
2. 3-Bromopyruvate
The alkylating agent 3-bromopyruvate (3-BrPA; Fig. 14-71) is another potent glycolytic inhibitor that displays activity both in vitro and in vivo (195, 385, 386). Anticancer activity of 3-BrPA seems to depend on irreversible adduction and inactivation of hexokinase II, the key enzyme maintaining high glycolytic fluxes in cancer cells (243). Moreover, 3-BrPA antagonizes ADP-stimulated mitochondrial respiratory rate and energy production, leading to energy crisis by depletion of cellular ATP in hepatoma and leukemia cell lines. The role of ROS-mediated mitochondrial dysregulation in 3-BrPA induced cell death has recently been examined in hepatoma cells (190). 3-BrPA-mediated cell death occurred through a signaling pathway initiated by ATP depletion, followed by ROS production, loss of mitochondrial membrane potential, PARP-1 hyperactivation, and AIF nuclear translocation. The potent antiglycolytic effects of 3-BrPA on rats bearing RMT mammary tumors have been demonstrated by examining reduction of [18F]2-fluoro-2-deoxy-D-glucose uptake after intravenous administration of 3-BrPA, and therapeutic efficacy of 3-BrPA has been demonstrated in various animal models including the rabbit VX2 model of liver cancer (195, 243, 354).
3. Dichloroacetate
Another prooxidant metabolic modulator that has shown promising anticancer activity in preclinical animal models is the pyruvate analogue dichloroacetate (DCA; Fig. 14-72), an inhibitor of mitochondrial pyruvate dehydrogenase kinase isoforms (PDK1 and PDK2) (186, 251, 295). PDK1 is transcriptionally controlled by HIF1α and antagonizes mitochondrial energy production by inhibitory phosphorylation of its substrate pyruvate dehydrogenase (PDH) enhancing glycolytic flux into lactate (37). In cancer cells, this shift towards respiration favors depolarization of the mitochondrial membrane with induction of ROS production and release of proapoptotic factors. DCA-induced generation of mitochondrially-derived ROS is also thought to activate the redox-sensitive K+ channel Kv1.5 in the plasma membrane, involved in caspase activation and induction of cancer cell apoptosis through reduction of intracellular potassium ion concentrations. Importantly, molecular inhibition of PDK2 by siRNA mimics DCA anticancer effects. In a rat implantation model of lung cancer, DCA has shown significant therapeutic efficacy (37). Rational design of novel PDK inhibitors such as the PDK2 inhibitor AZD7545 is facilitated through the availability of crystal structures for PDK1, PDK2, and PDK3 (186).
The inhibitory activity of DCA on lactate production brought about by its ability to shift the metabolism of pyruvate from glycolysis towards oxidation in the mitochondria has led to human trials of DCA administered as an oral drug for the treatment of congenital lactic acidosis associated with mitochondrial encephalomyopathy. Safety and efficacy of DCA-based anticancer chemotherapy are currently assessed in multiple ongoing clinical trials. A Phase II study tests DCA in malignant gliomas and glioblastoma multiforme patients (ClinicalTrials.gov Identifier: NCT00540176) based on the ability of DCA to readily cross the blood brain barrier. A Phase I single arm trial combines radiotherapy and temozolomide with DCA in patients with newly diagnosed glioblastoma multiforme tumors (ClinicalTrials.gov Identifier: NCT00703859).
4. Oxythiamine
Recently, it has been demonstrated that the transketolase isoform transketolase-like protein1 (TKTL1), a key enzyme of the pentosephosphate pathway, is significantly overexpressed in human tumors, and TKTL1 expression correlates with poor patient outcome and more aggressive tumor progression (112). Genetic and pharmacological target validation confirms TKTL1 expression as an important factor in driving tumor cell growth and increasing tumor tolerance to oxidative stress (399). Indeed, small molecule TKTL1 inhibitors represent an emerging class of modulators of glucose metabolism with significant anticancer activity associated with the induction of cellular oxidative stress. Anticancer effects that result from inhibition of glucose flux through the pentose phosphate pathway may originate from impairment of NADPH-dependent cellular redox homeostasis, fatty acid synthesis, and ribose-dependent nucleic acid synthesis (289). Oxythiamine (Fig. 14-73), a thiamine antagonist and potent transketolase inhibitor, displays significant anticancer activity in Ehrlich ascites tumor cells implanted in mice (284). Structure-based design of synthetic transketolase inhibitors displaying improved anticancer activity is now an active area of anticancer drug discovery (342).
B. Prooxidant intervention targeting mitochondria
Cancer cell mitochondria are attractive targets for anticancer drug discovery (285, 344). Indeed, functional alterations of cancer cell mitochondria including hyperpolarization of transmembrane potential, excessive electron leakage, and an increased threshold for drug-induced permeability transition pore opening may provide the basis for cancer cell-specific pharmacological intervention, as reviewed extensively elsewhere (Fig. 15) (2, 273). It is now established that alterations in mitochondrial respiration and oxidative phosphorylation result in aberrant mitochondrial apoptotic control and survival signaling that favor tumorigenesis (133, 164). For example, mutations in genes coding for mitochondrial electron transport chain proteins can contribute to phenotypic changes associated with cancer cells including constitutive electron leakage and oxidative stress-induced mutagenesis underlying a mutator phenotype that facilitates rapid development of chemoresistance. It has recently been shown that succinate dehydrogenase subunit C (SDHC) mutations cause increased superoxide production, metabolic oxidative stress, and genomic instability (164). Strikingly, the gene encoding the D-subunit of succinate dehydrogenase (SDHD) can behave as a classic tumor suppressor (133). Drugs that kill cancer cells through mitochondrial mechanisms that involve the induction of cellular oxidative stress are therefore an important class of emerging redox chemotherapeutics discussed in the following sections.
FIG. 15.
Prooxidant anticancer intervention targeting mitochondria. Drugs that induce oxidative stress by modulation of mitochondrial targets include redox silent α-tocopherol derivatives, including α-tocopherylsuccinate ester (74) and its orally available esterase-resistant ether-derivative (75), 3,3′-diindolylmethane (76) and its potentiated derivative 5,5′-dibromo-3,3′-diindolylmethane (77), the 1,4-benzodiazepine-derivative Bz-423 (78), the quinazoline-derivative erastin (79), and RSL5 (80).
1. Targeting mitochondrial respiration: α-TOS, DIM, and Bz-423
An important mechanism of action of mitochondrially-targeted anticancer drugs is based on functional impairment of mitochondrial energy production leading to increased ROS generation through respiratory chain electron leakage and activation of the mitochondria-dependent death signaling pathways (277, 285). It has recently been shown that pharmacological interference with mitochondrial electron transport leading to increased superoxide radical anion generation, especially through electron leakage from complex I or III, represents a strategy to enhance apoptosis of human leukemia cells induced by anticancer agents (274). Indeed, leukemia cell sensitization to induction of apoptosis by doxorubicin or ionizing radiation was achieved by upregulation of mitochondrial electron leakage using nanomolar concentrations of rotenone, a specific inhibitor of mitochondrial electron transport complex I. In a similar way, increased cellular ROS levels detected in primary leukemia cells isolated from patients with chronic lymphocytic leukemia (CLL) compared with normal lymphocytes from healthy donors were further enhanced by rotenone, a result that could also be obtained using the antileukemia redox drug As2O3 capable of inhibiting mitochondrial respiration. It has already been mentioned that recent evidence supports a mechanistic role of complex III inhibition in anticancer activity of the dietary prooxidant factor benzyl-isothiocyanate (384).
It is important to note that cell death induced by oxidative stress-associated respiratory dysfunction can occur by multiple death pathways that may also include autophagy (16). Indeed, recent evidence suggests that autophagic cell death may occur in cancer cells as a consequence of elevated oxidative stress, pharmacologically induced by respiratory chain inhibitors. It has been suggested that mitochondrial electron-transport-chain inhibitors (rotenone targeting complex I; thenoyltrifluoroacetone targeting complex II) preferentially induce autophagic ROS-mediated cell death in transformed and cancer cell lines, whereas treatment of nontransformed primary cells with electron chain inhibitors does not trigger ROS-induced autophagy (66). Genetic evidence supports the involvement of ROS in autophagic cancer cell death since blocking SOD2 expression by siRNA in HeLa cells increased ROS generation, autophagy, and cell death induced by rotenone and thenoyltrifluoroacetone, whereas siRNA-mediated downregulation of the expression of autophagic genes (beclin 1 and ATG5) decreased rotenone- and TTFA-induced cell death.
a. α-TOS
Pharmacological use of traditional biochemical respiratory inhibitors for anticancer intervention is precluded by an unfavorable toxicological profile, and considerable research aims at the identification of drug-like molecules that modulate mitochondrial respiration while offering an acceptable therapeutic window. It has recently been demonstrated that redox inactive (‘redox silent’) vitamin E analogues increase mitochondrial electron leakage. Indeed, α-tocopherylsuccinate ester (α-TOS; Fig. 15-74) and its orally available esterase-resistant ether- (Fig. 15-75) or isosteric amide analogues have been shown to induce prooxidant, pro-apoptotic, mitochondriotoxic effects that selectively target cancer cells (257, 383, 401). In many cancer cell lines, redox silent vitamin E analogues rapidly induce oxidative stress via formation of superoxide radical anions from mitochondrial sources. Experimental evidence suggests that α-TOS impairs mitochondrial complex II (succinate dehydrogenase) function acting as an ubiquinone antagonist that competitively binds to the proximal (Qp) and distal (Qd) ubiquinone sites of complex II causing functional disruption with superoxide formation upstream of Bax-mediated mitochondrial apoptosis (88). In addition, α-TOS displays BH3 domain mimetic activity that may interfer with Bax/Bak inhibitory binding by Bcl-2/Bcl-xL, thereby facilitating Bax propapototic activity. Redox inactive vitamin E analogues display potent cancer cell apoptogenicity and potentiate anticancer activity of doxorubicin, cisplatin, and etoposide, most likely through a mechanism that involves mitochondrial superoxide leakage followed by glutathione depletion. Chemotherapeutic efficacy of these agents was demonstrated in numerous preclinical animal models including melanoma, colorectal, and prostate carcinoma xenografts (22, 257), but systemic toxicity and lack of antitumor activity of α-TOS were reported in an immunocompetent murine mesothelioma model (162).
The observation that mitochondria-dependent induction of oxidative stress potentially targets cancer cells with sufficient specificity suggests that even mitochondrial F1F0-ATP synthase (complex V) may be a valid anticancer target (172). Indeed, F1F0-ATP synthase inhibition using the prototypical inhibitor oligomycin has been shown to induce cell cycle arrest in response to rapid ATP depletion and cyclin D downregulation (126). However, no meaningful therapeutic window that would allow cancer cell specific induction of cell cycle arrest was established in these experiments using oligomycin. Surprisingly, recent experimental evidence supports feasibility of pharmacological F1F0-ATP synthase inhibition for prooxidant anticancer intervention that occurs with sufficient specificity.
b. 3,3′-Diindolylmethane
Strong experimental evidence suggests that 3,3′-diindolylmethane (DIM; Fig. 15-76), a chemopreventive dietary factor from Brassica vegetables, exerts prooxidant anticancer activity as a mitochondrial F1F0-ATP synthase inhibitor (131). A molecular pathway was established in human breast cancer cells linking initial F1F0-ATP synthase target modulation with DIM-induced cell cycle arrest: Mitochondrial dysfunction through F1F0-ATP-synthase inhibition was associated with rapid increase in mitochondrial ROS leakage upstream of p38 and JNK activation, followed by transcriptional upregulation of the tumor suppressor and cyclin-dependent kinase inhibitor p21 responsible for G1 cell cycle arrest through hypophosphorylation and activation of the Rb tumor suppressor. Interestingly, DIM-induced mitochondria-dependent ROS formation and induction of cell death through inhibition of F1F0-ATP synthase was also demonstrated in the protozoan parasite Leishmania donovani (298).
As observed with many pleiotropic chemopreventive dietary factors, DIM targets multiple cellular pathways involved in carcinogenesis and tumor progression (304). DIM displays estrogenic activity and acts as an activating ligand of the aryl hydrocarbon receptor inducing nuclear translocation and subsequent complex formation with the AhR nuclear translocator. Generation of more efficacious chemotherapeutic agents inspired by DIM is an active area of anticancer drug discovery, and pharmacological dissociation of F1F0-ATP synthase inhibition from ligand-based activation of multiple nuclear receptors may represent a promising strategy for DIM lead optimization (213, 304). For example, a synthetic DIM-derivative, 5,5′-dibromo-DIM (Fig. 15-77), downregulated cyclin D1 protein expression in MCF-7 and MDA-MB-231 breast cancer cells in a proteasome-dependent manner and displayed pronounced mitochondriotoxic activity (355).
In numerous murine xenograft models of human cancer, DIM displays potent chemopreventive and chemotherapeutic activity (304). Remarkably, DIM is an established adjuvant for recurrent respiratory papillomatosis in humans, a benign noninvasive tumor caused by infection with HPV type 6 and 11 (373). DIM may prevent laryngeal papilloma growth causing life-threatening airway obstruction, particularly in children born to mothers with vaginal condyloma, an indication that is subject of an ongoing Phase II clinical trial (ClinicalTrials.gov Identifier: NCT00591305). Pharmacokinetics and tolerability of oral DIM medication have been established in a recent Phase I clinical trial (291). Oral DIM is currently evaluated in a randomized, double-blind, placebo controlled Phase III clinical trial for the treatment of cervical dysplasia (ClinicalTrials.gov Identifier: NCT00212381). In this study, regression of cervical dysplasia by oral DIM is assessed in otherwise healthy women, and treatment response is examined as a function of HPV colonization. Moreover, DIM anticancer efficacy is examined in numerous clinical trials including nonmetastatic prostate cancer not responsive to prior hormone therapy (ClinicalTrials.gov Identifier: NCT00305747).
c. Bz-423
A similar molecular pathway that harnesses pharmacological ATP synthase inhibition has recently been described in studies involving a promising synthetic small molecule designed for antiproliferative intervention in psoriasis (172, 357). The 1,4-benzodiazepine-derivative Bz-423 (Fig. 15-78) is an experimental antipsoriatic drug known to modulate the mitochondrial F1F0-ATPase inducing the formation of superoxide from the mitochondrial respiratory chain (33, 171). In an elegant study, the complete signaling pathway that leads from impairment of ATPase activity to apoptotic cell death has recently been elucidated (171). Bz-423-induced superoxide activates cytosolic ASK1 and its release from thioredoxin, followed by a mitogen-activated protein kinase cascade leading to the specific phosphorylation of JNK. JNK-activation of the proapoptotic effectors Bax and Bak then induces mitochondrial outer membrane permeabilization with release of cytochrome c, followed by caspase activation and cell death. Based on the hyperproliferative nature shared between psoriatic skin disease and tumorigenesis, it is tempting to speculate that the drug-like molecule Bz-423 represents a promising lead compound for the identification and development of other prooxidant agents that induce apoptosis in hyperproliferative cells through ligand-based inhibition of mitochondrial ATP synthase.
2. Targeting VDACs: Erastin
a. Erastin
A striking example of a redox-inactive mitochondrially targeted drug-like agent that upregulates cellular oxidative stress and preferentially induces cell death in cancer cells is provided by the recent discovery of the HRAS-oncogene-specific redox chemotherapeutic erastin. The synthetic quinazolinone-derivative 2-(1-{4-[2-(4-chloro-phenoxy)-acetyl]-piperazin-1-yl}-ethyl)-3-(2-ethoxyphenyl)-3H-quinazolin-4-one (erastin; Fig. 15-79) was recently identified as an active hit contained in a library of 24,000 compounds that were screened for preferential cytotoxic activity against human tumour cells containing the v-Ha-ras (Harvey rat sarcoma viral oncogene homologue, HRAS) over isogenic nontumorigenic cells lacking oncogenic RAS (85, 317, 388). Oncogene-selective lethality of erastin was associated with induction of a nonapoptotic oxidative cell death pathway that was blocked by antioxidants, including α-tocopherol, butylated hydroxytoluene, and β-carotene. Erastin displayed greater lethality in human tumor cells harboring mutations in the oncogenes HRAS, KRAS, or BRAF, suggesting a synthetic lethal mode of cytotoxicity, that is, pharmacological cell elimination as a function of oncogene and tumor suppressor gene expression patterns (85, 176, 350, 388, 393). Further investigations demonstrated that mitochondrial voltage-dependent anion channels (VDACs) are crucial molecular targets of erastin since RNA interference-mediated knockdown of VDAC2 or VDAC3 caused drug resistance. Moreover, direct binding of radiolabeled erastin to VDAC2 was demonstrated. Erastin–VDAC interaction alters outer mitochondrial membrane permeability, leading to mitochondrial functional impairment and induction of cellular oxidative stress. Based on these promising preliminary data, it may be concluded that redox-inactive VDAC ligands such as erastin represent a novel class of redox chemotherapeutics that induce oncogene-dependent oxidative cell death (317).
b. RSL5
Indeed, synthetic lethal screening has identified more small molecule prototype agents that activate a nonapoptotic oxidative cell death in oncogenic-RAS-harboring cancer cells. Interestingly, oxidative cell death by the prototype agent RSL5 (Fig. 15-80) occurred in a MEK- and iron-dependent manner and was blocked by RNA interference targeting voltage-dependent anion channel 3 (VDAC3), reminiscent of erastin–VDAC interactions (317, 393). A mechanistic link between expression of oncogenic RAS and preferential sensitivity to ROS-dependent cell death induced by VDAC-targeting small molecules was suggested by the finding that oncogenic RAS-transformed cells display increased iron content relative to their normal cell counterparts through upregulation of transferrin receptor 1 and downregulation of ferritin heavy chain 1 and ferritin light chain. Upregulation of cellular levels of redox active iron induced by oncogenic transformation may represent a mechanism of preferential redox sensitivity and may also explain sensitivity of cancer cells to iron ion-activated peroxide drugs such as artemisinins (as mentioned in Section II.A).
C. Targeting tumor hypoxia
It is now established that hypoxia is an important tumor phenotype that contributes to tumorigenesis and tumor progression (42, 339). Hypoxic tumors display enhanced malignancy, metastatic potential, and a degree of radio- and chemoresistance that can pose a formidable obstacle to chemotherapeutic intervention (102). Hypoxic conditions in tumors originate from insufficient tumor vascularization and oxygenation, selecting for genetic alterations and metabolic adaptations that favor tumorigenesis under adverse conditions of limited oxygen supply. Tumor hypoxia represents a phenotypic alteration charcteristic of tumor tissue and is therefore an important drug target for various classes of anticancer therapeutics currently in preclinical and clinical development. For example, recent studies have revealed that low concentrations of established nitric oxide-mimetic drugs, including glyceryl trinitrate and isosorbide dinitrate, can overcome hypoxia-induced drug resistance through activation of NO signaling involving activation of soluble guanylate cyclase, generation of cyclic GMP, and activation of cGMP-dependent protein kinase. Indeed, nitric oxide mimetics exert potent chemosensitization to doxorubicin as assessed in a mouse xenograft model of human prostate cancer (113, 332). The following section focuses on hypoxia-selective redox drugs, an important class of developmental redox chemotherapeutics (42, 364).
1. Hypoxia-activated redox chemotherapeutics: TPZ, AQ4N, and PR-104
Bioreductive activation of hypoxia-selective prodrugs initiates the formation of cytotoxic organic free radical and other reactive intermediates that kill cells under hypoxic conditions, but spare cells under conditions of normoxia due to oxygen-dependent electron transfer reactions that lead to inactivation of the cytotoxic free radical intermediate (42, 364). Importantly, dependence on bioreductive activation through enzymes upregulated in the hypoxic tumor environment including NADPH:cytochrome P450 reductase, NQO1, nitric oxide synthase, and xanthine oxidase may contribute to the therapeutic window achieved by this class of anticancer redox chemotherapeutics (24, 42, 312, 364).
a. Tirapazamine
The prototypic hypoxia-activated redox chemotherapeutic tirapazamine (3-amino-1,2,4-benzotriazine 1,4-dioxide, TPZ; Fig. 16-81) directly causes DNA strand breakage after single electron-reduction with formation of a cytotoxic drug-free radical. This free radical with high one-electron reduction potential first generates C1′-deoxyribose-located DNA radicals and then reacts with these radicals through transferring an oxygen atom, converting them into base-labile lesions in the complete absence of oxygen (159). According to the established chemical mechanism thought to underlie drug selectivity, normoxic conditions in untransformed tissue allow spontaneous electron transfer reaction between the oxygen-sensitive TPZ free radical and molecular oxygen, leading to formation of superoxide and regeneration of the drug molecule without DNA free radical damage. In clinical trials, TPZ has displayed modest therapeutic efficacy that could be improved based on careful phenotyping of tumors that display clinical parameters characteristic of hypoxia determined by PET imaging with [18F]-fluoromisonidazole (239). A very limited hypoxia-selectivity of cytotoxicity [as indicated by the hypoxic cytotoxicity ratio (HCR)in vivo: two- to threefold] was observed with this prototype agent under in vivo conditions, far below the level of selectivity achieved in cell culture experiments (HCRin vitro: up to 100-fold) (91). This functional differential translates into a pronounced attenuation of drug efficacy in patients that may originate from insuffient drug accumulation in the target tissue. In an attempt to overcome pharmacokinetic limitations associated with first generation hypoxia-selective redox chemotherapeutics, recent drug discovery has focused on the identification of novel agents with optimized extravascular transport and antitumor activity including 6-morpholinopropyloxy-1,2,4-benzotriazine 1,4-dioxide (Fig. 16-82) that display enhanced HCRs as determined in specialized three-dimensional cell culture models and tumor xenografts (145, 146, 148).
FIG. 16.
Redox chemotherapeutics targeting tumor hypoxia. Tumor tissue hypoxia (illustrated by the shaded triangular pO2 gradient) can be targeted by (I) hypoxia-selective redox drugs, an important class of developmental redox chemotherapeutics, including the bioreductively activated hypoxia-selective prodrug tirapazamine (81) and its improved derivative 6-morpholinopropyloxy-1,2,4-benzotriazine 1,4-dioxide (82), the hypoxia-activated topoisomerase II-inhibitor prodrug AQ4N (83), the nitrogen mustard prodrug PR-104 (84), and (II) the HIF antagonist PX-478 (85). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
b. AQ4N and PR-104
Other hypoxia-activated prodrugs with different hypoxia-activatable, cytotoxicity-determining pharmacophores such as AQ4N and PR-104 have advanced into clinical testing (42). AQ4N (banoxantrone; 1,4-bis{[2-(dimethylamino)ethyl]amino}-5,8-dihydroxyanthracene-9,10-dione bis-N-oxide, Fig. 16-83), is a noncytotoxic N-oxide prodrug preferentially converted in hypoxic tumor regions to the ditertiary cationic amine AQ4, a potent inhibitor of topoisomerase II displaying high affinity to DNA that limits diffusion of AQ4 beyond the tumor microenvironment (272). In a recent Phase I open-label, accelerated dose escalation study, AQ4N was well tolerated without toxicity at the maximum tolerated dose (768 mg/m2) (272). AQ4N is currently examined in a Phase I/II clinical trial in combination with radiotherapy and temozolomide in patients with newly diagnosed glioblastoma multiforme (ClinicalTrials.gov Identifier: NCT00394628). In another ongoing Phase I/II study, AQ4N is tested in patients with lymphoid neoplasms (ClinicalTrials.gov Identifier: NCT00109356).
The hypoxia-activated nitrogen mustard prodrug PR-104 (Fig. 16-84) is a water-soluble phosphate ester, first hydrolyzed systemically to the alcohol form. Subsequently, the electron-withdrawing nitro-group in para position to the mustard functional group is bioreductively transformed and thereby transformed into an electron-donating hydroxylamine- or amine-substituent that acts as a nucleophilic trigger for the activation of the alkylating mustard-group resulting in hypoxia-specific DNA crosslinking and cytotoxicity. It has also been demonstrated that PR-104 can be used in the context of Clostridium-directed enzyme prodrug therapy (CDEPT), a novel therapeutic strategy that targets hypoxic regions of solid tumors using a genetically engineered nonpathogenic strain of the bacterial genus Clostridium that expresses prodrug-activating nitroreductase only in hypoxic target tissues (220). A multicenter, open-label, dose escalation Phase I trial examines safety and pharmacokinetics of intravenous PR-104 given with prophylactic G-CSF in patients with solid tumors, and hypoxia in solid tumors is determined using using 18F-fluoromisonidazole PET imaging (ClinicalTrials.gov Identifier: NCT00616213). PR-104 is also tested in a Phase II trial in patients with previously untreated or relapsed small cell lung cancer (ClinicalTrials.gov Identifier: NCT00544674).
2. Targeting HIF-1α: PX-478
Obviously, the transcription factor subunit hypoxia-inducible factor-1alpha (HIF-1α), due to its established role as the master regulator of hypoxia-associated gene expression supporting malignant progression and chemoresistance, is a major molecular target for drug discovery that aims at redox and metabolic adaptations of tumors. HIF-1 activity is regulated by the availability of the HIF-1α subunit, which accumulates under hypoxic conditions. Under normoxia, HIF-1α is hydroxylated on prolines 402 and 564 and is targeted for ubiquitin-mediated degradation by interacting with the von Hippel–Lindau protein complex (pVHL) (368). It should be mentioned that another oxygen-regulated subunit (HIF-2α) may play an analogous proangiogenic role during embryogenesis and wound healing and may also be involved in tumor vascularization, as recently demonstrated in a mouse model of neuroblastoma (103, 226). Importantly, recent data indicate that HIF-2α may be expressed in tumor macrophages and neuroblastoma cells located in well-vascularized tumor regions maintaining tumor aggressiveness under normoxic conditions (226). Genetic target validation has been achieved in mutiple test systems (e.g., by demonstrating that targeting HIF-1α with shRNA via transferrin receptor-mediated endocytosis inhibits melanoma growth in vivo), an important pilot study that suggests feasibility of therapeutic anticancer intervention based on genetic antagonism of a redox target (222). Moreover, knockdown of HIF-2α by siRNA in cultured neuroblastoma cells, subsequently injected subcutaneously, delayed tumor growth in nude mice (150).
a. PX-478
Recently, a small molecule inhibitor of constitutive and hypoxia-induced HIF-1α levels and HIF activity, PX-478 (S-2-amino-3-[4′-N,N,-bis(2-chloroethyl)amino]phenyl propionic acid N-oxide dihydrochloride; Fig. 16-85), was identified based on activity screening for inhibitors of HIF transcriptional activity in a luciferase based reporter assay. PX-478 inhibits expression of HIF-1 target genes including VEGF and GLUT-1, displays potent anticancer activity in a multitude of xenograft models (368), and is also active as a radiosensitzer (269). Importantly, the antitumor response to PX-478 is positively correlated with tumor HIF-1α levels (368). In a recent study, it was demonstrated that hypoxia-induced VEGF formation was inhibited by PX-478, whereas baseline levels of VEGF in normoxia were unaffected, suggesting that PX-478 may not be associated with adverse drug reactions that could potentially result from complete blockade of VEGF-dependent angiogenesis in nontumor tissue (196). Moreover, PX-478 was shown to decrease levels of HIF-1α mRNA and to inhibit translation, suggesting that PX-478 is a multitargeted inhibitor of HIF-1α that does not act through HIF-1α binding (196). PX-478 has entered clinical testing, and the maximum tolerated dose of oral PX-478 is determined in patients with advanced solid tumors or lymphoma (ClinicalTrials.gov Identifier: NCT00522652).
V. Conclusions
A significant number of attractive molecular cancer targets, many of which are amenable to redox intervention by small molecule therapeutics, have now been identified and validated. Lead optimization of prototype redox chemotherapeutics from natural and synthetic sources has led to the development of advanced clinical candidates with pharmacophores that display attenuated and more targeted redox reactivity with less off-target toxicity. In addition, structure-based approaches have identifed redox-silent pharmacophores that target cancer cell redox status and signaling through ligand-based interactions. It seems particularly promising that prototype studies have demonstrated feasibility of gene-based redox intervention ranging from intratumoral siRNA injection to adenovirus-based gene therapy (25, 35, 340, 110, 150, 222, 319). These recent developments may therefore herald a new generation of gene-based therapeutics that modulate expression of crucial redox targets in cancer cells, as discussed throughout this review. The impressive number of ongoing clinical trials that examine therapeutic performance of these novel redox drugs in cancer patients demonstrates that redox chemotherapy has made the crucial transition from bench to bedside. Further translational research will be necessary to enhance the therapeutic benefit provided by early developmental candidates, but it is now evident that redox drugs represent a significant expansion of the chemotherapeutic armamentarium providing novel weapons that promise to impact the ongoing war on cancer.
Abbreviations Used
- 17-AAG
17-allylamino-17-demethoxygeldanamycin
- AP-1
activator protein 1
- APE/Ref-1
apurinic/apyrimidinic endonuclease/redox effector factor-1
- APL
acute promyelocytic leukemia
- ARE
antioxidant response element
- ASK1
apoptosis signal-regulating kinase 1
- 3-BrPA
3-bromopyruvate
- BSO
L-buthionine-S,R-sulfoximine
- CA
trans-cinnamic aldehyde (cinnamaldehyde)
- CDC25
cell division cycle 25 phosphatase
- CML
chronic myelogenous leukemia
- DADS
diallyldisulfide
- DATS
diallyltrisulfide
- DCA
dichloroacetic acid
- 2-DG
2-deoxy-D-glucose
- DIM
3,3′-diindolylmethane
- 17-DMAG
17-dimethylaminoethylamino-17-demethoxy-geldanamycin
- DMAPT
dimethylamino-parthenolide
- DSF
disulfiram
- ERE
estrogen responsive element
- ER
estrogen receptor
- GSSG
glutathione disulfide
- GSTπ
glutathione-S-transferase π
- HIF-1α
hypoxia-inducible factor-1α
- HO-1
heme oxygenase-1
- H2O2
hydrogen peroxide
- Keap1
Kelch-like ECH-associated protein 1
- 2-ME
2-methoxyestradiol
- MGd
motexafin gadolinium
- MnTBAP
manganese (III) tetrakis-(5,10,15,20)-benzoic acid porphyrin
- NFκB
nuclear factor κB
- NO
nitric oxide
- NQO1
NAD(P)H:quinone oxidoreductase
- Nrf2
nuclear factor-E2-related factor 2
- NSAID
nonsteroidal anti-inflammatory drug
- NSCLC
non-small cell lung cancer
- PEITC
β-phenyethyl-isothiocyanate
- PET
positron emission tomography
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RSS
reactive sulfur species
- SAR
structure activity relationship
- TEMPO
2, 2, 6, 6-tetramethylpiperidine-1-oxyl
- TKTL1
transketolase-like protein 1
- α-TOS
α-tocopherylsuccinate ester
- Trx
thioredoxin
- VDAC
voltage-dependent anion channel
- VEGF
vascular endothelial growth factor
Acknowledgments
Supported in part by grants from the National Institutes of Health [Award Number R01CA122484 from the National Cancer Institute, ES06694, Arizona Cancer Center Support Grant CA023074]. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Figure illustrations were produced using Servier Medical Art. www.servier.com
References
- 1.Adachi M. Sakamoto H. Kawamura R. Wang W. Imai K. Shinomura Y. Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer cells. Histol Histopathol. 2007;22:437–442. doi: 10.14670/HH-22.437. [DOI] [PubMed] [Google Scholar]
- 2.Adam–Vizi V. Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Adams BK. Cai J. Armstrong J. Herold M. Lu YJ. Sun A. Snyder JP. Liotta DC. Jones DP. Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad IM. Aykin-Burns N. Sim JE. Walsh SA. Higashikubo R. Buettner GR. Venkataraman S. Mackey MA. Flanagan SW. Oberley LW. Spitz DR. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 5.Alexandre J. Batteux F. Nicco C. Chereau C. Laurent A. Guillevin L. Weill B. Goldwasser F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41–48. doi: 10.1002/ijc.21685. [DOI] [PubMed] [Google Scholar]
- 6.Alexandre J. Hu Y. Lu W. Pelicano H. Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 7.Alexandre J. Nicco C. Chereau C. Laurent A. Weill B. Goldwasser F. Batteux F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst. 2006;98:236–244. doi: 10.1093/jnci/djj049. [DOI] [PubMed] [Google Scholar]
- 8.Allan JM. Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5:943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 9.Amato RJ. Jac J. Hernandez–McClain J. Motexafin gadolinium for the treatment of metastatic renal cell carcinoma: phase II study results. Clin Genitourin Cancer. 2008;6:73–78. doi: 10.3816/CGC.2008.n.011. [DOI] [PubMed] [Google Scholar]
- 10.Antosiewicz J. Herman–Antosiewicz A. Marynowski SW. Singh SV. c-Jun NH(2)-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006;66:5379–5386. doi: 10.1158/0008-5472.CAN-06-0356. [DOI] [PubMed] [Google Scholar]
- 11.Antosiewicz J. Ziolkowski W. Kar S. Powolny AA. Singh SV. Role of reactive oxygen intermediates in cellular responses to dietary cancer chemopreventive agents. Planta Med. 2008;74:1570–1579. doi: 10.1055/s-2008-1081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asayama S. Kasugai N. Kubota S. Nagaoka S. Kawakami H. Superoxide dismutase as a target enzyme for Fe-porphyrin-induced cell death. J Inorg Biochem. 2007;101:261–266. doi: 10.1016/j.jinorgbio.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Asgari MM. Maruti SS. White E. A large cohort study of nonsteroidal anti-inflammatory drug use and melanoma incidence. J Natl Cancer Inst. 2008;100:967–971. doi: 10.1093/jnci/djn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asher G. Dym O. Tsvetkov P. Adler J. Shaul Y. The crystal structure of NAD(P)H quinone oxidoreductase 1 in complex with its potent inhibitor dicoumarol. Biochemistry. 2006;45:6372–6378. doi: 10.1021/bi0600087. [DOI] [PubMed] [Google Scholar]
- 15.Aykin–Burns N. Ahmad IM. Zhu Y. Oberley LW. Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad MB. Chen Y. Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 17.Bael TE. Peterson BL. Gollob JA. Phase II trial of arsenic trioxide and ascorbic acid with temozolomide in patients with metastatic melanoma with or without central nervous system metastases. Melanoma Res. 2008;18:147–151. doi: 10.1097/CMR.0b013e3282f2a7ae. [DOI] [PubMed] [Google Scholar]
- 18.Bahlis NJ. McCafferty–Grad J. Jordan–McMurry I. Neil J. Reis I. Kharfan–Dabaja M. Eckman J. Goodman M. Fernandez HF. Boise LH. Lee KP. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2002;8:3658–3668. [PubMed] [Google Scholar]
- 19.Baker AF. Dragovich T. Tate WR. Ramanathan RK. Roe D. Hsu CH. Kirkpatrick DL. Powis G. The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med. 2006;147:83–90. doi: 10.1016/j.lab.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker AF. Koh MY. Williams RR. James B. Wang H. Tate WR. Gallegos A. Von Hoff DD. Han H. Powis G. Identification of thioredoxin-interacting protein 1 as a hypoxia-inducible factor 1alpha-induced gene in pancreatic cancer. Pancreas. 2008;36:178–186. doi: 10.1097/MPA.0b013e31815929fe. [DOI] [PubMed] [Google Scholar]
- 21.Baker AF. Landowski T. Dorr R. Tate WR. Gard JM. Tavenner BE. Dragovich T. Coon A. Powis G. The antitumor agent imexon activates antioxidant gene expression: Evidence for an oxidative stress response. Clin Cancer Res. 2007;13:3388–3394. doi: 10.1158/1078-0432.CCR-06-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnett KT. Fokum FD. Malafa MP. Vitamin E succinate inhibits colon cancer liver metastases. J Surg Res. 2002;106:292–298. doi: 10.1006/jsre.2002.6466. [DOI] [PubMed] [Google Scholar]
- 23.Beerheide W. Sim MM. Tan YJ. Bernard HU. Ting AE. Inactivation of the human papillomavirus-16 E6 oncoprotein by organic disulfides. Bioorg Med Chem. 2000;8:2549–2560. doi: 10.1016/s0968-0896(00)00193-0. [DOI] [PubMed] [Google Scholar]
- 24.Begleiter A. Leith MK. Patel D. Hasinoff BB. Role of NADPH cytochrome P450 reductase in activation of RH1. Cancer Chemother Pharmacol. 2007;60:713–723. doi: 10.1007/s00280-007-0417-8. [DOI] [PubMed] [Google Scholar]
- 25.Benlloch M. Mena S. Ferrer P. Obrador E. Asensi M. Pellicer JA. Carretero J. Ortega A. Estrela JM. Bcl-2 and Mn-SOD antisense oligodeoxynucleotides and a glutamine-enriched diet facilitate elimination of highly resistant B16 melanoma cells by tumor necrosis factor-alpha and chemotherapy. J Biol Chem. 2006;281:69–79. doi: 10.1074/jbc.M507471200. [DOI] [PubMed] [Google Scholar]
- 26.Bensaad K. Tsuruta A. Selak MA. Vidal MN. Nakano K. Bartrons R. Gottlieb E. Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 27.Berenson JR. Matous J. Swift RA. Mapes R. Morrison B. Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007;13:1762–1768. doi: 10.1158/1078-0432.CCR-06-1812. [DOI] [PubMed] [Google Scholar]
- 28.Berger TG. Dieckmann D. Efferth T. Schultz ES. Funk JO. Baur A. Schuler G. Artesunate in the treatment of metastatic uveal melanoma—First experiences. Oncol Rep. 2005;14:1599–1603. [PubMed] [Google Scholar]
- 29.Berkenblit A. Eder JP., Jr. Ryan DP. Seiden MV. Tatsuta N. Sherman ML. Dahl TA. Dezube BJ. Supko JG. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin Cancer Res. 2007;13:584–590. doi: 10.1158/1078-0432.CCR-06-0964. [DOI] [PubMed] [Google Scholar]
- 30.Berndtsson M. Hagg M. Panaretakis T. Havelka AM. Shoshan MC. Linder S. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int J Cancer. 2007;120:175–180. doi: 10.1002/ijc.22132. [DOI] [PubMed] [Google Scholar]
- 31.Bernhardt PV. Wilson GJ. Sharpe PC. Kalinowski DS. Richardson DR. Tuning the antiproliferative activity of biologically active iron chelators: Characterization of the coordination chemistry and biological efficacy of 2-acetylpyridine and 2-benzoylpyridine hydrazone ligands. J Biol Inorg Chem. 2008;13:107–119. doi: 10.1007/s00775-007-0300-4. [DOI] [PubMed] [Google Scholar]
- 32.Berry JM. Bradshaw TD. Fichtner I. Ren R. Schwalbe CH. Wells G. Chew EH. Stevens MF. Westwell AD. Quinols as novel therapeutic agents. 2.(1) 4-(1-arylsulfonylindol-2-yl)-4-hydroxycyclohexa-2,5-dien-1-ones and related agents as potent and selective antitumor agents. J Med Chem. 2005;48:639–644. doi: 10.1021/jm040859h. [DOI] [PubMed] [Google Scholar]
- 33.Bhagavathula N. Nerusu KC. Hanosh A. Aslam MN. Sundberg TB. Opipari AW., Jr. Johnson K. Kang S. Glick GD. Varani J. 7-Chloro-5-(4-hydroxyphenyl)-1-methyl-3-(naphthalen-2-ylmethyl)-4,5-dihydr o-1H-benzo[b][1,4]diazepin-2(3H)-one (Bz-423), a benzodiazepine, suppresses keratinocyte proliferation and has antipsoriatic activity in the human skin-severe, combined immunodeficient mouse transplant model. J Pharmacol Exp Ther. 2008;324:938–947. doi: 10.1124/jpet.107.130955. [DOI] [PubMed] [Google Scholar]
- 34.Blanchetot C. Boonstra J. The ROS-NOX connection in cancer and angiogenesis. Crit Rev Eukaryot Gene Expr. 2008;18:35–45. doi: 10.1615/critreveukargeneexpr.v18.i1.30. [DOI] [PubMed] [Google Scholar]
- 35.Blander G. de Oliveira RM. Conboy CM. Haigis M. Guarente L. Superoxide dismutase 1 knock-down induces senescence in human fibroblasts. J Biol Chem. 2003;278:38966–38969. doi: 10.1074/jbc.M307146200. [DOI] [PubMed] [Google Scholar]
- 36.Boivin B. Zhang S. Arbiser JL. Zhang ZY. Tonks NK. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc Natl Acad Sci USA. 2008;105:9959–9964. doi: 10.1073/pnas.0804336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnet S. Archer SL. Allalunis–Turner J. Haromy A. Beaulieu C. Thompson R. Lee CT. Lopaschuk GD. Puttagunta L. Bonnet S. Harry G. Hashimoto K. Porter CJ. Andrade MA. Thebaud B. Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Bradshaw TD. Matthews CS. Cookson J. Chew EH. Shah M. Bailey K. Monks A. Harris E. Westwell AD. Wells G. Laughton CA. Stevens MF. Elucidation of thioredoxin as a molecular target for antitumor quinols. Cancer Res. 2005;65:3911–3919. doi: 10.1158/0008-5472.CAN-04-4141. [DOI] [PubMed] [Google Scholar]
- 39.Brar SS. Grigg C. Wilson KS. Holder WD., Jr. Dreau D. Austin C. Foster M. Ghio AJ. Whorton AR. Stowell GW. Whittall LB. Whittle RR. White DP. Kennedy TP. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Mol Cancer Ther. 2004;3:1049–1060. [PubMed] [Google Scholar]
- 40.Brigelius–Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 41.Brown DP. Chin–Sinex H. Nie B. Mendonca MS. Wang M. Targeting superoxide dismutase 1 to overcome cisplatin resistance in human ovarian cancer. Cancer Chemother Pharmacol. 2009;63:723–730. doi: 10.1007/s00280-008-0791-x. [DOI] [PubMed] [Google Scholar]
- 42.Brown JM. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:297–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]
- 43.Brown KK. Eriksson SE. Arner ES. Hampton MB. Mitochondrial peroxiredoxin 3 is rapidly oxidized in cells treated with isothiocyanates. Free Radic Biol Med. 2008;45:494–502. doi: 10.1016/j.freeradbiomed.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Buerkle A. Weber WA. Imaging of tumor glucose utilization with positron emission tomography. Cancer Metastasis Rev. 2008;27:545–554. doi: 10.1007/s10555-008-9151-x. [DOI] [PubMed] [Google Scholar]
- 45.Burkitt MJ. Bishop HS. Milne L. Tsang SY. Provan GJ. Nobel CS. Orrenius S. Slater AF. Dithiocarbamate toxicity toward thymocytes involves their copper-catalyzed conversion to thiuram disulfides, which oxidize glutathione in a redox cycle without the release of reactive oxygen species. Arch Biochem Biophys. 1998;353:73–84. doi: 10.1006/abbi.1998.0618. [DOI] [PubMed] [Google Scholar]
- 46.Bush JA. Cheung KJ., Jr. Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 47.Cabello CM. Bair WB., 3rd Lamore SD. Ley S. Bause AS. Azimian S. Wondrak GT. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med. 2009;46:220–231. doi: 10.1016/j.freeradbiomed.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabello CM. Bair WB., 3rd Wondrak GT. Experimental therapeutics: Targeting the redox Achilles heel of cancer. Curr Opin Investig Drugs. 2007;8:1022–1037. [PubMed] [Google Scholar]
- 49.Callister ME. Pinhu L. Catley MC. Westwell AD. Newton R. Leaver SK. Quinlan GJ. Evans TW. Griffiths MJ. Burke–Gaffney A. PMX464, a thiol-reactive quinol and putative thioredoxin inhibitor, inhibits NF-kappaB-dependent proinflammatory activation of alveolar epithelial cells. Br J Pharmacol. 2008;155:661–672. doi: 10.1038/bjp.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camp ER. Summy J. Bauer TW. Liu W. Gallick GE. Ellis LM. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res. 2005;11:397–405. [PubMed] [Google Scholar]
- 51.Cano CE. Gommeaux J. Pietri S. Culcasi M. Garcia S. Seux M. Barelier S. Vasseur S. Spoto RP. Pebusque MJ. Dusetti NJ. Iovanna JL. Carrier A. Tumor protein 53-induced nuclear protein 1 is a major mediator of p53 antioxidant function. Cancer Res. 2009;69:219–226. doi: 10.1158/0008-5472.CAN-08-2320. [DOI] [PubMed] [Google Scholar]
- 52.Casini A. Scozzafava A. Supuran CT. Cysteine-modifying agents: a possible approach for effective anticancer and antiviral drugs. Environ Health Perspect. 2002;110:801–806. doi: 10.1289/ehp.02110s5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cen D. Brayton D. Shahandeh B. Meyskens FL., Jr. Farmer PJ. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47:6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 54.Chan K. Poon R. O'Brien PJ. Application of structure-activity relationships to investigate the molecular mechanisms of hepatocyte toxicity and electrophilic reactivity of alpha, beta-unsaturated aldehydes. J Appl Toxicol. 2008;28:1027–1039. doi: 10.1002/jat.1369. [DOI] [PubMed] [Google Scholar]
- 55.Chang HS. Bae SM. Kim YW. Kwak SY. Min HJ. Bae IJ. Lee YJ. Shin JC. Kim CK. Ahn WS. Comparison of diarsenic oxide and tetraarsenic oxide on anticancer effects: Relation to the apoptosis molecular pathway. Int J Oncol. 2007;30:1129–1135. [PubMed] [Google Scholar]
- 56.Chang J. Voorhees P. Kolesar J. Ahuja H. Sanchez F. Rodriguez G. Kim K. Werndli J. Bailey H. Kahl B. Phase II study of arsenic trioxide and ascorbic acid for relapsed or refractory lymphoid malignancies: A Wisconsin Oncology Network study. Hematol Oncol. 2009;27:11–16. doi: 10.1002/hon.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chatterji T. Gates KS. DNA cleavage by 7-methylbenzopentathiepin: a simple analog of the antitumor antibiotic varacin. Bioorg Med Chem Lett. 1998;8:535–538. doi: 10.1016/s0960-894x(98)00066-3. [DOI] [PubMed] [Google Scholar]
- 58.Chatterji T. Keerthi K. Gates KS. Generation of reactive oxygen species by a persulfide (BnSSH) Bioorg Med Chem Lett. 2005;15:3921–3924. doi: 10.1016/j.bmcl.2005.05.110. [DOI] [PubMed] [Google Scholar]
- 59.Chatterji T. Kizil M. Keerthi K. Chowdhury G. Pospisil T. Gates KS. Small molecules that mimic the thiol-triggered alkylating properties seen in the natural product leinamycin. J Am Chem Soc. 2003;125:4996–4997. doi: 10.1021/ja029169y. [DOI] [PubMed] [Google Scholar]
- 60.Chen D. Cui QC. Yang H. Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 61.Chen J. Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer. 2005;5:102–112. doi: 10.1038/nrc1547. [DOI] [PubMed] [Google Scholar]
- 62.Chen Q. Espey MG. Krishna MC. Mitchell JB. Corpe CP. Buettner GR. Shacter E. Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Q. Espey MG. Sun AY. Lee JH. Krishna MC. Shacter E. Choyke PL. Pooput C. Kirk KL. Buettner GR. Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Q. Espey MG. Sun AY. Pooput C. Kirk KL. Krishna MC. Khosh DB. Drisko J. Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y. Jungsuwadee P. Vore M. Butterfield DA. St Clair DK. Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y. McMillan–Ward E. Kong J. Israels SJ. Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 67.Chew EH. Lu J. Bradshaw TD. Holmgren A. Thioredoxin reductase inhibition by antitumor quinols: A quinol pharmacophore effect correlating to antiproliferative activity. FASEB J. 2008;22:2072–2083. doi: 10.1096/fj.07-101477. [DOI] [PubMed] [Google Scholar]
- 68.Choi HS. Shim JS. Kim JA. Kang SW. Kwon HJ. Discovery of gliotoxin as a new small molecule targeting thioredoxin redox system. Biochem Biophys Res Commun. 2007;359:523–528. doi: 10.1016/j.bbrc.2007.05.139. [DOI] [PubMed] [Google Scholar]
- 69.Chow MS. Liu LV. Solomon EI. Further insights into the mechanism of the reaction of activated bleomycin with DNA. Proc Natl Acad Sci USA. 2008;105:13241–13245. doi: 10.1073/pnas.0806378105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu F. O'Brian CA. PKC sulfhydryl targeting by disulfiram produces divergent isozymic regulatory responses that accord with the cancer preventive activity of the thiuram disulfide. Antioxid Redox Signal. 2005;7:855–862. doi: 10.1089/ars.2005.7.855. [DOI] [PubMed] [Google Scholar]
- 71.Clarke JD. Dashwood RH. Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clarke R. Liu MC. Bouker KB. Gu Z. Lee RY. Zhu Y. Skaar TC. Gomez B. O'Brien K. Wang Y. Hilakivi–Clarke LA. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 73.Cullen JJ. Hinkhouse MM. Grady M. Gaut AW. Liu J. Zhang YP. Weydert CJ. Domann FE. Oberley LW. Dicumarol inhibition of NADPH:quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res. 2003;63:5513–5520. [PubMed] [Google Scholar]
- 74.Danson S. Ward TH. Butler J. Ranson M. DT-diaphorase: A target for new anticancer drugs. Cancer Treat Rev. 2004;30:437–449. doi: 10.1016/j.ctrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Das A. Banik NL. Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007;110:1083–1095. doi: 10.1002/cncr.22888. [DOI] [PubMed] [Google Scholar]
- 76.Davis W., Jr. Ronai Z. Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther. 2001;296:1–6. [PubMed] [Google Scholar]
- 77.Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dehn DL. Siegel D. Zafar KS. Reigan P. Swann E. Moody CJ. Ross D. 5-Methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione, a mechanism-based inhibitor of NAD(P)H:quinone oxidoreductase 1, exhibits activity against human pancreatic cancer in vitro and in vivo. Mol Cancer Ther. 2006;5:1702–1709. doi: 10.1158/1535-7163.MCT-06-0105. [DOI] [PubMed] [Google Scholar]
- 79.Dell'Eva R. Pfeffer U. Vene R. Anfosso L. Forlani A. Albini A. Efferth T. Inhibition of angiogenesis in vivo and growth of Kaposi's sarcoma xenograft tumors by the anti-malarial artesunate. Biochem Pharmacol. 2004;68:2359–2366. doi: 10.1016/j.bcp.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 80.Dhillon N. Aggarwal BB. Newman RA. Wolff RA. Kunnumakkara AB. Abbruzzese JL. Ng CS. Badmaev V. Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 81.Diaz Z. Mann KK. Marcoux S. Kourelis M. Colombo M. Komarnitsky PB. Miller WH., Jr. A novel arsenical has antitumor activity toward As2O3-resistant and MRP1/ABCC1-overexpressing cell lines. Leukemia. 2008;22:1853–1863. doi: 10.1038/leu.2008.194. [DOI] [PubMed] [Google Scholar]
- 82.Disbrow GL. Baege AC. Kierpiec KA. Yuan H. Centeno JA. Thibodeaux CA. Hartmann D. Schlegel R. Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res. 2005;65:10854–10861. doi: 10.1158/0008-5472.CAN-05-1216. [DOI] [PubMed] [Google Scholar]
- 83.Doctrow SR. Huffman K. Marcus CB. Tocco G. Malfroy E. Adinolfi CA. Kruk H. Baker K. Lazarowych N. Mascarenhas J. Malfroy B. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J Med Chem. 2002;45:4549–4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 84.Doi K. Akaike T. Fujii S. Tanaka S. Ikebe N. Beppu T. Shibahara S. Ogawa M. Maeda H. Induction of haem oxygenase-1 nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. Br J Cancer. 1999;80:1945–1954. doi: 10.1038/sj.bjc.6690624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dolma S. Lessnick SL. Hahn WC. Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 86.Dominici S. Valentini M. Maellaro E. Del Bello B. Paolicchi A. Lorenzini E. Tongiani R. Comporti M. Pompella A. Redox modulation of cell surface protein thiols in U937 lymphoma cells: The role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27:623–635. doi: 10.1016/s0891-5849(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 87.Donate F. Juarez JC. Burnett ME. Manuia MM. Guan X. Shaw DE. Smith EL. Timucin C. Braunstein MJ. Batuman OA. Mazar AP. Identification of biomarkers for the antiangiogenic and antitumour activity of the superoxide dismutase 1 (SOD1) inhibitor tetrathiomolybdate (ATN-224) Br J Cancer. 2008;98:776–783. doi: 10.1038/sj.bjc.6604226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong LF. Low P. Dyason JC. Wang XF. Prochazka L. Witting PK. Freeman R. Swettenham E. Valis K. Liu J. Zobalova R. Turanek J. Spitz DR. Domann FE. Scheffler IE. Ralph SJ. Neuzil J. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene. 2008;27:4324–4335. doi: 10.1038/onc.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dragovich T. Gordon M. Mendelson D. Wong L. Modiano M. Chow HH. Samulitis B. O'Day S. Grenier K. Hersh E. Dorr R. Phase I trial of imexon in patients with advanced malignancy. J Clin Oncol. 2007;25:1779–1784. doi: 10.1200/JCO.2006.08.9672. [DOI] [PubMed] [Google Scholar]
- 90.Du J. Daniels DH. Asbury C. Venkataraman S. Liu J. Spitz DR. Oberley LW. Cullen JJ. Mitochondrial production of reactive oxygen species mediate dicumarol-induced cytotoxicity in cancer cells. J Biol Chem. 2006;281:37416–37426. doi: 10.1074/jbc.M605063200. [DOI] [PubMed] [Google Scholar]
- 91.Durand RE. Olive PL. Physiologic and cytotoxic effects of tirapazamine in tumor-bearing mice. Radiat Oncol Investig. 1997;5:213–219. doi: 10.1002/(SICI)1520-6823(1997)5:5<213::AID-ROI1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 92.Dvorakova K. Payne CM. Tome ME. Briehl MM. McClure T. Dorr RT. Induction of oxidative stress and apoptosis in myeloma cells by the aziridine-containing agent imexon. Biochem Pharmacol. 2000;60:749–758. doi: 10.1016/s0006-2952(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 93.Dvorakova K. Waltmire CN. Payne CM. Tome ME. Briehl MM. Dorr RT. Induction of mitochondrial changes in myeloma cells by imexon. Blood. 2001;97:3544–3551. doi: 10.1182/blood.v97.11.3544. [DOI] [PubMed] [Google Scholar]
- 94.Efferth T. Mechanistic perspectives for 1,2,4-trioxanes in anticancer therapy. Drug Resist Updat. 2005;8:85–97. doi: 10.1016/j.drup.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Efferth T. Molecular pharmacology and pharmacogenomics of artemisinin and its derivatives in cancer cells. Curr Drug Targets. 2006;7:407–421. doi: 10.2174/138945006776359412. [DOI] [PubMed] [Google Scholar]
- 96.Efferth T. Willmar Schwabe Award 2006: Antiplasmodial and antitumor activity of artemisinin—From bench to bedside. Planta Med. 2007;73:299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- 97.Efferth T. Briehl MM. Tome ME. Role of antioxidant genes for the activity of artesunate against tumor cells. Int J Oncol. 2003;23:1231–1235. [PubMed] [Google Scholar]
- 98.Evens AM. Balasubramanian L. Gordon LI. Motexafin gadolinium induces oxidative stress and apoptosis in hematologic malignancies. Curr Treat Options Oncol. 2005;6:289–296. doi: 10.1007/s11864-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 99.Fang J. Lu J. Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 100.Fang J. Sawa T. Akaike T. Akuta T. Sahoo SK. Khaled G. Hamada A. Maeda H. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003;63:3567–3574. [PubMed] [Google Scholar]
- 101.Fang J. Sawa T. Akaike T. Greish K. Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109:1–8. doi: 10.1002/ijc.11644. [DOI] [PubMed] [Google Scholar]
- 102.Fang JS. Gillies RD. Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin Cancer Biol. 2008;18:330–337. doi: 10.1016/j.semcancer.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Favier J. Lapointe S. Maliba R. Sirois MG. HIF2 alpha reduces growth rate but promotes angiogenesis in a mouse model of neuroblastoma. BMC Cancer. 2007;7:139. doi: 10.1186/1471-2407-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Filomeni G. Aquilano K. Civitareale P. Rotilio G. Ciriolo MR. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastoma cells. Free Radic Biol Med. 2005;39:345–354. doi: 10.1016/j.freeradbiomed.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 105.Filomeni G. Aquilano K. Rotilio G. Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63:5940–5949. [PubMed] [Google Scholar]
- 106.Filomeni G. Rotilio G. Ciriolo MR. Glutathione disulfide induces apoptosis in U937 cells by a redox-mediated p38 MAP kinase pathway. FASEB J. 2003;17:64–66. doi: 10.1096/fj.02-0105fje. [DOI] [PubMed] [Google Scholar]
- 107.Filomeni G. Turella P. Dupuis ML. Forini O. Ciriolo MR. Cianfriglia M. Pezzola S. Federici G. Caccuri AM. 6-(7-Nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol, a specific glutathione S-transferase inhibitor, overcomes the multidrug resistance (MDR)-associated protein 1-mediated MDR in small cell lung cancer. Mol Cancer Ther. 2008;7:371–379. doi: 10.1158/1535-7163.MCT-07-0487. [DOI] [PubMed] [Google Scholar]
- 108.Finch JS. Tome ME. Kwei KA. Bowden GT. Catalase reverses tumorigenicity in a malignant cell line by an epidermal growth factor receptor pathway. Free Radic Biol Med. 2006;40:863–875. doi: 10.1016/j.freeradbiomed.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 109.Findlay VJ. Townsend DM. Saavedra JE. Buzard GS. Citro ML. Keefer LK. Ji X. Tew KD. Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol Pharmacol. 2004;65:1070–1079. doi: 10.1124/mol.65.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fishel ML. He Y. Reed AM. Chin–Sinex H. Hutchins GD. Mendonca MS. Kelley MR. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair (Amst) 2008;7:177–186. doi: 10.1016/j.dnarep.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fojo T. Commentary: Novel therapies for cancer: Why dirty might be better. Oncologist. 2008;13:277–283. doi: 10.1634/theoncologist.2007-0090. [DOI] [PubMed] [Google Scholar]
- 112.Foldi M. Stickeler E. Bau L. Kretz O. Watermann D. Gitsch G. Kayser G. Zur Hausen A. Coy JF. Transketolase protein TKTL1 overexpression: A potential biomarker and therapeutic target in breast cancer. Oncol Rep. 2007;17:841–845. [PubMed] [Google Scholar]
- 113.Frederiksen LJ. Sullivan R. Maxwell LR. Macdonald–Goodfellow SK. Adams MA. Bennett BM. Siemens DR. Graham CH. Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clin Cancer Res. 2007;13:2199–2206. doi: 10.1158/1078-0432.CCR-06-1807. [DOI] [PubMed] [Google Scholar]
- 114.Fribley A. Zeng Q. Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fried L. Arbiser JL. The reactive oxygen-driven tumor: Relevance to melanoma. Pigment Cell Melanoma Res. 2008;21:117–122. doi: 10.1111/j.1755-148X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 116.Frohlich DA. McCabe MT. Arnold RS. Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–4362. doi: 10.1038/onc.2008.79. [DOI] [PubMed] [Google Scholar]
- 117.Fruehauf JP. Meyskens FL., Jr. Reactive oxygen species: A breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 118.Fruehauf JP. Trapp V. Reactive oxygen species: An Achilles' heel of melanoma? Expert Rev Anticancer Ther. 2008;8:1751–1757. doi: 10.1586/14737140.8.11.1751. [DOI] [PubMed] [Google Scholar]
- 119.Fruehauf JP. Zonis S. al–Bassam M. Kyshtoobayeva A. Dasgupta C. Milovanovic T. Parker RJ. Buzaid AC. Selective and synergistic activity of L-S,R-buthionine sulfoximine on malignant melanoma is accompanied by decreased expression of glutathione-S-transferase. Pigment Cell Res. 1997;10:236–249. doi: 10.1111/j.1600-0749.1997.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 120.Fry FH. Holme AL. Giles NM. Giles GI. Collins C. Holt K. Pariagh S. Gelbrich T. Hursthouse MB. Gutowski NJ. Jacob C. Multifunctional redox catalysts as selective enhancers of oxidative stress. Org Biomol Chem. 2005;3:2579–2587. doi: 10.1039/b502197a. [DOI] [PubMed] [Google Scholar]
- 121.Fukuyo Y. Inoue M. Nakajima T. Higashikubo R. Horikoshi NT. Hunt C. Usheva A. Freeman ML. Horikoshi N. Oxidative stress plays a critical role in inactivating mutant BRAF by geldanamycin derivatives. Cancer Res. 2008;68:6324–6330. doi: 10.1158/0008-5472.CAN-07-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gallegos A. Gasdaska JR. Taylor CW. Paine–Murrieta GD. Goodman D. Gasdaska PY. Berggren M. Briehl MM. Powis G. Transfection with human thioredoxin increases cell proliferation and a dominant-negative mutant thioredoxin reverses the transformed phenotype of human breast cancer cells. Cancer Res. 1996;56:5765–5770. [PubMed] [Google Scholar]
- 123.Garcia–Pineres AJ. Castro V. Mora G. Schmidt TJ. Strunck E. Pahl HL. Merfort I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 124.Garuti L. Roberti M. Pizzirani D. Synthetic small molecule Cdc25 phosphatases inhibitors. Curr Med Chem. 2008;15:573–580. doi: 10.2174/092986708783769722. [DOI] [PubMed] [Google Scholar]
- 125.Gatenby RA. Gillies RJ. Glycolysis in cancer: A potential target for therapy. Int J Biochem Cell Biol. 2007;39:1358–1366. doi: 10.1016/j.biocel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 126.Gemin A. Sweet S. Preston TJ. Singh G. Regulation of the cell cycle in response to inhibition of mitochondrial generated energy. Biochem Biophys Res Commun. 2005;332:1122–1132. doi: 10.1016/j.bbrc.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 127.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 128.Giles NM. Gutowski NJ. Giles GI. Jacob C. Redox catalysts as sensitisers towards oxidative stress. FEBS Lett. 2003;535:179–182. doi: 10.1016/s0014-5793(02)03890-5. [DOI] [PubMed] [Google Scholar]
- 129.Gius D. Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal. 2006;8:1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 130.Gong X. Kole L. Iskander K. Jaiswal AK. NRH:quinone oxidoreductase 2 and NAD(P)H:quinone oxidoreductase 1 protect tumor suppressor p53 against 20s proteasomal degradation leading to stabilization and activation of p53. Cancer Res. 2007;67:5380–5388. doi: 10.1158/0008-5472.CAN-07-0323. [DOI] [PubMed] [Google Scholar]
- 131.Gong Y. Sohn H. Xue L. Firestone GL. Bjeldanes LF. 3,3′-Diindolylmethane is a novel mitochondrial H(+)-ATP synthase inhibitor that can induce p21(Cip1/Waf1) expression by induction of oxidative stress in human breast cancer cells. Cancer Res. 2006;66:4880–4887. doi: 10.1158/0008-5472.CAN-05-4162. [DOI] [PubMed] [Google Scholar]
- 132.Goplen D. Wang J. Enger PO. Tysnes BB. Terzis AJ. Laerum OD. Bjerkvig R. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006;66:9895–9902. doi: 10.1158/0008-5472.CAN-05-4589. [DOI] [PubMed] [Google Scholar]
- 133.Gottlieb E. Tomlinson IP. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 134.Govindarajan B. Sligh JE. Vincent BJ. Li M. Canter JA. Nickoloff BJ. Rodenburg RJ. Smeitink JA. Oberley L. Zhang Y. Slingerland J. Arnold RS. Lambeth JD. Cohen C. Hilenski L. Griendling K. Martinez–Diez M. Cuezva JM. Arbiser JL. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grad JM. Bahlis NJ. Reis I. Oshiro MM. Dalton WS. Boise LH. Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells. Blood. 2001;98:805–813. doi: 10.1182/blood.v98.3.805. [DOI] [PubMed] [Google Scholar]
- 136.Greer A. On the origin of cytotoxicity of the natural product varacin. A novel example of a pentathiepin reaction that provides evidence for a triatomic sulfur intermediate. J Am Chem Soc. 2001;123:10379–10386. doi: 10.1021/ja016495p. [DOI] [PubMed] [Google Scholar]
- 137.Griffith OW. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982;257:13704–13712. [PubMed] [Google Scholar]
- 138.Gueven N. Luff J. Peng C. Hosokawa K. Bottle SE. Lavin MF. Dramatic extension of tumor latency and correction of neurobehavioral phenotype in Atm-mutant mice with a nitroxide antioxidant. Free Radic Biol Med. 2006;41:992–1000. doi: 10.1016/j.freeradbiomed.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 139.Guo J. Parise RA. Joseph E. Lan J. Pan SS. Joo B. Egorin MJ. Wipf P. Lazo JS. Eiseman JL. Pharmacology and antitumor activity of a quinolinedione Cdc25 phosphatase inhibitor DA3003-1 (NSC 663284) Anticancer Res. 2007;27:3067–3073. [PubMed] [Google Scholar]
- 140.Guo W. Reigan P. Siegel D. Zirrolli J. Gustafson D. Ross D. The bioreduction of a series of benzoquinone ansamycins by NAD(P)H:quinone oxidoreductase 1 to more potent heat shock protein 90 inhibitors, the hydroquinone ansamycins. Mol Pharmacol. 2006;70:1194–1203. doi: 10.1124/mol.106.025643. [DOI] [PubMed] [Google Scholar]
- 141.Gupta A. Rosenberger SF. Bowden GT. Increased ROS levels contribute to elevated transcription factor and MAP kinase activities in malignantly progressed mouse keratinocyte cell lines. Carcinogenesis. 1999;20:2063–2073. doi: 10.1093/carcin/20.11.2063. [DOI] [PubMed] [Google Scholar]
- 142.Guzman ML. Rossi RM. Neelakantan S. Li X. Corbett CA. Hassane DC. Becker MW. Bennett JM. Sullivan E. Lachowicz JL. Vaughan A. Sweeney CJ. Matthews W. Carroll M. Liesveld JL. Crooks PA. Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hail N., Jr. Cortes M. Drake EN. Spallholz JE. Cancer chemoprevention: a radical perspective. Free Radic Biol Med. 2008;45:97–110. doi: 10.1016/j.freeradbiomed.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 144.Hashemy SI. Ungerstedt JS. Zahedi Avval F. Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J Biol Chem. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- 145.Hay MP. Hicks KO. Pchalek K. Lee HH. Blaser A. Pruijn FB. Anderson RF. Shinde SS. Wilson WR. Denny WA. Tricyclic [1,2,4]triazine 1,4-dioxides as hypoxia selective cytotoxins. J Med Chem. 2008;51:6853–6865. doi: 10.1021/jm800967h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hay MP. Pchalek K. Pruijn FB. Hicks KO. Siim BG. Anderson RF. Shinde SS. Phillips V. Denny WA. Wilson WR. Hypoxia-selective 3-alkyl 1,2,4-benzotriazine 1,4-dioxides:The influence of hydrogen bond donors on extravascular transport and antitumor activity. J Med Chem. 2007;50:6654–6664. doi: 10.1021/jm701037w. [DOI] [PubMed] [Google Scholar]
- 147.Hibi T. Nii H. Nakatsu T. Kimura A. Kato H. Hiratake J. Oda J. Crystal structure of gamma-glutamylcysteine synthetase: Insights into the mechanism of catalysis by a key enzyme for glutathione homeostasis. Proc Natl Acad Sci USA. 2004;101:15052–15057. doi: 10.1073/pnas.0403277101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hicks KO. Pruijn FB. Secomb TW. Hay MP. Hsu R. Brown JM. Denny WA. Dewhirst MW. Wilson WR. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J Natl Cancer Inst. 2006;98:1118–1128. doi: 10.1093/jnci/djj306. [DOI] [PubMed] [Google Scholar]
- 149.Hoke EM. Maylock CA. Shacter E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic Biol Med. 2005;39:403–411. doi: 10.1016/j.freeradbiomed.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 150.Holmquist–Mengelbier L. Fredlund E. Lofstedt T. Noguera R. Navarro S. Nilsson H. Pietras A. Vallon–Christersson J. Borg A. Gradin K. Poellinger L. Pahlman S. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 151.Hong F. Freeman ML. Liebler DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 152.Hong SH. Kim J. Kim JM. Lee SY. Shin DS. Son KH. Han DC. Sung YK. Kwon BM. Apoptosis induction of 2′-hydroxycinnamaldehyde as a proteasome inhibitor is associated with ER stress and mitochondrial perturbation in cancer cells. Biochem Pharmacol. 2007;74:557–565. doi: 10.1016/j.bcp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 153.Hosono T. Fukao T. Ogihara J. Ito Y. Shiba H. Seki T. Ariga T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J Biol Chem. 2005;280:41487–41493. doi: 10.1074/jbc.M507127200. [DOI] [PubMed] [Google Scholar]
- 154.Hosono T. Hosono–Fukao T. Inada K. Tanaka R. Yamada H. Iitsuka Y. Seki T. Hasegawa I. Ariga T. Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells. Carcinogenesis. 2008;29:1400–1406. doi: 10.1093/carcin/bgn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang M. Maynard A. Turpin JA. Graham L. Janini GM. Covell DG. Rice WG. Anti-HIV agents that selectively target retroviral nucleocapsid protein zinc fingers without affecting cellular zinc finger proteins. J Med Chem. 1998;41:1371–1381. doi: 10.1021/jm9708543. [DOI] [PubMed] [Google Scholar]
- 156.Huang P. Feng L. Oldham EA. Keating MJ. Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 157.Huang XJ. Ma ZQ. Zhang WP. Lu YB. Wei EQ. Dihydroartemisinin exerts cytotoxic effects and inhibits hypoxia inducible factor-1alpha activation in C6 glioma cells. J Pharm Pharmacol. 2007;59:849–856. doi: 10.1211/jpp.59.6.0011. [DOI] [PubMed] [Google Scholar]
- 158.Hurne AM. Chai CL. Moerman K. Waring P. Influx of calcium through a redox-sensitive plasma membrane channel in thymocytes causes early necrotic cell death induced by the epipolythiodioxopiperazine toxins. J Biol Chem. 2002;277:31631–31638. doi: 10.1074/jbc.M201699200. [DOI] [PubMed] [Google Scholar]
- 159.Hwang JT. Greenberg MM. Fuchs T. Gates KS. Reaction of the hypoxia-selective antitumor agent tirapazamine with a C1'-radical in single-stranded and double-stranded DNA: The drug and its metabolites can serve as surrogates for molecular oxygen in radical-mediated DNA damage reactions. Biochemistry. 1999;38:14248–14255. doi: 10.1021/bi991488n. [DOI] [PubMed] [Google Scholar]
- 160.Igarashi Y. Yabuta Y. Sekine A. Fujii K. Harada K. Oikawa T. Sato M. Furumai T. Oki T. Directed biosynthesis of fluorinated pseurotin A, synerazol and gliotoxin. J Antibiot (Tokyo) 2004;57:748–754. doi: 10.7164/antibiotics.57.748. [DOI] [PubMed] [Google Scholar]
- 161.Irani K. Xia Y. Zweier JL. Sollott SJ. Der CJ. Fearon ER. Sundaresan M. Finkel T. Goldschmidt–Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 162.Ireland DJ. Kissick HT. Beilharz MW. Alpha-Tocopheryl succinate: Toxicity and lack of anti-tumour activity in immuno-competent mice. Food Chem Toxicol. 2008;46:508–512. doi: 10.1016/j.fct.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 163.Isham CR. Tibodeau JD. Jin W. Xu R. Timm MM. Bible KC. Chaetocin: A promising new antimyeloma agent with in vitro and in vivo activity mediated via imposition of oxidative stress. Blood. 2007;109:2579–2588. doi: 10.1182/blood-2006-07-027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ishii T. Yasuda K. Akatsuka A. Hino O. Hartman PS. Ishii N. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res. 2005;65:203–209. [PubMed] [Google Scholar]
- 165.Iyengar BS. Dorr RT. Remers WA. Chemical basis for the biological activity of imexon and related cyanoaziridines. J Med Chem. 2004;47:218–223. doi: 10.1021/jm030225v. [DOI] [PubMed] [Google Scholar]
- 166.Jacob C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat Prod Rep. 2006;23:851–863. doi: 10.1039/b609523m. [DOI] [PubMed] [Google Scholar]
- 167.Jelluma N. Yang X. Stokoe D. Evan GI. Dansen TB. Haas–Kogan DA. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res. 2006;4:319–330. doi: 10.1158/1541-7786.MCR-05-0061. [DOI] [PubMed] [Google Scholar]
- 168.Jeon KI. Byun MS. Jue DM. Gold compound auranofin inhibits IkappaB kinase (IKK) by modifying Cys-179 of IKKbeta subunit. Exp Mol Med. 2003;35:61–66. doi: 10.1038/emm.2003.9. [DOI] [PubMed] [Google Scholar]
- 169.Jeong HW. Han DC. Son KH. Han MY. Lim JS. Ha JH. Lee CW. Kim HM. Kim HC. Kwon BM. Antitumor effect of the cinnamaldehyde derivative CB403 through the arrest of cell cycle progression in the G2/M phase. Biochem Pharmacol. 2003;65:1343–1350. doi: 10.1016/s0006-2952(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 170.Jing Y. Dai J. Chalmers-Redman RM. Tatton WG. Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 171.Johnson KM. Chen X. Boitano A. Swenson L. Opipari AW., Jr. Glick GD. Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem Biol. 2005;12:485–496. doi: 10.1016/j.chembiol.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 172.Johnson KM. Cleary J. Fierke CA. Opipari AW., Jr. Glick GD. Mechanistic basis for therapeutic targeting of the mitochondrial F1F0-ATPase. ACS Chem Biol. 2006;1:304–308. doi: 10.1021/cb600143j. [DOI] [PubMed] [Google Scholar]
- 173.Juarez JC. Betancourt O., Jr. Pirie–Shepherd SR. Guan X. Price ML. Shaw DE. Mazar AP. Donate F. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin Cancer Res. 2006;12:4974–4982. doi: 10.1158/1078-0432.CCR-06-0171. [DOI] [PubMed] [Google Scholar]
- 174.Juarez JC. Manuia M. Burnett ME. Betancourt O. Boivin B. Shaw DE. Tonks NK. Mazar AP. Donate F. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci USA. 2008;105:7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kachadourian R. Liochev SI. Cabelli DE. Patel MN. Fridovich I. Day BJ. 2-methoxyestradiol does not inhibit superoxide dismutase. Arch Biochem Biophys. 2001;392:349–353. doi: 10.1006/abbi.2001.2455. [DOI] [PubMed] [Google Scholar]
- 176.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 177.Kaiser M. Wittlin S. Nehrbass-Stuedli A. Dong Y. Wang X. Hemphill A. Matile H. Brun R. Vennerstrom JL. Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RBx11160) Antimicrob Agents Chemother. 2007;51:2991–2993. doi: 10.1128/AAC.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kakolyris S. Giatromanolaki A. Koukourakis M. Powis G. Souglakos J. Sivridis E. Georgoulias V. Gatter KC. Harris AL. Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res. 2001;7:3087–3091. [PubMed] [Google Scholar]
- 179.Kalinowski DS. Richardson DR. Future of toxicology—Iron chelators and differing modes of action and toxicity: The changing face of iron chelation therapy. Chem Res Toxicol. 2007;20:715–720. doi: 10.1021/tx700039c. [DOI] [PubMed] [Google Scholar]
- 180.Kallifatidis G. Rausch V. Baumann B. Apel A. Beckermann BM. Groth A. Mattern J. Li Z. Kolb A. Moldenhauer G. Altevogt P. Wirth T. Werner J. Schemmer P. Buchler MW. Salnikov AV. Herr I. Sulforaphane targets pancreatic tumor-initiating cells by NF-{kappa}B-induced anti-apoptotic signaling. Gut. 2009;58:949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- 181.Kamb A. Wee S. Lengauer C. Why is cancer drug discovery so difficult? Nat Rev Drug Discov. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 182.Kantarjian H. O'Brien S. Cortes J. Wierda W. Faderl S. Garcia–Manero G. Issa JP. Estey E. Keating M. Freireich EJ. Therapeutic advances in leukemia and myelodysplastic syndrome over the past 40 years. Cancer. 2008;113:1933–1952. doi: 10.1002/cncr.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kar S. Wang M. Yao W. Michejda CJ. Carr BI. PM-20, a novel inhibitor of Cdc25A, induces extracellular signal-regulated kinase 1/2 phosphorylation and inhibits hepatocellular carcinoma growth in vitro and in vivo. Mol Cancer Ther. 2006;5:1511–1519. doi: 10.1158/1535-7163.MCT-05-0485. [DOI] [PubMed] [Google Scholar]
- 184.Karunagaran D. Rashmi R. Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–129. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- 185.Kashfi K. Rigas B. The mechanism of action of nitric oxide-donating aspirin. Biochem Biophys Res Commun. 2007;358:1096–1101. doi: 10.1016/j.bbrc.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 186.Kato M. Li J. Chuang JL. Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kawiak A. Piosik J. Stasilojc G. Gwizdek-Wisniewska A. Marczak L. Stobiecki M. Bigda J. Lojkowska E. Induction of apoptosis by plumbagin through reactive oxygen species-mediated inhibition of topoisomerase II. Toxicol Appl Pharmacol. 2007;223:267–276. doi: 10.1016/j.taap.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 188.Kelner MJ. Alexander NM. Methylene blue directly oxidizes glutathione without the intermediate formation of hydrogen peroxide. J Biol Chem. 1985;260:15168–15171. [PubMed] [Google Scholar]
- 189.Kim H. Kim EH. Eom YW. Kim WH. Kwon TK. Lee SJ. Choi KS. Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of DR5. Cancer Res. 2006;66:1740–1750. doi: 10.1158/0008-5472.CAN-05-1568. [DOI] [PubMed] [Google Scholar]
- 190.Kim JS. Ahn KJ. Kim JA. Kim HM. Lee JD. Lee JM. Kim SJ. Park JH. Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr. 2008;40:607–613. doi: 10.1007/s10863-008-9188-0. [DOI] [PubMed] [Google Scholar]
- 191.Kirkpatrick DL. Ehrmantraut G. Stettner S. Kunkel M. Powis G. Redox active disulfides: The thioredoxin system as a drug target. Oncol Res. 1997;9:351–356. [PubMed] [Google Scholar]
- 192.Kirkpatrick DL. Kuperus M. Dowdeswell M. Potier N. Donald LJ. Kunkel M. Berggren M. Angulo M. Powis G. Mechanisms of inhibition of the thioredoxin growth factor system by antitumor 2-imidazolyl disulfides. Biochem Pharmacol. 1998;55:987–994. doi: 10.1016/s0006-2952(97)00597-2. [DOI] [PubMed] [Google Scholar]
- 193.Kirshner JR. He S. Balasubramanyam V. Kepros J. Yang CY. Zhang M. Du Z. Barsoum J. Bertin J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol Cancer Ther. 2008;7:2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 194.Kirszberg C. Rumjanek VM. Capella MA. Methylene blue is more toxic to erythroleukemic cells than to normal peripheral blood mononuclear cells: A possible use in chemotherapy. Cancer Chemother Pharmacol. 2005;56:659–665. doi: 10.1007/s00280-005-1014-3. [DOI] [PubMed] [Google Scholar]
- 195.Ko YH. Smith BL. Wang Y. Pomper MG. Rini DA. Torbenson MS. Hullihen J. Pedersen PL. Advanced cancers: Eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun. 2004;324:269–275. doi: 10.1016/j.bbrc.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 196.Koh MY. Spivak-Kroizman T. Venturini S. Welsh S. Williams RR. Kirkpatrick DL. Powis G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 197.Konorev EA. Kotamraju S. Zhao H. Kalivendi S. Joseph J. Kalyanaraman B. Paradoxical effects of metalloporphyrins on doxorubicin-induced apoptosis: Scavenging of reactive oxygen species versus induction of heme oxygenase-1. Free Radic Biol Med. 2002;33:988. doi: 10.1016/s0891-5849(02)00989-9. [DOI] [PubMed] [Google Scholar]
- 198.Kopnin PB. Agapova LS. Kopnin BP. Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Koptyra M. Falinski R. Nowicki MO. Stoklosa T. Majsterek I. Nieborowska-Skorska M. Blasiak J. Skorski T. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319–327. doi: 10.1182/blood-2005-07-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Krishna MC. DeGraff W. Hankovszky OH. Sar CP. Kalai T. Jeko J. Russo A. Mitchell JB. Hideg K. Studies of structure-activity relationship of nitroxide free radicals and their precursors as modifiers against oxidative damage. J Med Chem. 1998;41:3477–3492. doi: 10.1021/jm9802160. [DOI] [PubMed] [Google Scholar]
- 201.Kuo PL. Chen CY. Hsu YL. Isoobtusilactone A induces cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells. Cancer Res. 2007;67:7406–7420. doi: 10.1158/0008-5472.CAN-07-1089. [DOI] [PubMed] [Google Scholar]
- 202.Kurtoglu M. Gao N. Shang J. Maher JC. Lehrman MA. Wangpaichitr M. Savaraj N. Lane AN. Lampidis TJ. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther. 2007;6:3049–3058. doi: 10.1158/1535-7163.MCT-07-0310. [DOI] [PubMed] [Google Scholar]
- 203.Kwok BH. Koh B. Ndubuisi MI. Elofsson M. Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 204.Kwok JC. Richardson DR. The iron metabolism of neoplastic cells: Alterations that facilitate proliferation? Crit Rev Oncol Hematol. 2002;42:65–78. doi: 10.1016/s1040-8428(01)00213-x. [DOI] [PubMed] [Google Scholar]
- 205.Lai H. Singh NP. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006;231:43–48. doi: 10.1016/j.canlet.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 206.Lambert C. Li J. Jonscher K. Yang TC. Reigan P. Quintana M. Harvey J. Freed BM. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol Chem. 2007;282:19666–19675. doi: 10.1074/jbc.M611527200. [DOI] [PubMed] [Google Scholar]
- 207.Lampidis TJ. Kurtoglu M. Maher JC. Liu H. Krishan A. Sheft V. Szymanski S. Fokt I. Rudnicki WR. Ginalski K. Lesyng B. Priebe W. Efficacy of 2-halogen substituted D-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”. Cancer Chemother Pharmacol. 2006;58:725–734. doi: 10.1007/s00280-006-0207-8. [DOI] [PubMed] [Google Scholar]
- 208.Lau A. Villeneuve NF. Sun Z. Wong PK. Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lau JP. Weatherdon KL. Skalski V. Hedley DW. Effects of gemcitabine on APE/ref-1 endonuclease activity in pancreatic cancer cells, and the therapeutic potential of antisense oligonucleotides. Br J Cancer. 2004;91:1166–1173. doi: 10.1038/sj.bjc.6602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Laurent A. Nicco C. Chereau C. Goulvestre C. Alexandre J. Alves A. Levy E. Goldwasser F. Panis Y. Soubrane O. Weill B. Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- 211.Lavecchia A. Coluccia A. Di Giovanni C. Novellino E. Cdc25B phosphatase inhibitors in cancer therapy: Latest developments, trends and medicinal chemistry perspective. Anticancer Agents Med Chem. 2008;8:843–856. doi: 10.2174/187152008786847783. [DOI] [PubMed] [Google Scholar]
- 212.Lavecchia A. Cosconati S. Limongelli V. Novellino E. Modeling of Cdc25B dual specifity protein phosphatase inhibitors: Docking of ligands and enzymatic inhibition mechanism. ChemMedChem. 2006;1:540–550. doi: 10.1002/cmdc.200500092. [DOI] [PubMed] [Google Scholar]
- 213.Lei P. Abdelrahim M. Cho SD. Liu S. Chintharlapalli S. Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-jun N-terminal kinase. Carcinogenesis. 2008;29:1139–1147. doi: 10.1093/carcin/bgn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 215.Leslie NR. Bennett D. Lindsay YE. Stewart H. Gray A. Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lewis A. Ough M. Li L. Hinkhouse MM. Ritchie JM. Spitz DR. Cullen JJ. Treatment of pancreatic cancer cells with dicumarol induces cytotoxicity and oxidative stress. Clin Cancer Res. 2004;10:4550–4-558. doi: 10.1158/1078-0432.CCR-03-0667. [DOI] [PubMed] [Google Scholar]
- 217.Li PC. Lam E. Roos WP. Zdzienicka MZ. Kaina B. Efferth T. Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res. 2008;68:4347–4351. doi: 10.1158/0008-5472.CAN-07-2970. [DOI] [PubMed] [Google Scholar]
- 218.Lillig CH. Berndt C. Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 219.Link EM. Costa DC. Lui D. Ell PJ. Blower PJ. Spittle MF. Targeting disseminated melanoma with radiolabelled methylene blue: Comparative bio-distribution studies in man and animals. Acta Oncol. 1996;35:331–341. doi: 10.3109/02841869609101650. [DOI] [PubMed] [Google Scholar]
- 220.Liu SC. Ahn GO. Kioi M. Dorie MJ. Patterson AV. Brown JM. Optimized clostridium-directed enzyme prodrug therapy improves the antitumor activity of the novel DNA cross-linking agent PR-104. Cancer Res. 2008;68:7995–8003. doi: 10.1158/0008-5472.CAN-08-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 222.Liu Y. Tao J. Li Y. Yang J. Yu Y. Wang M. Xu X. Huang C. Huang W. Dong J. Li L. Liu J. Shen G. Tu Y. Targeting hypoxia-inducible factor-1alpha with Tf-PEI-shRNA complex via transferrin receptor-mediated endocytosis inhibits melanoma growth. Mol Ther. 2009;17:269–277. doi: 10.1038/mt.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu Z. Du ZY. Huang ZS. Lee KS. Gu LQ. Inhibition of thioredoxin reductase by curcumin analogs. Biosci Biotechnol Biochem. 2008;72:2214–2218. doi: 10.1271/bbb.80229. [DOI] [PubMed] [Google Scholar]
- 224.Lixia G. Fei Y. Jiajia J. Jianhui L. Triethylene tetramine, a novel ligand of G-quadruplex, induces senescence of MCF-7 cells. Biotechnol Lett. 2008;30:47–53. doi: 10.1007/s10529-007-9513-4. [DOI] [PubMed] [Google Scholar]
- 225.Loewe R. Valero T. Kremling S. Pratscher B. Kunstfeld R. Pehamberger H. Petzelbauer P. Dimethylfumarate impairs melanoma growth and metastasis. Cancer Res. 2006;66:11888–11896. doi: 10.1158/0008-5472.CAN-06-2397. [DOI] [PubMed] [Google Scholar]
- 226.Lofstedt T. Fredlund E. Holmquist–Mengelbier L. Pietras A. Ovenberger M. Poellinger L. Pahlman S. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6:919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- 227.Lopez–Lazaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res 52 Suppl. 2008;1:S103–127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 228.Lowndes SA. Adams A. Timms A. Fisher N. Smythe J. Watt SM. Joel S. Donate F. Hayward C. Reich S. Middleton M. Mazar A. Harris AL. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin Cancer Res. 2008;14:7526–7534. doi: 10.1158/1078-0432.CCR-08-0315. [DOI] [PubMed] [Google Scholar]
- 229.Lu J. Chew EH. Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci USA. 2007;104:12288–12293. doi: 10.1073/pnas.0701549104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Luo M. Delaplane S. Jiang A. Reed A. He Y. Fishel M. Nyland RL., 2nd Borch RF. Qiao X. Georgiadis MM. Kelley MR. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of Ape1. Antioxid Redox Signal. 2008;10:1853–1867. doi: 10.1089/ars.2008.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Luo M. Kelley MR. Inhibition of the human apurinic/apyrimidinic endonuclease (APE1) repair activity and sensitization of breast cancer cells to DNA alkylating agents with lucanthone. Anticancer Res. 2004;24:2127–2134. [PubMed] [Google Scholar]
- 232.Ma B. Goh BC. Tan EH. Lam KC. Soo R. Leong SS. Wang LZ. Mo F. Chan AT. Zee B. Mok T. A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest New Drugs. 2008;26:169–173. doi: 10.1007/s10637-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 233.Mackenzie MJ. Saltman D. Hirte H. Low J. Johnson C. Pond G. Moore MJ. A Phase II study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) and gemcitabine in advanced pancreatic carcinoma. A trial of the Princess Margaret Hospital Phase II consortium. Invest New Drugs. 2007;25:553–558. doi: 10.1007/s10637-007-9066-3. [DOI] [PubMed] [Google Scholar]
- 234.Madhusudan S. Smart F. Shrimpton P. Parsons JL. Gardiner L. Houlbrook S. Talbot DC. Hammonds T. Freemont PA. Sternberg MJ. Dianov GL. Hickson ID. Isolation of a small molecule inhibitor of DNA base excision repair. Nucleic Acids Res. 2005;33:4711–4724. doi: 10.1093/nar/gki781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Maeda H. Hori S. Ohizumi H. Segawa T. Kakehi Y. Ogawa O. Kakizuka A. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and L-buthionine-sulfoximine. Cell Death Differ. 2004;11:737–746. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 236.Magda D. Lecane P. Miller RA. Lepp C. Miles D. Mesfin M. Biaglow JE. Ho VV. Chawannakul D. Nagpal S. Karaman MW. Hacia JG. Motexafin gadolinium disrupts zinc metabolism in human cancer cell lines. Cancer Res. 2005;65:3837–3845. doi: 10.1158/0008-5472.CAN-04-4099. [DOI] [PubMed] [Google Scholar]
- 237.Magda D. Miller RA. Motexafin gadolinium: A novel redox active drug for cancer therapy. Semin Cancer Biol. 2006;16:466–476. doi: 10.1016/j.semcancer.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 238.Mao X. Yu CR. Li WH. Li WX. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008;18:879–888. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]
- 239.Marcu L. Olver I. Tirapazamine: from bench to clinical trials. Curr Clin Pharmacol. 2006;1:71–79. doi: 10.2174/157488406775268192. [DOI] [PubMed] [Google Scholar]
- 240.Marty M. Espie M. Llombart A. Monnier A. Rapoport BL. Stahalova V. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006;17:614–622. doi: 10.1093/annonc/mdj134. [DOI] [PubMed] [Google Scholar]
- 241.Marzano C. Gandin V. Folda A. Scutari G. Bindoli A. Rigobello MP. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic Biol Med. 2007;42:872–881. doi: 10.1016/j.freeradbiomed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 242.Maschek G. Savaraj N. Priebe W. Braunschweiger P. Hamilton K. Tidmarsh GF. De Young LR. Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 243.Mathupala SP. Ko YH. Pedersen PL. Hexokinase II: Cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Matoba S. Kang JG. Patino WD. Wragg A. Boehm M. Gavrilova O. Hurley PJ. Bunz F. Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 245.Mays JR. Weller Roska RL. Sarfaraz S. Mukhtar H. Rajski SR. Identification, synthesis, and enzymology of non-natural glucosinolate chemopreventive candidates. Chembiochem. 2008;9:729–747. doi: 10.1002/cbic.200700586. [DOI] [PubMed] [Google Scholar]
- 246.Mehta MP. Shapiro WR. Phan SC. Gervais R. Carrie C. Chabot P. Patchell RA. Glantz MJ. Recht L. Langer C. Sur RK. Roa WH. Mahe MA. Fortin A. Nieder C. Meyers CA. Smith JA. Miller RA. Renschler MF. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: Results of a Phase III trial. Int J Radiat Oncol Biol Phys. 2008;73:1069–1076. doi: 10.1016/j.ijrobp.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 247.Meuillet EJ. Mahadevan D. Berggren M. Coon A. Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: A mechanism for the functional loss of PTEN's tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 248.Meyskens FL., Jr Farmer P. Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14:148–154. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 249.Meyskens FL., Jr Farmer PJ. Yang S. Anton–Culver H. New perspectives on melanoma pathogenesis and chemoprevention. Recent Results Cancer Res. 2007;174:191–195. doi: 10.1007/978-3-540-37696-5_16. [DOI] [PubMed] [Google Scholar]
- 250.Mi L. Xiao Z. Hood BL. Dakshanamurthy S. Wang X. Govind S. Conrads TP. Veenstra TD. Chung FL. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Michelakis ED. Webster L. Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mitsushita J. Lambeth JD. Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- 253.Moore JC. Lai H. Li JR. Ren RL. McDougall JA. Singh NP. Chou CK. Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett. 1995;98:83–87. [PubMed] [Google Scholar]
- 254.Mukherjee A. Huber K. Evans H. Lakhani N. Martin S. A cellular and molecular investigation of the action of PMX464, a putative thioredoxin inhibitor, in normal and colorectal cancer cell lines. Br J Pharmacol. 2007;151:1167–1175. doi: 10.1038/sj.bjp.0707342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mukherjee A. Martin SG. The thioredoxin system: A key target in tumour and endothelial cells. Br J Radiol 81 Spec No. 2008;1:S57–68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- 256.Nakase I. Lai H. Singh NP. Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 257.Neuzil J. Tomasetti M. Zhao Y. Dong LF. Birringer M. Wang XF. Low P. Wu K. Salvatore BA. Ralph SJ. Vitamin E analogs, a novel group of “mitocans,” as anticancer agents: the importance of being redox-silent. Mol Pharmacol. 2007;71:1185–1199. doi: 10.1124/mol.106.030122. [DOI] [PubMed] [Google Scholar]
- 258.Nguyen C. Teo JL. Matsuda A. Eguchi M. Chi EY. Henderson WR., Jr. Kahn M. Chemogenomic identification of Ref-1/AP-1 as a therapeutic target for asthma. Proc Natl Acad Sci USA. 2003;100:1169–1173. doi: 10.1073/pnas.0437889100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nguyen P. Awwad RT. Smart DD. Spitz DR. Gius D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006;236:164–174. doi: 10.1016/j.canlet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 260.Nicolaou KC. Smith AL. Yue EW. Chemistry and biology of natural and designed enediynes. Proc Natl Acad Sci USA. 1993;90:5881–5888. doi: 10.1073/pnas.90.13.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nolan KA. Zhao H. Faulder PF. Frenkel AD. Timson DJ. Siegel D. Ross D. Burke TR., Jr. Stratford IJ. Bryce RA. Coumarin-based inhibitors of human NAD(P)H:quinone oxidoreductase-1. Identification, structure-activity, off-target effects and in vitro human pancreatic cancer toxicity. J Med Chem. 2007;50:6316–6325. doi: 10.1021/jm070472p. [DOI] [PubMed] [Google Scholar]
- 262.Nonn L. Berggren M. Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003;1:682–689. [PubMed] [Google Scholar]
- 263.Nowis D. Bugajski M. Winiarska M. Bil J. Szokalska A. Salwa P. Issat T. Was H. Jozkowicz A. Dulak J. Stoklosa T. Golab J. Zinc protoporphyrin IX, a heme oxygenase-1 inhibitor, demonstrates potent antitumor effects but is unable to potentiate antitumor effects of chemotherapeutics in mice. BMC Cancer. 2008;8:197. doi: 10.1186/1471-2407-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nyman ES. Hynninen PH. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J Photochem Photobiol B. 2004;73:1–28. doi: 10.1016/j.jphotobiol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 265.Oh S. Kim BJ. Singh NP. Lai H. Sasaki T. Synthesis and anticancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Lett. 2009;274:33–39. doi: 10.1016/j.canlet.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 266.Oh SY. Sohn YW. Park JW. Park HJ. Jeon HM. Kim TK. Lee JS. Jung JE. Jin X. Chung YG. Choi YK. You S. Lee JB. Kim H. Selective cell death of oncogenic Akt-transduced brain cancer cells by etoposide through reactive oxygen species mediated damage. Mol Cancer Ther. 2007;6:2178–2187. doi: 10.1158/1535-7163.MCT-07-0111. [DOI] [PubMed] [Google Scholar]
- 267.Oka D. Nishimura K. Shiba M. Nakai Y. Arai Y. Nakayama M. Takayama H. Inoue H. Okuyama A. Nonomura N. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-kappaB. Int J Cancer. 2007;120:2576–2581. doi: 10.1002/ijc.22570. [DOI] [PubMed] [Google Scholar]
- 268.Opsenica I. Opsenica D. Smith KS. Milhous WK. Solaja BA. Chemical stability of the peroxide bond enables diversified synthesis of potent tetraoxane antimalarials. J Med Chem. 2008;51:2261–2266. doi: 10.1021/jm701417a. [DOI] [PubMed] [Google Scholar]
- 269.Palayoor ST. Mitchell JB. Cerna D. Degraff W. John–Aryankalayil M. Coleman CN. PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer. 2008;123:2430–2437. doi: 10.1002/ijc.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pan JG. Mak TW. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE. 2007;2007:pe14. doi: 10.1126/stke.3812007pe14. [DOI] [PubMed] [Google Scholar]
- 271.Pan XQ. Harday J. Electromicroscopic observations on gliotoxin-induced apoptosis of cancer cells in culture and human cancer xenografts in transplanted SCID mice. In vivo. 2007;21:259–265. [PubMed] [Google Scholar]
- 272.Papadopoulos KP. Goel S. Beeram M. Wong A. Desai K. Haigentz M. Milian ML. Mani S. Tolcher A. Lalani AS. Sarantopoulos J. A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin Cancer Res. 2008;14:7110–7115. doi: 10.1158/1078-0432.CCR-08-0483. [DOI] [PubMed] [Google Scholar]
- 273.Pedersen PL. The cancer cell's “power plants” as promising therapeutic targets: An overview. J Bioenerg Biomembr. 2007;39:1–12. doi: 10.1007/s10863-007-9070-5. [DOI] [PubMed] [Google Scholar]
- 274.Pelicano H. Feng L. Zhou Y. Carew JS. Hileman EO. Plunkett W. Keating MJ. Huang P. Inhibition of mitochondrial respiration: A novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 275.Pelicano H. Martin DS. Xu RH. Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 276.Perry BN. Govindarajan B. Bhandarkar SS. Knaus UG. Valo M. Sturk C. Carrillo CO. Sohn A. Cerimele F. Dumont D. Losken A. Williams J. Brown LF. Tan X. Ioffe E. Yancopoulos GD. Arbiser JL. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol. 2006;126:2316–2322. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- 277.Pham NA. Hedley DW. Respiratory chain-generated oxidative stress following treatment of leukemic blasts with DNA-damaging agents. Exp Cell Res. 2001;264:345–352. doi: 10.1006/excr.2000.5148. [DOI] [PubMed] [Google Scholar]
- 278.Pourpak A. Meyers RO. Samulitis BK. Sherry Chow HH. Kepler CY. Raymond MA. Hersh E. Dorr RT. Preclinical antitumor activity, pharmacokinetics and pharmacodynamics of imexon in mice. Anticancer Drugs. 2006;17:1179–1184. doi: 10.1097/01.cad.0000236305.43209.f0. [DOI] [PubMed] [Google Scholar]
- 279.Powis G. Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 280.Powis G. Wipf P. Lynch SM. Birmingham A. Kirkpatrick DL. Molecular pharmacology and antitumor activity of palmarumycin-based inhibitors of thioredoxin reductase. Mol Cancer Ther. 2006;5:630–636. doi: 10.1158/1535-7163.MCT-05-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Powolny AA. Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Prakash C. Sharma R. Gleave M. Nedderman A. In vitro screening techniques for reactive metabolites for minimizing bioactivation potential in drug discovery. Curr Drug Metab. 2008;9:952–964. doi: 10.2174/138920008786485209. [DOI] [PubMed] [Google Scholar]
- 283.Quintas–Cardama A. Verstovsek S. Freireich E. Kantarjian H. Chen YW. Zingaro R. Chemical and clinical development of darinaparsin, a novel organic arsenic derivative. Anticancer Agents Med Chem. 2008;8:904–909. doi: 10.2174/187152008786847666. [DOI] [PubMed] [Google Scholar]
- 284.Rais B. Comin B. Puigjaner J. Brandes JL. Creppy E. Saboureau D. Ennamany R. Lee WN. Boros LG. Cascante M. Oxythiamine and dehydroepiandrosterone induce a G1 phase cycle arrest in Ehrlich's tumor cells through inhibition of the pentose cycle. FEBS Lett. 1999;456:113–118. doi: 10.1016/s0014-5793(99)00924-2. [DOI] [PubMed] [Google Scholar]
- 285.Ralph SJ. Neuzil J. Mitochondria as targets for cancer therapy. Mol Nutr Food Res. 2009;53:9–28. doi: 10.1002/mnfr.200800044. [DOI] [PubMed] [Google Scholar]
- 286.Ralser M. Wamelink MM. Struys EA. Joppich C. Krobitsch S. Jakobs C. Lehrach H. A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth. Proc Natl Acad Sci USA. 2008;105:17807–17811. doi: 10.1073/pnas.0803090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ramanathan B. Jan KY. Chen CH. Hour TC. Yu HJ. Pu YS. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65:8455–8460. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- 288.Ramanathan RK. Fakih M. Mani S. Deutsch M. Perez RP. Ritter MA. Eiseman JL. Ivy SP. Trump DL. Belani CP. Parise RA. Potter DM. Egorin MJ. Phase I and pharmacokinetic study of the novel redox-active agent, motexafin gadolinium, with concurrent radiation therapy in patients with locally advanced pancreatic or biliary cancers. Cancer Chemother Pharmacol. 2006;57:465–474. doi: 10.1007/s00280-005-0071-y. [DOI] [PubMed] [Google Scholar]
- 289.Ramos-Montoya A. Lee WN. Bassilian S. Lim S. Trebukhina RV. Kazhyna MV. Ciudad CJ. Noe V. Centelles JJ. Cascante M. Pentose phosphate cycle oxidative and nonoxidative balance: A new vulnerable target for overcoming drug resistance in cancer. Int J Cancer. 2006;119:2733–2741. doi: 10.1002/ijc.22227. [DOI] [PubMed] [Google Scholar]
- 290.Rao VA. Klein SR. Agama KK. Toyoda E. Adachi N. Pommier Y. Shacter EB. The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells. Cancer Res. 2009;69:948–957. doi: 10.1158/0008-5472.CAN-08-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Reed GA. Sunega JM. Sullivan DK. Gray JC. Mayo MS. Crowell JA. Hurwitz A. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:2619–2624. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Reigan P. Colucci MA. Siegel D. Chilloux A. Moody CJ. Ross D. Development of indolequinone mechanism-based inhibitors of NAD(P)H:quinone oxidoreductase 1 (NQO1): NQO1 inhibition and growth inhibitory activity in human pancreatic MIA PaCa-2 cancer cells. Biochemistry. 2007;46:5941–5950. doi: 10.1021/bi700008y. [DOI] [PubMed] [Google Scholar]
- 293.Rice L. Phoenix DA. Wainwright M. Waring JJ. Effect of increasing methylation on the ability of methylene blue to cause diaphorase-catalyzsed oxidation of NADH. Biochem Soc Trans. 1998;26:S319. doi: 10.1042/bst026s319. [DOI] [PubMed] [Google Scholar]
- 294.Rigas B. Novel agents for cancer prevention based on nitric oxide. Biochem Soc Trans. 2007;35:1364–1368. doi: 10.1042/BST0351364. [DOI] [PubMed] [Google Scholar]
- 295.Roche TE. Hiromasa Y. Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia, and cancer. Cell Mol Life Sci. 2007;64:830–849. doi: 10.1007/s00018-007-6380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Rodrigues MS. Reddy MM. Sattler M. Cell cycle regulation by oncogenic tyrosine kinases in myeloid neoplasias: from molecular redox mechanisms to health implications. Antioxid Redox Signal. 2008;10:1813–1848. doi: 10.1089/ars.2008.2071. [DOI] [PubMed] [Google Scholar]
- 297.Ross D. Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol. 2004;382:115–144. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 298.Roy A. Ganguly A. BoseDasgupta S. Das BB. Pal C. Jaisankar P. Majumder HK. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol. 2008;74:1292–1307. doi: 10.1124/mol.108.050161. [DOI] [PubMed] [Google Scholar]
- 299.Rozenblat S. Grossman S. Bergman M. Gottlieb H. Cohen Y. Dovrat S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem Pharmacol. 2008;75:369–382. doi: 10.1016/j.bcp.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 300.Rudolph J. Cdc25 phosphatases: Structure, specificity, and mechanism. Biochemistry. 2007;46:3595–3604. doi: 10.1021/bi700026j. [DOI] [PubMed] [Google Scholar]
- 301.Ruscoe JE. Rosario LA. Wang T. Gate L. Arifoglu P. Wolf CR. Henderson CJ. Ronai Z. Tew KD. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001;298:339–345. [PubMed] [Google Scholar]
- 302.Saavedra JE. Srinivasan A. Buzard GS. Davies KM. Waterhouse DJ. Inami K. Wilde TC. Citro ML. Cuellar M. Deschamps JR. Parrish D. Shami PJ. Findlay VJ. Townsend DM. Tew KD. Singh S. Jia L. Ji X. Keefer LK. PABA/NO as an anticancer lead: Analogue synthesis, structure revision, solution chemistry, reactivity toward glutathione, and in vitro activity. J Med Chem. 2006;49:1157–1164. doi: 10.1021/jm050700k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Sablina AA. Budanov AV. Ilyinskaya GV. Agapova LS. Kravchenko JE. Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Safe S. Papineni S. Chintharlapalli S. Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Lett. 2008;269:326–338. doi: 10.1016/j.canlet.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Samlowski WE. Petersen R. Cuzzocrea S. Macarthur H. Burton D. McGregor JR. Salvemini D. A nonpeptidyl mimic of superoxide dismutase, M40403, inhibits dose-limiting hypotension associated with interleukin-2 and increases its antitumor effects. Nat Med. 2003;9:750–755. doi: 10.1038/nm874. [DOI] [PubMed] [Google Scholar]
- 307.Sannella AR. Casini A. Gabbiani C. Messori L. Bilia AR. Vincieri FF. Majori G. Severini C. New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: Mechanistic and pharmacological implications. FEBS Lett. 2008;582:844–847. doi: 10.1016/j.febslet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 308.Sathisha MP. Revankar VK. Pai KS. Synthesis, structure, electrochemistry, and spectral characterization of bis-isatin thiocarbohydrazone metal complexes and their antitumor activity against ehrlich ascites carcinoma in swiss albino mice. Met Based Drugs. 2008;2008:362105. doi: 10.1155/2008/362105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Sattler M. Verma S. Shrikhande G. Byrne CH. Pride YB. Winkler T. Greenfield EA. Salgia R. Griffin JD. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 310.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 311.Schito ML. Goel A. Song Y. Inman JK. Fattah RJ. Rice WG. Turpin JA. Sher A. Appella E. In vivo antiviral activity of novel human immunodeficiency virus type 1 nucleocapsid p7 zinc finger inhibitors in a transgenic murine model. AIDS Res Hum Retroviruses. 2003;19:91–101. doi: 10.1089/088922203762688595. [DOI] [PubMed] [Google Scholar]
- 312.Seow HA. Penketh PG. Shyam K. Rockwell S. Sartorelli AC. 1,2-Bis(methylsulfonyl)-1-(2-chloroethyl)-2-[[1-(4-nitrophenyl)ethoxy]carbonyl]hydrazine: An anticancer agent targeting hypoxic cells. Proc Natl Acad Sci USA. 2005;102:9282–9287. doi: 10.1073/pnas.0409013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Shankar S. Chen Q. Ganapathy S. Singh KP. Srivastava RK. Diallyl trisulfide increases the effectiveness of TRAIL and inhibits prostate cancer growth in an orthotopic model: molecular mechanisms. Mol Cancer Ther. 2008;7:2328–2338. doi: 10.1158/1535-7163.MCT-08-0216. [DOI] [PubMed] [Google Scholar]
- 314.Shankar S. Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- 315.Shinohara M. Shang WH. Kubodera M. Harada S. Mitsushita J. Kato M. Miyazaki H. Sumimoto H. Kamata T. Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J Biol Chem. 2007;282:17640–17648. doi: 10.1074/jbc.M609450200. [DOI] [PubMed] [Google Scholar]
- 316.Siegel D. Gustafson DL. Dehn DL. Han JY. Boonchoong P. Berliner LJ. Ross D. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 317.Simamura E. Shimada H. Hatta T. Hirai K. Mitochondrial voltage-dependent anion channels (VDACs) as novel pharmacological targets for anticancer agents. J Bioenerg Biomembr. 2008;40:213–217. doi: 10.1007/s10863-008-9158-6. [DOI] [PubMed] [Google Scholar]
- 318.Simons AL. Ahmad IM. Mattson DM. Dornfeld KJ. Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Singh A. Boldin–Adamsky S. Thimmulappa RK. Rath SK. Ashush H. Coulter J. Blackford A. Goodman SN. Bunz F. Watson WH. Gabrielson E. Feinstein E. Biswal S. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Singh A. Misra V. Thimmulappa RK. Lee H. Ames S. Hoque MO. Herman JG. Baylin SB. Sidransky D. Gabrielson E. Brock MV. Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Singh NP. Lai HC. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004;24:2277–2280. [PubMed] [Google Scholar]
- 322.Sivaramakrishnan S. Gates KS. Possible chemical mechanisms underlying the antitumor activity of S-deoxyleinamycin. Bioorg Med Chem Lett. 2008;18:3076–3080. doi: 10.1016/j.bmcl.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Siwak DR. Shishodia S. Aggarwal BB. Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 324.Solit DB. Osman I. Polsky D. Panageas KS. Daud A. Goydos JS. Teitcher J. Wolchok JD. Germino FJ. Krown SE. Coit D. Rosen N. Chapman PB. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Spitz DR. Sim JE. Ridnour LA. Galoforo SS. Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann NY Acad Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 326.Stasi R. Gemtuzumab ozogamicin: An anti-CD33 immunoconjugate for the treatment of acute myeloid leukaemia. Expert Opin Biol Ther. 2008;8:527–540. doi: 10.1517/14712598.8.4.527. [DOI] [PubMed] [Google Scholar]
- 327.Stephenson CM. Levin RD. Lis CG. Phase 1 trial of high-dose intravenous vitamin C treatment for patients with cancer. J Am Osteopath Assoc. 2007;107:212–213. [PubMed] [Google Scholar]
- 328.Stoltzman CA. Peterson CW. Breen KT. Muoio DM. Billin AN. Ayer DE. Glucose sensing by MondoA:Mlx complexes: A role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci USA. 2008;105:6912–6917. doi: 10.1073/pnas.0712199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Stravopodis DJ. Margaritis LH. Voutsinas GE. Drug-mediated targeted disruption of multiple protein activities through functional inhibition of the Hsp90 chaperone complex. Curr Med Chem. 2007;14:3122–3138. doi: 10.2174/092986707782793925. [DOI] [PubMed] [Google Scholar]
- 330.Suh YA. Arnold RS. Lassegue B. Shi J. Xu X. Sorescu D. Chung AB. Griendling KK. Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 331.Suliman HB. Carraway MS. Ali AS. Reynolds CM. Welty–Wolf KE. Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sullivan R. Graham CH. Chemosensitization of cancer by nitric oxide. Curr Pharm Des. 2008;14:1113–1123. doi: 10.2174/138161208784246225. [DOI] [PubMed] [Google Scholar]
- 333.Suy S. Mitchell JB. Samuni A. Mueller S. Kasid U. Nitroxide tempo, a small molecule, induces apoptosis in prostate carcinoma cells and suppresses tumor growth in athymic mice. Cancer. 2005;103:1302–1313. doi: 10.1002/cncr.20898. [DOI] [PubMed] [Google Scholar]
- 334.Sweeney CJ. Mehrotra S. Sadaria MR. Kumar S. Shortle NH. Roman Y. Sheridan C. Campbell RA. Murry DJ. Badve S. Nakshatri H. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther. 2005;4:1004–1012. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- 335.Szatrowski TP. Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 336.Tagg SL. Foster PA. Leese MP. Potter BV. Reed MJ. Purohit A. Newman SP. 2-Methoxyoestradiol-3,17-O,O-bis-sulphamate and 2-deoxy-D-glucose in combination: A potential treatment for breast and prostate cancer. Br J Cancer. 2008;99:1842–1848. doi: 10.1038/sj.bjc.6604752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Tang Y. Dong Y. Vennerstrom JL. Synthetic peroxides as antimalarials. Med Res Rev. 2004;24:425–448. doi: 10.1002/med.10066. [DOI] [PubMed] [Google Scholar]
- 338.Tarumoto T. Nagai T. Ohmine K. Miyoshi T. Nakamura M. Kondo T. Mitsugi K. Nakano S. Muroi K. Komatsu N. Ozawa K. Ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell line. Exp Hematol. 2004;32:375–381. doi: 10.1016/j.exphem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 339.Tatum JL. Kelloff GJ. Gillies RJ. Arbeit JM. Brown JM. Chao KS. Chapman JD. Eckelman WC. Fyles AW. Giaccia AJ. Hill RP. Koch CJ. Krishna MC. Krohn KA. Lewis JS. Mason RP. Melillo G. Padhani AR. Powis G. Rajendran JG. Reba R. Robinson SP. Semenza GL. Swartz HM. Vaupel P. Yang D. Croft B. Hoffman J. Liu G. Stone H. Sullivan D. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 340.Teoh ML. Sun W. Smith BJ. Oberley LW. Cullen JJ. Modulation of reactive oxygen species in pancreatic cancer. Clin Cancer Res. 2007;13:7441–7450. doi: 10.1158/1078-0432.CCR-07-0851. [DOI] [PubMed] [Google Scholar]
- 341.Thimmulappa RK. Mai KH. Srisuma S. Kensler TW. Yamamoto M. Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 342.Thomas AA. Le Huerou Y. De Meese J. Gunawardana I. Kaplan T. Romoff TT. Gonzales SS. Condroski K. Boyd SA. Ballard J. Bernat B. DeWolf W. Han M. Lee P. Lemieux C. Pedersen R. Pheneger J. Poch G. Smith D. Sullivan F. Weiler S. Wright SK. Lin J. Brandhuber B. Vigers G. Synthesis, in vitro and in vivo activity of thiamine antagonist transketolase inhibitors. Bioorg Med Chem Lett. 2008;18:2206–2210. doi: 10.1016/j.bmcl.2007.11.101. [DOI] [PubMed] [Google Scholar]
- 343.Tibodeau J. Benson L. Isham C. Owen W. Bible K. The anticancer agent chaetocin is a competitive substrate and inhibitor of thioredoxin reductase. Antioxid Redox Signal. 2009;11:1097–1106. doi: 10.1089/ars.2008.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Toogood PL. Mitochondrial drugs. Curr Opin Chem Biol. 2008;12:457–463. doi: 10.1016/j.cbpa.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 345.Townsend DM. Findlay VL. Tew KD. Glutathione S-transferases as regulators of kinase pathways and anticancer drug targets. Methods Enzymol. 2005;401:287–307. doi: 10.1016/S0076-6879(05)01019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Townsend DM. He L. Hutchens S. Garrett TE. Pazoles CJ. Tew KD. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res. 2008;68:2870–2877. doi: 10.1158/0008-5472.CAN-07-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Townsend DM. Tew KD. Pharmacology of a mimetic of glutathione disulfide. Biomed Pharmacother. 2009;63:75–78. doi: 10.1016/j.biopha.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Toyokuni S. Okamoto K. Yodoi J. Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 349.Trachootham D. Lu W. Ogasawara MA. Nilsa RD. Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Trachootham D. Zhou Y. Zhang H. Demizu Y. Chen Z. Pelicano H. Chiao PJ. Achanta G. Arlinghaus RB. Liu J. Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 351.Tsou HR. Overbeek–Klumpers EG. Hallett WA. Reich MF. Floyd MB. Johnson BD. Michalak RS. Nilakantan R. Discafani C. Golas J. Rabindran SK. Shen R. Shi X. Wang YF. Upeslacis J. Wissner A. Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J Med Chem. 2005;48:1107–1131. doi: 10.1021/jm040159c. [DOI] [PubMed] [Google Scholar]
- 352.Vad NM. Yount G. Moore D. Weidanz J. Moridani MY. Biochemical mechanism of acetaminophen (APAP) induced toxicity in melanoma cell lines. J Pharm Sci. 2009;98:1409–1429. doi: 10.1002/jps.21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Vad NM. Yount G. Moridani MY. Biochemical mechanism of acetylsalicylic acid (Aspirin) selective toxicity toward melanoma cell lines. Melanoma Res. 2008;18:386–399. doi: 10.1097/CMR.0b013e3283107df7. [DOI] [PubMed] [Google Scholar]
- 354.Vali M. Vossen JA. Buijs M. Engles JM. Liapi E. Ventura VP. Khwaja A. Acha-Ngwodo O. Shanmugasundaram G. Syed L. Wahl RL. Geschwind JF. Targeting of VX2 rabbit liver tumor by selective delivery of 3-bromopyruvate: A biodistribution and survival study. J Pharmacol Exp Ther. 2008;327:32–37. doi: 10.1124/jpet.108.141093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Vanderlaag K. Samudio I. Burghardt R. Barhoumi R. Safe S. Inhibition of breast cancer cell growth and induction of cell death by 1,1-bis(3′-indolyl)methane (DIM) and 5,5′-dibromoDIM. Cancer Lett. 2006;236:198–212. doi: 10.1016/j.canlet.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 356.Vaquero EC. Edderkaoui M. Pandol SJ. Gukovsky I. Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 357.Varani J. Bhagavathula N. Nerusu KC. Sherzer H. Fay K. Boitano AE. Glick GD. Johnson K. Kang S. Opipari AW., Jr. A novel benzodiazepine selectively inhibits keratinocyte proliferation and reduces retinoid-induced epidermal hyperplasia in organ-cultured human skin. J Pharmacol Exp Ther. 2005;313:56–63. doi: 10.1124/jpet.104.075929. [DOI] [PubMed] [Google Scholar]
- 358.Verrax J. Stockis J. Tison A. Taper HS. Calderon PB. Oxidative stress by ascorbate/menadione association kills K562 human chronic myelogenous leukaemia cells and inhibits its tumour growth in nude mice. Biochem Pharmacol. 2006;72:671–680. doi: 10.1016/j.bcp.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 359.von Eschenbach AC. NCI sets goal of eliminating suffering and death due to cancer by 2015. J Natl Med Assoc. 2003;95:637–639. [PMC free article] [PubMed] [Google Scholar]
- 360.Wang LH. Yang XY. Zhang X. An P. Kim HJ. Huang J. Clarke R. Osborne CK. Inman JK. Appella E. Farrar WL. Disruption of estrogen receptor DNA-binding domain and related intramolecular communication restores tamoxifen sensitivity in resistant breast cancer. Cancer Cell. 2006;10:487–499. doi: 10.1016/j.ccr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 361.Wang LH. Yang XY. Zhang X. Mihalic K. Fan YX. Xiao W. Howard OM. Appella E. Maynard AT. Farrar WL. Suppression of breast cancer by chemical modulation of vulnerable zinc fingers in estrogen receptor. Nat Med. 2004;10:40–47. doi: 10.1038/nm969. [DOI] [PubMed] [Google Scholar]
- 362.Wang XJ. Sun Z. Villeneuve NF. Zhang S. Zhao F. Li Y. Chen W. Yi X. Zheng W. Wondrak GT. Wong PK. Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wang ZY. Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 364.Wardman P. Electron transfer and oxidative stress as key factors in the design of drugs selectively active in hypoxia. Curr Med Chem. 2001;8:739–761. doi: 10.2174/0929867013372959. [DOI] [PubMed] [Google Scholar]
- 365.Waring P. Sjaarda A. Lin QH. Gliotoxin inactivates alcohol dehydrogenase by either covalent modification or free radical damage mediated by redox cycling. Biochem Pharmacol. 1995;49:1195–1201. doi: 10.1016/0006-2952(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 366.Webster KA. Prentice H. Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid Redox Signal. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- 367.Wells WW. Rocque PA. Xu DP. Meyer EB. Charamella LJ. Dimitrov NV. Ascorbic acid and cell survival of adriamycin resistant and sensitive MCF-7 breast tumor cells. Free Radic Biol Med. 1995;18:699–708. doi: 10.1016/0891-5849(94)00188-p. [DOI] [PubMed] [Google Scholar]
- 368.Welsh S. Williams R. Kirkpatrick L. Paine-Murrieta G. Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2004;3:233–244. [PubMed] [Google Scholar]
- 369.Welsh SJ. Bellamy WT. Briehl MM. Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089–5095. [PubMed] [Google Scholar]
- 370.Welsh SJ. Williams RR. Birmingham A. Newman DJ. Kirkpatrick DL. Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- 371.Weydert CJ. Waugh TA. Ritchie JM. Iyer KS. Smith JL. Li L. Spitz DR. Oberley LW. Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth. Free Radic Biol Med. 2006;41:226–237. doi: 10.1016/j.freeradbiomed.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 372.Whitnall M. Howard J. Ponka P. Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Wiatrak BJ. Overview of recurrent respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg. 2003;11:433–441. doi: 10.1097/00020840-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 374.Willoughby JA., Sr. Sundar SN. Cheung M. Tin AS. Modiano J. Firestone GL. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem. 2009;284:2203–2213. doi: 10.1074/jbc.M804491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Winski SL. Faig M. Bianchet MA. Siegel D. Swann E. Fung K. Duncan MW. Moody CJ. Amzel LM. Ross D. Characterization of a mechanism-based inhibitor of NAD(P)H:quinone oxidoreductase 1 by biochemical, X-ray crystallographic, and mass spectrometric approaches. Biochemistry. 2001;40:15135–15142. doi: 10.1021/bi011324i. [DOI] [PubMed] [Google Scholar]
- 376.Wissner A. Mansour TS. The development of HKI-272 and related compounds for the treatment of cancer. Arch Pharm (Weinheim) 2008;341:465–477. doi: 10.1002/ardp.200800009. [DOI] [PubMed] [Google Scholar]
- 377.Wissner A. Overbeek E. Reich MF. Floyd MB. Johnson BD. Mamuya N. Rosfjord EC. Discafani C. Davis R. Shi X. Rabindran SK. Gruber BC. Ye F. Hallett WA. Nilakantan R. Shen R. Wang YF. Greenberger LM. Tsou HR. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2) J Med Chem. 2003;46:49–63. doi: 10.1021/jm020241c. [DOI] [PubMed] [Google Scholar]
- 378.Wolchok JD. Williams L. Pinto JT. Fleisher M. Krown SE. Hwu WJ. Livingston PO. Chang C. Chapman PB. Phase I trial of high dose paracetamol and carmustine in patients with metastatic melanoma. Melanoma Res. 2003;13:189–196. doi: 10.1097/00008390-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 379.Wondrak GT. NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Free Radic Biol Med. 2007;43:178–190. doi: 10.1016/j.freeradbiomed.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Wondrak GT. Cabello CM. Villeneuve NF. Zhang S. Ley S. Li Y. Sun Z. Zhang DD. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radic Biol Med. 2008;45:385–395. doi: 10.1016/j.freeradbiomed.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wondrak GT. Jacobson MK. Jacobson EL. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci. 2006;5:215–237. doi: 10.1039/b504573h. [DOI] [PubMed] [Google Scholar]
- 382.Wu FY. Sun TP. Vitamin K3 induces cell cycle arrest and cell death by inhibiting Cdc25 phosphatase. Eur J Cancer. 1999;35:1388–1393. doi: 10.1016/s0959-8049(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 383.Wu Y. Zu K. Ni J. Yeh S. Kasi D. James NS. Chemler S. Ip C. Cellular and molecular effects of alpha-tocopheryloxybutyrate: Lessons for the design of vitamin E analog for cancer prevention. Anticancer Res. 2004;24:3795–3802. [PubMed] [Google Scholar]
- 384.Xiao D. Powolny AA. Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–30163. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Xu RH. Pelicano H. Zhang H. Giles FJ. Keating MJ. Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005;19:2153–2158. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- 386.Xu RH. Pelicano H. Zhou Y. Carew JS. Feng L. Bhalla KN. Keating MJ. Huang P. Inhibition of glycolysis in cancer cells: A novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–621. [PubMed] [Google Scholar]
- 387.Xu Y. Shao Y. Voorhees JJ. Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J Biol Chem. 2006;281:27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Yagoda N. von Rechenberg M. Zaganjor E. Bauer AJ. Yang WS. Fridman DJ. Wolpaw AJ. Smukste I. Peltier JM. Boniface JJ. Smith R. Lessnick SL. Sahasrabudhe S. Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Yamaura M. Mitsushita J. Furuta S. Kiniwa Y. Ashida A. Goto Y. Shang WH. Kubodera M. Kato M. Takata M. Saida T. Kamata T. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- 390.Yancik R. Population aging and cancer: A cross-national concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 391.Yang S. Irani K. Heffron SE. Jurnak F. Meyskens FL., Jr. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther. 2005;4:1923–1935. doi: 10.1158/1535-7163.MCT-05-0229. [DOI] [PubMed] [Google Scholar]
- 392.Yang S. Meyskens FL. Apurinic/apyrimidinic endonuclease/redox effector factor-1(APE/Ref-1): a unique target for the prevention and treatment of human melanoma. Antioxid Redox Signal. 2009;11:639–650. doi: 10.1089/ars.2008.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Yang WS. Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Yoshida A. Takemura H. Inoue H. Miyashita T. Ueda T. Inhibition of glutathione synthesis overcomes Bcl-2-mediated topoisomerase inhibitor resistance and induces nonapoptotic cell death via mitochondrial-independent pathway. Cancer Res. 2006;66:5772–5780. doi: 10.1158/0008-5472.CAN-05-3916. [DOI] [PubMed] [Google Scholar]
- 395.Zahedi Avval F. Berndt C. Pramanik A. Holmgren A. Mechanism of inhibition of ribonucleotide reductase with motexafin gadolinium (MGd) Biochem Biophys Res Commun. 2009;379:775–779. doi: 10.1016/j.bbrc.2008.12.128. [DOI] [PubMed] [Google Scholar]
- 396.Zahler WL. Clel WW. A specific and sensitive assay for disulfides. J Biol Chem. 1968;243:716–719. [PubMed] [Google Scholar]
- 397.Zhang H. Trachootham D. Lu W. Carew J. Giles FJ. Keating MJ. Arlinghaus RB. Huang P. Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia. 2008;22:1191–1199. doi: 10.1038/leu.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zhang J. Yang PL. Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 399.Zhang S. Yang JH. Guo CK. Cai PC. Gene silencing of TKTL1 by RNAi inhibits cell proliferation in human hepatoma cells. Cancer Lett. 2007;253:108–114. doi: 10.1016/j.canlet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 400.Zhang ZM. Yang XY. Deng SH. Xu W. Gao HQ. Anti-tumor effects of polybutylcyanoacrylate nanoparticles of diallyl trisulfide on orthotopic transplantation tumor model of hepatocellular carcinoma in BALB/c nude mice. Chin Med J (Engl) 2007;120:1336–1342. [PubMed] [Google Scholar]
- 401.Zhao Y. Neuzil J. Wu K. Vitamin E analogues as mitochondria-targeting compounds: From the bench to the bedside? Mol Nutr Food Res. 2009;53:129–139. doi: 10.1002/mnfr.200800045. [DOI] [PubMed] [Google Scholar]
- 402.Zheng Y. Shi Y. Tian C. Jiang C. Jin H. Chen J. Almasan A. Tang H. Chen Q. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene. 2004;23:1239–1247. doi: 10.1038/sj.onc.1207205. [DOI] [PMC free article] [PubMed] [Google Scholar]