Abstract
In this study, we hypothesize that supplementation of suture repair of the anterior cruciate ligament (ACL) with platelet-rich plasma (PRP) will improve the biomechanics of the repair. Six 30-kg pigs underwent bilateral suture repair of the ACL. One side was treated with suture repair alone, while the contralateral side was treated with suture repair augmented with PRP. After 14 weeks in vivo, anterior–posterior (AP) knee laxity and the tensile properties of the repaired ligament were measured. The addition of PRP to the suture repairs did not improve AP knee laxity at 30° (p =0.73) or 60° (p =0.65). It also did not improve the maximum tensile load (p =0.64) or linear stiffness (p =0.42) of the ACL repairs after 14 weeks in vivo. The model had 80% power to detect a 30% improvement of biomechanical properties with PRP; thus, we are confident that a clinically meaningful effect as a result of adding PRP is unlikely. Use of PRP alone to supplement suture repair of the ACL is ineffective in this animal model.
Keywords: ACL, suture repair, platelet-rich plasma, ligament, healing, pig model
Complete rupture of the ACL has become an increasingly common injury, especially among young athletes. Because the ACL does not readily heal, the current gold standard for treatment is ACL reconstruction, or replacement of the torn ligament with an autograft of tendon. However, there has been recent interest in discovering methods to stimulate repair and/or regeneration of the ACL rather than replacing it. Successful ACL repair has the theoretical advantages of preserving the broad insertion sites and the proprioceptive nerve fibers of the native ACL, as well as being a less invasive procedure compared to ACL reconstruction.
Previous results of attempted primary ACL repair in large animal models have been universally poor. Animal models of ACL transection and repair have been studied in rabbits,1 monkeys,2 and dogs.2–4 In canines, untreated ACL transections showed no evidence of healing and incomplete healing in the repaired ligaments,2,4 even in partial tears.5,6 These results were also seen in rhesus monkeys (45% return of strength following ACL repair at 1 year).2 However, recent studies have shown that the use of a collagen-platelet scaffold can enhance the mechanical properties of the healing ACL after 4 weeks in vivo.7 Fourteen weeks was selected as the end time point, as prior work in large animal models of ACL reconstruction have found that the structural and mechanical properties of the graft are improving by this time point.8,9 Therefore, better improvements at this time point may be indicative of a level of functional healing; whereas earlier time points may not progress successfully.8,9
Traditional orthopedic teaching promotes the concept that “kids heal faster than adults” for bone fractures. Studies of bone healing in animal models appear to support this viewpoint.3 However, studies of partial ACL transection and patellar tendon healing suggest that the scar formed in skeletally immature animals is actually weaker that that formed in skeletally mature animals.4,9 As patients with open physes are vulnerable to ACL injury, and stand to have the longest period of disability if premature osteoarthritis occurs, it is clinically important to study techniques of ACL treatment in this harsher skeletally immature model.
Prior work has examined healing in the human ACL after rupture10 and compared it with the medial collateral ligament (MCL), a ligament that heals clinically.11,12 Using histology and immunohistochemistry techniques, it has been demonstrated that the ACL cells within the ligament proliferate and the ligament revascularizes after rupture.10–12 Collagen production also continues within the ACL up to 1 year out from injury.13 However, the platelet-rich provisional scaffold found in the wound site of other ligaments, such as the MCL, was not found in the wound site of the ACL, where the two ligament ends were simply washing around in synovial fluid and unable to reconnect.10 Thus, it appeared that although the cells and vascularity of the ACL were capable of mounting a histologic healing response, there was no filling of the wound site with platelets.
Platelets are known to release several cytokines important in wound healing, including TGF-b1 and PDGF-AB,14,15 which also are important in stimulating collagen production by ligament cells.16,17 Based on these findings, we currently hypothesize that this lack of concentrated platelets between the two ends of the torn ACL is a key mechanism behind the failure of the ACL to heal.
The objective of the current study was to determine if supplementation of the suture repair with platelet-rich plasma (PRP) alone would improve anterior–posterior (AP) laxity of the knee and the structural properties of the repaired ligament. The primary outcome variables that were measured were AP knee laxity and the linear stiffness and maximum load to failure of the repair tissue. Secondary outcome measures included safety measures [including changes in range of motion of the knee, thigh circumference, and systemic white blood cell (WBC) and synovial fluid cell counts] as well as secondary biomechanical measures of displacement at failure and maximum tensile load. Successfully proving this hypothesis would be viewed as significant progress toward an all-autologous clinical treatment method of ACL repair. Because there are recent reports by other groups using this technique in human patients, we were hopeful that this study would provide support for this clinical practice.
MATERIALS AND METHODS
Experimental Design
Before beginning this study, it was approved by the Institutional Animal Care and Use Committee. Six 30-kg female skeletally immature 4-month-old Yorkshire pigs underwent bilateral ACL transection and suture repair. All animals were treated with suture repair alone in one knee (SUTURE), and suture repair augmented with platelet-rich plasma (SUTURE-PRP) on the contralateral knee (n =6). The knees receiving each treatment were block randomized preoperatively. All animals were euthanized after 14 weeks.
Manufacture of PRP
At the time of surgery, 54 cc of whole blood was drawn from each animal through an 18-gauge needle. The blood was aspirated into tubes containing 6 mL of acid-citrate dextrose (ACD) and transferred into a centrifugation chamber and centrifuged at 1200 × g for 4 min. The upper 7 cc of the centrifuged blood was aspirated, 2 mL of ACD added and the mixture placed into a second chamber for a second spin for 9 min at 1000 × g to create a platelet pellet. The pellet was then resuspended in 10 cc of autologous platelet-poor plasma, and the mixture recentrifuged at 1200 × g for 2 min to remove the majority of the remaining erythrocytes. The PRP was aspirated from the supernatant (approximately 7 cc yield from 60 cc of whole blood). Platelet concentrations were determined using a Coulter Ac.T diff 2tm Analyzer (Hialeah, FL). The protocol had been used in the past on porcine blood to achieve platelet enrichment of at least twice that of the whole blood.15
Surgical Procedure
Once anesthetized, the pigs were weighed and placed in the supine position on the operating room table. Preoperative measurements of knee flexion and extension, and Lachman examinations were performed on both knees. WBC count and platelet count were also recorded for each animal preoperatively. Both hind limbs were shaved, prepared with chlorhexidine followed by betadyne paint, and sterilely draped. To expose the ACL, a 4-cm incision was made over the medial border of the patellar tendon. The incision was extended through the synovium using electrocautery. The fat pad was released from its proximal attachment and partially resected to expose the intermeniscal ligament. A Lachman maneuver, in which AP directed shear loads were applied to the tibia with respect to the femur, was performed prior to releasing the ACL to verify knee stability. Two #1 Vicryl sutures were secured in the distal ACL stump using a modified Kessler stitch. The ACL was transected completely at the junction of the middle and proximal thirds using a No. 12 blade. Complete transection was verified visually and then with a Lachman exam: all ACL deficient knees were positive, with no significant end point. Knees were irrigated with 500 cc of sterile saline before suture anchor placement.
A suture repair of the ACL was then performed. An absorbable suture anchor (TwinFix AB 5.0 Suture Anchor with DuraBraid Suture (USP#2); Smith and Nephew, Inc., Andover, MA) was placed at the back of the femoral notch to provide femoral fixation for the repair. The sutures from the anchor were tied with standard surgical knots to the sutures in the tibial stump to make a four-stranded repair. The sutures were tied with the knees in resting flexion (approximately 70°). In the SUTURE group, the knee was then closed. In the SUTURE-PRP group, 3 cc of PRP (with a minimum of twice the systemic platelet count) were injected around the suture material to fill the notch, and clot stimulation was provided by the exposed collagen of the ACL and adjacent tissue within the notch. In the knees treated with suture anchor repair alone, a blood clot formed in the notch after suture anchor placement. The incisions were closed in multiple layers with absorbable sutures. Animals were maintained under anesthesia for 1 h after placement of the PRP to allow time for full gelation.
The animals were not restrained postoperatively, and were allowed ad libitum activity. Buprenex 0.01 mg/kg IM once and a Fentanyl patch 1–4 μg/kg transdermal were provided for postoperative analgesia. All animals were weight bearing on their hind limbs within 24 h after surgery. After 14 weeks in vivo, the animals were anesthetized, blood was drawn for a systemic WBC count, and synovial fluid was aspirated to measure intraarticular WBC concentration. Measures of knee flexion and extension, and Lachman examinations were performed on both knees. Animals were euthanized using Fatal Plus (Vortech Pharmaceuticals, Dearborn, MI) at 1 cc/10 lbs. The limbs were disarticulated at the hip and ankle and the knees were frozen at −20°C until mechanical testing.
Biomechanical Testing
The specimens were removed from the freezer approximately 24 h prior to testing and thawed to room temperature. All extraneous muscle and soft tissue were carefully removed from the joint leaving the knee capsule intact. Specimens were kept moist throughout the test protocol with a wrapping of normal saline-soaked gauze. In preparation for potting, the long bones were transected at middiaphysis. Two pairs of drywall screws were inserted bicortically and perpendicular to each other to ensure stable fixation when the bones were potted. Sections of 3.8-cm diameter, schedule 40 PVC pipe were cut to 14-cm lengths. These “cannons” had eight 1-cm holes drilled through the wall to enhance fixation of the bone within the cannon. Each bone was rigidly embedded in a urethane potting compound (Smooth On, Easton, PA)18 leaving approximately 5 cm of exposed bone to the joint line. The bones were oriented such that the long axes of the bone and cannon were coaxial.
Cyclic AP laxity testing was performed with the knee flexed at both 30 and 60°. Knees were supported on custom fixtures and rigidly attached to either an Instron 8521S servohydraulic load frame (Instron Corporation, Norwood, MA) or an MTS 810 servohydraulic load frame (MTS Systems Corporation, Eden Prairie, MN) (Fig. 1). For AP laxity testing, fully reversed, sinusoidal AP-directed shear loads of ±20 N were applied at 0.0833 Hz for 12 cycles. Load and displacement data were acquired at 20 Hz and plotted using Excel to give a load–displacement curve (Fig. 2). During the AP laxity tests, axial rotation was locked in the neutral position, whereas the varus–valgus angulation and the coronal plane translations were left unconstrained.
Figure 1.
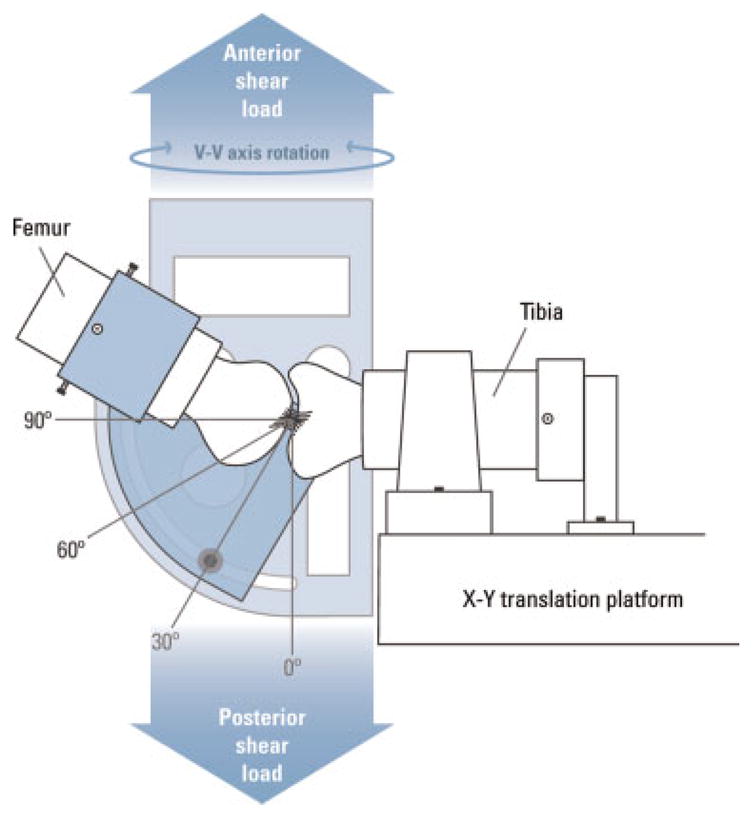
AP laxity jig. The femoral shaft is secured in the upper fixture, which can be rotated to place the knee between 0 and 90° of flexion for testing. All testing in this experiment was performed with the knee at 60° of flexion.
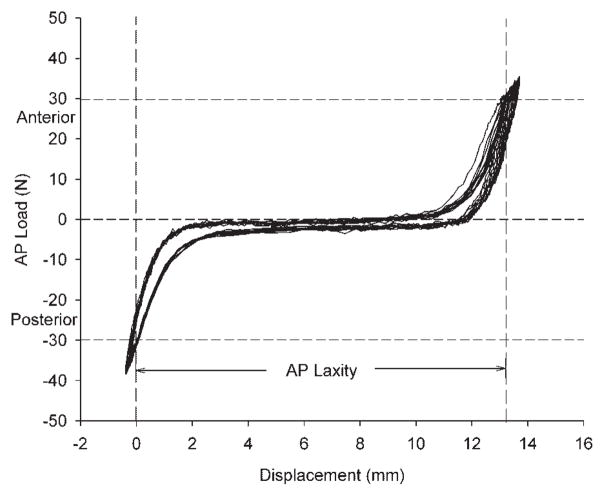
Figure 2.
Sample graph of the AP laxity testing load versus displacement data. The data shown here are for an ACL repair with sutures +platelet-rich plasma (SUTURE-PRP group). AP laxity was measured as the distance between −30 N (posterior) and +30 N (anterior) shear loads. The laxity test shown here was performed with the knee in 60° of flexion, and the resulting AP laxity value for this specimen was 13.2 mm.
Following the AP laxity testing, the knees were positioned for tensile failure testing. The joint capsule, menisci, collateral ligaments, and the PCL were dissected from the joint leaving the ACL scar mass intact. The tibia and femur were positioned so that the mechanical axis of the ACL was collinear with the load axis of the material test system. The knee flexion angle was initially set at 30°. The tibia was mounted to the base of the MTS via a sliding X-Y platform. The femur was unconstrained to rotation. This enabled the specimen to seek its own position so that the load was distributed over the cross-section of the healing ligament when the tensile load was applied. A ramp at 20 mm/min was performed19 and the load–displacement data were recorded at 100 Hz. From the MTS load–displacement tracing, the failure load, failure displacement, and the linear stiffness were determined.
Statistical Methods
Repeated-measures analysis of variance (ANOVA) with a mixed model approach was used with animal as the subject factor and knee as the repeated-measures factor to compare physical examination, laboratory, tensile/mechanical, and AP laxity variables between treatment groups and to determine 95% confidence intervals.20 Fieller’s theorem was applied to calculate the 95% confidence intervals (CIs) for the mechanical testing ratios of SUTURE-PRP to SUTURE alone (because ratios are nonlinear) and to determine the ratios differed significantly from a null value of 1.0.21 All statistical analyses were performed using the SPSS 16.0 software (SPSS Inc., Chicago, IL). The level of significance was set at a two-tailed α-level of p <0.05. The sample size of five animals provided 80% power (α =0.05, β=0.20) to detect a mean difference of 30% between SUTURE versus SUTURE-PRP with respect to mechanical properties and AP laxity assuming an interanimal standard deviation of 20% (standardized effect size =1.5).22
RESULTS
At the time of testing, one of the ligaments in the SUTURE-PRP group had a strength value over 3 SDs higher than the rest of the group and very similar to our prior intact control ligaments. On retrospective review of an MRI scan done at 4 weeks on this knee, the ACL appeared to be intact on the sagittal sections and did not have any of the characteristic features of early healing seen in the multiple other 4-week MRI evaluations we had done prior to this study (specifically, we saw no stump retraction or revascularization and the T2 signal in the ACL was very low suggesting mature ligament tissue). We hypothesize that this ACL was not completely transected at the time of the original surgery, and therefore, this animal was excluded from the remaining analyses.
PRP Preparation
The PRP centrifugation technique used in this experiment resulted in an average platelet enrichment of 2.83 ± 0.53X (range =2.2X to 3.5X). This process resulted in enrichment of the WBCs as well, with an average enrichment of 1.95 ± 0.34 (mean ± SD, n =5).
Physical Examination and Laboratory Parameters
No significant differences were observed in preoperative or postoperative flexion (132 ± 11 degrees in the SUTURE group and 129 ± 11 degrees in the SUTURE-PRP group; mean ± SD; p =0.85) or extension (33 ± 6 degrees in the SUTURE group and 30 ± 6 degrees in the SUTURE-PRP group; p =0.88) between the repaired groups. Average extension gained was 11 ± 16 degrees in the SUTURE group and 12 ± 14 degrees in the SUTURE-PRP group, whereas flexion increased on average by 7 ± 8 degrees and 8 ± 3 degrees in the two groups, respectively. The preoperative Lachman exam values were also similar (2.8 ± 0.4 mm in the SUTURE group and 2.8 ± 0.4 in the SUTURE-PRP group; p =0.90), as were the postoperative values for this parameter (4.3 ± 2.0 mm in the SUTURE group and 5.4 ± 2.8 mm in the SUTURE-PRP group; p =0.49).
Systematic/Synovial Fluid Platelet Counts
There was no significant increase in the systemic WBC count or platelet count during the 14 week in vivo period for either group (p >0.95). Synovial fluid from the knees at the 14-week time point was clear and yellow and had an mean WBC count of 1.1 ± 1.6 × 103/μL in the SUTURE group and 0.2 ± 0.5 ± 103/μL in the SUTURE-PRP group, with no significant difference detected between groups (p =0.37).
Gross Observations
Upon removal of the capsule and meniscal tissue for mechanical testing of the ACL repair constructs, there was noted to be ACL repair tissue in all specimens. There was no difference in the amount of tissue observed between groups. There was no significant synovitis in any of the knees, and no cartilage or meniscal injuries were seen in any of the animals.
Mechanical Properties
There was no improvement in AP laxity of the knees with the addition of PRP to the suture repairs after 14 weeks in vivo when AP laxity was tested at either 30 or 60 degrees of knee flexion (Table 1). The 95% CIs for the two groups had significant overlap at both 30 degrees (8.8 to 14.5 mm for SUTURE and 7.6 to 13.3 for SUTURE-PRP) and 60 degrees (10.8 to 16.8 mm for the SUTURE group and 11.1 to 17.1 for SUTURE-PRP; Table 2).
Table 1.
Results of Mechanical Testing and AP Laxity Based on ACL Treatment
| Variable | SUTURE (five knees) | SUTURE-PRP (five knees) | Mean Difference | p |
|---|---|---|---|---|
| AP laxity 30° (mm) | 11.7 ± 2.2 | 10.5 ± 3.2 | −1.2 ± 4.3 | 0.56 |
| AP laxity 60° (mm) | 13.8 ± 3.3 | 14.1 ± 2.4 | 0.3 ± 3.6 | 0.88 |
| Linear stiffness (N) | 13.5 ± 6.7 | 18.2 ± 12.3 | 4.7 ± 9.5 | 0.33 |
| Maximum tensile load (N) | 81.0 ± 43.2 | 104.0 ± 90.4 | 23.0 ± 63.9 | 0.47 |
| Displacement at failure (mm) | 5.5 ± 0.9 | 4.2 ± 1.7 | −1.3 ± 1.8 | 0.18 |
| Energy at failure (N × mm) | 233 ± 137 | 318 ± 236 | 85 ± 160 | 0.30 |
Data are mean ± SD. PRP = platelet-rich plasma. Repeated-measures ANOVA indicated no significant difference between knees treated with SUTURE versus the contralateral side treated with SUTURE-PRP for any of the six variables.
Table 2.
Mechanical Testing and Laxity: Assessment of PRP Treatment Effect
| Variable | SUTURE-PRP/SUTURE | 95% CIa | p |
|---|---|---|---|
| AP laxity 30° (mm) | 0.94 | 0.49–1.39 | 0.73 |
| AP laxity 60° (mm) | 1.06 | 0.71–1.41 | 0.65 |
| Linear stiffness (N) | 1.35 | 0.29–2.41 | 0.42 |
| Maximum tensile load (N) | 1.19 | 0.13–2.25 | 0.64 |
| Displacement at failure (mm) | 0.77 | 0.36–1.19 | 0.20 |
| Energy at failure (N × mm) | 1.36 | 0.39–2.34 | 0.26 |
PRP = collagen–platelet-rich plasma; CI =confidence interval.
Based on Fieller’s theorem with a test to determine if the observed ratio differs from 1.
There was no significant difference in the linear stiffness, maximum load, displacement at failure, or energy to failure between the two groups (Table 1). The 95% CIs had significant overlap for stiffness (2.7 to 24.2 N/mm for SUTURE and 7.4 to 28.9 for SUTURE-PRP), as well as maximum load (3.2 to 158.8 N for SUTURE and 26.2 to 181.8 for SUTURE-PRP). The maximum load to failure in both groups remained approximately 10% of age-matched intact porcine ACL maximum loads, which are approximately 1000 N (data not shown). The 95% CIs also had significant overlap for displacement at failure (4.1 to 6.9 mm for suture and 2.8 to 5.6 mm for SUTURE-PRP) and energy to failure (18 to 448 for SUTURE and 103 to 534 for SUTURE-PRP; Table 2).
DISCUSSION
The addition of PRP alone to a suture repair of the ACL was not sufficient to enhance any of the outcome measures evaluated here, including AP laxity of the knee and the tensile properties of the repair. It should be noted that the experimental design was designed with 80% power to detect a 30% improvement in biomechanical results. Therefore, it is unlikely that a clinically meaningful result was missed.
In this study, the porcine model was chosen because of its size, its dependence on the ACL for function,23 its similarity with human gait biomechanics,24 and the similarity of the baseline coagulation values and platelet sedimentation characteristics to human blood.25 Platelet counts in the pig averaged 482 ± 95 k/mm3, a value just above the normal range reported for humans (150 to 450 k/mm3).25 However, the Yorkshire pig grows at an extremely fast rate, and in this study the animals almost doubled in size from 30 to 60 kg over the three months of the study and during this same period, the maximum load of the intact ACL almost triples (data not shown). Therefore, the chief limitation of this model is the fact that it may be problematic to use for a study designed to go longer than 3 months. The use of minipigs obviates this rapid growth issue, but in our experience, there are two problems that prevent the minipig from being a good animal for large-scale testing. First, the purchase price of these animals is typically 10 times greater than that of the Yorkshire pig, and second, age-, weight-, and gender-matched groups of animals are more difficult to find due to their limited availability compared to the young Yorkshire animals. As noted in the results, the animals used in this study had the advantages of very little interanimal variation in range of motion, joint size or laxity, and weight at the beginning of the study. Thus, for initial studies designed to go 3 months or less, the skeletally immature Yorkshire pig continues to be a good model for relatively large-scale studies, whereas the minipig may be more conducive to, longer term studies with fewer experimental groups.
In addition, the use of a skeletally immature large animal model likely provides the harshest testing environment for ligament healing studies. Prior work examining partial ACL transection and patellar tendon healing suggest that the scar formed in skeletally immature animals is functionally inferior to that that formed in skeletally mature animals.4,9 Thus, if we can eventually be successful in stimulating healing in this more difficult population, the results may be more effectively translated to the mature knee; whereas development of techniques in the mature knee may not hold up in the skeletally immature knee.
An additional limitation of this model is that is that bilateral surgeries were performed on each animal. Thus, there may be effects on activity limitations imposed by this model may be more severe than that seen in a unilateral model. To mitigate this concern, animals were monitored closely for any signs of persistent ambulatory defects during the first 2 weeks after surgery. No evidence of limp, disinterest in food or water or lack of activity were noted to persist longer than 1 week in any of the animals.
The final limitation of this model is that although it is less expensive than the minipig models, large animal studies still remain inherently more costly than studies with smaller animals. Therefore, the number of animals that can be included in each group is far less than might be accomplished with a mouse or rabbit model with the same budget. As in this study, this can lead to a relatively low power to detect small differences between groups. However, the number of animals for this study was calculated based on a power analysis for the percent difference between groups that we felt would be clinically significant (30% improvement) and the previously determined standard deviation for primary suture repair in the pig model (20%). Using these values, our standardized effect size was 1.5. Our experimental sample size of five animals provided 80% power to detect this size of a difference for each of the failure and laxity variables based on a paired t-test and our prior standard deviations in this model. Our actual standard deviations in the model were higher than 20%, and thus our power lower for the outcome measures that estimated in the sample size calculations—for example, the power to detect a 30% difference in AP laxity at 30° was only 71%, whereas at 60° it remained over 80%. Post hoc analysis demonstrated that particularly for failure load, the power was relatively low (26%) to determine if the observed 23 N higher mean obtained in the PRP group was significant. Based on the observed variability, and a subsequent effect size of 0.5 (using the lowest observed SD and the mean difference between groups), we would have needed approximately 28–32 animals to achieve 80% power for detecting whether this relatively small difference was statistically significant.
However, this model has actually fairly low variability within groups compared to the knee laxity differences that would need to be achieved to make primary repair clinically relevant. For example, the standard deviation in AP laxity was 2.2 mm at 30° and 3.3 mm at 60° for the suture repair group, whereas the difference in AP laxity between the suture repair group and the AP laxity at 60° of flexion in a historical group of intact pig ACLs ligaments was 8.9 mm.19 To achieve a clinically significant improvement of 6 mm of laxity (thus bringing the repaired knee to within 3 mm of the intact knee laxity), the effect size would be approximately 6 mm/3 mm or 2.0, and based on repeated-measures ANOVA with knee as the repeated-measures fixed factor, a sample size of five pigs provided 80% probability to detect this magnitude of a difference. Thus, we are confident in concluding that effects this large or larger as a result of adding PRP are unlikely. With the data obtained in this study, we can also now predict that with similar standard deviations in future studies, this model will provide 90% power to detect a difference of 6 mm in AP laxity if six animals are in each group, and 95% power if n =7. Thus, this may be a useful model in future studies given the relatively low standard deviations, likely made possible by the availability of age-, gender- and weight-matched animals.
This is the second study using the porcine complete ACL transection model. In the first study, collagen–PRP hydrogels were used to stimulate repair of the ACL and the results evaluated at 4 weeks after surgery. In that study, significant increases in maximum load and linear stiffness with the addition of a collagen–PRP hydrogel were reported. In this study, using PRP alone without the collagen scaffold, no significant effects using PRP were noted on the same mechanical properties. The maximum load of the suture-only repairs in this study, at 3 months, was almost twice as high as that previously reported at 4 weeks, suggesting some additional gain in strength may occur even in the ACLs repaired with sutures and no collagen or PRP. However, the mean maximum load at 14 weeks for ACLs treated with suture and PRP did not achieve the same value as that previously reported for collagen–PRP-enhanced repairs at 4 weeks (103 ± 27 N).
The immature pig knee has several clinically important similarities with the human condition with regard to ACL injury and healing. There was no significant spontaneous improvement in knee laxity with suture repair over the 3 months, a finding similar to that in humans after ACL repair. In addition, the addition of PRP alone did not significantly improve healing of the ligament. In comparing these results with historic controls, the AP laxity of the knee at 14 weeks after suture repair of the ACL with or without PRP was almost three times higher than that previously reported for intact knees19 (4.9 ± 0.9 mm, mean ± SD; p <0.0001; Table 1), but remained well below that previously reported in the ACL deficient porcine knee (32 mm; n =6; p <0.0001).19 Even with the addition of PRP, there was little evidence in this group of the two ends of the ACL reconnected with a sufficient scar mass. This is consistent with prior reports suggesting that even after acute ACL injury in human patients, the bleeding from the ligament and surrounding tissues is not enough to encapsulate the ends of the ligament in a fibrin clot.10 In this study, it would appear the same is true, and that the fibrin and platelets of the PRP need to be immobilized by a scaffold material within the ACL wound site to be effective as was seen in the prior short-term study in this model using a collagen scaffold to “capture” the platelets in the wound site.7
In conclusion, the skeletally immature Yorkshire pig represents an animal model that is conducive to studies of stimulating ACL repair. The knee is large enough for standard surgical equipment and techniques to be used, these animals are readily available in age-, weight-, and gender-matched groups to minimize heterogeneity. In addition, the biomechanics of the knee and response to suture repair are similar to that of the human knee. Thus, for short-term (<3 month) studies of ACL repair, the skeletally immature pig model is recommended. However, the use of PRP alone as an adjunct to suture repair did not improve ACL healing in this model, and currently there is no translational animal model evidence to support its use. We hypothesize that PRP would need to be combined with a scaffold to stabilize it within the wound site in order for it to be effective.
Acknowledgments
The authors would like to acknowledge Jessica Hootnick, David Spenciner, David Paller, and Ryan Rich for their assistance with this project. In addition, funding was received from NIH Grants AR054099 (M.M.M.) and AR049199 (B.C.F.).
References
- 1.Hefti FL, Kress A, Fasel J, et al. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73:373–383. [PubMed] [Google Scholar]
- 2.Cabaud HE, Rodkey WG, Feagin JA. Experimental studies of acute anterior cruciate ligament injury and repair. Am J Sports Med. 1979;7:18–22. doi: 10.1177/036354657900700105. [DOI] [PubMed] [Google Scholar]
- 3.O’Donoghue DH, Frank GR, Jeter GL, et al. Repair and reconstruction of the anterior cruciate ligament in dogs. Factors influencing long-term results. J Bone Joint Surg Am. 1971;53:710–718. [PubMed] [Google Scholar]
- 4.O’Donoghue DH, Rockwood CA, Jr, Frank GR, et al. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48:503–519. [PubMed] [Google Scholar]
- 5.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 6.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 7.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 8.Hunt P, Scheffler SU, Unterhauser FN, et al. A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch Orthop Trauma Surg. 2005;125:238–248. doi: 10.1007/s00402-004-0643-z. [DOI] [PubMed] [Google Scholar]
- 9.Weiler A, Peters G, Maurer J, et al. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A. two-year study in sheep. Am J Sports Med. 2001;29:751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]
- 10.Murray MM, Martin SD, Martin TL, et al. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Woo SL, Vogrin TM, Abramowitch SD. Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg. 2000;8:364–372. doi: 10.5435/00124635-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Frank C, Amiel D, Akeson WH. Healing of the medial collateral ligament of the knee. A. morphological and biochemical assessment in rabbits. Acta Orthop Scand. 1983;54:917–923. doi: 10.3109/17453678308992934. [DOI] [PubMed] [Google Scholar]
- 13.Spindler KP, Clark SW, Nanney LB, Davidson JM. Expression of collagen and matrix metalloproteinases in ruptured human anterior cruciate ligament: an in situ hybridization study. J Orthop Res. 1996;14:857–861. doi: 10.1002/jor.1100140603. [DOI] [PubMed] [Google Scholar]
- 14.Fufa D, Shealy B, Jacobson M, et al. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson M, Fufa D, Abreu EL, et al. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008;16:370–378. doi: 10.1111/j.1524-475X.2008.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawase T, Okuda K, Wolff LF, et al. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol. 2003;74:858–864. doi: 10.1902/jop.2003.74.6.858. [DOI] [PubMed] [Google Scholar]
- 17.Meaney Murray M, Rice K, Wright RJ, et al. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238–244. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 18.Spenciner D, Greene D, Paiva J, et al. The multidirectional bending properties of the human lumbar intervertebral disc. Spine J. 2006;6:248–257. doi: 10.1016/j.spinee.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Fleming BC, Spindler KP, Carey JL, et al. Can suture repair of acl transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vittinghoff E, Glidden D, Shiboski S, et al. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. New York: Springer; 2005. [Google Scholar]
- 21.Cox C. Fieller’s theorem, the likelihood and the delta method. Biometrics. 1990;46:709–718. [Google Scholar]
- 22.Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. New York: W.H. Freeman; 1995. [Google Scholar]
- 23.Boguszewski DV, Shearn JT, Wagner CT, et al. Effect of anterior translation on total knee force in a porcine model. Stanford, CA: International Society of Tendons and Ligaments (ISLT); 2008. [Google Scholar]
- 24.Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]
- 25.Mueller XM, Tevaearai HT, Jegger D, et al. Are standard human coagulation tests suitable in pigs and calves during extracorporeal circulation? Artif Organs. 2001;25:579–584. doi: 10.1046/j.1525-1594.2001.025007579.x. [DOI] [PubMed] [Google Scholar]