Abstract
The establishment and maintenance of stable, long-term male-female relationships, or pair bonds, are marked by high levels of mutual attraction, selective preference for the partner, and high rates of sociosexual behavior. Central oxytocin (OT) affects social preference and partner-directed social behavior in rodents, but the role of this neuropeptide has yet to be studied in heterosexual primate relationships. The present study evaluated whether the OT system plays a role in the dynamics of social behavior and partner preference during the first three weeks of cohabitation in male and female marmosets, Callithrix penicillata. OT activity was stimulated by intranasal administration of OT, and inhibited by oral administration of a non-peptide OT-receptor antagonist (L-368,899; Merck). Social behavior throughout the pairing varied as a function of OT treatment. Compared to controls, marmosets initiated huddling with their social partner more often after OT treatments but reduced proximity and huddling after OT antagonist treatments. OT antagonist treatment also eliminated food sharing between partners. During the 24-h preference test, all marmosets interacted more with an opposite-sex stranger than with the partner. By the third-week preference test, marmosets interacted with the partner and stranger equally with the exception that intranasal-OT treatments facilitated initial partner-seeking behavior over initial contact with the stranger. Our findings demonstrate that pharmacological manipulations of OT activity alter partner-directed social behavior during pair interactions, suggesting that central OT may facilitate the process of pair-bond formation and social relationships in marmoset monkeys.
Most primate species are highly social, and a number of species form stable, long-term male-female relationships with strong social attachments, referred to as pair-bonds (Kleiman, 1977; Fuentes, 1999). Pair-bond formation and maintenance are marked by mutual attraction, high rates of selective sociosexual behavior between the pair, and aggression toward unfamiliar conspecifics. Curiously, the neural mechanisms underlying these social relationships in primates have only received limited consideration (Bales et al., 2007) and consequently are not well understood. In this present paper, we used several behavioral assays along with manipulation of oxytocin (OT) activity to examine the underlying neural mechanisms that facilitate the social behavior required to form and maintain stable, long-term pair-bonds in primates.
OT is a neuromodulator that facilitates affiliation, including those behavioral patterns that mediate social relationships in pair-bonding voles (Insel and Young, 2001; Young and Wang, 2004). Cohabitation for 24–48 h between male and female prairie voles is sufficient for animals to develop a social preference such that they are more aggressive to same-sex intruders and prefer to affiliate with the familiar partner compared to an unfamiliar opposite-sex conspecific (Williams et al., 1992; Winslow et al., 1993a; Insel and Hulihan, 1995). The length of cohabitation required to form partner preferences is reduced in female prairie voles if the pair mates (Williams et al., 1992; Insel et al., 1995) or if exogenous OT is infused into the brain (Winslow et al., 1993b; Williams et al., 1994; Insel and Hulihan, 1995). Central (intracerebroventricular; ICV) administration of an OT antagonist before a socialization and mating period inhibits preference formation (Insel and Hulihan, 1995). While earlier studies examined the effects of OT on pair bonding almost exclusively in female prairie voles, Cho et al. (1999) noted that ICV OT can induce a partner preference and OT antagonist and OT/OT antagonist treatment can inhibit preference formation in male and female prairie voles if the testing paradigm is consistent. Thus for pair-bonding voles, central administrations of OT can act as a substitute for mating by facilitating partner preference formation, and blocking the mating-induced rise in OT inhibits the formation of partner preference.
Like socially monogamous voles, marmoset and tamarin monkeys establish and maintain long-term male-female social relationships (captivity: Rothe, 1975; Epple, 1977; Woodcock, 1982; Evans and Poole, 1983; Savage et al., 1988; Schaffner et al., 1995; wild: Soini, 1987; Ferrari and Lopes Ferrari, 1989; Digby, 1995) characterized by partner-directed social behavior (Kleiman, 1977; Schaffner et al., 1995), social preference for a familiar partner (Epple, 1990; Inglett et al., 1990; cf., Buchanan-Smith and Jordan, 1992), and intrasexual aggression (Epple, 1977; Epple, 1978; French and Snowdon, 1981; French and Inglett, 1989). Behaviorally, pair formation and maintenance are marked by high levels of social interactions including food sharing and allogrooming, coordinated activity patterns, and initially high rates of mating behavior, followed by reduced mating activity but continued high rates of social interaction and contact (Evans and Poole, 1983; Buchanan-Smith and Jordan, 1992; Schaffner et al., 1995). Despite research on the behavior and social aspects of heterosexual relationships in marmosets, the neuroendocrinology of pairing has yet to be examined.
Some research evaluating oxytocinergic neural circuitry in primates has identified OT neuroanatomy in brain regions that facilitate social behavior. OT immunoreactive (ir) cells and fibers in marmosets and OT receptors in humans and other nonhuman primates are localized in the same critical brain regions that facilitate social behavior and attachment in rodents (Loup et al., 1991; Wang et al., 1997; Boccia et al., 2001; Schorscher-Petcu et al., 2009) suggesting that OT may have a function in the establishment and maintenance of social relationships in primates. Recently, OT and OT antagonists have been delivered into the brain by several peripheral routes (e.g., intranasal OT: Born et al., 2002; Heinrichs et al., 2003; Parker et al., 2005; Ditzen et al., 2009; per os (p.o.) and intravenous OT-receptor antagonist: Thompson et al., 1997; Boccia et al., 2007). Small neuropeptides administered intranasally enter the brain via the olfactory and trigeminal neural pathways (reviewed in Hanson and Frey, 2008) and are detected in the cerebrospinal fluid (CSF) within 10-min and remain elevated in CSF levels for over 120-min (Born et al., 2002). L-368,899 (Merck), a nonpeptide OT receptor antagonist, is detected in plasma within minutes of oral administration and remains in the circulation for ten hours, at moderate doses (Thompson et al., 1997). Moreover, L-368,899 can cross the blood-brain barrier and is detectable in the CSF within an hour of entrance in the blood, lasting for several hours (Boccia et al., 2007). Both intranasal OT and peripheral OT antagonist administrations modify centrally regulated functions such as social behavior and neuroendocrine stress responses in human and nonhuman primates (Heinrichs et al., 2003; Parker et al., 2005; Boccia et al. 2007; Ditzen et al., 2009). These findings suggest that the peripheral administration of OT and specific OT antagonist compounds constitutes a valid method for studying the central effects of OT in primates using noninvasive procedures.
We examined the hypothesis that the OT system modulates sociosexual relationships in marmosets using selective sociosexual behavior during cohabitation and social preference as two behavioral indices for pair bond formation. First, to the extent that OT activity mediates the selective social behavior of new pairs, then marmosets should display more social behavior and increased contact with a new social partner following OT treatment and less following OT antagonist treatment relative to the control treatment. Second, if OT activity affects the attraction aspect of a pair-bond in marmosets, then marmosets should show a greater preference for a pairmate over a stranger when administered OT and reduced or no preference when administered OT antagonist. To the extent that OT exerts its effects on sociality in marmosets as it does in voles, then female marmosets should be more sensitive to OT manipulations than males.
Materials and methods
Subjects
Subjects were five adult male and five adult female black-pencilled marmosets (Callithrix penicillata). All marmosets were housed in colony rooms at the Callitrichid Research Center (CRC) at the University of Nebraska at Omaha (UNO). All males were vasectomized, and all females received 0.15 mL IM injections of estrumate, a synthetic prostaglandin analogue causing functional and morphological regression of the corpus luteum (luteolysis) 2–5 days after treatment, three days before each pairing to synchronize females’ ovarian cycles.
Colony rooms at the CRC were maintained at a temperature range of 19.0 – 22.0°C and a 12h:12h light-dark cycle. All housing enclosures, during paired- and individually-housed periods, were wire-meshed cages (0.9 × 0.8 × 2.0 m) and equipped with branched, nest boxes, and other assorted enrichment items. All housing enclosures were furnished with opaque panels to prevent any visual contact between groups. All other dietary and husbandry information were consistent with CRC protocol and can be reviewed in Schaffner et al. (1995). The UNO/UNMC Institutional Animal Care and Use Committee reviewed and approved all procedures for this study (Protocol #: 07-073-11-FC). The CRC is a registered research facility with the U.S.D.A., and is accredited by the Association of Zoos and Aquaria (AZA). All appropriate guidelines for housing and conducting research with animals were followed.
Procedure
We used a repeated measures factorial design examining pair-bond formation under five different conditions (see Table 1); two in which males received either OT or OT antagonist, two in which females received either OT or OT antagonist, and one trial in which neither partner received experimental treatments. In the control condition, pair-bond formation was evaluated under normal (placebo) conditions for both male and female partners. (see Table 1). In the four other conditions, the role of OT on the dynamic of social pairing was assessed by enhancing (intranasal administrations of OT) or reducing (oral administration of OT antagonist) OT activity in either the male or female partner (see Table 1). Both administration routes were optimized to ensure that each compound entered the brain, while minimizing disruption to the animals (see procedures below). Each animal was exposed to all conditions, and the order of treatment varied across animals in order to counterbalance any treatment order effects and pairing order effects on behavior. Pair-bond formation and maintenance were studied for a three-week period during which behavioral observations and social preference tests were completed. After the pairing period, animals were separated and individually-housed (but with acoustic and olfactory contact with conspecifics) for a one-week, wash-out period. Afterward, animals were placed into a new condition and paired with a new partner until all animals had been exposed to all conditions. Marmosets were only paired once to a particular partner to prevent issues of multiple pairing under different OT manipulations. The opposite-sex strangers in the preference tests never were previous partners, excluding the final pairing. This was the case for one animal in each OT treatment condition; therefore, any effects that repeated exposure may have had on social behavior during the preference tests were consistent across all OT treatment conditions.
Table 1.
Oxytocin treatment combinations for marmosets and their partners
| Condition | Male Treatment | Female Treatment |
|---|---|---|
| Placebo Control | C | C |
| Male OT Agonist | OT+ | - |
| Male OT Antagonist | OT− | - |
| Female OT Agonist | - | OT+ |
| Female OT Antagonist | - | OT− |
Note. Diagram identifying treatment conditions for males and females including placebo control (C), oxytocin-treated (OT+), oxytocin antagonist- treated (OT−), and untreated partners (−).
Intranasal administration of oxytocin
OT (synthesized and provided by Dr. Maurice Manning, Medical College of Ohio, University of Toledo) was administered intranasally once per day throughout the pairing period, and the OT dose was based on human and nonhuman primate literature (Epperson et al., 1996; Heinrichs et al., 2003; Parker et al., 2005). Each animal received 50µg (~23 IU) of OT/100 µl saline solution, as described in Parker et al. (2005), at 0830 h, 30 min before the pair formation and prior to each morning behavioral observation. This yielded a dose of approximately 150 µg/kg since the average weight of both males and females was approximately 300g. The administration required manual restraint of the marmoset for less than 2 min. OT was administered by pipetting 50 µl of solution in each nostril, with 30 sec between administrations, alternating between the left and right nostril. OT-treated marmosets also received an untreated food item following the protocol of the OT antagonist treatments.
Oral administration of oxytocin antagonist
The OT antagonist (L-368,899; Merck; provided by Dr. Peter Williams, Dept. Medicinal Chemistry, Merck) is readily absorbed after oral administration, survives passage through the gut, crosses the blood-brain barrier, and is present in both CSF and brain areas known to contain neurons with OT receptors (Thompson et al., 1997; Boccia et al., 2007). L-368,899 was administered orally at a dose of 20 mg/kg in a preferred food item 90 min (0730 h) before the pairing and daily during the pairing period (three weeks). In order to control for handling effects associated with the intranasal administration of OT, animals receiving OT antagonist were also manually restrained and administered 100 µl of intranasal saline, following the same protocol of the OT treatment.
Control treatments
Control animals received both oral and intranasal placebos. In order to control for handling effects associated with the intranasal administrations of OT, control animals were also manually restrained and administered 100 µl of intranasal saline, following the OT treatment protocol. Control animals also received an untreated, preferred food item each day without administration of agents, following the protocol of the OT antagonist treatment.
Sociosexual behavior during cohabitation
After OT treatment conditions were established, males and females were placed together in paired-housing. Two observations were completed on the first day of pairing, one 40-min morning (between 0900–1000 h) and one 20-min (between 1400–1600 h) afternoon observation. Thereafter, one 20-min morning and one 20-min afternoon behavioral observations were complete each day during the three weeks of pairing. Animals were given several minutes to habituate to the presence of observers prior to the onset of observations. We recorded several behavioral patterns that can be defined as social, territorial/communicative, aggressive, and sexual categories (see Table 2), as well as the spatial proximity of pairmates using Noldus® Observer. The social and sexual behaviors were selected as behavioral markers for the establishment and maintenance of a social partnership as described by previous literature (Evans and Poole, 1983; Buchanan-Smith and Jordan, 1992; Schaffner et al., 1995). The territorial/communicative and aggressive behaviors were selected as control behavior as these behaviors do not directly track the development of marmoset social relationships; rather these behaviors represent territorial intergroup communication and agonistic intragroup interactions (French and Snowdon, 1981; Norcross and Newman, 1997).
Table 2.
Ethogram
| Behavior | Definition |
|---|---|
| Social behavior | |
| Approach | moving to a distance of < 10 cm from partner |
| Leave | moving to a distance of > 10 cm from partner |
| Proximitya | duration that male-female pair spend with < 10 cm apart |
| Huddlingb | sitting or resting in side-by-side contact with pair |
| Solicit grooming | orientation of body or head to present for grooming |
| Allogroomingb | manipulating another’s coat with teeth or hands |
| Food sharing | offering or passively releasing a food item to another animal |
| Male mating behavior | |
| Mount | adult male grasps adult female’s back and thrusts pelvis with erect phallus |
| Attempted Mount | adult male places one or two hands on hind of female without thrusting |
| Copulation | adult male licks erect phallus after mounting |
| Sexual solicitation behavior | |
| Genital sniff | placing nose on or near another animal’s genital region |
| OMD | open-mouth displays such as lip-smacking or tongue-flicking |
| Territorial/communicative behavior | |
| Phee calling | loud, single or multiple syllable whistle |
| Scent marking | anogenital rubbing across substrate often preceded by gnawing surface |
| Aggressive behavior | |
| Erh-erh vocals | bout of quick, guttural chucks in the context of an aggression interaction |
| Piloerection | erect pelage and arched back |
| Genital displaying | raising tail and displaying genitals while looking a directed target |
| Food stealing | aggressively taking or fighting over a food item from another animal |
| Fighting | biting, scratching, hitting, or chasing another animal |
Note. Behaviors observed for all occurrences,
duration, or
both.
Partner preference testing
After 24 h and three weeks of cohabitation, a social preference test was conducted by placing the treated marmoset into a T-shaped apparatus and allowing the marmoset to freely associate with either its current partner, an opposite-sex stranger, or to spend time in a neutral area (no animals) for a 20 min trial (Inglett et al., 1990). The T-apparatus consisted of three individual cages (33 × 33 × 33 cm) holding the partner, the stranger, and an empty cage as well as a large T-shaped cage. All animals were placed into the preference room for several minutes prior to testing to become familiarized with the apparatus and the novel room. During the familiarization period, barriers were used to obscure visual contact between the animals. Afterward, small cages containing the current partner and opposite-sex stranger were attached to opposite sides of the test apparatus with a physical barrier at the center of the T-apparatus to prevent visual contact of same-sex conspecifics. The treated marmoset was then placed into the testing apparatus, and all behavioral interactions and proximity between the treated marmoset and either the partner or stranger were recorded.
Statistical analyses
Sociosexual behavior in the pairs was evaluated comparing the pattern of behaviors over each week, during morning versus afternoon, by sex, and by OT treatment condition. A weekly composite score for each social behavior was computed by averaging observed behaviors during the morning and afternoon observations throughout each of the three weeks of pairing, except for food shares which were totaled for the three weeks. A repeated measures factorial analysis was completed for each behavioral composite score for each week with OT treatment conditions, length of cohabitation (by week), time of day (within-subjects) and sex (between-subjects) as independent variables. In addition, behavioral data from the social preference tests were analyzed with a mixed-model analysis of variance or Friedman’s ANOVA, with OT treatment conditions and type of social stimulus (within-subjects) and sex (between-subjects) as independent variables. If main effects or interactions were significant, Bonferroni’s t-test or Wilcoxon signed-rank test was used for all post-hoc testing. In addition, repeated measures factorial analyses were completed to evaluate the effects of treatment order and pairing order on social behavior. All alpha levels were set at P < .05.
Results
Selective sociosexual behavior during cohabitation
Social behavior throughout the three weeks of cohabitation varied as a function of OT treatments for both male and female marmosets, see Figs. 1 a–c. Marmosets actively sought social contact with a partner at different rates depending on treatment condition, measured by initiating close proximity [see Fig. 1a; F(2,18) = 7.03, p < 0.01] and huddling with partner, see Fig. 1b; F(2,18) = 16.96, p < 0 .001. Compared to control conditions, marmosets started huddling with a social partner more often during OT administrations, but reduced close proximity and tended to reduce huddling when administered the OT antagonist. The frequency that an individual shared food with a social partner depended on OT treatment condition, see Fig. 1c; F(2,18) = 5.14, p < 0.05. Both males and females shared food at equal rates with their partner under the control condition and following administered OT, but OT antagonist-treated marmosets all but refused to share food with their partner. Rates of all social behaviors declined over the three weeks of pairing [F’s(2,16) > 3.73, p’s < 0.05], however, this decline was not altered by OT treatment conditions.
Fig. 1.
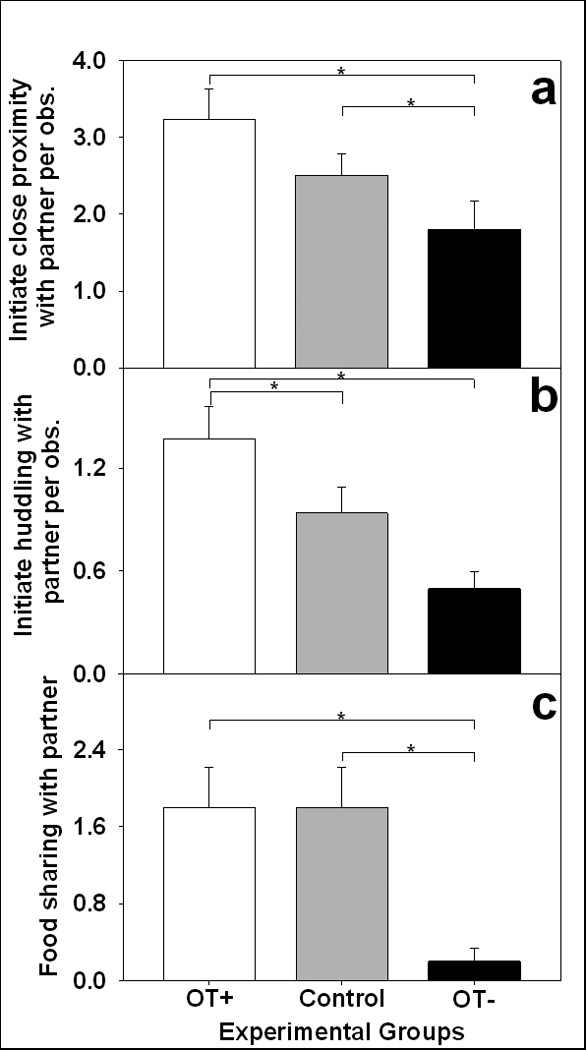
Social behavior during the first three weeks of pairing as a function of OT treatment. (a) The mean frequency (± s.e.m.) of initiations of close proximity with the partner per observation as a function of the treatment condition. (b) The mean frequency (± s.e.m.) of initiations of huddling with the partner per observation as a function of the treatment condition. (c) The mean number (± s.e.m.) of food shares with the partner throughout the pairing as a function of the treatment condition. White bar indicates intranasal OT treatment (OT+), grey bars indicate control treatment (Control), and black bars indicate the oral OT antagonist treatment (OT−). * p’s < 0.05.
All pairs mated during the three-week cohabitation period. Howbeit, male mating behavior, and sexual solicitations by male and female marmosets, did not vary as a function of treatment condition, p’s > 0.30, see Table. S.1 in Supplemental Data. As predicted, territorial behavior (p’s > 0.07) and aggression toward the new social partner (p’s > 0.21) did not vary between OT treatment conditions, see Table. S.1 in Supplemental Data. There was no main effect for treatment order [F’s(2,18) < 1.10, p’s > 0.36] or pairing order [F’s(4,32) < 2.12, p’s > 0.10] on social behavior.
24-h partner preference test
Both male and female marmosets displayed a preference to interact with the opposite-sex stranger more often than with their new social partner or the neutral cage during the 24-h preference tests, regardless of treatment condition (Figs. 2a–c). Marmosets varied the time spent with [F(2,16) = 10.03, p < 0.001], order of first contact with [F(2,16) = 7.96, p < 0.005], and amount of approaches to [F(2,16) = 16.96, p < 0.001] each of the stimulus types. Marmosets spent more time with the stranger than either their partner or the neutral cage (see Fig. 2a), approached the stranger sooner than the neutral cage and tended to approach the stranger sooner than their partner (see Fig. 2b), and approached the stranger more often than either their partner or the neutral cage (see Fig. 2c). None of the patterns of social preference at 24 h differed as a function of OT treatment condition or sex of the treated animal. All marmosets displayed more sexual solicitations (open-mouth displays) towards the stranger (M = 4.10, SE = 1.21) than toward the partner (M = 0.57, SE = 0.37), F(1,8) = 6.73, p < 0.05. Aggressive behaviors occurred too infrequent to quantify.
Fig. 2.
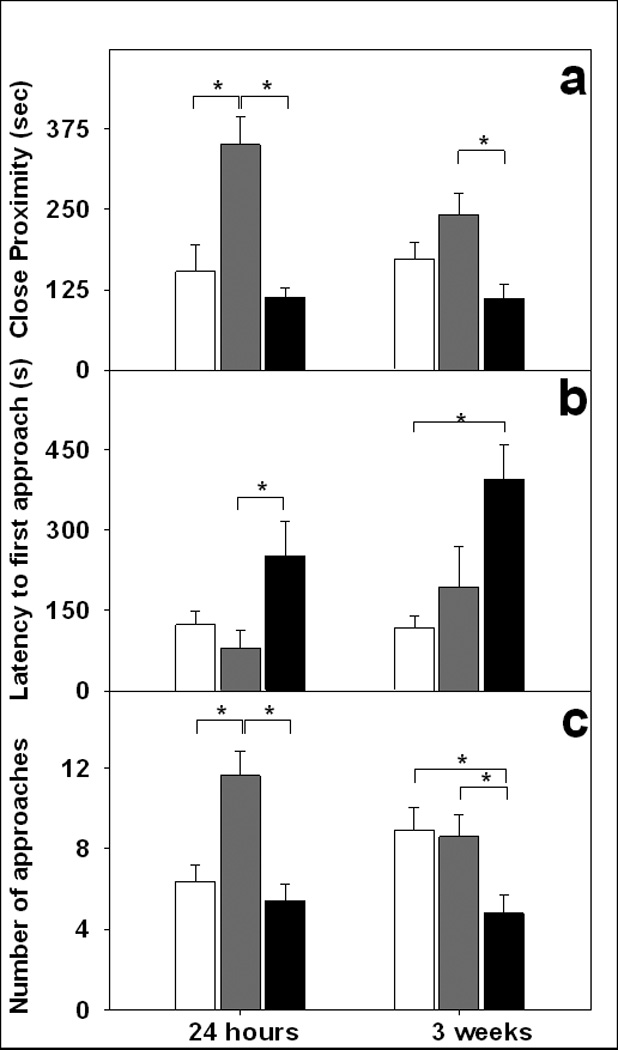
Social behavior during the 24-h and 3-week partner preference tests. The behaviors include (a) the mean duration (± s.e.m.) of time spent in close proximity, (b) the latency to first contact the social stimuli, and (c) the mean number (± s.e.m.) of approaches or initiations of close proximity during the 20 min preference test. White bar indicates interactions with the partner, grey bars indicate interactions with the opposite-sex stranger, and black bars indicate behavior while alone near the neutral, empty cage.
3-week partner preference test
During the 3-week preference test, marmosets no longer exhibited a preference for interacting with the stranger, and these patterns were similar across OT treatment conditions. Rather, they spent more time proximal to the stranger than near the neutral cage, approached their partner more quickly than the neutral cage, and approached the partner and the stranger more frequently than the neutral cage (see Figs. 2a–c). However, the order in which male and female marmosets first established contact with their partner and the stranger varied as a function of OT treatment condition [χ2(8) = 23.35, p < 0.005] but not by the sex of the treated animal, see Fig. 3. OT-treated marmosets established contact with their partner before interacting with either the stranger or spending time near the neutral cage. In contrast, OT antagonist-treated marmosets and controls did not always establish contact with their partner before engaging with the stranger. Moreover, OT-treated marmosets established contact with their partner sooner compared to OT antagonist-treated marmosets or controls. There was a difference in frequency with which marmosets approached the neutral cage [χ2(8) = 21.41, p < 0.01] such that marmosets treated with the OT antagonist approached the neutral cage about twice as many times as marmosets intranasally-treated with OT (see Fig. 4). Still, all marmosets displayed sexual solicitations (open-mouth displays) more toward the stranger (M = 2.30, SE = 0.83) than toward the partner (M = 0.03, SE = 0.03), F(1,8) = 7.49, p < 0.05. Aggressive behaviors occurred too infrequent to quantify.
Fig. 3.
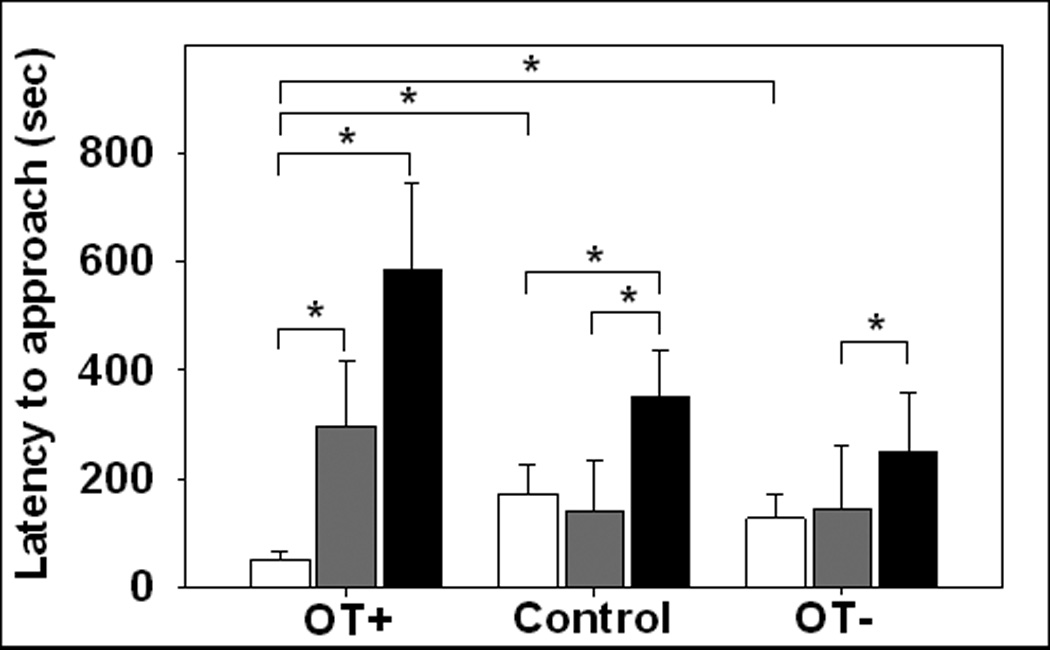
The latency to first approach each social stimuli during the 3-week preference test controlling for OT manipulation (OT treatment (OT+), control treatment (Control), and OT antagonist treatment (OT−)). White bar indicates interactions with the partner, grey bars indicate interactions with the opposite-sex stranger, and black bars indicate behavior while alone near the neutral, empty cage.
Fig 4.
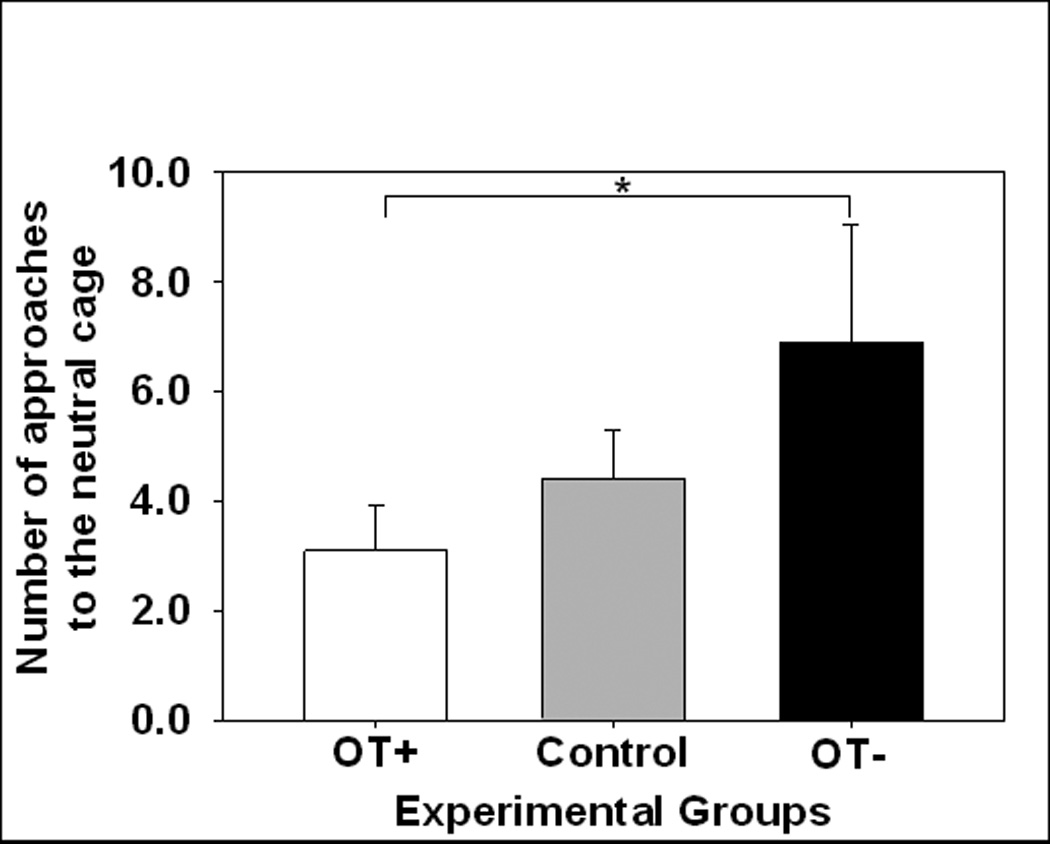
The mean number (± s.e.m.) of approaches to the neutral cage during the three week preference test. White bar indicates intranasal OT treatment (OT+), grey bars indicate control treatment (Control), and black bars indicate the oral OT antagonist treatment (OT−).
Discussion
Social pairs are established and maintained through selective social behavior, mutual attraction, and intruder aggression, and the neurobiology of a paired individual can dictate the expression of behaviors required for prolonged social bonds. In the current study, treatment conditions designed to alter central OT activity influenced behavior patterns requisite for the formation and maintenance of the social bond in heterosexual marmoset pairs. The OT treatment effects appeared to be specific to social behavior, since neither general activity nor sexual and aggressive behaviors were altered. These results suggest that the oxytocinergic neural circuits underlying social behavior are influential in pair bond formation in marmosets.
The social bond between male and female marmosets and tamarins is reflected, in part, by the extensive amount of time that the new pair spends in close physical contact and the high levels of sociosexual behavior (captivity: Rothe, 1975; Epple, 1977; Woodcock, 1982; Evans and Poole, 1983; Savage et al., 1988; Schaffner et al., 1995; Cleveland and Snowdon, 1982; wild: Soini, 1987; Ferrari and Lopes Ferrari, 1989; Digby, 1995). In general, the formation of social relationships is characterized by intense social contact and sexual interactions, and over time, the frequency of these sociosexual behaviors decreases to a level that is maintained throughout the duration of the pairing. Our study documented similar results; furthermore, OT manipulations altered the frequency of social contact and behavior. OT-treated males and females sought contact with their partner more during the course of the pairing, even as the general trend of social contact and interaction decreased between pairs. The opposite trend was observed for OT antagonist-treated marmosets such that social contact and food sharing were less frequent than in pairs formed under control conditions. It seems that suppression of OT activity through OT antagonist treatments had a stronger impact on social behavior than did the OT treatments. Marmosets are inherently social within the context of a new social partnership (see above citations), and behavior of newly paired marmosets (e.g., allogrooming and sex) should activate the oxytocinergic system, as in other mammals (Ross et al., 2009; Dunbar, 2010). Suppression of endogenous OT activity by the OT antagonist seems to modify social behavior more effectively than elevation of an already active oxytocinergic system by exogenous OT.
Sexual behavior did not vary depending on OT manipulations. The effects of OT on sexual behavior and receptivity in male and female rodents (e.g., rats, mice, and prairie vole) have been noted in a number of articles (reviewed in Carter, 1992; Argiolas and Melis, 2004). The main findings are such that peripheral and low doses of centrally administered OT facilitates sexual behavior of males and females while central administrations of OT antagonist reduce sexual behavior, suggesting OT promotes sexual arousal of rodents (Arletti et al., 1985; Arletti and Bertolini, 1985; Melis et al., 1986; Argiolas et al., 1988; Witt et al., 1990; Argiolas and Melis, 2004). Conversely, high doses of centrally administered OT reduce sexual behaviors, indicating a role of elevated OT in sexual satiety (Stoneham et al., 1985; Argiolas et al., 1987; Witt et al., 1990; Mahalati et al., 1991). In addition, mating can facilitate a partner preference in prairie voles (Williams et al., 1992; Insel et al., 1995). Even though all marmoset pairs mated during the three-week pairing period, marmosets did not show an explicit social preference, with the exception that intranasal OT administrations facilitated initial partner-seeking behavior. OT does not seem to affect sexual behavior of marmosets as it does in rodents, at least under the conditions of this study.
There is considerable interspecific variability in the social, biological, and temporal features of pair bonding. The role of OT in pair bonding has been evaluated predominantly in the socially monogamous prairie vole, where partner preference can emerge within a 24-hour period of social interaction (Williams et al., 1992; Winslow et al., 1993b) or after a few hours if mating occurs (Williams et al., 1992; Insel et al., 1995). Central injections of OT can mimic the effects of long cohabitation and mating, reducing the period of cohabitation required to establish a partner preference in male and female prairie voles (Winslow et al., 1993b; Williams et al., 1994; Insel and Hulihan, 1995; cf. Cho et al., 1999). However, pair bonding in marmosets and other pair-bonded primate species develops over the course of several weeks (Schaffner, 1996). Roberts et al. (1999) noted that male common marmoset (Callithrix jacchus) demonstrate a preference for unfamiliar females over new female partners after 24-h of cohabitation, and both male and female marmosets display a partner preference by the third- and sixth-week of cohabitation.
In the current study, male and female marmosets also showed a preference for the stranger during the 24-h preference test. During the third-week preference test, marmosets interacted equally with their partner and the opposite-sex stranger, with the exception that OT-treated marmosets established first contact faster with their partner than the stranger. Since our controls did not demonstrate a preference during the three week preference test, it may be worth prolonging the time course of future work to assess the partner preference aspect of pair bonding and the effects of OT. Nevertheless, it is clear that OT has more immediate effects on the selective social contact and behavioral aspects of pair bonding in marmosets. OT-treated male and female marmosets initiated contact more often during cohabitation, while OT antagonist-treated marmosets exhibited less affiliation and contact. Although social preferences in marmosets develop over a longer time period than in prairie voles, the formation of these social bonds in marmosets also appears to be influenced by OT activity. Thus, the effect of OT on social relationships lasts well beyond the short period of cohabitation that is typically studied in socially monogamous vole models.
Beyond the social choice of the three-chamber preference paradigm, subjects are also exposed to a novel environment (potentially stressful) and an intruder test (potentially inducing aggressive responses). First, novel environments activate the hypothalamic-pituitary-adrenal (HPA) axis and induce anxiety-like behavior in marmosets (e.g. Smith et al., 1998; Shepherd & French, 1999). In addition, OT influences and is influenced by HPA axis activity (reviewed in DeVries et al., 2003). During the three-week preference test, OT-antagonist treated marmosets approached the neutral cage more often than OT-treated marmosets. Thus, either the OT antagonist treatment increased or the OT treatment reduced solitary-seeking behavior. This may be a reflection of OT modifying the sociability of treated marmosets or anxiety responses associated with the novel environment and interactions with an unfamiliar conspecific. Further research is warranted to the dual role that OT has in facilitation of affiliation and attenuation of anxiety in marmosets. Second, while intrasexual aggression is high between paired marmosets and intruders (Epple, 1977; Epple, 1978; French and Snowdon, 1981; French and Inglett, 1989), intersexual aggression is less common as noted in other partner preference studies with Callitrichine primates (Epple, 1990; Inglett et al., 1990; cf., Buchanan-Smith and Jordan, 1992). In conjunction with these findings, there were only a few aggressive bouts observed during the three-week preference test with most directed at the stranger, though not significantly more.
The effects of OT on pair-bonding behavior during cohabitation and the preference tests did not differ between male and female marmosets. Earlier reports in prairie voles have led to the hypothesis that among the peptides that influence pair-bonding behavior, OT only influences females while another neurohypophysial hormone, vasopressin, modulates male pair bonding (reviewed by Nair and Young, 2006). However, female prairie voles have been almost exclusively utilized to evaluate the function of OT in these prior studies, potentially due to the known involvement of OT in mother–infant bonding (as denoted by Young et al., 2008). Further, most of the evidence evaluating this hypothesis deploys different paradigms (e.g., cohabitation duration, social stimulation, and OT manipulations) for males and females making it difficult for direct comparisons. Under parallel conditions, OT manipulations at lower and higher doses of OT, OT antagonist, or both alters partner-directed social behavior and partner preferences in male and female prairie voles, similarly (Cho et al., 1999). Therefore, it is likely that OT regulates pair bonding in both male and female voles. Further research such as a dose-response curve is warranted to determine the effectiveness of OT to induce pair bonding in male and female marmosets and the true extent of any sex differences. Moreover, since vasopressin modulates pair bonding in male and female prairie voles (reviewed by Nair and Young, 2006; Young et al., 2008), its role should be consisted in the social bonding of newly-paired primates.
One limitation of this study is the inability to state with certainty that our treatments altered central OT function, such as measuring CSF levels of OT or examining OT receptor occupancy by the OT antagonist. However, there is empirical evidence that OT manipulations similar or identical to those employed in our study influence brain regions mediating social behavior. OT antagonist L-368,899 circulates in the blood within minutes and last for over 14 h following oral administrations in rats and dogs (Thompson et al., 1997). Furthermore, within an hour of entering the circulatory system, the antagonist crossed the blood-brain barrier and entered the CSF of Rhesus macaques (Macaca mulatta; Boccia et al., 2007). Boccia and colleagues noted that this antagonist accumulated in brain areas that Gimple and Fahrenholtz (2001) identified to contain neurons with OT receptors (i.e., hypothalamus, septum, orbitofrontal cortex, amygdala and hippocampus), but not other brain regions 1-h following peripheral administration. In addition, peripheral administration of L-368,899 to macaques has pronounced inhibitory effects on behavioral systems known to be regulated by OT, such as maternal and sexual behavior. Small peptides (like OT) administered intranasally are transported to the CSF in humans within 10 min of administration (Born et al., 2002) and to various brain regions in rats (Ross et al., 2004) and squirrel monkeys (Balin et al., 1986). Intranasal OT administration also reduces the magnitude of the plasma ACTH surge after a stressor (Parker et al., 2005). Therefore, even though we did not directly measure central OT activity during our treatments, there is good reason to believe that the behavioral effects of our treatments reflect the modification of the oxytocinergic neural system. In addition, these two routes were chosen to optimize entrance of each compound into the brain, while minimizing disruption to the animals.
The most conspicuous and persistent sociosexual relationship within socially monogamous groups is that between the breeding male and female, and for over a decade, the influence of central OT activity on the social bond established within this dyad has been evaluated in monogamous rodents. The current study suggests that the affiliative behavior and close spatial proximity that promote this long-term relationship are influenced by the OT activity. Interestingly, sexual interactions were not affected by modifying OT activity, suggesting that the proximate mechanisms underlying social and sexual interactions in male-female marmoset pairs are independent. Therefore, we suggest that oxytocinergic neural circuits influence the strategies and behavioral interactions that male and female marmosets employ to establish and maintain a social relationship.
Supplementary Material
ACKNOWLEDGEMENTS
Special thanks to Shelton Hendricks, Claudia Rauter, and Stephanie Matthews for their comments and recommendations. Thanks also to Heather Jensen and Liz Gunkelman for providing excellent husbandry and general care for the animals and Emily Harrison, Amanda Ciurej, and Kayleigh Fehncke for their assistance with data collection. We would also like to thank Maurice Manning (University of Toledo) and Peter Williams (Merck & Co., Inc.) for their professional courtesies offering material support. The research was supported in part by funds from the National Institutes of Health (HD-42882), National Science Foundation (NSF) (IBN 00-91030), NSF Graduate Research Fellowship Program, University Committee on Research and Creative Activity at the University of Nebraska at Omaha, and American Society of Primatologists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argiolas A, Collu M, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur. J. Pharmacol. 1988;149:389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol. Behav. 2004;83:309–317. doi: 10.1016/j.physbeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Vargiu L, Gessa GL. d(CH2)5Tyr(Me)-[Orn8]vasotocin, a potent oxytocin antagonist, antagonizes penile erection and yawning induced by oxytocin and apomorphine, but not by ACTH-(1–24) Eur. J. Pharmacol. 1987;134:221–224. doi: 10.1016/0014-2999(87)90168-3. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bazzani C, Castelli M. Oxytocin improves male copulatory performance in rats. Horm. Behav. 1985;191:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bertolini A. Oxytocin stimulates lordosis behavior in female rats. Neuropeptides. 1985;6:247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catanac C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J. Comp. Neurol. 1986;251:260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Goursaud A-PS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,8997®, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in nonhuman primates. Horm. Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Panicker AK, Pedersen CA, Petrusz P. Oxytocin receptors in nonhuman primate brain visualized with monoclonal antibody. Neuroreport. 2001;128:1723–1726. doi: 10.1097/00001756-200106130-00041. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Buchanan-Smith HM, Jordan TR. An experimental investigation of the pair bond in the callitrichid monkey, Saguinus labiatus. Int. J. Primatol. 1992;13:51–72. [Google Scholar]
- Carter CS. Oxytocin and sexual behavior. Neurosci. Biobehav. R. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113:1071–1080. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cleveland J, Snowdon CT. The complex vocal repertoire of the adult cotton-top tamarin (Saguinus oedipus) Z. Tierpsychol. 1982;58:231–270. [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Digby LJ. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995;36:361–375. [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiat. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neurosci. Biobehav. R. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Epperson CN, McDougle CJ, Price LH. Intranasal oxytocin in obsessive-compulsive disorder. Biol. Psychiat. 1996;40:547–549. doi: 10.1016/0006-3223(96)00120-5. [DOI] [PubMed] [Google Scholar]
- Epple G. Notes on the establishment and maintenance of the pair bond in Saguinus fuscicollis. In: Kleiman DG, editor. The biology and conservation of the Callitrichidae. Washington, D. C: Smithson-Jan Institution Press; 1977. pp. 251–270. [Google Scholar]
- Epple G. Lack of effect of castration on scent marking, displays and aggression in a South American primate (Saguinus fuscicollis) Horm. Behav. 1978;11:139–150. doi: 10.1016/0018-506x(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Epple G. Sex differences in partner preference in mated pairs of saddle-back tamarins (Saguinus fuscicollis) Behav. Ecol. Sociobiol. 1990;27:455–459. [Google Scholar]
- Evans S, Poole TB. Pair-bond formation and breeding success in the common marmoset Callithrix jacchus jacchus. Int. J. Primatol. 1983;4:83–97. [Google Scholar]
- Ferrari SF, Lopes Ferrari MA. A re-evaluation of the social organization of the Callithrichidae, with reference to the ecological differences between genera. Folia Primatol. 1989;52:132–147. doi: 10.1159/000156392. [DOI] [PubMed] [Google Scholar]
- French JA, Inglett BJ. Female-female aggression and male indifference in response to unfamiliar intruders in lion tamarins. Anim. Behav. 1989;37:487–497. [Google Scholar]
- French JA, Snowdon CT. Sexual dimorphism in response to unfamiliar intruders in the tamarin, Saguinus oedipus. Anim. Behav. 1981;29:822–829. [Google Scholar]
- Fuentes A. Re-evaluating primate monogamy. Am. Anthropol. 1999;100:890–907. [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001;81:630–668. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Hanson L, Frey W. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9 Suppl 3:S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiat. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Inglett BJ, French JA, Dethlefs TM. Patterns of social preference across different social contexts in golden lion tamarins (Leontopithecus rosalia) J. Comp. Psychol. 1990;104:131–139. doi: 10.1037/0735-7036.104.2.131. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: Behavioral consequences. Physiol. Behav. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young L. The neurobiology of attachment. Nat. Rev. Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q. Rev. Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubios-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain: An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Mahalati K, Okanoya K, Witt DM, Carter CS. Oxytocin inhibits male sexual behavior in prairie voles. Pharmacol. Biochem. Behav. 1991;39:219–222. doi: 10.1016/0091-3057(91)90426-3. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A, Gessa GL. Oxytocin-induced penile erection and 662 yawning: Site of action in the brain. Brain Res. 1986;398:259–265. doi: 10.1016/0006-8993(86)91485-x. [DOI] [PubMed] [Google Scholar]
- Nair HP, Young LJ. Vasopressin and pair-bond formation: Genes to brain to behavior. Physiol. Behav. 2006;21:146–152. doi: 10.1152/physiol.00049.2005. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Social context affects phee call production by nonreproductive common marmosets (Callithrix jacchus) Am. J. Primatol. 1997;43:135–146. doi: 10.1002/(SICI)1098-2345(1997)43:2<135::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Uri EM, Newman JD. Delayed pair bond formation in common marmosets: Sex is not enough. Am. J. Primatol. 1999;49:93. [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH., 2nd Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: A non-invasive treatment strategy for multiple sclerosis. J. Neuroimmunol. 2004;151:66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Rothe H. Some aspects of sexuality and reproduction in groups of captive marmosets (Callithrix jacchus) Z. Tierpsychol. 1975;35:253–273. doi: 10.1111/j.1439-0310.1975.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Savage A, Ziegler TE, Snowdon CT. Sociosexual development, pair bond formation, and mechanisms of fertility suppression in female cotton-top tamarins (Saguinus oedipus oedipus) Am. J. Primatol. 1988;14:345–359. doi: 10.1002/ajp.1350140404. [DOI] [PubMed] [Google Scholar]
- Schaffner CM. Ph.D. University of Nebraska at Omaha; 1996. Social and endocrine factors in the establishment and maintenance of sociosexual relationships in Wied's black tufted-ear marmosets (Callithrix kuhli) [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in Wied's black tufted-ear marmosets (Callithrix kuhlii) Am. J. Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Shepherd RE, French JA. Comparative analysis of sociality in lion tamarins (Leontopithecus rosalia) and marmosets (Callithrix kuhli): Responses to separation from long-term pairmates. J. Comp. Psychol. 1999;113:24–32. [Google Scholar]
- Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci. Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhlii) Horm. Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Soini P. Sociosexual behavior of a free-ranging Cebuella pygmaea (Callitrichidae, platyrrhini) troop during postpartum estrus of its reproductive female. Am. J. Primatol. 1987;13:223–230. doi: 10.1002/ajp.1350130302. [DOI] [PubMed] [Google Scholar]
- Stoneham MD, Everitt BJ, Hansen S, Lightman SL, Todd K. Oxytocin and sexual behavior in the male rat and rabbit. J. Endocrinol. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Thompson KL, Vincent SH, Miller RR, Colletti AE, Alvaro RF, Wallace MA, Feeney WP, Chiu S-HL. Pharmacokinetics and disposition of the oxytocin receptor antagonist L-368,899 in rats and dogs. Drug Metab. Dispos. 1997;25:1113–1118. [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synapse. 1997;27:14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel TR. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann. N. Y. Acad. Sci. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaught CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J. Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993a;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Shapiro LE, Carter CS, Insel TR. Oxytocin and complex social behaviors: Species comparisons. Psychopharmacol. Bull. 1993b;29:409–414. [PubMed] [Google Scholar]
- Witt DM, Carter CS, Walton DM. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster) Pharmacol. Biochem. Behav. 1990;37:63–69. doi: 10.1016/0091-3057(90)90042-g. [DOI] [PubMed] [Google Scholar]
- Woodcock AJ. The first weeks of cohabitation of newly-formed heterosexual pairs of common marmoset (Callithrix jacchus) Folia Primatol. 1982;37:228–254. doi: 10.1159/000156035. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp. Biochem. Phys. C. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair-bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


