Abstract
Purpose
Docosahexaenoic acid (DHA), an n-3 fatty acid is the major polyunsaturate in rod outer segments. The effect of long term n-3 fatty acid deficiency on rod and cone phototransduction was investigated in the rhesus monkey.
Methods
From birth to ≅ 9 years rhesus monkeys were fed an n-3 deficient diet (n=9) known to reduce retinal DHA by 80%. Monkeys in the control group (n=12) received either 8% α-linolenic acid (ALA) or 0.6% DHA both of which support normal retinal DHA levels. None of the diets contained carotenoids. Photoactivation kinetics were assessed from the rate of increase, and a P3 model fit of the ERG a-wave. Maximal cone amplitude and sensitivity were measured from the cone a-wave at 4 ms. The rod photoresponse and rod recovery were derived using a paired flash method.
Results
Rod sensitivity was reduced by 40% in the n-3 deficient monkeys at 9 but not 4.5 years. The onset of the rising phase of the photoresponse was significantly delayed (p<0.004) at 9 years. Rod recovery was delayed by 20% in n-3 deficient monkeys at both ages, but only for bright saturating flashes. Cone phototransduction was not altered by n-3 deficiency
Conclusions
Long-term dietary n-3 deficiency in the rhesus monkey was associated with two changes in retinal function. First, there was a delay in rod recovery that has remained relatively constant throughout life. Second, there was an age dependent loss in rod phototransduction sensitivity; the lack of dietary carotenoids may have contributed to this decline.
Introduction
Docosahexaenoic acid (DHA or 22:6n-3), an n-3 fatty acid with 6 double bonds, is the major polyunsaturated fatty acid in rod outer segments (ROS). The highest concentration of DHA is found in the retina, particularly within the ROS where DHA accounts for up to 60% of phosphatidylethanolamine (PE) acyl chains.1 PE is the major phospholipid class of the outer bilipid layer of ROS discs. The cone-dominated retinas of the goldfish and chick are also highly enriched in DHA.1,2 The effects of dietary n-3 deficiency on retinal lipid composition have been well described.3-5 In rhesus monkeys, dietary n-3 deficiency during gestation and/or infancy results in up to an 80% reduction in retinal DHA. The loss of DHA is compensated for by increases in the long chain polyunsaturated n-6 fatty acids (e.g. 22:5n-6, 20:4n-6) such that the overall level of polyunsaturation remains the same.
The most consistent change in retinal function in n-3 deficient rodents, as measured with the ERG, is a reduction in ERG amplitude.6 An age dependent loss of rod phototransduction sensitivity in n-3 deficient guinea pigs was also reported.7 Weisinger et al7 described a non-linear function that related the reduction in rod sensitivity to retinal DHA levels, but only at 16 weeks of age, by which time guinea pigs are adults. In contrast, there were no significant losses of rod sensitivity from n-3 deficient guinea pigs at 6 or 11 weeks of age, despite 35-40% reductions in retinal DHA at these time points. At 16 weeks, similar reductions in retinal DHA levels were associated with significantly reduced rod sensitivity. The results indicate lower retinal DHA in combination with aging alters rod photoreceptor function in the guinea pig.
In our earlier studies of the rhesus monkey, the most consistent change in retinal function of n-3 deficient animals was a delay in rod recovery as measured from either ERG a- or b-waves, a change seen starting in infancy.5,8 However, we found no alteration in rod phototransduction sensitivity in n-3 deficient rhesus monkeys at 4.5 years of age when these animals were juveniles or sub-adults.8 Given the interaction between age and n-3 deficiency in guinea pigs, the purpose of the present study was to investigate the effect of long-term n-3 deficiency on phototransduction mechanisms in our rhesus monkeys, now mature adults aged 9.2 ± 1.2 years.
The monkeys examined in the present study are of particular interest as their diets were also free of carotenoids, including the xanthophylls, lutein and zeaxanthin, which form the macular pigment. In a previous study of monkeys fed the same diets as we used, no lutein or zeaxanthin were present in serum and there was no detectable macular pigment.9 In that study, monkeys fed the carotenoid free diet, regardless of their n-3 dietary intake, had altered RPE cell density profiles compared with chow fed monkeys fed a diet containing adequate levels of carotenoids and n-3 fatty acids.10 Although the effects of varying dietary carotenoid levels were not examined in the monkeys described here, the effect of long term deficiency of both carotenoids and n-3 fatty acids on retinal function and aging is of particular interest. These two nutrients have been identified as risk factors for age-related macular degeneration (AMD) and are being evaluated in a large-scale trial of AMD progression11 (www.areds2.org).
Materials and Methods
Animals and Diets
All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center and were conducted in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. From birth, twenty-one rhesus monkeys (Macaca mulatta) were fed either an n-3 deficient SAF (safflower oil-based) diet (n=9), or one of two n-3 sufficient diets, SOY diet (soy-oil based; n=7) or DHA (n=5) that support equivalent retinal DHA levels. 8 The content and administration of these diets have been described in detail previously.8 They contained all essential nutrients and supported normal growth and health. At the time of ERG studies reported herein, the mean ages of the groups (mean ± SD) were 9.7 ± 1.7, 9.0 ± 0.6 and 9.3 ± 0.5 years for the SAF, SOY and DHA groups, respectively.
Animal Preparation
Monkeys were anesthetized with intramuscular injection of ketamine, xylazine and atropine (10:1:0.4 mg/kg). Anesthesia was maintained with the same drugs (5:0.5:0.4 mg/kg) at 30- to 50-minute intervals as required. Ketamine and atropine were administered 5 minutes before xylazine injection. Supplemental O2 was delivered via nasal canula at 0.5 L/min, and heart rate and O2 saturation were monitored by pulse oximetry. Rectal temperature was maintained between 37.0°C and 38.5°C by water-circulation heated pads placed either side of the animal. After completion of the ERG studies, a triple antibiotic (Vetropolycin; Dechra Pharmaceuticals, Overland Park, KS) was placed on the eyes and an analgesic (ketaprofen, 20 mg/kg) given intramuscularly. Animals were recovered in a darkened room before being returned to their home cage.
Before ERG recording, pupils were dilated with phenylephrine (2.5%) and tropicamide (1%). The cornea was anaesthetized with proparacaine (1%) and lubricated with methylcellulose (1%) before insertion of a bipolar Burian-Allen contact lens electrode. A subdermal needle electrode placed in the back served as ground. Flash artifacts were minimized by covering the silvered speculum of the Burian-Allen electrode with black ink from a permanent marker and placing a collar made from black plastic around the top edge of the speculum. Animals were dark adapted for 30 min prior to ERG recording. ERGs were amplified (10 000) and filtered (-3dB at 0.1Hz & 1 kHz) before being sampled at 5 kHz with a 12-bit A/D converter and stored for off-line analysis.
Light Stimulus
Flash stimulation was provided by two high-intensity photoflash units (2405CX and a modified 1205 CX power supplies with 205 flash units: Speedotron; Chicago, Ill) mounted on a customized 35-cm Ganzfeldt. Flash intensities (cd • s/m2) were measured by a photometer with a scotopic filter set to integration mode (PR1980A-PL, Photo Research, Chatsworth CA). Retinal illuminance in scotopic trolands was calculated from the respective flash energies and pupil size.12
Rod and Cone Isolation
ERGs with mixed rod and cone contributions were recorded to achromatic flashes with retinal illuminances from 1.4 to 5.8 log scot td • s in approximately 0.25-log steps. Rod isolated ERGs were obtained by subtracting a dark-adapted cone-isolated ERGs from the mixed rod/cone responses. Dark adapted cone ERGs were obtained with a paired flash protocol; the first flash saturated the rods for a defined interval, during which a second flash produced a isolated cone response.13 For retinal illuminances under 3.3 log scot td • s, each flash was presented 0.5 second after a 3.3 log scot td • s flash. At higher intensities, identical paired flashes were separated by 0.8 to 3 seconds. These intervals ensured that the second flash occurred after complete cone recovery but before the onset of rod recovery. The interval between flash pairs (IFPI) ranged from 1 to 6 minutes to allow full rod recovery.
Photoactivation: Linear Photocurrent Range
A P3 model was used to fit the leading edges of ERG a-waves.13 Derived parameters were: S ((scot td • s)-1 s-2), the sensitivity parameter that scales retinal illuminance; td (s), the delay due to the filter and finite duration of the flash; and RmaxP3 (μV), the maximal response. Parameter values were determined from the ensemble fit of the P3 model to the leading edges of the ERG a-waves for retinal illuminances up to 3.6 log scot td • s. The “leading edge” was determined to be all points with amplitude less than 80% of the a-wave peak for each flash intensity. The 80% value was chosen to avoid the influence of the post-receptoral components that contribute to the a-wave near its peak.14 During fitting, RmaxP3 was fixed at the maximal a-wave amplitude obtained and S and td were varied. Cone a-wave amplitude was measured at 4 ms after stimulus to avoid contamination from post-photoreceptoral components that contribute to the monkey cone a-wave by 5 ms post stimulus in response to a bright flash.14 The plot of cone a-wave amplitude against flash intensity was well described by the exponential function:
| (1) |
where C (μV) is cone a-wave amplitude in response to flash intensity I (scot td • s). Derived parameters were Cmax (μV), maximal cone a-wave amplitude, and the rate constant k (scot td • s)-1. Half maximal amplitude occurs at intensity CIk (scot td • s) given by 0.69/k.
Photoactivation: Photocurrent Rate Saturation
For flash intensities greater than 3.6 log scot td • s, S declined as the rate of increase of the ERG a-wave approached a limiting value, a direct consequence of rate saturation of the circulating photocurrent.15 Rate saturation was characterized by plotting the maximum rate of increase of each ERG a-wave response as a function of flash intensity.16 The maximum rate of increase was obtained by differentiating the ERG a-wave with respect to time, after the response was first low pass filtered (-3dB at 500Hz). The peak of this differential indicates both the maximum rate of change in photocurrent (dR/dt|max) at a given flash intensity, and the time at which this maximum occurs. Differential peaks were well defined for flash intensities above 2.8 log scot td • s. The maximum rates of increase were normalized by dividing each function by RmaxP3. The plot of normalized maximum rate of increase as a function of flash intensity was well described by the function:16
| (2) |
where dR/dt|max (s-1) = maximum rate of increase of the ERG a-wave generated by a flash intensity of ‘I’ scot td • s. The derived parameters were dR/dt|SAT (s-1), the saturating value of the maximum rate of increase; and IK (log scot td • s) the flash intensity at 70% of dR/dt|SAT. The parameter dR/dt|SAT defines the lower limit for the maximum value of the hydrolytic rate constant of cGMP-phosphodiesterase (PDE), the effector in the phototransduction G-protein cascade.16
Photoresponse activation and inactivation – Subsaturating flashes
At the high flash intensities needed for describing phototransduction kinetics (above) and rod recovery (below), activated rhodopsin molecules are produced in large excess relative to transducin, resulting in saturation of the G-protein signaling cascade. Such saturation may minimize any differences in rhodopsin and transducin activation kinetics that result from n-3 deficiency.17 In order to overcome problems associated with G-protein saturation, the rod photoresponse to a dim flash was mapped with the paired flash method.18 The dim test flash (4.2 [0.62 log] scot td • s) activated approximately 1 in 20 000 rhodopsin molecules, well below the bleach required to saturate all available G-proteins. The test flash was followed by a probe flash (3.3 log scot td • s) at interstimulus intervals (ISI) ranging from 30 to 800 ms. Both test and probe flashes were short wavelength (Wratten 47B filter, λmax = 449 nm; Eastman Kodak, Rochester, NY). The rod-isolated probe response was derived from paired probe flashes as just described.
The normalized rod photoresponse was calculated by dividing the derived photoresponse amplitudes by the maximum amplitude of the rod-isolated probe only response. The following model19 provided a good description of the rod photoresponse for all monkeys tested:
| (3a) |
with
| (3b) |
where t0 (ms) is the time to the onset of the falling phase and τrec (ms) is the time constant of the falling phase. Rise(t) was the normalized version of equation 1 with derived parameters SN, tdN: Here N denotes the normalized versions of the parameters. For all monkeys, n was fixed at 2 and τrec at 190 ms (mean of values when floated), which provided good fits for all monkeys. The parameters SN, tdN and t0 were allowed to vary during the fitting process. The following parameters were also derived: AP (no units) is peak of the normalized response, TP (ms) is time to peak, TR50 (ms) is time to reach 50% of the peak and TF50 (ms) is the time for the photoresponse to fall to 50% of the peak.
Photoinactivation – Saturating flashes
We previously reported a 30% delay in rod recovery in SAF monkeys compared with SOY/DHA monkeys when these animals were 4.5 years of age.8 As the purpose was to determine the effects of aging on these animals, rod recovery was measured to saturating flashes of 2.8, 4.4 and 5.2 log scot td • s using a paired flash protocol. The 2.8 log flash was added for this study as it provides an intermediate between subsaturating flash above (0.62 log scot td • s) and the two highest flash intensities used previously.
Identical flashes were presented with ISIs ranging from 0.5 second to 210 seconds. The time between flash pairs was 20, 90 or 210 seconds for the 2.8, 4.4 and 5.2 log scot td • s flashes respectively. Rod isolated ERGs were derived as described earlier. Rod recovery was described by the function:13
| (4) |
where Rmax P31 and Rmax P32 are the amplitudes (μV) of the first and second flashes respectively. The derived parameters were Tc, the time (seconds) to the initiation of rod recovery and τ, the time constant of recovery (seconds).
Results
The SOY and DHA groups had equivalent results across all analyses and were combined to form a single n-3 sufficient group for comparison with the SAF group.
Photoactivation: Saturating Flashes
Figure 1A shows the ensemble fit of the P3 model13 to rod isolated ERG a-waves from a representative SOY monkey. Rod phototransduction sensitivity, S derived from the P3 model, was 40% lower in SAF monkeys, a significant reduction (p<0.02) compared with the SOY/DHA monkeys (Fig 1B & Table 1). Figure 1B highlights that phototransduction sensitivity decreased in the SAF monkeys between 4.5 and 9.2 years but remained constant in the SOY/DHA animals. There was no significant effect of diet for the other rod phototransduction parameters, RmaxP3 or td at either 4.5 years8 or 9.2 years (Table 1). The value of S at 4.5 years is higher than we previously reported,8 and is a direct result of using a simpler P3 model that does not incorporate a parameter for rod capacitance. Fig 1C shows scotopic cone responses from the same SOY monkey as shown in Fig 1A. Cone a-wave amplitude was measured at 4 ms after stimulus (vertical dashed line, Fig 1D) to avoid post-photoreceptoral contributions.14 There was a trend for smaller cone a-wave amplitudes in the SAF group (Fig 1D), although the difference was not significant (p=0.15). There was no difference in cone sensitivity between diet groups (Table 1).
Figure 1.
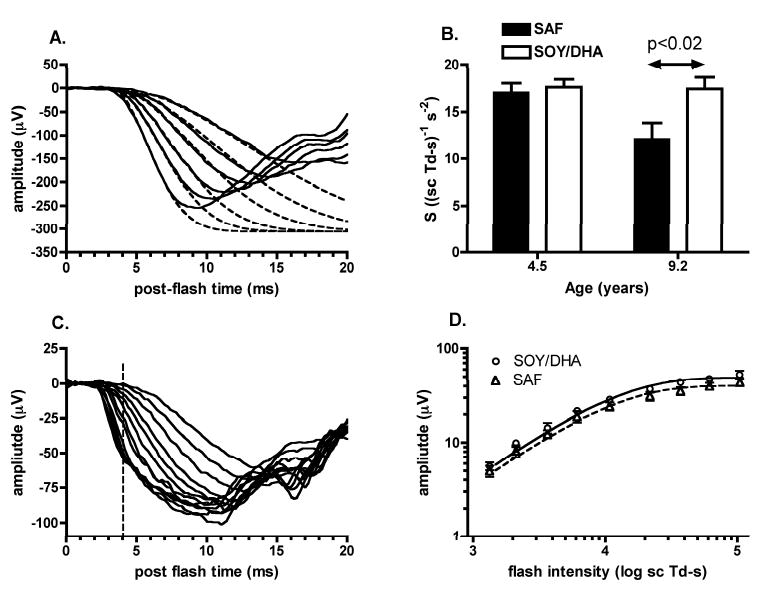
Age dependent loss in rod phototransduction sensitivity (A) Rod isolated ERG a-waves (solid lines) and fitted P3 model (dashed lines) for flash intensities from 2.4 to 3.5 log scot td • s (top to bottom). Derived phototransduction parameters for this SOY monkey were S = 18.5 (scot td • s)-1 s-2, RmaxP3 = -306.3 μV and td=3.1 ms. (B) Mean rod phototransduction sensitivity for SAF and combined SOY/DHA groups at 4.5 and 9.2 years of age. (C) Scotopic cone ERG a-waves for flash intensities from 2.1 – 4.8 log scot td • s. (D) Mean cone a-wave amplitude measured at 4 ms post stimulus (vertical dashed line in C) plotted against flash intensity. Lines show mean of the fits of equation 1 from each monkey. Error bars indicate SEM.
Table 1.
Photoactivation parameters at 9.2 years
| Parameter | SAF | SOY/DHA |
|---|---|---|
| P3 model fit of ERG a-wave | ||
| RmaxP3 (μV) | 246.3 ± 75.6 | 272.5 ± 57.9 |
| S (scot td • s)-1 s-2) | 12.1 ± 4.9* | 17.4 ± 4.2* |
| td (ms) | 2.56 ± 0.28 | 2.92 ± 0.22 |
| Cone ERG a-wave | ||
| Cmax (μV) | 40.2 ± 3.3 | 48.7 ± 4.4 |
| CIk (log scot td • s) | 3.908 ± 0.049 | 3.902 ± 0.040 |
| Maximum Rate of Increase | ||
| dR/dt|SAT (s-1) | 557 ± 31 | 576 ± 27 |
| IK (log scot td • s) | 4.536 ± 0.149† | 4.390 ± 0.124† |
Data are expressed as the mean ± S.D.
p<0.02;
p<0.04
Figure 2A shows the differentials of the leading edges of rod-isolated ERG a-waves from a SOY monkey for a select range of flash intensities. The maximum rate or increase was calculated from the peak of the differential as indicated by the arrows for two flash intensities. The plot of maximum rate of increase as a function of flash intensity for SAF monkeys was shifted right relative to the SOY/DHA monkeys (Fig 2B). This right shift results in a 40% increase (p<0.04) in IK, the amount of light needed to generate 70% of dR/dtSAT for SAF monkeys (Table 1), consistent with the 40% reduction in S. There was no significant effect of diet for dR/dtSAT, the saturating value for the maximum rate of increase (Table 1), nor for the latencies to reach maximum rates of increase (p<0.33: data not shown). There also was no effect of diet on maximum rate of increase at 4.5 years (unpublished data). Mean dR/dtSAT was 576 s-1 in the SOY/DHA monkeys, comparable with the value of 608 s-1 reported in humans.16
Figure 2.
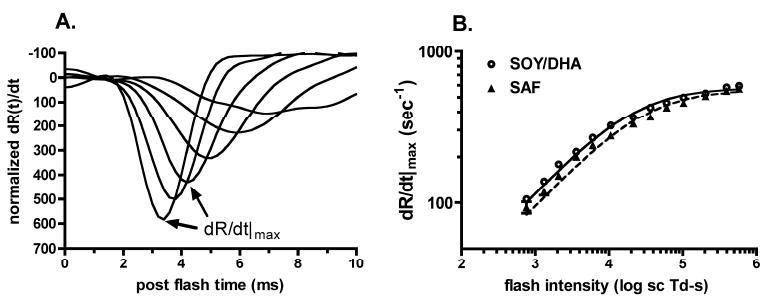
Maximum rate of increase of ERG a-wave (A) Waveforms show the differential of ERG a-waves normalized to RmaxP3 for selected flash intensities from 3.0 to 5.7 log scot td • s. The maximum rate of increase for a given flash intensity is defined by the peak of the waveform, as indicated by the arrows. (B) Mean maximum rates of increase of ERG a-waves plotted as a function of flash intensity for SAF and SOY/DHA groups. Curves: mean of fits of equation 2 for each monkey in the SAF and SOY/DHA diet groups respectively. Error bars, SEM.
Photoactivation/inactivation: Sub-saturating Flashes
The rising phase of the normalized rod photoresponse to a sub-saturating flash was delayed in the SAF group compared with the SOY/DHA group (Fig 3). The rod photoresponses of the two diet groups rise in parallel; however, the initiation of the rising phase occurs later in the SAF animals (Fig 3 & Table 2). As a result of delayed photoresponse initiation, SAF monkeys required 56.8 ms to reach 50% of the peak photoresponse, some 8.4 ms longer (p<0.002) than the 48.4 ms needed by the SOY/DHA monkeys (Table 2). The size and time of the photoresponse peak and the kinetics of the falling phase were not altered by diet (Table 2 & Fig 3 inset). These results indicate a slowing in the activation kinetics of the dim flash photoresponse in the SAF monkeys but no effect of diet on deactivation kinetics.
Figure 3.
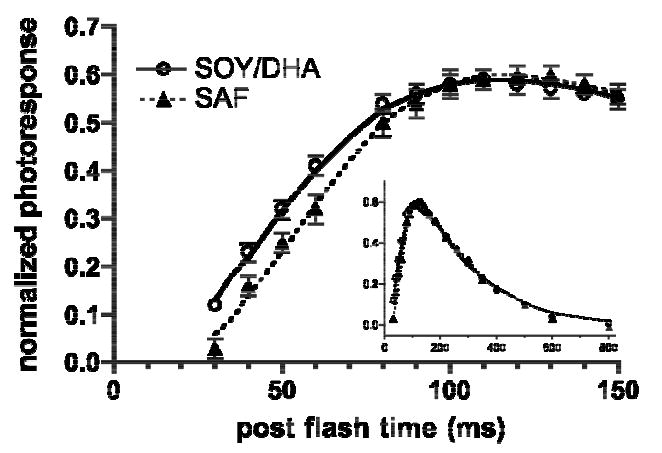
Mean normalized rod photoresponses to a 4.2 scot td • s test flash at discrete time points. Curves: means of the fits of equation 3 in each monkey. Errors bars indicate SEM. Inset: the entire derived rod photoresponses in the two diet groups.
Table 2.
Rod photoresponse parameters
| Parameter | SAF | SOY/DHA |
|---|---|---|
| SN ((scot td • s)-1 s-2) | 57.0 ± 4.9 | 52.0 ± 17.3 |
| tdN (ms) | 14.5 ± 6.2* | 2.6 ± 5.1* |
| to (ms) | 78.2 ± 29.1 | 73.1 ± 29.5 |
| AP (no units) | 0.60 ± 0.06 | 0.60 ± 0.05 |
| TRise50 (ms) | 56.8 ± 4.5† | 48.4 ± 5.0† |
| TPeak (ms) | 116.4 ± 3.4 | 112.0 ± 9.8 |
| TFall50 (ms) | 298.8 ± 8.9 | 295.2 ± 14.4 |
Data are expressed as the mean ± S.D.
p<0.0004;
p<0.002
The results from the experiments summarized in Figures 1-3 and Tables 1-2 indicate an interaction between long-term dietary n-3 deficiency and aging that alters the kinetics of rod phototransduction activation, as none of these effects were present in our previous study at 4.5 years of age.
Photoinactivation: Saturating Flashes
SAF monkeys had slower rod recovery than the SOY/DHA monkeys in response to both the 4.4 log scot td • s (Fig 4) and 5.2 log scot td • s (not shown) flashes. The time constants of recovery were 22% and 17% longer in the SAF monkeys for the 4.4 and 5.2 log scot td • s flashes respectively, although there was only a significant effect of diet (p<0.04) only at the lower intensity (Fig 5A). In contrast to the high flash intensities, the kinetics of rod recovery were essentially identical between the two diet groups in response to the 2.8 log scot td • s flash (Fig 4 inset & Fig 5). There was no significant effect of diet on Tc, the time to the initiation of rod recovery for any flash intensity (Fig 5B).
Figure 4.
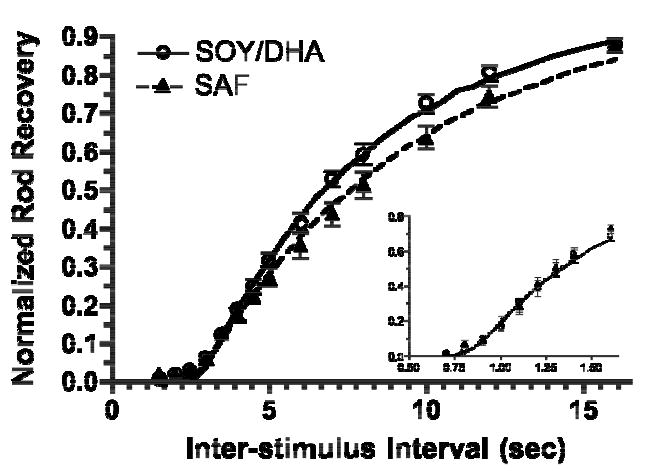
Mean normalized rod recovery to a 4.4 log scot td • s flash for a range of interstimulus intervals. Curves: the means of the fits of equation 4 from each monkey. Errors bars indicate SEM. Inset: normalized rod recovery to 2.8 log scot td • s flash.
Figure 5.
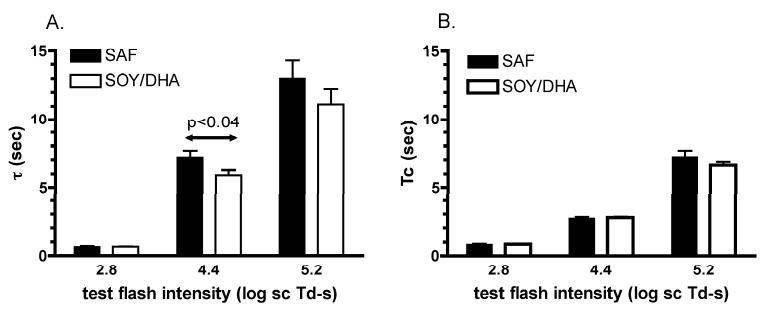
The means of τ (A) and Tc (B) for the two diet groups at each test flash intensity.
The apparent interaction between flash intensity and diet on rod recovery may be accounted for by examining the change in S with flash intensity. S was derived using flash intensities from 2.0 to 3.6 log scot td • s, a range over which S remained relatively constant for each monkey. At higher flash intensities, S declines as a direct consequence of rate saturation of the phototransduction proteins.15 This rate saturation is clearly seen in Fig 2B where the maximum rate of increase of the ERG a-wave reaches a limiting value at very high intensities. The data in Figure 2B can be transformed into a plot of sensitivity against flash intensity, which is well described by the function:16
| (5) |
where phototransduction SI (scot td • s)-1 varies as a function of flash intensity, I (scot td • s). Figure 6 shows the plots of equation 5 for the diet monkeys using the mean values of IK and S (for So) from Table 1. In SAF monkeys, the value of S was reduced by 35%, 24% and 13% relative to the SOY/DHA monkeys for the 2.8, 4.4 and 5.2 log scot td • s flashes, respectively (Fig 6). A reduction in S means a less efficient phototransduction cascade resulting in closure of fewer cation channels and therefore quicker rod recovery. In our previous study of these monkeys at 4.5 years of age, rod recovery was delayed by 30% for flash intensities between 4.4 and 5.2 log scot td • s. If we assume that the 30% delay in rod recovery has remained constant, then the reduced rod phototransduction sensitivity at lower intensities in the SAF monkeys may mask this slower rod recovery. That is, the 35% reduction rod sensitivity in SAF monkeys for the 2.8 log scot td • s flash is counteracted by the underlying 30 % delay in rod recovery. At higher intensities, the relative difference in rod phototransduction sensitivity between SAF and SOY/DHA monkeys is lower, and the delay in rod recovery in the SAF animals becomes more obvious. Therefore, rod recovery was still delayed by 17-22% in the SAF monkeys despite reduced phototransduction sensitivity in these animals. Based on the reductions in sensitivity and measured delays in rod recovery at 9.2 years it seems likely that the deficit in rod recovery in SAF monkeys has remained relatively constant with age.
Figure 6.
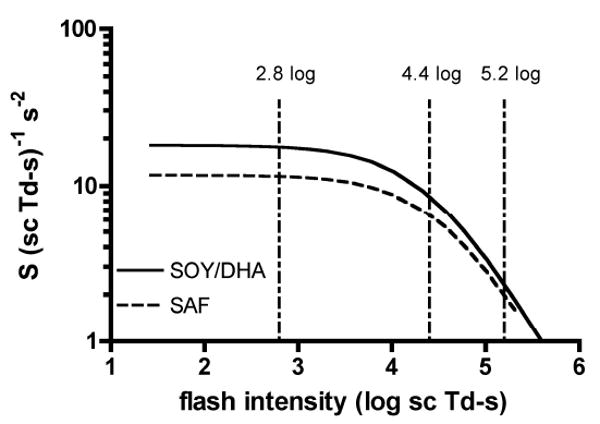
Variation in rod phototransduction sensitivity, S as a function of flash intensity in the SOY/DHA (solid) and SAF (dashed) monkeys. Curved lines were created from equation 5 with So and IK for each diet group listed in Table 1. Vertical lines indicate the test flash intensities used for measuring rod recovery.
Discussion
Functional Effects of n-3 deficiency
The present study extends our investigation into the effects of dietary n-3 deficiency on retinal function of the rhesus monkey into adulthood. At 9.2 years of age, dietary n-3 deficiency in the rhesus monkey is associated with two distinct changes in retinal function. Firstly, rod recovery (measured from ERG a- or b-wave) is delayed by approximately 20-30%, and this delay has remained relatively constant from infancy (Neuringer M, et al. IOVS 1997;38:ARVO Abstract 3451) through juvenile development8 and into adulthood (current study). Secondly, there is an age dependent loss in rod phototransduction sensitivity.
The monkeys in present study were born to mothers fed the same SAF, SOY and DHA diets from 2 months before conception and throughout gestation. In a previous study, infant monkeys born to mothers fed a SAF diet similar to one used here had a 50% reduction in retinal DHA perinatally.5 Therefore, the delay in rod recovery is directly related to n-3 deficiency since this functional change is present from infancy by which time retinal DHA levels are already dramatically reduced in SAF monkeys. DHA plays an important role in the function of certain ion channels,20,21 and we have previously hypothesized that the delays in rod recovery may result from alteration in calcium influx through the cGMP gated ion channels in rod photoreceptors.8
The reduction in rod sensitivity in SAF monkeys at 9.2 years of age indicates an interaction with aging, since rod phototransduction was not altered when these animals were tested at 4.5 years. The loss of rod sensitivity cannot be attributed to a reduction in retinal DHA alone, given the profound reductions in retinal DHA by two years of age in monkeys fed the same SAF diet used here.5 Our results in monkey are similar to the age dependent loss in rod sensitivity in n-3 deficient guinea pigs.7 The age dependent loss of rod phototransduction sensitivity in both species indicates a slow process, related to n-3 deficiency but not dependent on the absence of DHA alone.
Effect on n-3 Deficiency on Molecular Mechanisms of Phototransduction
The rod phototransduction sensitivity parameter, S, provides a measure of the gain and/or efficiency of the phototransduction cascade. Phototransduction is initiated with photon capture by rhodopsin, which rapidly isomerizes to metarhodopsin II (MII). MII binds the G-protein, transducin, which exchanges GTP for GDP. Subsequently, transducin splits into Gβγ and GαGTP, the latter of which activates the effector protein, PDE. Activated PDE hydrolyzes cGMP and the resulting fall in cytosolic cGMP causes the cGMP gated cation channels to close, and the photoreceptor to hyperpolarize.
The 40% reduction in S in the SAF monkeys indicates that one or more the activation steps of phototransduction is altered in these animals. A reduction in rod sensitivity could occur secondary to changes in rhodopsin density, but rhodopsin levels are either unaffected or increased by dietary n-3 deficiency in rats.22,23 The parameter dR/dt|SAT which defines the lower limit for the maximum value of the hydrolytic rate constant of PDE would be altered by changes in either the complement of PDE or its maximal rate of cGMP hydrolysis.16 The parameter dR/dt|SAT was not altered by diet indicating that the altered mechanisms in phototransduction occur before cGMP hydrolysis by PDE. In native rat rod disk membranes in which DHA was lowered 80% by diet, rhodopsin activation was slowed by 20% and the rate of MII-GαGTP coupling was slowed by 16%.17 The net result was a slowing in the rate of MII-GαGTP formation by 38% in native rod disk membranes from n-3 deficient rats. This delay in MII-GαGTP formation is remarkably similar to the 40% reduction in phototransduction sensitivity in our n-3 deficient monkeys. These combined results indicate that the lower phototransduction sensitivity in n-3 deficient animals is most likely due to slowing in the rate of rhodopsin activation and MII-GαGTP formation.
DHA rich lipids impart important physical properties that provide the optimal membrane environment for rhodopsin function and diffusion of the phototransduction proteins.24 Recent evidence suggests DHA may play an even more important role in rhodopsin function. DHA is selectively and tightly bound by rhodopsin and the direct protein-lipid interaction between the two are important to the optimal function of rhodopsin.24,25 It has also been proposed that DHA may act as a ligand for rhodopsin.26
Cone sensitivity was not altered in the SAF monkeys indicating that rods are more susceptible to n-3 deficiency. Lipid analyses of cone dominant retinas from fish and birds indicate that cones, like rods, have high levels of DHA1,2. Why rods might be more susceptible than cones to long-term n-3 deficiency is an intriguing question. The phototransduction proteins in rods and cones are broadly the same, albeit with different splice variants or isomers.27 Rods and cones have very different outer segment structures but it is not obvious how these differences might differentially affect susceptibility to n-3 deficiency. Cones are less sensitive than rods and the amplification constant of cones is at least 20 fold lower than rods.28 This difference in amplification constant may provide a clue to the greater susceptibility of rods to n-3 deficiency. The higher amplification in rods means that rhodopsin needs to activate the G-protein tranducin at least 20 times faster than the same reaction in cones. Therefore, rods may be more susceptible to the change in biophysical properties that slow this reaction in n-3 deficient membranes.
While the results in isolated disk membranes of n-3 deficient rats indicate that the reduction in phototransduction sensitivity in n-3 deficient animals can be explained by changes in the efficiency and kinetics of the initial steps of the phototransduction cascade, the question remains as to why such changes are age dependent (see below).
Effects of Carotenoid Deficiency
A possible contributing factor in the present study is that all diets were carotenoid free. In a previous study of monkeys fed the same diets as in this study, lutein and zeaxanthin were absent in serum and macular pigment was undetectable.9 Monkeys fed the xanthophyll free diets had altered RPE cell density profiles with a prominent dip at the fovea, regardless of their n-3 dietary intake.10 By comparison, in control monkeys fed a standard stock diet with adequate levels of lutein, zeaxanthin and n-3 fatty acids, RPE cell density peaked at the foveal center.10 Monkeys fed the xanthophyll free, n-3 deficient (SAF) diet also had a 22-30% increase in RPE cell density and a 50% reduction in ROS cell density compared with monkeys fed either the control or xanthophyll free, n-3 adequate (SOY/DHA) diets.10,29 However, these changes in RPE and rod outer segment densities in SAF monkeys would not explain the 40% decrease in rod sensitivity observed in the present study. Indeed a reduction in rod outer segment density would be consistent with a reduction in RmaxP3, but there was only a small (10%) non-significant reduction in this parameter in the SAF monkeys. Thus, the anatomical changes in the retina of monkeys fed xanthophyll and/or n-3 deficient diets cannot account for the reduction in rod sensitivity in the SAF monkeys. The results from this series of studies suggest a complex relationship in the retina between n-3 fatty acids, probably DHA and the xanthophylls of the macula pigment.10
Both DHA and the xanthophylls are thought to serve important antioxidant, anti-inflammatory and neuroprotective roles in the retina. 30,31 For example, within the RPE, DHA serves as a precursor to the synthesis of neuroprotection D1 (NPD1).32 NPD1 inhibits oxidative stress-induced apoptosis mediated by A2E, a byproduct of phototransduction and major fluorophore of lipofuscin that becomes toxic when accumulated within the retina.33 NPD1 regulation has been suggested as a possible important factor in some retinal diseases, particularly macular degeneration. Besides their antioxidant properties, the xanthophylls also serve to filter potentially damaging high energy short-wavelength light before it reaches the retina.31 Therefore, the lack of macular pigments might increase oxidative damage, and the reduction in retinal DHA in the SAF monkeys along with RPE remodeling could lower the availability of NPD1 to ameliorate oxidative stress. In addition, lower rhodopsin density through protein oxidation or disruption of membrane biophysical properties through lipid oxidation could both lower the efficiency of phototransduction and, therefore, reduce sensitivity. Whether protein and/or lipid oxidation in the rod outer segment underlies the age-related loss of phototransduction sensitivity in n-3 deficient animals awaits further investigation.
Dietary n-3 and carotenoid deficiency: a model of premature retinal senescence?
In the present study, monkeys fed an n-3 deficient, carotenoid free diet had an age dependent loss in rod sensitivity with an approximately 40% reduction between 4.5 and 9.2 years of age. All diets in the present study were carotenoid free, so the age-related loss in rod sensitivity in the SAF monkeys could be due to the combination of dietary carotenoid and n-3 deficiency. Further studies will be needed to address whether n-3 deficiency or carotenoid deficiency alone or in combination underlie the loss of rod sensitivity with age in the monkey. With a 1:3 year ratio for monkey/human comparison, these monkeys at 9.2 years would correspond to only 28 years of age in humans. Human studies have reported reductions in rod sensitivity with age, 34,35,36 although this age affect is lessened when light absorption of the aging lens is taken into account.36 Two studies of non-human primates support the possibility of accelerated aging of the retina in monkeys fed a carotenoid free diet. In monkeys fed the same diets used in the present study, higher perifoveal lipofuscin was found in all xanthophyll free monkeys, but particularly in the n-3 deficient animals (Leung IY, et al. IOVS 2006;47:ARVO E-abstract 2882). Additionally, drusen are common at a much earlier age in carotenoid free monkeys compared with animals fed a standard diet (Neuringer M. et al. IOVS 2003;44:ARVO E-Abstract 4949). Accelerated aging or early onset of AMD in the n-3 deficient/carotenoid free monkeys would be of particular interest given that these two compounds may reduce the risk of AMD11,37 and both are being evaluated in a multicenter controlled randomized trial of AMD progression (www.areds2.org).
Acknowledgments
The authors thank Andrea Peck for assistance with ERG recordings.
Grants: NEI (EY13199), NICHD (DK29930), NCRR (RR000163) and The Foundation Fighting Blindness
Bibliography
- 1.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GJ, Connor WE, Corliss JD, Lin DS. Rapid modulation of the n-3 docosahexaenoic acid levels in the brain and retina of the newly hatched chick. J Lipid Res. 1989;30:433–441. [PubMed] [Google Scholar]
- 3.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary à-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 4.Weisinger HS, Vingrys AJ, Sinclair AJ. Dietary manipulation of long-chain polyunsaturated fatty acids in the retina and brain of guinea pigs. Lipids. 1995;30:471–473. doi: 10.1007/BF02536307. [DOI] [PubMed] [Google Scholar]
- 5.Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega-3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeffrey BG, Weisinger HS, Neuringer M, Mitchell DC. The role of docosahexaenoic acid in retinal function. Lipids. 2001;36:859–871. doi: 10.1007/s11745-001-0796-3. [DOI] [PubMed] [Google Scholar]
- 7.Weisinger HS, Vingrys AJ, Bui BV, Sinclair AJ. Effects of dietary n-3 fatty acid deficiency and repletion in the guinea pig retina. Invest Ophthalmol Vis Sci. 1999;40:327–338. [PubMed] [Google Scholar]
- 8.Jeffrey BG, Mitchell DC, Gibson RA, Neuringer M. n-3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys. Invest Ophthalmol Vis Sci. 2002;43:2806–2814. [PubMed] [Google Scholar]
- 9.Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas. I. Effects of lutein or zeaxanthin supplements on serum and macular pigment of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2004;45:3234–3243. doi: 10.1167/iovs.02-1243. [DOI] [PubMed] [Google Scholar]
- 10.Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004;45:3244–56. doi: 10.1167/iovs.02-1233. [DOI] [PubMed] [Google Scholar]
- 11.Coleman H, Chew E. Nutritional supplementation in age related macular degeneration. Curr Opin Ophthalmol. 2007;18:220–223. doi: 10.1097/ICU.0b013e32814a586b. [DOI] [PubMed] [Google Scholar]
- 12.Wyszecki G, Stiles WS. Color Science: Concepts and methods, quantitative data and formulae. 2nd. New York: John Wiley & Sons; 1982. [Google Scholar]
- 13.Birch DG, Hood DC, Nusinowitz S, Pepperberg DR. Abnormal activation and inactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the pro-23-his mutation. Invest Ophthalmol Vis Sci. 1995;36:1603–1614. [PubMed] [Google Scholar]
- 14.Robson JG, Saszik SM, Ahmed J, Frishman LJ. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol. 2003;547:509–30. doi: 10.1113/jphysiol.2002.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobbs WH, Pugh EN., Jr Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. J Physiol. 1987;394:529–72. doi: 10.1113/jphysiol.1987.sp016884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breton ME, Schueller AW, Lamb TD, Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. [PubMed] [Google Scholar]
- 17.Niu SL, Mitchell DC, Lim SY, Wen ZM, Kim HY, Salem N, Jr, Litman BJ. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem. 2004;279:31098–104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- 18.Pepperberg DR, Birch DG, Hood DC. Photoresponses of human rods in vivo derived from paired-flash electroretinograms. Vis Neurosci. 1997;14:73–82. doi: 10.1017/s0952523800008774. [DOI] [PubMed] [Google Scholar]
- 19.Friedburg C, Thomas MM, Lamb TD. Time course of the flash response of dark- and light-adapted human rod photoreceptors derived from the electroretinogram. J Physiol. 2001;534:217–42. doi: 10.1111/j.1469-7793.2001.t01-1-00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leaf A. Omega-3 fatty acids and prevention of ventricular fibrillation. Prostaglandins, Leukotr Essent Fatty Acids. 1995;52:197–198. doi: 10.1016/0952-3278(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 21.Vreugdenhil M, Bruehl C, Voskuyl RA, Kang J, Leaf A, Wadman WJ. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc Natl Acad Sci U S A. 1996;93:12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush RA, Malnoe A, Reme CE, Williams TP. Dietary deficiency of N-3 fatty acids alters rhodopsin content and function in the rat retina. Invest Ophthalmol Vis Sci. 1994;35:91–100. [PubMed] [Google Scholar]
- 23.Reme CE, Malnoe A, Jung HH, Wei JQ, Munz K. Effect of dietary fish oil on acute light induced photoreceptor damage in the rat retina. Invest Ophthalmol Vis Sci. 1994;35:78–90. [PubMed] [Google Scholar]
- 24.Feller SE, Gawrisch K. Properties of docosahexaenoic-acid-containing lipids and their influence on the function of rhodopsin. Curr Opin Struct Biol. 2005;15:416–22. doi: 10.1016/j.sbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc Natl Acad Sci U S A. 2006;103:4888–93. doi: 10.1073/pnas.0508352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Turco EB, Jackson FR, Parkins N, Gordon WC. Strong association of unesterified [3H]docosahexaenoic acid and [3H-docosahexaenoyl]phosphatidate to rhodopsin during in vivo labeling of frog retinal rod outer segments. Neurochem Res. 2000;25:695–703. doi: 10.1023/a:1007571305987. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Yau KW. Phototransduction in mouse rods and cones. Eur J Physiol. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura S, Tachibanaki S. Rod and cone photoreceptors: Molecular basis of the difference in their physiology. Comp Biochem Physiol A Mol Integr Physiol. 2008;150:369–377. doi: 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- 29.Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Max Snodderly D. Nutritional manipulation of primate retinas. IV. Effects of n--3 fatty acids, lutein, and zeaxanthin on S-cones and rods in the foveal region. Exp Eye Res. 2005;81:513–29. doi: 10.1016/j.exer.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 30.SanGiovanni JP, Chew EY. The role of omega-3 long-chian polyunsaturated fatty acids in health and disease of the retina. Prog Retina Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Ann Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007;104:13152–7. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cideciyan AV, Jacobson SG. An alternative phototransduction model for human rod and cone ERG a-waves: normal parameters and variation with age. Vision Res. 1996;36:2609–2621. doi: 10.1016/0042-6989(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 35.Birch DG, Hood DC, Locke KG, Hoffman DR, Tzekov RT. Quantitative electroretinogram measures of phototransduction in cone and rod photoreceptors: normal aging, progression with disease, and test-retest variability. Arch Ophthalmol. 2002;120:1045–51. doi: 10.1001/archopht.120.8.1045. [DOI] [PubMed] [Google Scholar]
- 36.Jackson GR, McGwin G, Jr, Phillips JM, Owsley C. Impact of aging and age-related maculopathy on activation of the a-wave of the rod mediated electroretinogram. Invest Ophthalmol Vis Sci. 2004;45:3271–78. doi: 10.1167/iovs.04-0019. [DOI] [PubMed] [Google Scholar]
- 37.Chong EWT, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Dietary ω-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration. A systematic review and meta-analysis. Arch Ophthalmol. 2008;126:826–833. doi: 10.1001/archopht.126.6.826. [DOI] [PubMed] [Google Scholar]


