Summary
Swim stress regulates forebrain 5-hydroxytryptamine (5-HT) release in a complex manner and its effects are initiated in the serotonergic dorsal raphe nucleus (DRN). The purpose of this study was to examine the effects of swim stress on the physiology of DRN neurons in conjunction with 5-HT immunohistochemistry. Basic membrane properties, 5-HT1A and 5-HT1B receptor-mediated responses and glutamatergic excitatory postsynaptic currents (EPSCs) were measured using whole-cell patch clamp techniques. Rats were forced to swim for 15 min and 24 h later DRN brain slices were prepared for electrophysiology. Swim stress altered the resting membrane potential, input resistance and action potential duration of DRN neurons in a neurochemical-specific manner. Swim stress selectively elevated glutamate EPSC frequency in 5-HT DRN neurons. Swim stress non-selectively reduced EPSC amplitude in all DRN cells. Swim stress elevated the 5-HT1B receptor-mediated inhibition of glutamatergic synaptic activity that selectively targeted 5-HT cells. Non-5-HT DRN neurons appeared to be particularly responsive to the effects of a milder handling stress. Handling elevated EPSC frequency, reduced EPSC decay time and enhanced a 5-HT1B receptor-mediated inhibition of mEPSC frequency selectively in non-5-HT DRN cells. These results indicate that swim stress has both direct, i.e., changes in membrane characteristics, and indirect effects, i.e., via glutamatergic afferents, on DRN neurons. These results also indicate that there are distinct local glutamatergic afferents to neurochemically specific populations of DRN neurons, and furthermore that these distinct afferents are differentially regulated by swim stress. These cellular changes may contribute to the complex effects of swim stress on 5-HT neurotransmission and/or the behavioral changes underlying the forced swimming test model of depression.
Keywords: Serotonin, Glutamate, 5-HT receptors, AMPA/kainate receptors, Electrophysiology, Rat
1. Introduction
Swim stress is known to regulate the 5-hydroxytryptamine (5-HT) system in a complex manner. It produces bidirectional, region-specific effects on 5-HT release in the forebrain (Kirby et al., 1995). In addition to its regulation of the 5-HT system, swim stress, as demonstrated in the forced swimming test (FST), initiates behavioral changes 24 h later which are selectively altered by antidepressant treatment (see Porsolt et al., 1977). For this reason, the FST has been used extensively as an antidepressant screen and an animal model of depression. Earlier work in our laboratory also demonstrated that there is a correlation between changes in these antidepressant-sensitive behaviors in the FST and changes in forebrain 5-HT (Kirby et al., 1997; Kirby and Lucki, 1997). The purpose of this study was to examine the cellular mechanisms whereby swim stress regulates the DRN 5-HT system. To achieve this goal, we compared the cellular properties, receptor-mediated responses and glutamatergic synaptic activity of dorsal raphe nucleus (DRN) neurons in brain slices prepared from naïve rats or those with prior exposure to swim stress or control handling using whole-cell patch clamp recording techniques. Our hypothesis was that the complex effects of swim stress on the 5-HT system are initiated by alterations of the cellular properties, receptor-mediated responses, and/or modulation by glutamatergic afferent inputs to forebrain-projecting 5-HT DRN neurons. We conducted these electrophysiology studies in the DRN 24 h following swim stress exposure. Previous studies showed that while there is an acute serotonergic response to swim stress (Kirby et al., 1995), this response adapts when subjects are re-exposed to swim stress 24 h later (Kirby and Lucki, 1998; Price et al., 2002). This study therefore examines the potential cellular mechanisms underlying this serotonergic adaptation to the stressor and investigates cellular changes in the 5-HT system at a time-point in which the FST model demonstrates behavioral sensitivity to antidepressant drug treatment.
2. Methods
2.1. Subjects
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) initially weighing 75–150 g were housed 2–3 per cage on a 12 h light schedule (lights on at 0700 h) in a temperature-controlled (20 °C) colony room. Rats were given access to standard rat chow and water ad libitum. Animal protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Swim stress
Rats were placed in a cylindrical glass tank (46 cm tall × 20 cm diameter) of 21–22 °C water filled to a depth of 30 cm for 15 min. Handled control subjects were picked up twice to mimic the handling exposure of swim stress subjects (first to place them in the swim tank and 15 min later to return them to the home cage). Naïve controls were not handled. The use of two control groups, unstressed naïve subjects and handled subjects that differ from the swim stress group only by exposure to the swim tank, allows for a more complete picture of the effects of the complex swim stressor.
2.3. Slice preparation
Twenty-four hours following the swim stress or handling control, rats were rapidly decapitated and the head placed in ice cold artificial cerebrospinal fluid (ACSF) in which sucrose (248 mM) was substituted for NaCl. Naïve controls were sacrificed without prior handling. The brain was rapidly removed and trimmed to isolate the brainstem region. Slices 200 μm thick were cut throughout the rostro-caudal extent of the DRN using a Leica microslicer (Leica, Allendale, NJ) or Vibratome 3000 plus (Vibratome, St. Louis, MO) and placed in a holding vial containing ACSF at 34–36 °C bubbled with 95%O2/5%CO2 for 1 h. After 1 h the slices were kept in room temperature ACSF bubbled with 95%O2/5%CO2. The composition of the ACSF was (mM), NaCl 124, KCl 2.5, NaH2PO4 2, CaCl2 2.5, Dextrose 10 and NaHCO3 26.
2.4. Electrophysiological recording
Slices were transferred to a recording chamber (Warner Instruments, Hamden, CT) and continuously perfused with ACSF at 1.5–2ml/min at 32–34 °C maintained by an in-line solution heater (TC-324, Warner Instruments). Raphe neurons were visualized using a Nikon E600 upright microscope fitted with a 40× water-immersion objective, differential interference contrast (DIC) and infrared filter (Optical Apparatus, Ardmore, PA). The image from the microscope was enhanced using a CCD camera and displayed on a computer monitor. Whole-cell recording pipettes were fashioned on a P-97 micropipette puller (Sutter Instruments, Novato, CA) using borosilicate glass capillary tubing (1.2 mm OD, 0.69 mm ID; Warner Instruments). The resistance of the electrodes was 4–8MΩ when filled with an intracellular solution of (in mM) Kgluconate 130, NaCl 5, MgCl2 1, EGTA 0.02, HEPES 10, Naphosphocreatinine 10, MgATP 2, Na2GTP 0.5, 0.1% Biocytin, pH 7.3.
All chemicals for making the ACSF and electrolyte solution were obtained from Sigma-Aldrich (St. Louis, MO). A 100 μM stock solution of the 5-HT1/5/7 agonist, 5-carboxamidotryptamine maleate (5-CT; Sigma-Aldrich) was prepared in distilled water, stored at −80 °C and diluted on the day of the experiment to its final concentration of 100 nM in the ACSF.
2.5. Experimental protocols
All recordings in this study were made in the ventromedial subdivision of the DRN that contains the densest cluster of 5-HT neurons in the nucleus. A visualized cell was approached with the electrode, a gigaohm seal established and the cell membrane ruptured to obtain a whole-cell recording using either an Axoclamp 2A, Axopatch 200B (Axon Instruments, Foster City, CA) or HEKA patch clamp EPC-10 amplifier (HEKA Elecktronik, Pfalz, Germany). Series resistance was monitored throughout the experiment. If the series resistance of the electrode was unstable or exceeded four times the electrode resistance, the cell was discarded. For the Axoclamp and Axopatch amplifiers, signals were digitized by Digidata 1320-series analog-to-digital converters and stored on-line using pClamp 7/8/9 software. For the HEKA amplifier, signals were stored on-line using Pulse software. The liquid junction potential was approximately 10 mV between the pipette solution and the ACSF and was not subtracted from the data obtained.
Cell characteristics were recorded from DRN neurons in current clamp mode. The cell characteristics and their response to 5-CT were analyzed with pClamp software. The cell characteristics measured were resting membrane potential, input resistance, time constant (tau), action potential and afterhyperpolarization potential characteristics (Beck et al., 2004).
Glutamatergic synaptic activity was recorded from DRN neurons in voltage clamp mode (Vm = −70mV). Synaptic activity was analyzed with MiniAnalysis software (Synaptosoft, Decatur, GA). Synaptic events over a 1-min period were analyzed for frequency (Hz), amplitude (pA), baseline holding current (mV), rise time (ms; calculated from 10–90% of peak amplitude) and decay time (ms; calculated by averaging 200 events randomly selected by MiniAnalysis and fitting a single exponential function from 10–90% of the decay phase). For the synaptic activity protocol, both spontaneous EPSCs (sEPSCs) and miniature spontaneous EPSCs (mEPSCs) were recorded. Spontaneous EPSCs represent action potential-dependent, i.e., glutamate neurons in the slice that are spontaneously active, and non-action potential-dependent events. Miniature spontaneous EPSCs, recorded in the presence of the Na+ channel blocker tetrodotoxin (TTX; 1 μM), are non-action potential-dependent events. First, sEPSCs were recorded for 6 min. Next, TTX was added and mEPSCs were recorded for 6 min. 5-CT was then added to the perfusion bath and mEPSCs recorded for 6 min. Membrane hyperpolarization (represented by an increase in outward current in voltage clamp conditions) in response to 5-CT has been shown to be mediated by 5-HT1A receptors (Williams et al., 1988; Beck et al., 2004). Inhibition of mEPSC frequency by 5-CT has been shown in our laboratory to be mediated by the 5-HT1B receptor (Lemos et al., 2006). In some cells, the AMPA/kainate glutamate receptor-mediation of EPSCs was verified at the end of the experiment with the addition of the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM) or 6,7-dinitroquinoxaline-2,3(1 H,4H)-dione (DNQX; 20 μM). Under these conditions, CNQX or DNQX eliminated all EPSC activity.
2.6. Immunohistochemistry
If a recorded cell could not be identified as 5-HT or non-5-HT containing by immunohistochemistry it was not included in the data set for this study. Standard immunofluorescence procedures were used to visualize the filled cell and neurotransmitter content. Slices were fixed by submersion in 4% paraformaldehyde prepared in 0.1 M phosphate buffer (PB; pH 7.4) overnight and then stored in 30% sucrose. Sections were incubated with rabbit α 5-HT antibody (1:2000; ImmunoStar, Hudson, WI) for 1 week at 4 °C or mouse α tryptophan hydroxylase antibody (1:200; Sigma-Aldrich) overnight at 4 °C. Subsequently, immunohistochemical labeling was visualized using a Alexa 488 conjugated donkey α rabbit secondary antiserum (1:100; Molecular Probes, Eugene, OR) or FITC conjugated donkey α mouse secondary antiserum (1:100; Jackson ImmunoResearch, West Grove PA) for 60 min at room temperature. The biocytin in the patched cell was visualized using streptavidin-conjugated Alexa 633 (1:100; Molecular Probes) for 60 min at room temperature. Between incubations slices were rinsed with PB solutions (3 × 10 min). Sections were mounted with ProLong Antifade Kit (Molecular Probes) on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and visualized using a Leica DMR fluorescence microscope and a Leica DMIRE2 (Leica Microsystems, Exton, PA) or Olympus FV300 confocal microscope (Olympus America, Mellville, NY). Images were captured using a digital camera and Openlab 3.09 software (Improvision, Lexington, MA) on the fluorescence microscope and a digital camera and Leica Confocal software (Version 2.5, Leica) or FluoView software (Version 2.1, Olympus). When using the confocal microscope sequential collection, i.e., Alexa 633 separately from FITC/Alexa 488, images of 0.6 μm thickness were acquired at the level of the cell body of the biocytin-labeled neuron. The laser power and emission filters were adjusted for both the red and green fluorophor so that there was minimal possibility of a false positive result. This was done by exciting at the optimal wavelength for the green fluorophor and detecting using the emission spectra for the red fluorophor and vice versa. Images were adjusted for optimal color balance and contrast using Adobe Photoshop 6.0 software (Adobe, San Jose, CA).
2.7. Data analysis
Membrane characteristics were compared between different cell populations by unpaired Student's t-test or Mann–Whitney Rank Sum tests when the populations were not normally distributed. The effect of swim stress and neurochemical identity (5-HT or non-5-HT) on receptor-mediated responses or synaptic event characteristics (frequency, amplitude, rise and decay times) of sEPSCs and mEPSCs were analyzed by two-way factorial or repeated measures analysis of variance (ANOVA). When statistical significance was found with two-way ANOVA, separate one-way ANOVAs were conducted (factorial or repeated measures ANOVAs). Student–Newman–Keuls tests were used for follow-up pairwise comparisons between groups. The frequency of EPSC events in individual cells is also illustrated as a cumulative inter-event interval probability graph (Fig. 1). The non-parametric Kolmogorov–Smirnov (K–S) test was used to compare sEPSC, mEPSC and mEPSC+5-CT distributions. A probability of p<0.05 was considered significant. Most data are reported as mean ± ⦵ SEM. The exception is EPSC rise time, whose frequency distributions were skewed (non-normal distribution). As a consequence, rise time data are presented as median ± SEM.
Figure 1.

Swim stress enhances glutamatergic EPSC frequency in a 5-HT DRN neuron. Panels Ai, Bi and Ci show raw traces of sEPSCs, mEPSCs (recorded in the presence of TTX, 1 μM) and mEPSCs+5-CT (100 nM) recorded from a 5-HT cell in subjects pre-exposed to swim stress (A), handling (B) or naïve control conditions (C). Panels Aiii, Biii and Ciii are fluorescent photomicrographs demonstrating that the recorded cells contain 5-HT. The recorded biocytin-filled cells are shown in red, tryptophan hydroxylase-IR is shown in green and the merged panels demonstrating double labeling appear yellow. Cumulative inter-event interval probability graphs for each cell (Aii, Bii and Cii) illustrate a shift to the right as EPSC frequency is inhibited by TTX (mEPSCs) and further by the addition of 5-CT, an effect previously demonstrated to be mediated by the 5-HT1B receptor (Lemos et al., 2006). Data from these cells exemplify the finding that EPSC frequency is higher in animals pre-exposed to swim stress than in control conditions. EPSCs are mediated by AMPA/kainate glutamate receptors as they are completely suppressed by the non-NMDA glutamate receptor antagonists CNQX or DNQX (20 μM) under these conditions (data not shown).
3. Results
3.1. Membrane characteristics
Table 1 illustrates the effect of swim stress on membrane characteristics of 5-HT- and non-5-HT-containing DRN neurons. Only four cellular characteristics were altered by the behavioral manipulations in 5-HT neurons, i.e., resting membrane potential, input resistance and action potential threshold and duration. Interestingly, there were no changes by any of the behavioral manipulations in one of the hallmark characteristics of 5-HT containing neurons, i.e., the afterhyperpolarization.
Table 1.
Effect of swim stress on membrane characteristics in 5-HT (panel A) and non-5-HT DRN neurons (panel B).
| RMP (mV) | IR (MΩ) | Tau (ms) | AP thresh. (mV) | AP amp. (mV) | AP dur. (ms) | AHP amp. (mV) | AHP dur. (t1/2; ms) | |
|---|---|---|---|---|---|---|---|---|
| A. 5-HT DRN neurons: membrane characteristics | ||||||||
| Swim (31) | −65.2 ± 2.2 | 506.5 ± 29.4† | 46.2 ± 2.7 | −27.4 ± 1.0† | 68.2 ± 2.7 | 1.6 ± 0.1† | 15.2 ± 0.9 | 127.3 ± 9.3 |
| Handled (22) | −69.7 ± 1.8† | 536.8 ± 38.4† | 45.1 ± 4.6 | −28.3 ± 0.9† | 73.9 ± 3.0 | 1.9 ± 0.2 | 16.1 ± 1.4 | 128.0 ± 16.4 |
| Naïve (32) | −63.5 ± 1.8 | 640.2 ± 33.7 | 51.6 ± 3.1 | −21.5 ± 1.8 | 68.9 ± 1.5 | 2.0 ± 0.1 | 16.4 ± 0.9 | 133.5 ± 5.6 |
| B. Non 5-HT DRN neurons: membrane characteristics | ||||||||
| Swim (15) | −60.6 ± 2.2† | 513.5 ± 36.1 | 42.0 ± 3.2 | −30.4 ± 1.2*† | 70.5 ± 4.1 | 1.5 ± 0.2 | 13.8 ± 1.4 | 139.1 ± 13.5 |
| Handled (14) | −62.1 ± 2.8 | 510.2 ± 49.6 | 43.3 ± 4.7 | −26.7 ± 1.1 | 77.6 ± 2.5† | 1.5 ± 0.2 | 15.2 ± 1.4 | 117.1 ± 20.1 |
| Naïve (18) | −68.4 ± 1.8 | 544.6 ± 39.2 | 41.9 ± 2.7 | −24.1 ± 2.6 | 63.3 ± 3.5 | 1.5 ± 0.1 | 14.4 ± 1.0 | 119.4 ± 10.7 |
Data are expressed as a mean ± SEM. Number of recorded cells is listed within parentheses for each treatment group. In the swim stress group, 5-HT cells were recorded in 28 brain slices from 16 rats and non-5-HT cells were recorded in 14 brain slices from 11 rats. In the handled group, 5-HT cells were recorded in 22 brain slices from 12 rats and non-5-HT cells recorded in 14 brain slices from 11 rats. In the naïve group, 5-HT cells were recorded in 29 brain slices from 27 rats and non-5-HT cells recorded in 17 brain slices from 15 rats. Pairwise comparisons were made by unpaired Student's t-test. Asterisks (*) indicate a significant difference from handled controls. The dagger (†) indicates a significant difference from naïve controls. In addition to the main effects illustrated in the table, in the handled group, resting membrane potential was significantly lower in 5-HT than non-5-HT cells. Furthermore, in the naïve group, both the action potential duration and tau were significantly larger in 5-HT than non-5-HT cells. Statistical significance is defined as p<0.05. Abbreviations: RMP, resting membrane potential; IR, input resistance; AP, action potential; AHP, afterhyperpolarization potential; thresh., threshold; amp., amplitude; dur., duration.
In 5-HT neurons swim stress decreased input resistance, action potential threshold and action potential duration. Handling produced a hyperpolarization of membrane potential, but also decreased input resistance and action potential threshold. These concomitant changes indicate that 5-HT neuron membrane characteristics are sensitive to behavioral manipulations. The decreased action potential threshold could lead to an enhanced excitability of the 5-HT neurons.
The behavioral manipulations did not produce as many alterations in cellular characteristics in the non-5-HT-containing neurons. Swim stress depolarized the resting membrane potential of the non-5-HT neurons and reduced the action potential threshold, changes that could also lead to an enhanced excitability of these neurons. In swim stress pretreated subjects, the difference between resting membrane potential and action potential threshold was significantly greater in non-5-HT than 5-HT neurons, thus swim stress enhancement of neuronal excitability was greater in the non-5-HT neuronal population. For a summary of all the statistical results see Table 1.
3.2. Synaptic activity
Table 2 illustrates the effect of swim stress on EPSC synaptic activity of 5-HT- and non-5-HT-containing DRN neurons. Fig. 1 shows EPSC synaptic activity recordings (panels Ai, Bi and Ci) from three cells that were subsequently identified to be serotonergic (tryptophan hydroxylase-IR positive) by immunohistochemistry (Aiii, Biii and Ciii). EPSC frequency was higher in the swim stress-pretreated subject (panel A) than in the handled (panel B) or naïve control (panel C). Cumulative inter-event interval probability graphs for each cell (Aii, Bii and Cii) illustrate a significant shift to the right as EPSC frequency is inhibited by TTX (mEPSCs) (swim stress: Z = 3.83, p<0.001; handled: Z = 1.90, p<0.01; naïve: Z = 2.89, p<0.001) and further by the addition of 5-CT (swim stress: Z = 3.63, p<0.001; handled: Z = 2.38, p<0.001; naïve: Z = 4.16, p<0.001). The 5-CT inhibition of mEPSCs is an effect previously demonstrated to be mediated by the 5-HT1B receptor (Lemos et al., 2006). EPSCs are mediated by non-NMDA glutamate receptors as mEPSC frequency is completely suppressed by the AMPA/kainate receptor antagonists DNQX (20 μM) or 6-cyano-7-nitroquinoxaline-2,3-dione (20 μM) (data not shown).
Table 2.
Effect of swim stress on glutamatergic EPSC frequency, amplitude, rise and decay time in 5-HT (panel A) and non-5-HT (panel B) DRN neurons.
| Frequency (Hz) | Amplitude (pA) | Rise time (ms) | Decay time (ms) | |||||
|---|---|---|---|---|---|---|---|---|
| sEPSC | mEPSC | sEPSC | mEPSC | sEPSC | mEPSC | sEPSC | mEPSC | |
| A. 5-HT DRN neurons: EPSC characteristics | ||||||||
| Swim stress (28) | 20.2±2.4* | 15.5±2.2* | 18.0±1.0† | 16.3±0.9 | 0.9±0.1 | 1.0±0.1 | 2.8±0.2 | 3.0±0.2 |
| Handled (20) | 8.0±1.0 | 6.9±0.7 | 19.2±1.0 | 17.9±1.0 | 0.8±0.0 | 0.9±0.1 | 2.5±0.1 | 2.8±0.2 |
| Naïve (21) | 10.0±1.4 | 7.2±1.0 | 22.0±1.1 | 19.6±1.1 | 0.9±0.1 | 0.9±0.0 | 3.2±0.3 | 3.4±0.3 |
| B. Non 5-HT DRN neurons: EPSC characteristics | ||||||||
| Swim stress (11) | 10.6±1.8 | 8.4±1.5 | 15.9±1.2† | 15.9±1.0† | 1.0±0.1 | 1.0±0.1 | 3.2±0.4 | 3.3±0.3 |
| Handled (16) | 20.0±3.5# | 15.8±2.6# | 18.4±1.2 | 17.7±1.3† | 0.9±0.1 | 1.0±0.1 | 2.3±0.2# | 2.6±0.2 |
| Naïve (19) | 12.5±2.6 | 7.8±1.7 | 20.9±1.2 | 20.8±1.1 | 0.8±0.0 | 0.9±0.1 | 2.8±0.1 | 3.0±0.2 |
Data are expressed as mean (frequency, amplitude, decay time) or median (rise time)±SEM. Number of recorded cells is listed within parentheses for each treatment group. In the swim stress group, 5-HT cells were recorded in 28 brain slices from 23 rats and non-5-HT cells recorded in 11 brain slices from 11 rats. In the handled group, 5-HT cells were recorded in 20 brain slices from 14 rats and non-5-HT cells recorded in 16 brain slices from 13 rats. In the naïve group, 5-HT cells were recorded in 21 brain slices from 17 rats and non-5-HT cells recorded in 19 brain slices from 16 rats. Pairwise comparisons were made by Student–-Newman–-Keuls test. The asterisk (*) indicates differences from both naïve and handled controls. The dagger (†) indicates differences from naïve controls. The number sign (#) indicates differences from naïve and swim groups. In addition to the main effects illustrated in the table, in the swim stress group, EPSC frequency was significantly higher in 5-HT than non-5-HT cells whereas in the handled group, EPSC frequency was significantly lower in 5-HT than non-5-HT cells. Furthermore, sEPSC frequency was significantly higher than mEPSC frequency in all groups except non-5-HT cells from handled subjects. Statistical significance is defined as p<0.05.
Fig. 2 (and Table 2) shows mean sEPSC and mEPSC frequency in 5-HT (panel A) and non-5-HT cells (panel B) recorded from swim stress-preexposed (black bars), handled controls (grey bars) and naïve controls (white bars). Fig. 2 illustrates two major findings: (a) swim stress modulated EPSC frequency in a neurochemical-specific manner (i.e. 5-HT versus non-5-HT) and (b) TTX inhibited EPSC frequency in a neurochemical-independent manner. The main effect of swim stress was neurochemically dependent, thus there was a significant interaction between these factors (sEPSC: F(2,109) = 9.73, p<0.001; mEPSC: F(2,109) = 8.71, p<0.001). Swim stress selectively elevated EPSC frequency in 5-HT cells (F(2,66) = 11.98, p<0.001). In contrast, handling but not swim stress elevated EPSC frequency in non-5-HT cells (F(2,43) = 3.83, p<0.05). Follow-up pairwise comparisons by Student–Newman–Keuls tests demonstrated that swim stress significantly elevated sEPSC and mEPSC frequency in the 5-HT group above both control groups (p<0.001). In contrast, handling significantly elevated sEPSC (p<0.001) and mEPSC frequencies (p<0.05) above both swim stress subjects and naïve controls in the non-5-HT group. TTX reduced EPSC frequency significantly in all groups tested, regardless of neurochemical identity (5-HT: (F(1,66) = 30.03, p<0.001); non-5-HT: (F(1,43) = 19.62, p<0.001).
Figure 2.
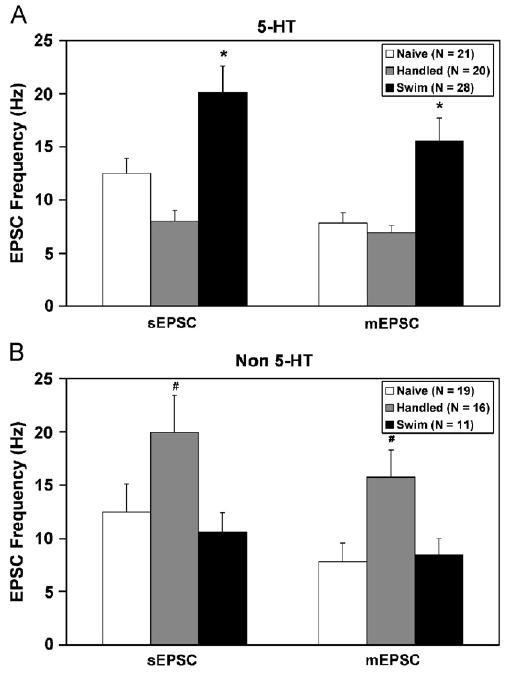
Swim stress elevates glutamatergic EPSC frequency selectively in 5-HT cells. The frequencies of sEPSCs and mEPSCs are shown in 5-HT (panel A) and non-5-HT cells (panel B) from naïve (white bars), handled (grey bars) and swim stress subjects (black bars). Data are expressed as mean±SEM. Pairwise comparisons were made by Student–Newman–Keuls test. The asterisk (*) indicates differences from naïve and handled controls. The number sign (#) indicates differences from naïve and swim stress subjects. In addition to the main effects illustrated in the figure, in the swim stress group, EPSC frequency was higher in 5-HT than non-5-HT cells whereas in the handled group, EPSC frequency was lower in 5-HT than non-5-HT cells. Furthermore, sEPSC frequency was significantly higher than mEPSC frequency in all groups except non-5-HT cells from handled subjects. Statistical significance is defined as p<0.05.
Fig. 3 (and Table 2) shows mean sEPSC and mEPSC amplitude in 5-HT (panel A) and non-5-HT cells (panel B) recorded from swim stress-preexposed (black bars), handled controls (grey bars) and naïve controls (white bars). Swim stress reduced EPSC amplitude in both 5-HT (F(2,66) = 3.71, p<0.05) and non-5-HT cells (F(2,43) = 4.72, p<0.05). Follow-up pairwise comparisons by Student–Newman–Keuls tests demonstrated that swim stress sEPSC amplitude was significantly smaller than in naïve controls in the 5-HT and non-5-HT groups (p<0.05). In the non-5-HT group, both the handled and swim stress groups had smaller mEPSC amplitude than in naïve controls (p<0.05).
Figure 3.

Swim stress reduces glutamatergic EPSC amplitude in both 5-HT and non-5-HT cells. The amplitudes of sEPSCs and mEPSCs are shown in 5-HT (panels A) and non-5-HT cells (panels B) from naïve (white bars), handled (grey bars) and swim stress subjects (black bars). Data are expressed as mean±SEM. Pairwise comparisons were made by Student–Newman–Keuls test. The dagger (†) indicates differences from naïve controls. Statistical significance is defined as p<0.05.
Fig. 4 (and Table 2) shows mean sEPSC and mEPSC decay time in 5-HT (panel A) and non-5-HT cells (panel B) recorded from swim stress-preexposed (black bars), handled controls (grey bars) and naïve controls (white bars). The major finding was that there was a selective effect of handling on EPSC decay time in non-5-HT cells (F(2,43) = 4.05, p<0.05). Pairwise comparisons demonstrated that handling significantly reduced sEPSC decay time in non-5-HT cells relative to both naïve controls and to swim stress subjects (p<0.05).
Figure 4.

Swim stress increases glutamatergic EPSC decay time in non-5-HT DRN cells. The decay times (10–90%) of sEPSCs and mEPSCs are shown in 5-HT (panel A) and non-5-HT cells (panel B) from naïve (white bars), handled (grey bars) and swim stress subjects (black bars). To illustrate this effect of swim stress on sEPSC decay time, panel C shows sEPSCs (averaged from 200 events) recorded in two non-5-HT DRN cells: one from the handled (grey line) and the other from the swim stress group (black line). In panel C the sEPSC from the handled subject has a decay time of 2.4 ms and the sEPSC from the swim stress subject has a decay time of 3.4 ms. Data are expressed as mean±SEM. Pairwise comparisons were made by Student–Newman–Keuls test. The number sign (#) indicates differences from naïve and swim stress subjects. Statistical significance is defined as p<0.05.
Table 2 also details median EPSC rise time in 5-HT (panel A) and non-5-HT cells (panel B) from naïve, handled and swim stress subjects. Analyses of median EPSC rise time data indicated no main effects of swim stress or neurochemical identity.
Fig. 5 shows the 5-HT1A receptor-mediated (panel A) and 5-HT1B receptor-mediated effect (panel B) in 5-HT and non-5-HT cells recorded from swim stress-preexposed (black bars), handled controls (grey bars) and naïve controls (white bars). The 5-CT, a non-specific 5-HT1/5/7 agonist, was used to produce both responses. The 5-CT stimulation of outward current has been shown to be mediated by the 5-HT1A receptor (Williams et al., 1988; Beck et al., 2004). The 5-CT inhibition of mEPSC frequency has been shown to be mediated by the 5-HT1B receptor (Lemos et al., 2006). Even though there appears to be a greater magnitude of outward current produced by 5-CT in the swim stress group, the two-way ANOVA demonstrated no significant effects of any treatment (swim stress, handling, naïve) (F(2,95) = 1.96, p = 0.15) or neurochemical identity (F(1,95) = 0.14, n.s.) on the 5-HT1A receptor-mediated effect. In addition, within the 5-HT group, one-way ANOVA also did not demonstrate a significant treatment effect (p = 0.10). Baseline holding currents for the different treatment groups and cell types were as follows: swim stress 5-HT (−11.7 ± 4.3), handled 5-HT (2.7 ± 6.3), naïve 5-HT (−10.7 ± 4.4), swim stress non-5-HT (−15.0 ± 7.7), handled non-5-HT (−15.6 ± 6.6) and naïve non-5-HT (−11.9 ± 6.0). None of these holding currents were different among the treatment groups, thus the effect of 5-CT on this measure can be interpreted as mediated solely by 5-HT1A receptor activation. For the 5-HT1B receptor-mediated effect, there was a main effect of treatment (swim stress, handling, naïve) (F(2,90) = 5.29, p<0.01), no main effect of neurochemical identity and a significant interaction (F(2,90) = 6.19, p<0.01). Swim stress significantly elevated the 5-HT1B receptor-mediated inhibition of glutamatergic synaptic activity targeting 5-HT cells when compared to either control group (p<0.05). Handling significantly elevated the 5-HT1B receptor-mediated effect in glutamatergic afferents targeting non-5-HT cells (p<0.001).
Figure 5.
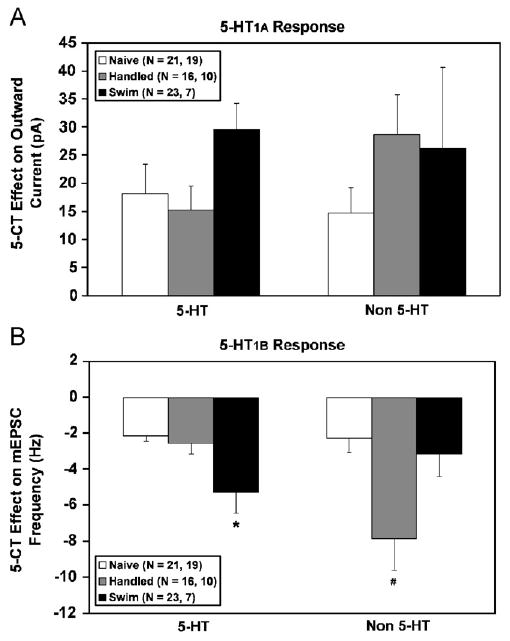
Swim stress elevates a 5-HT1B receptor-mediated response in glutamate afferents selectively targeting 5-HT cells. A 5-HT1A receptor-mediated (panel A) and 5-HT1B receptor-mediated effect (panel B) is shown in 5-HT and non-5-HT cells recorded from naïve (white bars), handled (grey bars) and swim stress subjects (black bars). 5-CT, a non-specific 5-HT1/5/7 agonist, was used to produce both effects. The 5-CT stimulation of outward current has been shown to be mediated by the 5-HT1A receptor (Williams et al., 1988; Beck et al., 2004). The 5-CT inhibition of mEPSC frequency has been shown to be mediated by the 5-HT1B receptor (Lemos et al., 2006). Data are expressed as mean ± SEM. Pairwise comparisons were made by Student–Newman–Keuls test. The asterisk (*) indicates differences from naïve and handled controls. The number sign (#) indicates differences from naïve and swim stress subjects. In addition to the main effects illustrated in the figure, within the handled control group, the 5-HT1B receptor-mediated effect was significantly greater in non-5-HT than 5-HT cells. Statistical significance is defined as p<0.05.
4. Discussion
This study examined the membrane properties, receptor-mediated effects and glutamatergic synaptic activity in specific populations of DRN cells 24 h following exposure to a swim stress. The major finding was that swim stress exposure had both direct and indirect effects on cells of the serotonergic DRN. In general, the effects of swim stress on membrane characteristics were non-specific as many effects were also produced by a milder handling stress. In contrast, the indirect effects of swim stress on DRN cells via modulation of excitatory glutamatergic afferents were stressor-specific. Specifically, swim stress increased the sensitivity of inhibitory 5-HT1B receptors on glutamatergic afferents that selectively target 5-HT DRN neurons. Swim stress also elevated presynaptic glutamate synaptic activity to 5-HT but not non-5-HT DRN cells. This finding implies that there are distinct local glutamatergic afferents to neurochemically-specific populations of DRN neurons, and furthermore that these distinct afferents are differentially regulated by stress. Swim stress non-selectively decreased glutamatergic EPSC amplitude, an indication either of decreased postsynaptic non-NMDA glutamate receptor density on 5-HT and non-5-HT DRN cells or a reflection of stress-induced changes in the input resistance of these cells. Non-5-HT DRN neurons appeared to be more sensitive to the effects of a milder handling stress. Handling elevated presynaptic glutamate release, altered the kinetics of postsynaptic non-NMDA glutamate receptors and enhanced the 5-HT1B receptor-mediated inhibition of EPSC frequency in non-5-HT DRN cells. All of these direct and indirect effects of swim stress exposure potentially alter the ability of DRN cells to process incoming signals and distribute them to their distinct forebrain targets, potentially contributing to the complex effects of swim stress on forebrain 5-HT release that has previously been observed in vivo (Kirby et al., 1995).
Both swim stress and handling altered different aspects of DRN membrane properties, sometimes in distinct neuronal populations. Swim stress lowered the action potential threshold in all cells and handling lowered action potential threshold in 5-HT cells. At the same time, swim stress also elevated resting membrane potential in non-5-HT cells whereas handling lowered resting membrane potential in 5-HT cells. The net effect of these changes was that both swim and handling reduced the difference between resting membrane potential and action potential threshold, particularly in non-5-HT neurons. This effect would serve to significantly reduce the amount of depolarization required to elicit neuronal firing in non-5-HT neurons, and thus enhance cell excitability. Interestingly, these effects on action potential threshold were concomitant with a reduction of input resistance in 5-HT cells. This finding may indicate an increase in ionic conductance that is active during the depolarization prior to activation of the action potential, such as a persistent sodium current (Crill, 1996). Whereas handling reduced resting membrane potential and input resistance in 5-HT neurons, indicating a potential activation of a potassium conductance as well, swim stress only reduced input resistance in these neurons without changing resting membrane potential. This swim stress effect could be attributed to a change in chloride current, particularly in light of evidence that swim stress and the stress neurohormone corticotropin-releasing factor can modulate GABAergic neurotransmission in the DRN (Roche et al., 2003; Nunan et al., 2004; Freeman-Daniels et al., 2006).
In addition to these direct effects of swim stress on DRN neurons, we also found significant indirect effects via local glutamatergic afferents to these neurons. While swim stress had no significant effect on a 5-HT1A receptor-mediated response, it had differential effects on the 5-HT1B receptor-mediated response in different populations of DRN neurons. Stimulation of the 5-HT1B receptor decreases presynaptic glutamate release at DRN neurons (Lemos et al., 2006), indicating that the receptor is located on presynaptic glutamate terminals rather than on the recorded DRN neuron. Presynaptic modulation of TTX-insensitive glutamate release (mEPSC activity) by the 5-HT1B receptor has also been demonstrated in cells of the hypoglossal nucleus (Singer et al., 1996; Bouryi and Lewis, 2003), suprachiasmatic nucleus (Pickard et al., 1999), nucleus accumbens (Muramatsu et al., 1998) and cortex (Boeijinga and Boddeke, 1996; Golembiowska and Dziubina, 2002; Laurent et al., 2002), as well as in 5-HT cells of the caudal raphe (Li and Bayliss, 1998). The current and earlier findings of our laboratory further demonstrate that 5-HT1B receptor-induced reduction in glutamate release in the DRN occurs in the presence of TTX (Lemos et al., 2006). These data indicate that this presynaptic modulation in the DRN can occur in the absence of action potentials, altering the spontaneous fusion and release of glutamate vesicles from the presynaptic terminal. Our findings also indicate that swim stress enhanced the 5-HT1B effect in glutamate afferents that target 5-HT DRN neurons. In contrast, the milder handling stress enhanced that response in glutamate afferents targeting non-5-HTcells. The importance of DRN 5-HT1B autoreceptors in the regulation of 5-HT neurotransmission is well established (Davidson and Stamford, 1995; Adell et al., 2001). Our studies highlight the additional importance of 5-HT1B heteroreceptors located on glutamate terminals that subsequently can regulate 5-HT neurotransmission within the DRN. It is noteworthy that stress effects on the 5-HT1B receptor occurred in the same cell populations that showed stress-enhanced presynaptic glutamate release, with swim stress targeting 5-HT neurons and handling targeting non-5-HT neurons. Enhancement of the inhibitory 5-HT1B receptor on glutamate terminals may be an adaptive response to limit enhanced glutamate release under these conditions. These data thus provide evidence for discrete populations of glutamate afferents that (1) target distinct neurochemical populations of DRN neurons and (2) are differentially sensitive to stressors.
As previously described, swim stress altered presynaptic glutamate release in the absence of 5-HT1B receptor stimulation. Swim stress elevated presynaptic release of glutamate from local afferents synapsing on 5-HT DRN neurons, potentially enhancing their excitability. This finding may provide a cellular mechanism to explain earlier findings of adaptation of the 5-HT system to repeated swim stress in vivo (Kirby and Lucki, 1998). While acute swim stress reduces 5-HT release in the lateral septum, a region that receives input from ventromedial 5-HT DRN neurons (Kohler et al., 1982; Waselus et al., 2006), a second swim stress 24 h later has no effect (Kirby and Lucki, 1998). If glutamatergic afferents to this population of 5-HT DRN cells are stimulated 24 h after a swim stress, this excitatory modulation could offset the inhibition of 5-HT that is normally seen after acute swim stress exposure, resulting in the observed adaptation: no net change in 5-HT neurotransmission. This stimulatory effect of swim stress on glutamate EPSCs was not observed in non-5-HT DRN neurons. Instead, non-5-HT DRN neurons appeared to be more sensitive to the effects of the milder handling stress. The handling procedure itself elevates EPSC frequency at non-5-HT cells, an effect not observed in response to the swim stress. Though considered a relatively subtle stressor, handling has been shown to have neurobiological as well as behavioral effects (Andrews et al., 1992; Pierce and Raper, 1995). While handled subjects more appropriately control for exposure to the swim stress than do naïve subjects, it is important to interpret handling itself as a mildly stressful procedure. Nonetheless, these data provide additional evidence for separate populations of glutamate afferents that target different cell types within the DRN and are differentially regulated by swim stress. In the slice preparation, many of the glutamate afferents are likely intrinsic to the slice, originating in either the periaqueductal grey or the raphe nuclei (Jolas and Aghajanian, 1997; Akanwa and Beck, 2006). Intrinsic glutamate release is evidenced by the fact that approximately 25% of EPSCs recorded in vitro are TTX-sensitive and therefore dependent on neuronal activity. However, there is also evidence that glutamate terminals disconnected from their cell bodies can generate spontaneous glutamate release, as would be the case for many afferents severed by the brain slice preparation (Drewe et al., 1988). In this case, longer projecting afferents from the areas such as the medial prefrontal cortex may also contribute to glutamate release in this preparation. In addition, 5-HT1B receptor mRNA has been demonstrated in medial prefrontal cortex (Bruinvels et al., 1994; Doucet et al., 1995). Therefore, it is possible that the 5-HT1B heteroreceptor-expressing glutamate afferents to the DRN demonstrated in our studies originate in areas such as the medial prefrontal cortex. While forebrain glutamatergic regulation of the DRN is well known (Celada et al., 2001, 2002), evidence for local glutamatergic regulation of DRN neurons has also been demonstrated previously by anatomical and electrophysiological studies (Kalen et al., 1985; Jolas and Aghajanian, 1997; Lee et al., 2003). Lee et al. (2003) also showed that some glutamatergic afferent circuits selectively target cells within distinct subdivisions of the dorsal raphe. The current study extends this finding, demonstrating that there are specific glutamatergic afferent circuits that target neurochemically distinct neuronal populations within the same DRN subdivision. Interestingly, a recent ultrastructural study in our laboratory provided evidence for two populations of DRN glutamate afferents with cortical versus subcortical origins that synapse on distinct dendritic domains of both 5-HT and non-5-HT DRN cells (Commons et al., 2005). Nonetheless, these different studies all provide evidence that glutamatergic control of DRN neurons has exquisite selectivity and can be fine-tuned to modulate the output of neurochemically and regionally distinct populations of neurons with potentially different targets and functional roles.
The finding that distinct glutamate circuits can be differentially affected by stress has been observed previously (Gilad et al., 1990; Moghaddam, 1993; Wang et al., 2005). These studies showed preferential release of glutamate in particular brain regions in response to an acute stressor. Our findings extend this observation to the cellular level, providing evidence that differential glutamate release can occur at the level of individual cell populations within the same brain region. In this way, the glutamate system provides a mechanism whereby stressors can selectively activate or dampen activity of neurochemically distinct neuronal populations with potentially different behavioral or physiological functions in the stress response.
In addition to swim stress effects on presynaptic glutamate release, potential postsynaptic effects on AMPA/kainite glutamate receptors were also observed. We found that swim stress reduced EPSC amplitude in all DRN neurons examined, regardless of neurochemical identity. This effect could indicate a general suppression of postsynaptic AMPA/kainate receptor sensitivity or density throughout the DRN. An alternative explanation is that this reduction in EPSC amplitude is a direct result of the reduction of input resistance produced by both swim stress and handling. According to Ohm's law, a reduction of input resistance will mean that when a channel opens and a given current is passed, there will be a smaller voltage drop across the membrane. Under voltage clamp conditions, this effect should be seen as smaller amplitude EPSCs. In fact, swim stress produced a 21% reduction in input resistance and a corresponding 18% reduction in sEPSC amplitude in 5-HT DRN neurons, with handling producing slightly lesser reductions in both of these measures. Postsynaptic glutamate receptor kinetics were affected in non-5-HT cells by handling. Handling prolonged the decay time of the glutamate receptor–ionophore in non-5-HT but not 5-HT cells. This increase in decay time would allow more Na+ influx through the ionophore and potentially a prolonged excitation of the postsynaptic non-5-HTcell. Taken together, these findings indicate that swim stress has primarily presynaptic effects on glutamate release whereas handling effects on non-5-HT neurons occur at both the pre- and postsynaptic levels.
These findings also highlight the concept of differential stressor responsivity in different populations of DRN neurons. While 5-HT neurons were more responsive to swim stress, showing changes in presynaptic glutamate release and 5-HT1B receptor-modulated glutamate release, non-5-HT neurons were more responsive to handling stress, showing changes in presynaptic glutamate release, 5-HT1B receptor-modulated glutamate release and postsynaptic glutamate receptor kinetics. Previous in vivo microdialysis studies have also shown stressor-specific changes in 5-HT release within the raphe nuclei and in forebrain targets of the raphe nuclei (Adell et al., 1997; Kirby et al., 1997). These in vivo effects may be initiated by differential stressor-sensitivity of individual populations of DRN neurons to excitatory glutamate input as shown in this study.
The potential clinical significance of these findings relates to the fact that the neurophysiological DRN changes described in the current study occur 24 h following a swim stress, at a time when antidepressant-sensitive behavioral changes are observed in the FST model. The FST was developed to model depression-like behavior in rodents and therefore either swim stress-induced changes in DRN neuronal properties or swim stress effects on glutamate synaptic activity could contribute to FST behaviors. To test this, in future studies we will attempt to reverse these neurophysiological changes with antidepressants that also successfully reverse the depression-like behaviors in the FST model. The fact that serotonergic neurotransmission plays a critical role in the FST is already established by numerous studies showing that manipulation of 5-HT, by 5-HT depletion, serotonin-selective reuptake inhibitors (SSRIs), or selective 5-HT receptor subtype ligands, modulates antidepressant sensitive behaviors in the model (for review see Lucki, 1997; Cryan et al., 2005). Glutamate neurotransmission via AMPA receptors has also been shown to play a role in the model as AMPA receptor potentiators possess antidepressant-like effects in the FST (Bai et al., 2001; Li et al., 2001; Nakamura and Tanaka, 2001) and also enhance the potency of more traditional antidepressants including SSRIs in the FST (Li et al., 2003). Given our finding that swim stress potentially reduces DRN AMPA receptor number or sensitivity, AMPA receptor potentiators may produce their antidepressant-like effects by reversing this. In addition, AMPA receptor expression in the brain is enhanced by chronic antidepressant treatment and thus is a potential neural adaptation which may contribute to the well-known delayed therapeutic response to antidepressants (Martinez-Turrillas et al., 2002). Taken together, these results describe changes in glutamatergic afferents to DRN neurons in response to swim stress that may contribute to the serotonergic response to swim stress as well as to the antidepressant-sensitive behavioral responses to swim stress in the FST model. These data also suggest that AMPA receptors or AMPA receptors in combination with 5-HT neurotransmission may be useful targets for novel antidepressant treatments.
Acknowledgments
Role of the funding sources: This work was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD) and the National Institute of Mental Health (MH 63301), grants issued to Dr. Kirby. Additional support was provided by NIMH (MH 60773, MH 63078, MH 75047) and the Office of Naval Research (N00014-03-1-0311), grants issued to Dr. Beck. NARSAD, the NIMH and the Office of Naval Research had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of interest: All authors declare that they have no conflicts of interest.
References
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Artigas F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Akanwa AC, Beck SG. GABA and glutamate innervation of dorsal and median raphe visualized using glutamate and GABA vesicular transporters. Soc Neurosci Abs. 2006;36:722.3. [Google Scholar]
- Andrews N, Zharkovsky A, File SE. Acute handling stress downregulates benzodiazepine receptors: reversal by diazepam. Eur J Pharmacol. 1992;210:247–251. doi: 10.1016/0014-2999(92)90411-v. [DOI] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. J Neurophysiol. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeijinga PH, Boddeke HW. Activation of 5-HT1B receptors suppresses low but not high frequency synaptic transmission in the rat subicular cortex in vitro. Brain Res. 1996;721:59–65. doi: 10.1016/0006-8993(96)00149-7. [DOI] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-HT of glutamatergic inputs from the raphe pallidus to rat hypoglossal motoneurones, in vitro. J Physiol. 2003;553:1019–1031. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafsson JA, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1Dalpha, 5-HT1E, and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Martin-Ruiz R, Casanovas JM, Artigas F. Control of the serotonergic system by the medial prefrontal cortex: potential role in the etiology of PTSD and depressive disorders. Neurotox Res. 2002;4:409–419. doi: 10.1080/10298420290030550. [DOI] [PubMed] [Google Scholar]
- Commons KG, Beck SG, Bey VW. Two populations of glutamatergic axons in the rat dorsal raphe nucleus defined by the vesicular glutamate transporters 1 and 2. Eur J Neurosci. 2005;21:1577–1586. doi: 10.1111/j.1460-9568.2005.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson C, Stamford JA. Evidence that 5-hydroxytryptamine release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br J Pharmacol. 1995;114:1107–1109. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet E, Pohl M, Fattaccini CM, Adrien J, Mestikawy SE, Hamon M. In situ hybridization evidence for the synthesis of 5-HT1B receptor in serotoninergic neurons of anterior raphe nuclei in the rat brain. Synapse. 1995;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- Drewe JA, Childs GV, Kunze DL. Synaptic transmission between dissociated adult mammalian neurons and attached synaptic boutons. Science. 1988;241:1810–1813. doi: 10.1126/science.2459774. [DOI] [PubMed] [Google Scholar]
- Freeman-Daniels EL, Lemos JC, Nunan JD, Beck SG, Kirby LG. Receptor characterization of corticotropin-releasing factor (CRF) effects on serotonin dorsal raphe neurons. Soc Neurosci Abs. 2006:58.4. [Google Scholar]
- Gilad GM, Gilad VH, Wyatt RJ, Tizabi Y. Region-selective stress-induced increase of glutamate uptake and release in rat forebrain. Brain Res. 1990;525:335–338. doi: 10.1016/0006-8993(90)90886-g. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Dziubina A. Inhibition of amino acid release by 5-HT1B receptor agonist in the rat prefrontal cortex. Pol J Pharmacol. 2002;54:625–631. [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Kalen P, Karlson M, Wiklund L. Possible excitatory amino acid afferents to nucleus raphe dorsalis of the rat investigated with retrograde wheat germ agglutinin and D- [3 H]aspartate tracing. Brain Res. 1985;360:285–297. doi: 10.1016/0006-8993(85)91244-2. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J Pharmacol Exp Ther. 1997;282:967–976. [PubMed] [Google Scholar]
- Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1995;682:189–196. doi: 10.1016/0006-8993(95)00349-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V, Steinbusch H. The distribution and origin of serotonin-containing fibers in the septal area: a combined immunohistochemical and fluorescent retrograde tracing study in the rat. J Comp Neurol. 1982;209:91–111. doi: 10.1002/cne.902090109. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Pan YZ, Ma X, Lamy C, Akanwa AC, Beck SG. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci. 2006;24:3415–3430. doi: 10.1111/j.1460-9568.2006.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator ( LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Li X, Witkin JM, Need AB, Skolnick P. Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell Mol Neurobiol. 2003;23:419–430. doi: 10.1023/a:1023648923447. [DOI] [PubMed] [Google Scholar]
- Li YW, Bayliss DA. Presynaptic inhibition by 5-HT1B receptors of glutamatergic synaptic inputs onto serotonergic caudal raphe neurones in rat. J Physiol. 1998;510(Part 1):121–134. doi: 10.1111/j.1469-7793.1998.121bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Martinez-Turrillas R, Frechilla D, Del RJ. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43:1230–1237. doi: 10.1016/s0028-3908(02)00299-x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Lapiz MD, Tanaka E, Grenhoff J. Serotonin inhibits synaptic glutamate currents in rat nucleus accumbens neurons via presynaptic 5-HT1B receptors. Eur J Neurosci. 1998;10:2371–2379. doi: 10.1046/j.1460-9568.1998.00248.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Tanaka Y. Antidepressant-like effects of aniracetam in aged rats and its mode of action. Psychopharmacology (Berl) 2001;158:205–212. doi: 10.1007/s002130100849. [DOI] [PubMed] [Google Scholar]
- Nunan JD, Pan YZ, Akanwa A, Beck SG, Kirby LG. Corticotropin-releasing factor increases presynaptic GABA release onto dorsal raphe neurons. Soc Neurosci Abs. 2004:760.4. [Google Scholar]
- Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, Sollars PJ. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J Neurosci. 1999;19:4034–4045. doi: 10.1523/JNEUROSCI.19-10-04034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce TL, Raper C. The effects of laboratory handling procedures on naloxone-precipitated withdrawal behavior in morphine-dependent rats. J Pharmacol Toxicol Methods. 1995;34:149–155. doi: 10.1016/1056-8719(95)00060-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Lepichon M, Jalfre M. Depression-new animal-model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 2002;162:406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- Roche M, Commons KG, Peoples A, Valentino RJ. Circuitry underlying regulation of the serotonergic system by swim stress. J Neurosci. 2003;23:970–977. doi: 10.1523/JNEUROSCI.23-03-00970.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat moto-neurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Galvez JP, Valentino RJ, Van Bockstaele EJ. Differential projections of dorsal raphe nucleus neurons to the lateral septum and striatum. J Chem Neuroanatomy. 2006;31:233–242. doi: 10.1016/j.jchemneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Williams JT, Colmers WF, Pan ZZ. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci. 1988;8:3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


